Abstract
Purpose
Retinal pigment epithelium (RPE) expresses GPR109A, a receptor for the vitamin niacin and the ketone body β-hydroxybutyrate (β-HB). Because diabetes results in elevated levels of β-HB, here we studied expression of the receptor in diabetic retina. We also investigated its functional relevance in RPE.
Methods
Retinal expression of GPR109A in diabetic mice and postmortem human eyes was evaluated by quantitative PCR (qPCR). ARPE-19 cells and primary wild-type and Gpr109a−/− mouse RPE cells were exposed to TNF-α in the presence or absence of niacin or β-HB, followed by analysis of IL-6 and Ccl2 expression via real-time qPCR and ELISA.
Results
GPR109A expression was increased in diabetic mouse and human retina. TNF-α increased the expression and secretion of IL-6 and Ccl2 in ARPE-19 cells. Niacin and β-HB suppressed these effects, implicating GPR109A as the target responsible for mediation of the observed effects. Primary RPE cells from wild-type mice behaved similarly. In contrast, GPR109A ligands failed to suppress TNF-α–induced expression and secretion of IL-6 and Ccl2 in primary RPE cells from Gpr109a−/− mice, confirming that the observed anti-inflammatory effects were mediated specifically by Gpr109a.
Conclusions
GPR109A plays an anti-inflammatory role in RPE and its expression is upregulated in diabetes. Inflammation is a key causative factor in the pathogenesis of diabetic retinopathy. We speculate that the increased expression of GPR109A and elevation of its ligand β-HB in diabetes are mechanisms by which the tissue attempts to fight inflammation in this disease. Pharmacological activation of GPR109A may therefore have therapeutic potential in clinical management of diabetic retinopathy.
GPR109A, a G-protein coupled receptor for β-hydroxybutyrate, plays an anti-inflammatory role in RPE and its expression is upregulated in diabetes in mice and humans.
Introduction
GPR109A is the G-protein–coupled receptor responsible for mediating the antilipolytic actions of niacin (nicotinic acid), a B-complex vitamin and also a drug used widely to lower blood lipid levels.1 β-hydroxybutyrate (β-HB) is the physiologic ligand for this receptor.2 GPR109A expression was initially thought to be limited to adipocytes, the cell type in which its antilipolytic functions are most warranted, and immune cells.3–5 Recent reports, however, have described expression of the receptor in a number of other cell types, including hepatocytes6 and epithelial cells of the small intestine and colon.7,8 In addition, we demonstrated GPR109A expression in the retinal pigment epithelium (RPE), localized specifically to the basolateral membrane.9 Although GPR109A is most noted functionally for its antilipolytic effects in adipocytes, recent studies suggest that activation of the receptor also is associated with novel immunomodulatory responses.10–12 We have characterized expression of GPR109A in RPE; however, the functional significance of receptor expression in this cell type remains unknown.
RPE performs a number of vital functions crucial to the overall health of the retina.13 Additionally, it is known to play a critical role in regulation of the immune/inflammatory response in retina by producing and secreting a number of proinflammatory cytokines, as well as a few counteractive immunosuppressive molecules.14 Hence, the regulatory role played by RPE in modulation of the immune response in retina is essential under normal physiologic conditions, and becomes increasingly important in degenerative retinal diseases, such as age-related macular degeneration and diabetic retinopathy, in which chronic, subclinical inflammation is believed to play a major causative role.15,16 Interestingly, however, whether GPR109A expressed in RPE might play a role in regulation of the inflammatory response mounted by these cells has not been studied. The possibility that the RPE-specific expression of GPR109A may be capable of directly influencing the inflammatory environment in retina has tremendous implications in terms of the development of novel therapeutics aimed at protecting this tissue against damage induced by inflammation under a broad spectrum of pathological conditions. This is particularly relevant to diabetic retinopathy, because uncontrolled diabetes is associated with elevated levels of β-HB, an agonist for the receptor.
