Abstract
In 1995, in the Biochemical Society Transactions, Mundy published the first review on CLIMP-63 (cytoskeleton-linking membrane protein 63) or CKPA4 (cytoskeleton-associated protein 4), initially just p63. Here we review the following 20 years of research on this still mysterious protein. CLIMP-63 is a type II transmembrane protein, the cytosolic domain of which has the capacity to bind microtubules whereas the luminal domain can form homo-oligomeric complexes, not only with neighbouring molecules but also, in trans, with CLIMP-63 molecules on the other side of the endoplasmic reticulum (ER) lumen, thus promoting the formation of ER sheets. CLIMP-63 however also appears to have a life at the cell surface where it acts as a ligand-activated receptor. The still rudimentary information of how CLIMP-63 fulfills these different roles, what these are exactly and how post-translational modifications control them, will be discussed.
Keywords: cytoskeleton-linking membrane protein (CLIMP-63)/ cytoskeleton-associated protein 4 (CKAP4), palmitoyl-acyltransferase 2 (has a DHHC motif) [DHHC2], endoplasmic reticulum (ER) cytoskeleton anchor, nuclear translocation, palmitoylation, plasma membrane receptor, rough endoplasmic reticulum (RER)
Introduction
CLIMP-63 (cytoskeleton-linking membrane protein 63), also known as CKAP4 (cytoskeleton-associated protein 4) was discovered independently by two groups in the early 1990s. Mundy and Warren [1] found CLIMP-63 as the most palmitoylated protein during mitosis. Schweizer et al. [2] identified CLIMP-63 as the antigen of monoclonal antibodies generated against a subcellular fraction enriched in Golgi membranes. Studies by both groups suggested that CLIMP-63 affects endoplasmic reticulum (ER) to Golgi transport and might interfere with coatamer assembly [3]. These findings were however challenged and CLIMP-63 has since been established as an abundant ER protein.
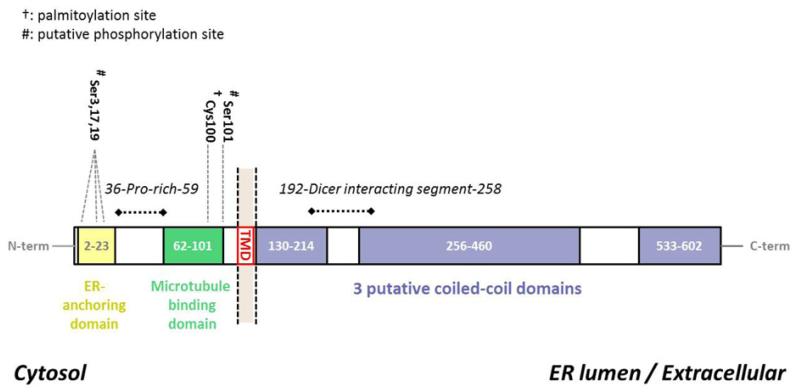
CLIMP-63 is a non-glycosylated, devoid of any N-glycosylation consensus motif [1], type II transmembrane protein present in higher eukaryotes, expressed as a single isoform. No homologues appear to be present in bacteria, yeasts, worms and insects. In human, it has a 106 amino acid cytoplasmic tail, a single transmembrane domain and a large extra-cytoplasmic segment of 474 residues, leading to a total molecular mass of about 63 kDa [2] (Figure 1). The cytosolic tail contains a proline-rich region followed by seven glycine, six serine and eight alanine repeats. Even though repeats may lead to genomic sequencing aberrations, multiple sequence analysis indicates that the cytoplasmic domain is the most variable region of CLIMP-63. As an example, rodent CLIMP-63 lacks a 15 amino acid stretch in the G-S-A repeat region. Software predictions also suggest that the cytosolic domain of CLIMP-63 is highly disordered (IUpred software [4]) whereas its ER luminal domain contains 3–5 coiled coil domains (COILS software [5]).
Figure 1. Topology of the protein CLIMP-63.
CLIMP-63 is a type II single transmembrane protein. In the cytosol, it has an ER-anchoring sequence and a domain interacting with microtubules. In the ER lumen, CLIMP-63 has several coiled-coil domains that are required for its multimerization.
Using immunofluorescence and immunoelectron microscopy, CLIMP-63 was proposed to localize to the ER–Golgi intermediate compartment (ERGIC) [6]. Later it was reassessed by the same group using different antibodies and improved techniques and it was established that CLIMP-63 is resident protein of the rough ER (RER) [7]. The RER indeed consists of membrane sheets and cisternae where ribosomes are anchored, ensuring synthesis of membrane and secreted proteins, whereas the smooth ER (SER) is a meshwork of membrane tubules that extent through the cell cortex [8]. Initial morphological studies on the endogenous protein reported on the absence of CLIMP-63 at the plasma membrane [9]. Previous functional studies however reported a role for CLIMP-63 as a receptor for extracellular ligands indicating that a subpopulation of the protein might reside at the cell surface [10-12].