The purpose of the present study was to investigate the effect of diabetes on GPR109A expression and the functional role of the receptor in RPE. For GPR109A to be useful as a therapeutic target for combating inflammation in retina, particularly under diabetic conditions, two critical factors must be shown to be true: preservation of receptor expression in retina under diabetic conditions and an anti-inflammatory role of this receptor in this tissue. Here we show, using two different mouse models of type 1 diabetes, the streptozotocin-induced diabetic mouse and the Ins2Akita/+ (Akita) mouse, that RPE-specific expression of GPR109A not only remains intact under diabetic conditions but is actually upregulated in diabetic retina. This is also true in a mouse model of type 2 diabetes (db/db mouse). These findings were corroborated by analysis of GPR109A expression in postmortem human eyes obtained from nondiabetic and diabetic patients. Additionally, we show that activation of GPR109A in cultured RPE cells by its agonists, nicotinic acid and β-HB, suppresses TNF-α–induced production of the proinflammatory cytokines IL-6 and Ccl2, thus demonstrating an anti-inflammatory role for this receptor in retina. Collectively, these data provide compelling support for future studies to explore the role of GPR109A in diabetes and its potential use as a therapeutic target for combating retinal inflammation.
Materials and Methods
Materials
ARPE-19 cells, a human retinal pigment epithelial cell line, were obtained from American Type Culture Collection (Manassas, VA). All cell culture media and secondary antibodies for immunofluorescence studies were from Invitrogen (Grand Island, NY). Preparation of the rabbit polyclonal anti-GPR109A antibody has been described.9 Mouse and human TNF-α and ELISA assay kits for human IL-6, human chemokine (C-C motif) ligand 2 (Ccl2), mouse IL-6, and mouse Ccl2 were from R & D Systems (Minneapolis, MN). Pertussis toxin (PTX) was from EMD Chemicals (Gibbstown, NJ). Reagents for RT-PCR and quantitative real-time PCR were obtained from Applied Biosystems (Foster City, CA).
Animals
Gpr109a−/− mice have been described previously.8 Breeding pairs of Gpr109a+/− mice were used such that Gpr109a+/+ and Gpr109a−/− mice were obtained from the same litter. The genotypes of the animals used in the study were confirmed by PCR using specific primer pairs.17 Nuclear factor (NF)-κB transgenic mice have also been described previously.7 Normal male C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and used for induction of diabetes using streptozotocin per our previously published method.18 Ins2Akita/+ (Akita) mice also were purchased from the Jackson Laboratory. Akita mice were bred and genotyped following our previously published protocol.19 Female Akita mice were excluded from this study. Male BKS.C g-m +/+Leprdb/J (db/db) mice, aged 12 weeks, and age-matched wild-type C57BLKS/J mice were also obtained from Jackson Laboratory. Histologic processing of mouse eyes was performed following our previously published protocol.18 The care and use of all animals adhered to institutional guidelines for the humane treatment of animals and to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Postmortem Retina Specimens
Human retina samples from anonymous donors were obtained from the Georgia Eye Bank (Atlanta, GA). The following selection criteria, adapted from that of Barber et al.,20 were applied: adults younger than 75 years, with insulin-requiring diabetes or no diabetes (control), no life-support measures, and no chemotherapy. All eyes were enucleated 8 hours or less after time of death; retina samples were then obtained and frozen at −80°C.
Cell Culture
ARPE-19 cells were maintained as described previously.9 RPE cells (mRPE) were isolated from 3-week-old Gpr109a+/+, Gpr109a−/−, and NF-κB mice for establishment of primary cultures per our published protocol.9 mRPE cells were maintained in culture as described previously.9 Passage-2 mRPE cells were used for all experiments.
Generation of a GPR109A-Overexpressing ARPE-19 Cell Line
Human GPR109A was subcloned from GPR109A-pCMV-SPORT6 (cDNA clone MGC:65,083 IMAGE:5,747,743) into pCDH-CMV-MCS-EF1-Puro vector. Recombinant lentivirus was produced by cotransfection into 293FT cells with GPR109A-pCDH and control pCDH vector and three other helper vectors (ViraPower Lentiviral Expression System, Invitrogen), using Lipofectamine 2000 transfection reagent. Lentiviral supernatants were harvested at 72 hours after transfection and filtered through a 0.45-μm membrane. ARPE-19 cells were then infected with lentivirus for 24 hours. The cells were selected for puromycin resistance (5 μg/mL) for several weeks, and maintained in medium containing 0.5 μg/mL puromycin. Stable overexpression of GPR109A was confirmed by RT-PCR, fluorescence-activated cell sorting, and 3H-nicotinate (NA) binding assay. cAMP assay was used to confirm functional activity of GPR109A expressed in these cells following our previously published method.9
Cell Treatments
To determine the effect of GPR109A activation on the expression and secretion of the proinflammatory cytokines IL-6 and Ccl2, ARPE-19 cells were seeded at a density of 0.2 × 106 cells/well in 6-well culture plates. Twenty-four hours after seeding, cells were pretreated with NA (1 mM) or β-HB (5 mM) for 1 hour, followed by exposure to TNF-α (10 ng/mL) for 24 hours. Nicotinate and β-HB were present also during TNF-α treatment. mRPE cells isolated from wild-type and Gpr109a−/− mouse retinas were treated identically as described above. For experiments in which PTX was used, we followed a method very similar to that of Digby et al.10 In brief, ARPE-19 cells were incubated with PTX (200 ng/mL) for 18 hours, followed by treatment with TNF-α (10 ng/mL) with or without NA (1 mM) or β-HB (5 mM) for 4 hours. Following cell treatments, the culture medium was collected and used for ELISA. Cells were harvested and total RNA prepared for use in RT-PCR and qPCR experiments.
RT-PCR and Real-Time qPCR
qPCR was used to quantify the steady-state levels of GPR109A mRNA in retina in streptozotocin-induced diabetic mice, Akita mice, and db/db mice at various time intervals of postonset diabetes (n = 6 per group). Age-matched, nondiabetic mice of similar genetic background served as controls for each group (n = 6 per group). qPCR was used also to evaluate the expression levels of GPR109A in postmortem human retina samples. Following genotyping and isolation of mRPE from wild-type and Gpr109a−/− mice, RT-PCR was carried out under optimal conditions using primers specific for mouse GPR109A.9 The molecular identity of the PCR products was confirmed by sequencing. Real-time qPCR was used to monitor steady-state levels of GPR109A, IL-6, and Ccl2 mRNAs in ARPE-19 and mRPE cell cultures, as well as in whole eyes obtained from wild-type and Gpr109a−/− mice. The sequences of the primers used in these reactions are shown in Table 1. Hypoxanthine phosphoribosyltransferase 1, 18S, or glyceraldehyde phosphate dehydrogenase was used as the internal control for PCR reactions. Each RT-PCR experiment was repeated at least three times. Real-time qPCR amplifications, using detection chemistry (SYBR Green; Applied Biosystems), were run in triplicate on 96-well reaction plates.
Table 1.
Primer Sequences Used for qPCR
|
Gene |
Primer Sequence |
Expected Product Size |
| Human GPR109A | Fwd: TGCCGCCCTTCCTGATGGACA | 177 |
| Rev: TGTTCAGGGCGTGGTGGGGA | ||
| Human IL-6 | Fwd: AACCTGAACCTTCCAAAGATGG | 159 |
| Rev: TCTGGCTTGTTCCTCACTACT | ||
| Human Ccl2 | Fwd: TCGCTCAGCCAGATGCAATCAATG | 195 |
| Rev: TGGAATCCTGAACCCACTTCTGCT | ||
| Mouse GPR109A | Fwd: GGCGTGGTGCAGTGAGCAGT | 232 |
| Rev: GGCCCACGGACAGGCTAGGT | ||
| Mouse IL-6 | Fwd: CTACCCCAATTTCCAATGCT | 187 |
| Rev: ACCACAGTGAGGAATGTCCA | ||
| Mouse Ccl2 | Fwd: TGCTACTCATTCACCAGCAA | 152 |
| Rev: GTCTGGACCCATTCCTTCTT | ||
| GAPDH | Fwd: AGCCTCCCGCTTCGCTCTCT | 140 |
| Rev: CCAGGCGCCCAATACGACCA |
IL-6 and Ccl2 ELISA
The secretion of IL-6 and Ccl2 proteins into the culture medium by ARPE-19 or mRPE cells was analyzed using commercially available ELISA assay kits (R & D Systems) for human and mouse IL-6 and Ccl2, respectively, following the manufacturer's directions. ELISA plates were read spectrophotometrically at 450 nm using a SpectraMAX 190 plate reader (Molecular Devices, Sunnyvale, CA).
Immunofluorescence
Immunofluorescence techniques were used to localize GPR109A protein in mRPE cells as described previously.9
NF-κB–Luciferase Reporter Assay
NF-κB–luciferase reporter assay was done using mRPE cells prepared from mice carrying the NF-κB–luciferase transgene under the control of the β-actin promoter following our previously described method.8 In brief, mRPE from NF-κB transgenic mice were pretreated with NA (1 mM) or β-HB (5 mM) for 30 minutes, followed by exposure to TNF-α (10 ng/mL). Nicotinate and β-HB were present also during TNF-α treatment. Total treatment time was 4 hours. Cells were then collected, and the lysates used for measurement of luciferase activity.