CLIMP-63 and ER architecture
CLIMP-63 is a very stable, abundant, protein of the ER
As shown in various cell types, CLIMP-63 is an abundant protein, with copy numbers per cell varying from 100000 to 400000 possibly, reflecting the percentage of the cellular volume occupied by the RER [13-15]. Moreover, CLIMP-63 is very stable, with a reported half-life varying from 20 h in human embryonic kidney (HEK)293 [16], 42 h in HeLa and up to 156 h in C2C12 [13] as determined using MS-based approaches or 35S metabolic labelling. Immunofluorescence analysis indicates that despite its abundance, CLIMP-63 preferentially localizes to the perinuclear ER but is excluded of the nuclear envelope [17,18]. This restrictive behaviour might be due to the fact that CLIMP-63 forms large oligomers that cannot diffuse from the RER to the outer nuclear membrane [18]. Localization to the ER, as opposed to other cellular organelles depends on both the luminal and the cytoplasmic domains and even possibly the transmembrane domain as indicated by truncation studies in which removal of any of the domains led to significant mislocalization. A variant of CLIMP-63 lacking the entire cytoplasmic tail was mainly observed at the plasma membrane [9]. In the tail, amino acids 2–23, in particular pairs formed by positively charged amino acid and a glycine (R7-G8, K10-G11, K21-G22) might act as a preponderant motif for folding of the protein and ER localization [9]. Variants of CLIMP-63 lacking the entire luminal segment or only portions of it were also found at the plasma membrane [9,18] or in the ER including the nuclear envelope [18]. Altogether, these studies indicate that multiple regions of the protein are involved in specifying the predominantly perinuclear ER localization.
CLIMP-63 oligomerization and ER morphology
Early on, it was observed that CLIMP-63 has the capacity to form clusters in the ER in a luminal domain-dependent manner [2,9]. Multimerization of the luminal domain is probably responsible for the slow mobility observed for CLIMP-63 in ER membrane, which is in the range of that of ER-bound ribosomes, as determined by FRAP [18]. Indeed a mutant of CLIMP-63 lacking the luminal domain showed increased mobility and was able to diffuse from the RER to the outer nuclear membrane [18].
That CLIMP-63 can multimerize was further supported by sedimentation experiments where cells were solubilized in TX-100 at different pHs and then ultracentrifuged, showing that CLIMP-63 remains insoluble in TX-100 at neutral pH and below [9]. Formation of CLIMP-63 multimers is mediated by its luminal domain since dimers could be observed using sucrose gradient centrifugation followed by non-reducing SDS/PAGE of cells expressing a variant lacking major part of the tail but not the transmembrane domain [2,9]. Moreover, analytical ultra-centrifugation showed that recombinant CLIMP-63 luminal domain forms large complexes probably stabilized by electrostatic interactions as indicated by its high content in charged residues and the sensitivity to the complexes to NaCl [18]. Consistently, far ultraviolet CD (circular dichroism) spectroscopic analysis of its secondary structure content and its thermal unfolding indicated this domain is almost entirely α-helical and adopts a coiled-coil conformation [18]. Analytical ultracentrifugation did not allow determining the stoichiometry of the complex due to the heterogeneity of the sample. The heterogeneity in size, also visible by EM, led the authors to speculate that the luminal domain of CLIMP-63 could potentially assemble both in the parallel and in the antiparallel orientation and that specific proteins in the ER would control the multiplicity/orientation of the oligomeric association [18]. Hauri and co-workers [18] also proposed that ‘the luminal segments of CLIMP-63 molecules of opposing cisternal membranes may bind to each other in an antiparallel or parallel manner … an arrangement that would help to keep ER cisternae flat’.
Consistent with this hypothesis, silencing CLIMP-63 was found to be sufficient to reduce the intraluminal space of ER sheets as shown by EM [19] (Figure 2A). Whereas these observations are consistent with CLIMP-63 playing the role of an ER spacer, it was unfortunately not investigated whether expression of the double luminal CLIMP-63 mutant, which harbours a tandem of luminal domains [18], in CLIMP-63 depleted cells, would lead to ER sheets with increased sheet width. Overexpression of CLIMP-63 was however found to greatly increase the abundance of ER sheets, an effect that could be counter-balanced by co-overexpression of reticulon proteins, which promote membrane bending and ER tubule formation [19,20]. Thus adjusting the relative expression of CLIMP-63 and tubule-promoting ER proteins appears to control the sheet-to-tubule ratio, presumably to control ER function upon demand, i.e. increase in protein secretion compared with lipid synthesis for example.
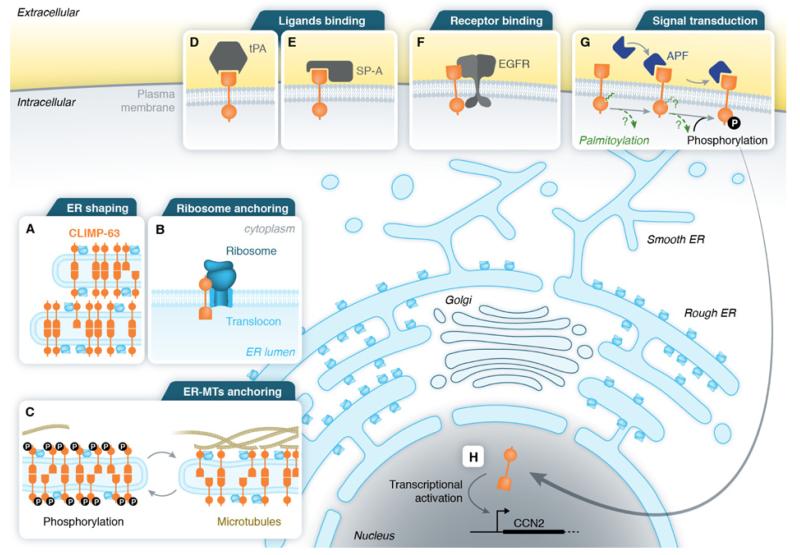
Figure 2. CLIMP-63 cellular functions and interactions.