Data Analysis
All experiments were repeated two to four times with independent cell preparations. qPCR and ELISA measurements were made in duplicate in each experiment. Data are presented as means ± SD. Statistical significance was determined by Student's t-test.
Results
Gpr109a Expression in Diabetic Retina
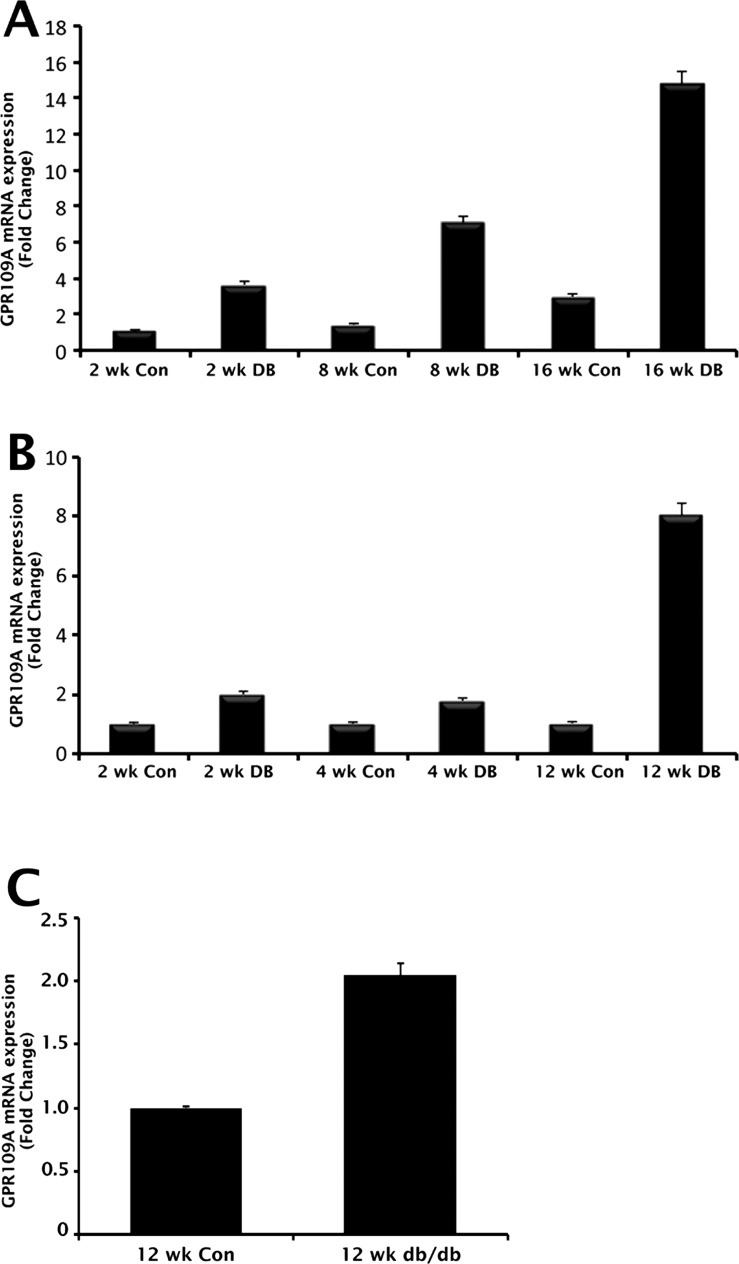
β-HB is the first, and at present, only endogenous ligand to be identified for GPR109A in noncolonic tissues.2 We have shown that butyrate, produced by bacterial fermentation of dietary fiber, serves as the principal physiologic ligand for the receptor in the colon7; however, butyrate is not likely to be physiologically relevant to retina. Even though β-HB is the likely physiologic GPR109A agonist in retina, the levels of this ligand in circulation are too low to activate the receptor under normal conditions. Under normal fed state, the circulating levels of β-HB are in the range of 50 to 100 μM, whereas the effective dose necessary for half-maximal activation of the receptor is approximately 700 μM.2 The blood levels of this metabolite increase considerably during starvation and also in individuals who are on ketogenic diets to concentrations sufficient to activate the receptor. Uncontrolled diabetes is associated with ketoacidosis, a clinical condition in which circulating levels of β-HB may reach millimolar concentrations, enough to activate the receptor maximally. As such, we are keenly interested in understanding how the receptor is regulated in diabetes. Therefore, we examined the temporal pattern of GPR109A expression in retinal samples obtained from two commonly used rodent models of type 1 diabetes, the streptozotocin-induced diabetic mouse and the Ins2Akita/+ (Akita) mouse, by qPCR. Nondiabetic animals of comparable age served as controls. Before animals were killed, body weight and blood glucose measurements were obtained. We reported previously on mean body weight and blood glucose levels in streptozotocin-induced diabetic and Akita animals at various intervals of time postonset of diabetes.18,19 Body weight and blood glucose measurements obtained from the animals used in the present study did not differ significantly from those previously published values (data not shown). Gpr109a mRNA expression was increased in samples of retina obtained from streptozotocin-induced diabetic animals in comparison with those obtained from nondiabetic, age-matched control animals at all time points tested (Fig. 1A). Significant increases in Gpr109a expression (approximately 4-fold) were observed in diabetic animals as early as 2 weeks postonset of diabetes. The mRNA expression continued to increase exponentially with the duration/severity of diabetes in these animals, such that by 16-weeks postonset of diabetes, Gpr109a mRNA expression was approximately 10-fold higher in diabetic animals compared with corresponding age-matched, nondiabetic controls. Similar increases in Gpr109a expression were observed in diabetic Akita animals (Fig. 1B). Early diabetes (2–4 weeks postonset) in Akita mice was associated with an approximately 2-fold increase in Gpr109a expression. By 12 weeks postonset of diabetes, the increase in Gpr109a expression in diabetic Akita animals was approximately 9-fold higher than in age-matched, nondiabetic control animals.
Figure 1.
Upregulation of Gpr109a expression in diabetic mouse retina. qPCR was used to evaluate Gpr109a mRNA expression in retinas obtained from (A) streptozotocin (STZ)-induced diabetic mice, (B) Ins2Akita/+ (Akita) diabetic mice, and (C) db/db diabetic mice at various intervals of time postonset of diabetes. Age-matched, nondiabetic mice of appropriate genetic background were used as controls.
Diabetic ketoacidosis is traditionally considered a key clinical feature of type 1 (insulin-dependent) diabetes mellitus. Although less common, ketoacidosis can occur in patients with type 2 diabetes.21,22 Hence, type 2 diabetics may also be faced with elevated circulating levels of β-HB. Therefore, we examined Gpr109a expression in RNA samples obtained from the eyes of db/db mice, a widely used mouse model of insulin resistance, a key feature of type 2 diabetes. Age-matched, nondiabetic mice of similar genetic background were used as controls. Mean blood glucose level of db/db mice was 369 ± 42 mg/dL; the corresponding value in control mice was 95 ± 5 mg/dL. qPCR analysis of Gpr109a mRNA levels revealed an approximately 2-fold increase in db/db mouse retina (Fig. 1C).
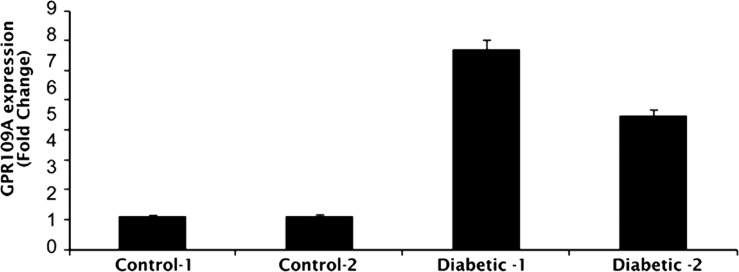
To determine whether similar increases in GPR109A expression occur in diabetes in human patients, qPCR was used to study GPR109A mRNA expression in postmortem retina samples obtained from human patients. Table 2 summarizes the clinical details of each donor. GPR109A expression was increased 4- to 6-fold in retinas obtained from diabetic donors (Fig. 2), supporting the data obtained using diabetic animals; however, as the number of human donor retina samples available for use in these studies was limited, studies with additional samples are needed to confirm the present finding and establish statistical relevance.
Table 2.