(A) CLIMP-63 is an abundant RER resident protein and its multimerization maintains the ER sheets thickness. (B) CLIMP-63 is involved in ribosome anchoring in the RER. (C) Non-phosphorylated CLIMP-63 binds to microtubules whereas phosphorylation of the CLIMP-63 tail leads to release of the interaction with microtubules. (D) CLIMP-63 is a receptor for tPA at the plasma membrane. (E) CLIMP-63 is a receptor for SP-A at the plasma membrane in pneumocytes II. (F) CLIMP-63 interacts with EGF-R in absence of EGF. (G) At the plasma membrane, CLIMP-63 acts as a receptor for APF. After binding APF, CLIMP-63 might undergo depalmitoylation, phosphorylation and translocation/trafficking to the nucleus. (H) In the nucleus, CLIMP-63 acts as a transcription factor for the gene CCN2. Translocation/trafficking routes for CLIMP-63 from the ER to the plasma membrane and its possible way back to the ER or into the nucleus remain completely unknown.
CLIMP-63-microtubule interactions
The first proposed function of CLIMP-63 was its ability to bind microtubules, hence its name cytoskeleton-linking membrane protein. Overexpression of wild-type (WT) CLIMP-63 was reported to induce a rearrangement of the ER and microtubule networks with tubular bundles of membranes that co-aligned with microtubules, [7,17,21]. Interestingly, overexpression of a truncate CLIMP-63, lacking the cytosolic domain, still led to ER rearrangement, but left the microtubule network intact [17]. The cytosolic tail of CLIMP-63 alone was able to bind microtubules, promote tubulin polymerization in vitro but not bundling in vivo [17]. The minimal identified sequence necessary for binding microtubules, while conserving ER localization, was found to be residues 62–101 [21]. Interestingly, this domain shares some homology with polycystin-1, also shown to induce ER rearrangement into membrane bundles [22]. Although it is well known that ER membranes undergo massive cytoskeleton-dependent rearrangement during the cell cycle [23,24], it is presently unclear whether CLIMP-63 is involved in this process.
Given the described affect of CLIMP-63 on the architecture of the ER and microtubule networks, its association with microtubules must be regulated. Hauri and co-workers [21] indeed found that microtubule binding was negatively regulated by phosphorylation. Using a variant of CLIMP-63 lacking the last C-terminal luminal coiled-coil domain and 32P–labelling, the authors observed CLIMP-63 phosphorylation, in particular during mitosis. Consistently phosphor-mimicking mutants at positions 3, 17 and 19 were unable to bind microtubules, inducing collapse of the ER around the nucleus [25] (Figure 2C). Ser3, Ser19 and Ser101 are potential protein kinase C (PKC) sites whereas Ser17 could be modified by casein kinase II (CKII) [21]. CLIMP-63 phosphorylation might thus allow rapid ER collapse at the entry of mitosis, bypassing the slow process of microtubule depolymerization.
CLIMP-63 probably neither acts alone nor is the only ER–cytoskeleton anchoring protein. Indeed, the long form of the SNARE protein syntaxin 5 was found to interact with CLIMP-63 and its overexpression induced co-alignment of the ER membranes with microtubules [26]. Similarly, overexpression of the valosin-containing protein/p97-interacting membrane protein (VIMP), which can also interact with CLIMP-63, led to alterations in ER architecture [27]. Finally, in rat hippocampal neurons, a direct interaction between CLIMP-63 and the cytosolic microtubule-binding protein MAP2 has been observed [28] and this interaction was proposed to control the microtubule-dependent positioning and concentration of RER membranes in the somato-dendritic compartment of neurons, the RER being absent from axons. Interestingly, through its effect on ER architecture, CLIMP-63 controls ER export kinetics of synaptic receptors [29]. In particular trafficking of AMPA-type glutamate receptors from the ER to the dendritic plasma membrane is rate-limited by their mobility in the ER, showing a dwell time of several hours in low diffusion and high complexity local ER regions beneath dendritic spines. Upon stimulation by selective agonist of glutamate receptor, PKC activity is triggered. Consistently, expression of a phospho-mimic CLIMP-63 mutant led to increased ER complexity, thus further reducing receptor mobility in the ER. By affecting ER architecture, CLIMP-63 was proposed to influence dendritic branching.
CLIMP-63–translocon interactions
High-resolution native electrophoresis combined MS revealed that CLIMP-63 is recruited early during the assembly of ribosome–translocon complexes [30] (Figure 2B). Moreover, expressing a CLIMP-63 mutant lacking the microtubule-binding domain or silencing CLIMP-63 led to a significant increase in the mobility of the translocon complex [31], which is rather static under control conditions [32]. Association of CLIMP-63 with ER-bound ribosomes is further supported by the observation that treatment of cells with puromycin (which disassembles the polysomes) leads to the re-localization of CLIMP-63 from the RER to the entire ER, with the exception of the nuclear membrane [19].
Unconventional ER-functions of CLIMP-63
CLIMP-63 was recently reported to interact with the ribonuclease dicer [16]. Unexpectedly given the well-documented role of dicer in cytosol [33], the interaction was mapped to the luminal domain of CLIMP-63 [amino acid (aa) 192–258]. The authors proposed that a subpopulation of dicer is imported into the ER, undergoes glycosylation (dicer accumulates in the ER lumen in the absence of CLIMP-63), binds to CLIMP-63 and is secreted with CLIMP-63 into the extracellular environment. The CLIMP-63–dicer complex showed pre-microRNA processing activity. Equally intriguingly, although consistent with the membrane topology, CLIMP-63 was proposed to act as a receptor for mRNAs to specific ER subdomains in a ribosome and translation independent manner [34].
CLIMP-63 at the plasma membrane
CLIMP-63 as a surface receptor
During the last decade, several studies report the presence of CLIMP-63 at the plasma membrane. CLIMP-63 was first identified, in a single report, as a receptor for tissue plasminogen activator (tPA), which increases the activation of cell-associated plasminogen in human vascular smooth muscle cells [10] (Figure 2D).
CLIMP-63 was then proposed to act as a plasma membrane receptor for the surfactant protein A (SP-A) which mediates surfactant turnover (Figure 2E), in rat pneumocytes type II and human A549 cells [11] as reviewed previously [35]. SP-A binds to CLIMP-63 in a calcium-dependent manner, is internalized via clathrin-coated pits, enhances extracellular liposome uptake and inhibits the ATP-stimulated secretion of phosphatidylcholine and surfactant lipids [35]. This function would be reinforced by a positive feedback loop since CLIMP-63 was observed to be enriched at the plasma membrane following SP-A or cAMP treatment, in a phosphoinositide 3-kinase (PI3K)-dependent manner [36].
CLIMP-63 was also found to be a receptor for alginate exopolysaccharides [37]. Treatment with anti-CLIMP-63 monoclonal antibodies or CLIMP-63 RNAi reduced the binding of alginate exopolysaccharides to human airway epithelial cells. Alginate exopolysaccharides are especially secreted by Pseudomonas aeruginosa during lung infection [37]. Since SP-A has an important role for the maintenance of innate immunity in the lung, P. aeruginosa might produce alginate exopolysaccharides to compete with SP-A for CLIMP-63 binding as a strategy to reduce the cellular response and avoid host defences. CLIMP-63 might thus be an interesting drug target to restore surfactant homoeostasis in P. aeruginosa infected patients.
Finally, CLIMP-63 was described as a high affinity receptor for a small sialoglycopeptide called anti-proliferative factor (APF; Figure 2G) [12]. APF is uniquely secreted by bladder epithelial cells of patients with interstitial cystitis disorder where the bladder epithelium undergoes ulceration or is inhibited in its growth. APF peptide sequence has 100% homology to the sixth transmembrane domain of frizzled-8 [38,39]. The APF induced inhibition of Akt (on Ser473 and The308) and GSK3β phosphorylation (Tyr216) and increase in β-catenin phosphorylation was found to depend on CLIMP-63 [40].
CLIMP-63 palmitoylation
CLIMP-63 was found to be a substrate of DHHC2 [palmitoyl-acyltransferase 2 (has a DHHC motif)] palmitoyl-transferase, a putative tumour suppressor [41,42] that localizes to the plasma membrane and in cytosolic vesicles [32,43]. S-Palmitoylation is the only reversible lipid modification and consists of the addition of a 16-carbon fatty acid chain on cytosolic cysteines [44]. In human, palmitoylation is mediated by 23 different multi-spanning integral membrane Palmitoyl-acyl transferases that contain a highly conserved DHHC motif, which catalyse the reaction and at least 3 acyl protein thioesterases which remove the palmitic acid moiety. CLIMP-63 was found to be a DHHC2 target in a MS-based screen after capture of palmitoylated proteins in DHHC2 silencing compared with mock control cells. Palmitoylation of CLIMP-63 occurs on a sole cytoplasmic cysteine (Cys100) [1,3,6,45]. Using immunofluorescence, the authors found that the population of endogenous CLIMP-63 residing at the plasma membrane was redistributed to the peripheral ER after DHHC2 knockdown [41]. In control cells, addition of APF led to the relocalization of CLIMP-63 from the plasma membrane to bright clusters in the nucleus observable by immunofluorescence [46]. This APF-induced CLIMP-63 relocalization neither occurs upon DHHC2 knockdown nor for a palmitoylation deficient CLIMP-63 mutant. In contrast, a phospho-mimic, palmitoylation-deficient CLIMP-63 mutant was uniquely observed in the nucleus [47]. These observations led to the proposal of a scenario where palmitoylation mediates the export of CLIMP-63 to the plasma membrane but upon APF binding, CLIMP-63 is depalmitoylated and phosphorylated leading to translocation to the nucleus (Figures 2G and 2H). How a transmembrane protein like CLIMP-63 can, in its full-length form [48], relocalize to the interior of the nucleus is very mysterious. Once in the nucleus, CLIMP-63 was found to bind DNA through its ectodomain [47,48]. More specifically, it was found to bind the proximal promoter of the CCN2 gene, which acts on cell proliferation, adhesion, migration, differentiation and survival.