Clinical Characteristics of Human Eye Donors
|
Subjects |
Age (y) |
Sex |
Race |
Diabetes Duration (y) |
Cause of Death |
| Control 1 | 64 | M | Caucasian | — | MVA/ABI |
| Control 2 | 74 | F | Caucasian | — | RF |
| Diabetic 1 | 58 | M | Caucasian | 30 | MI |
| Diabetic 2 | 62 | M | Caucasian | 14 | CVD |
ABI, anoxic brain injury; MVA, motor vehicle accident; RF, respiratory failure; MI, myocardial infarction; CVD, cardiovascular disease.
Figure 2.
Upregulation of GPR109A expression in postmortem human retina. qPCR was used to evaluate GPR109A expression in postmortem human retina samples from donors with insulin-dependent diabetes mellitus. Retina samples obtained from nondiabetic donors of similar age served as controls.
Nicotinate and β-HB Suppress IL-6 and Ccl2 Expression/Secretion in ARPE-19 Cells Exposed to TNF-α
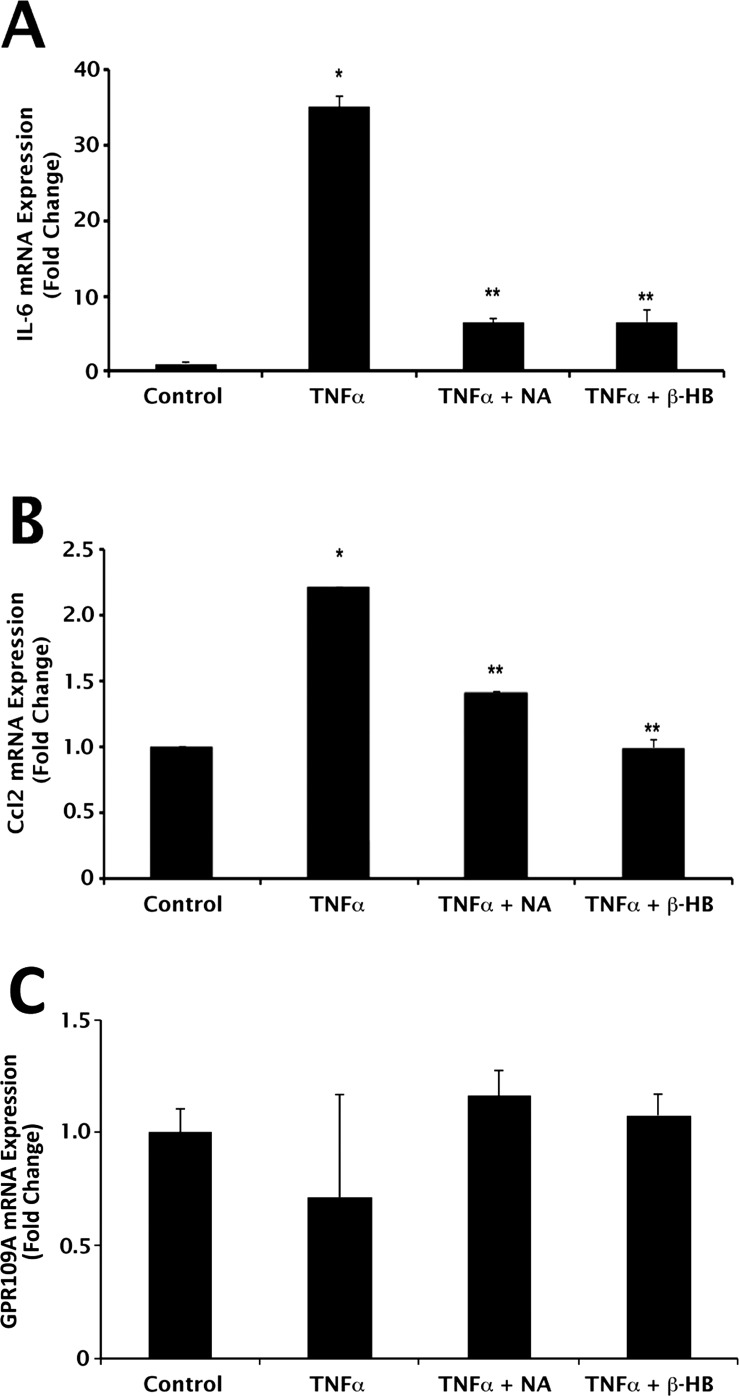
GPR109A activation elicits direct anti-inflammatory effects in some cell types.10,11 Whether the same is true for GPR109A expressed in RPE, we do not know. RPE is known to express and secrete the proinflammatory cytokines IL-6 and Ccl2.14 To determine the effects of the GPR109A ligands NA and β-HB on gene expression and secretion of IL-6 and Ccl2 in cultured human RPE cells, ARPE-19 cells were exposed to TNF-α (10 ng/mL) with or without the addition of NA (1 mM) or β-HB (5 mM), followed by qPCR and ELISA analyses of IL-6 and Ccl2 mRNA and protein expression. Incubation