DHHC2, which localizes to the plasma membrane, might however not be the only DHHC enzyme capable of modifying CLIMP-63. CLIMP-63 palmitoylation is indeed highly enhanced upon brefeldin-A treatment [1,6,45] and since this drug blocks ER to Golgi transport, palmitoylation can probably also occur in the early secretory pathway. Thus CLIMP-63 might undergo cycles of palmitoylation–depalmitoylation, by different sets of enzymes, as it moves from the ER to the plasma membrane. How palmitoylation of CLIMP-63 is regulated is unclear. Although it is not affected neither by its neighbouring serines (Ser99 and Ser101), nor by the sequence of the transmembrane domain, it appears to be sensitive to the distance between the target cysteine and the membrane (six residues in human CLIMP-63), leading to a decrease in palmitoylation when bringing the cysteine to the lipid head groups [45]. For unclear reasons, these experiments were performed on cytoplasmic truncation mutants of CLIMP-63 and therefore a role of the cytosolic domain cannot be excluded.
What the function CLIMP-63 palmitoylation in the early secretory pathway would be is unclear. It has been reported, in a study on aminoglycoside-induced cytotoxicity [49] that gentamicin induces multimerization of CLIMP-63 in a manner that requires its palmitoylation, suggesting that palmitoylation may regulate CLIMP-63 oligomerization. As a side note, gentamicin also induced association of overexpressed CLIMP-63 with 14-3-3θ, forming a complex that could be involved in gentamicin-induced cytotoxicity.
CLIMP-63 in diseases
CLIMP-63 has been related to various cancer prognoses. Overexpression of CLIMP-63 was observed in the majority of cholangio-cellular carcinoma and inter alia and correlated with distant and lymph node metastasis presences [50]. Prognosis accuracy was improved when considering protein expression levels of both CLIMP-63 and DHHC2 in hepatocellular carcinoma where carcinoma cells presented high amount of CLIMP-63 and low expression level of DHHC2. Monitoring both levels correlated with the overall survival and the cumulative disease recurrence prediction [51]. Finally, CLIMP-63 was shown to inhibit growth and metastasis of hepatocellular carcinoma by binding extracellular growth factor (EGF) receptor (EGF-R) and regulating EGF-R signalling [52]. In particular, expression of CLIMP-63 might reduce SMMC-7721 and MHCC–LM3 cell proliferation. Moreover expression of CLIMP-63 might reduce EGF-R activation, whereas CLIMP-63 knockdown increases EGF-induced EGF-R phosphorylation levels. Again suggestive of a plasma membrane population, upon EGF treatment, CLIMP-63 was found to dissociate from EGF-R [52] (Figure 2F).
Concluding remarks
CLIMP-63 is a stable and abundant transmembrane protein predominantly localized to the RER. There, through antiparallel homo-oligomerization in trans, it appears to act as a spacer that defines the width of ER sheets. On the cytosolic side of the ER, it can bind microtubules and thus anchors the RER to the cytoskeleton. Although already multifunctional in the ER, CLIMP-63 appears to have additional functions at the cell surface where it has been proposed to act as a receptor for different ligands (tPA, SP-A, APF and alginate exopolysaccharides). Moreover, ligand binding was proposed to trigger translocation to the lumen of the nucleus, a scenario that is potentially very interesting but would, in a simplistic view, require either a change in membrane topology of CLIMP-63 or extraction from the membrane. Thus CLIMP-63 appears to have multiple lives, at multiple cellular locations. Understanding the molecular mechanisms that govern and coordinate, in time and space, the function of this mysterious protein constitutes the challenge for the years to come.
Acknowledgments
The authors would like to thank Hans-Peter Hauri for helpful comments and discussions, Sarah Friebe, Maria Eugenia Zaballa and Jérôme Bürgi for critical reading of the manuscript and Laura Symul for generating Figure 2.
Funding
This work was supported by the European Research Council under the European Union’s Seventh Framework Program [grant numbers FP7/2007-2013 and 340260 PalmERa].
Abbreviations
- APF
anti-proliferative factor
- CKPA4
cytoskeleton-associated protein 4
- CLIMP-63
cytoskeleton-linking membrane protein
- DHHC2
palmitoyl-acyltransferase 2 (has a DHHC motif)
- EGF
extracellular growth factor
- EGF-R
EGF receptor
- ER
endoplasmic reticulum
- PKC
protein kinase C
- RER
rough ER
- SP-A
surfactant protein A
- tPA
tissue plasminogen activator
References
- 1.Mundy DI, Warren G. Mitosis and inhibition of intracellular transport stimulate palmitoylation of a 62-kD protein. J. Cell Biol. 1992;116:135–146. doi: 10.1083/jcb.116.1.135. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schweizer A, Ericsson M, Bächi T, Griffiths G, Hauri HP. Characterization of a novel 63 kDa membrane protein. Implications for the organization of the ER-to-Golgi pathway. J. Cell Sci. 1993;104:671–683. doi: 10.1242/jcs.104.3.671. [DOI] [PubMed] [Google Scholar]
- 3.Mundy DI. Protein palmitoylation in membrane trafficking. Biochem. Soc. Trans. 1995;23:572–576. doi: 10.1042/bst0230572. [DOI] [PubMed] [Google Scholar]
- 4.Dosztanyi Z, Csizmok V, Tompa P, Simon I. IUPred: web server for the prediction of intrinsically unstructured regions of proteins based on estimated energy content. Bioinformatics. 2005;21:3433–3434. doi: 10.1093/bioinformatics/bti541. CrossRef. [DOI] [PubMed] [Google Scholar]
- 5.Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. CrossRef. [DOI] [PubMed] [Google Scholar]
- 6.Schweizer A, Rohrer J, Jenö P, DeMaio A, Buchman TG, Hauri HP. A reversibly palmitoylated resident protein (p63) of an ER-Golgi intermediate compartment is related to a circulatory shock resuscitation protein. J. Cell Sci. 1993;104:685–694. doi: 10.1242/jcs.104.3.685. [DOI] [PubMed] [Google Scholar]
- 7.Schweizer A, Rohrer J, Slot JW, Geuze HJ, Kornfeld S. Reassessment of the subcellular localization of p63. J. Cell Sci. 1995;108:2477–2485. doi: 10.1242/jcs.108.6.2477. [DOI] [PubMed] [Google Scholar]
- 8.Goyal U, Blackstone C. Untangling the web: mechanisms underlying ER network formation. Biochim. Biophys. Acta. 2013;1833:2492–2498. doi: 10.1016/j.bbamcr.2013.04.009. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schweizer A, Rohrer J, Hauri HP, Kornfeld S. Retention of p63 in an ER-Golgi intermediate compartment depends on the presence of all three of its domains and on its ability to form oligomers. J. Cell Biol. 1994;126:25–39. doi: 10.1083/jcb.126.1.25. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Razzaq TM, Bass R, Vines DJ, Werner F, Whawell SA, Ellis V. Functional regulation of tissue plasminogen activator on the surface of vascular smooth muscle cells by the type-II transmembrane protein p63 (CKAP4) J. Biol. Chem. 2003;278:42679–42685. doi: 10.1074/jbc.M305695200. CrossRef. [DOI] [PubMed] [Google Scholar]
- 11.Gupta N, Manevich Y, Kazi AS, Tao JQ, Fisher AB, Bates SR. Identification and characterization of p63 (CKAP4/ERGIC-63/CLIMP-63), a surfactant protein A binding protein, on type II pneumocytes. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;291:L436–L446. doi: 10.1152/ajplung.00415.2005. CrossRef. [DOI] [PubMed] [Google Scholar]
- 12.Conrads TP, Tocci GM, Hood BL, Zhang CO, Guo L, Koch KR, Michejda CJ, Veenstra TD, Keay SK. CKAP4/p63 is a receptor for the frizzled-8 protein-related antiproliferative factor from interstitial cystitis patients. J. Biol. Chem. 2006;281:37836–37843. doi: 10.1074/jbc.M604581200. CrossRef. [DOI] [PubMed] [Google Scholar]
- 13.Foster LJ, de Hoog CL, Zhang Y, Zhang Y, Xie X, Mootha VK, Mann M. A mammalian organelle map by protein correlation profiling. Cell. 2006;125:187–199. doi: 10.1016/j.cell.2006.03.022. CrossRef. [DOI] [PubMed] [Google Scholar]
- 14.Nagaraj N, Wisniewski JR, Geiger T, Cox J, Kircher M, Kelso J, Pääbo S, Mann M. Deep proteome and transcriptome mapping of a human cancer cell line. Mol. Syst. Biol. 2011;7:548. doi: 10.1038/msb.2011.81. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck M, Schmidt A, Malmstroem J, Claassen M, Ori A, Szymborska A, Herzog F, Rinner O, Ellenberg J, Aebersold R. The quantitative proteome of a human cell line. Mol. Syst. Biol. 2011;7:549. doi: 10.1038/msb.2011.82. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pepin G, Perron MP, Provost P. Regulation of human Dicer by the resident ER membrane protein CLIMP-63. Nucleic Acids Res. 2012;40:11603–11617. doi: 10.1093/nar/gks903. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klopfenstein DR, Kappeler F, Hauri HP. A novel direct interaction of endoplasmic reticulum with microtubules. EMBO J. 1998;17:6168–6177. doi: 10.1093/emboj/17.21.6168. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klopfenstein DR, Klumperman J, Lustig A, Kammerer RA, Oorschot V, Hauri HP. Subdomain-specific localization of CLIMP-63 (p63) in the endoplasmic reticulum is mediated by its luminal alpha-helical segment. J. Cell Biol. 2001;153:1287–1300. doi: 10.1083/jcb.153.6.1287. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shibata Y, Shemesh T, Prinz WA, Palazzo AF, Kozlov MM, Rapoport TA. Mechanisms determining the morphology of the peripheral ER. Cell. 2010;143:774–788. doi: 10.1016/j.cell.2010.11.007. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006;124:573–586. doi: 10.1016/j.cell.2005.11.047. CrossRef. [DOI] [PubMed] [Google Scholar]
- 21.Vedrenne C, Klopfenstein DR, Hauri HP. Phosphorylation controls CLIMP-63-mediated anchoring of the endoplasmic reticulum to microtubules. Mol. Biol. Cell. 2005;16:1928–1937. doi: 10.1091/mbc.E04-07-0554. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao H, Sellin LK, Pütz M, Nickel C, Imgrund M, Gerke P, Nitschke R, Walz G, Kramer-Zucker AG. A short carboxy-terminal domain of polycystin-1 reorganizes the microtubular network and the endoplasmic reticulum. Exp. Cell Res. 2009;315:1157–1170. doi: 10.1016/j.yexcr.2009.01.027. CrossRef. [DOI] [PubMed] [Google Scholar]
- 23.Friedman JR, Webster BM, Mastronarde DN, Verhey KJ, Voeltz GK. ER sliding dynamics and ER-mitochondrial contacts occur on acetylated microtubules. J. Cell Biol. 2010;190:363–375. doi: 10.1083/jcb.200911024. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu L, Ladinsky MS, Kirchhausen T. Formation of the postmitotic nuclear envelope from extended ER cisternae precedes nuclear pore assembly. J. Cell Biol. 2011;194:425–440. doi: 10.1083/jcb.201012063. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vedrenne C, Hauri HP. Morphogenesis of the endoplasmic reticulum: beyond active membrane expansion. Traffic. 2006;7:639–646. doi: 10.1111/j.1600-0854.2006.00419.x. CrossRef. [DOI] [PubMed] [Google Scholar]
- 26.Miyazaki K, Wakana Y, Noda C, Arasaki K, Furuno A, Tagaya M. Contribution of the long form of syntaxin 5 to the organization of the endoplasmic reticulum. J. Cell Sci. 2012;125:5658–5666. doi: 10.1242/jcs.105304. CrossRef. [DOI] [PubMed] [Google Scholar]
- 27.Noda C, Kimura H, Arasaki K, Matsushita M, Yamamoto A, Wakana Y, Inoue H, Tagaya M. Valosin-containing protein-interacting membrane protein (VIMP) links the endoplasmic reticulum with microtubules in concert with cytoskeleton-linking membrane protein (CLIMP)-63. J. Biol. Chem. 2014;289:24304–24314. doi: 10.1074/jbc.M114.571372. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farah CA, Liazoghli D, Perreault S, Desjardins M, Guimont A, Anton A, Lauzon M, Kreibich G, Paiement J, Leclerc N. Interaction of microtubule-associated protein-2 and p63: a new link between microtubules and rough endoplasmic reticulum membranes in neurons. J. Biol. Chem. 2005;280:9439–9449. doi: 10.1074/jbc.M412304200. CrossRef. [DOI] [PubMed] [Google Scholar]
- 29.Cui-Wang T, Hanus C, Cui T, Helton T, Bourne J, Watson D, Harris KM, Ehlers MD. Local zones of endoplasmic reticulum complexity confine cargo in neuronal dendrites. Cell. 2012;148:309–321. doi: 10.1016/j.cell.2011.11.056. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dejgaard K, Theberge JF, Heath-Engel H, Chevet E, Tremblay ML, Thomas DY. Organization of the Sec61 translocon, studied by high resolution native electrophoresis. J. Proteome Res. 2010;9:1763–1771. doi: 10.1021/pr900900x. CrossRef. [DOI] [PubMed] [Google Scholar]
- 31.Nikonov AV, Hauri HP, Lauring B, Kreibich G. Climp-63-mediated binding of microtubules to the ER affects the lateral mobility of translocon complexes. J. Cell Sci. 2007;120:2248–2258. doi: 10.1242/jcs.008979. CrossRef. [DOI] [PubMed] [Google Scholar]
- 32.Greaves J, Carmichael JA, Chamberlain LH. The palmitoyl transferase DHHC2 targets a dynamic membrane cycling pathway: regulation by a C-terminal domain. Mol. Biol. Cell. 2011;22:1887–1895. doi: 10.1091/mbc.E10-11-0924. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paddison PJ. RNA interference in mammalian cell systems. Curr. Top Microbiol. Immunol. 2008;320:1–19. doi: 10.1007/978-3-540-75157-1_1. [DOI] [PubMed] [Google Scholar]
- 34.Cui XA, Zhang H, Palazzo AF. p180 promotes the ribosome-independent localization of a subset of mRNA to the endoplasmic reticulum. PLoS Biol. 2012;10:e1001336. doi: 10.1371/journal.pbio.1001336. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bates SR. P63 (CKAP4) as an SP-A receptor: implications for surfactant turnover. Cell Physiol. Biochem. 2010;25:41–54. doi: 10.1159/000272062. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kazi AS, Tao JQ, Feinstein SI, Zhang L, Fisher AB, Bates SR. Role of the PI3-kinase signaling pathway in trafficking of the surfactant protein A receptor P63 (CKAP4) on type II pneumocytes. Am. J. Physiol. Lung Cell Mol. Physiol. 2010;299:L794–L807. doi: 10.1152/ajplung.00372.2009. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barbier M, Martínez-Ramos I, Townsend P, Albertí S. Surfactant protein A blocks recognition of Pseudomonas aeruginosa by CKAP4/P63 on airway epithelial cells. J. Infect. Dis. 2012;206:1753–1762. doi: 10.1093/infdis/jis587. CrossRef. [DOI] [PubMed] [Google Scholar]
- 38.Keay SK, Szekely Z, Conrads TP, Veenstra TD, Barchi JJ, Jr, Zhang CO, Koch KR, Michejda CJ. An antiproliferative factor from interstitial cystitis patients is a frizzled 8 protein-related sialoglycopeptide. Proc. Natl. Acad. Sci. U.S.A. 2004;101:11803–11808. doi: 10.1073/pnas.0404509101. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim J, Freeman MR. Antiproliferative factor signaling and interstitial cystitis/painful bladder syndrome. Int. Neurourol. J. 2011;15:184–191. doi: 10.5213/inj.2011.15.4.184. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shahjee HM, Koch KR, Guo L, Zhang CO, Keay SK. Antiproliferative factor decreases Akt phosphorylation and alters gene expression via CKAP4 in T24 bladder carcinoma cells. J. Exp. Clin. Cancer Res. 2010;29:160. doi: 10.1186/1756-9966-29-160. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J, Planey SL, Ceballos C, Stevens SM, Jr, Keay SK, Zacharias DA. Identification of CKAP4/p63 as a major substrate of the palmitoyl acyltransferase DHHC2, a putative tumor suppressor, using a novel proteomics method. Mol. Cell Proteomics. 2008;7:1378–1388. doi: 10.1074/mcp.M800069-MCP200. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oyama T, Miyoshi Y, Koyama K, Nakagawa H, Yamori T, Ito T, Matsuda H, Arakawa H, Nakamura Y. Isolation of a novel gene on 8p21.3-22 whose expression is reduced significantly in human colorectal cancers with liver metastasis. Genes Chromosomes Cancer. 2000;29:9–15. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1001>3.0.co;2-#. CrossRef. [DOI] [PubMed] [Google Scholar]
- 43.Fukata Y, Dimitrov A, Boncompain G, Vielemeyer O, Perez F, Fukata M. Local palmitoylation cycles define activity-regulated postsynaptic subdomains. J. Cell Biol. 2013;202:145–161. doi: 10.1083/jcb.201302071. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jia L, Linder ME, Blumer KJ. Gi/o signaling and the palmitoyltransferase DHHC2 regulate palmitate cycling and shuttling of RGS7 family-binding protein. J. Biol. Chem. 2011;286:13695–13703. doi: 10.1074/jbc.M110.193763. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schweizer A, Rohrer J, Kornfeld S. Determination of the structural requirements for palmitoylation of p63. J. Biol. Chem. 1995;270:9638–9644. doi: 10.1074/jbc.270.16.9638. CrossRef. [DOI] [PubMed] [Google Scholar]
- 46.Planey SL, Keay SK, Zhang CO, Zacharias DA. Palmitoylation of cytoskeleton associated protein 4 by DHHC2 regulates antiproliferative factor-mediated signaling. Mol. Biol. Cell. 2009;20:1454–1463. doi: 10.1091/mbc.E08-08-0849. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zacharias DA, Mullen M, Planey SL. Antiproliferative factor-induced changes in phosphorylation and palmitoylation of cytoskeleton-associated protein-4 regulate its nuclear translocation and DNA binding. Int. J. Cell Biol. 2012;2012:150918. doi: 10.1155/2012/150918. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matika CA, Wasilewski M, Arnott JA, Planey SL. Antiproliferative factor regulates connective tissue growth factor (CTGF/CCN2) expression in T24 bladder carcinoma cells. Mol. Biol. Cell. 2012;23:1976–1985. doi: 10.1091/mbc.E11-08-0714. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karasawa T, Wang Q, David LL, Steyger PS. CLIMP-63 is a gentamicin-binding protein that is involved in drug-induced cytotoxicity. Cell Death Dis. 2010;1:e102. doi: 10.1038/cddis.2010.80. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li MH, Dong LW, Li SX, Tang GS, Pan YF, Zhang J, Wang H, Zhou HB, Tan YX, Hu HP, Wang HY. Expression of cytoskeleton-associated protein 4 is related to lymphatic metastasis and indicates prognosis of intrahepatic cholangiocarcinoma patients after surgery resection. Cancer Lett. 2013;337:248–253. doi: 10.1016/j.canlet.2013.05.003. CrossRef. [DOI] [PubMed] [Google Scholar]
- 51.Li SX, Tang GS, Zhou DX, Pan YF, Tan YX, Zhang J, Zhang B, Ding ZW, Liu LJ, Jiang TY, et al. Prognostic significance of cytoskeleton-associated membrane protein 4 and its palmitoyl acyltransferase DHHC2 in hepatocellular carcinoma. Cancer. 2014;120:1520–1531. doi: 10.1002/cncr.28593. CrossRef. [DOI] [PubMed] [Google Scholar]
- 52.Li SX, Liu LJ, Dong LW, Shi HG, Pan YF, Tan YX, Zhang J, Zhang B, Ding ZW, Jiang TY, et al. CKAP4 inhibited growth and metastasis of hepatocellular carcinoma through regulating EGFR signaling. Tumour Biol. 2014;35:7999–8005. doi: 10.1007/s13277-014-2000-3. CrossRef. [DOI] [PubMed] [Google Scholar]