times and concentrations of TNF-α, NA, and β-HB used in this study were determined empirically in preliminary experiments (data not shown). Exposure of cells to TNF-α resulted in significant increases in IL-6 (35.0 ± 1.4-fold) and Ccl2 (2.2 ± 0.002-fold) mRNA expression (Figs. 3A, 3B). Inclusion of NA in the culture medium attenuated significantly the ability of TNF-α to induce the expression of IL-6 and Ccl2 (82% and 36% decrease, respectively). A similar effect on IL-6 and Ccl2 expression was observed in the presence of β-HB (81% and 45% decrease, respectively. qPCR was used also to determine whether cell treatments had any effect on steady-state expression of GPR109A mRNA transcripts. GPR109A expression was not altered by exposure of cells to TNF-α, NA, or β-HB (Fig. 3C).
Figure 3.
Suppression of TNF-α–induced proinflammatory cytokine expression by NA and β-HB at mRNA level. qPCR analysis of (A) IL-6, (B) Ccl2, and (C) GPR109A mRNA expression in ARPE-19 cells exposed to TNF-α (10 ng/mL; 24-hour incubation) in the presence or absence of nicotinate (1 mM) or β-HB (5 mM). *P < 0.01 compared with control cells; **P < 0.01 compared with TNF-α–treated cells.
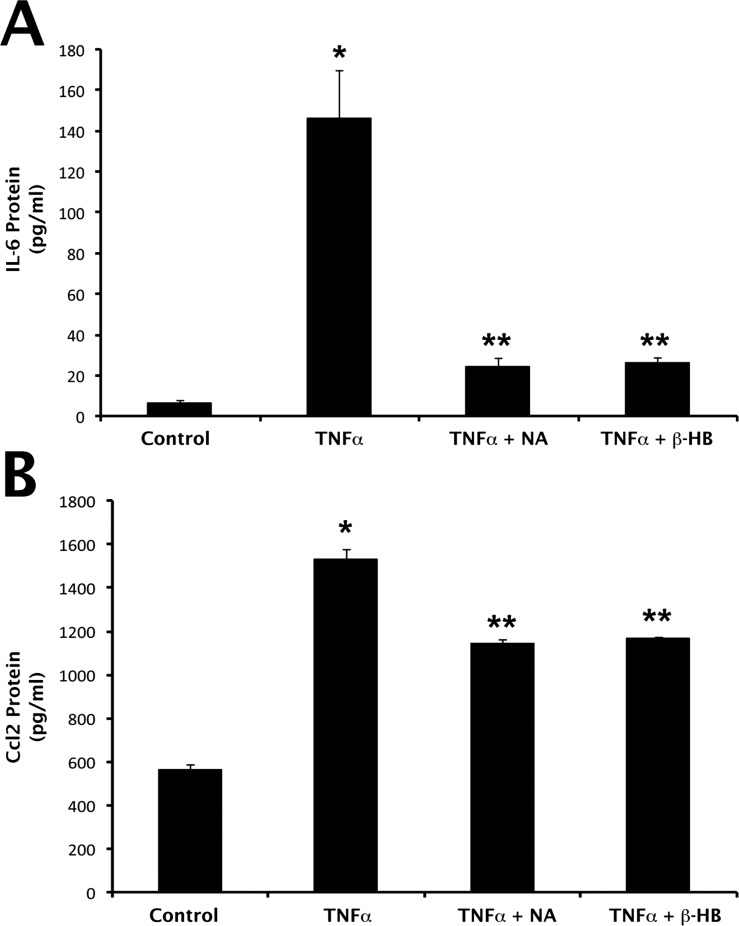
To determine whether the changes in IL-6 and Ccl2 mRNA expression in ARPE-19 cells exposed to TNF-α in the presence or absence of NA or β-HB were associated with corresponding alterations in IL-6 and Ccl2 protein secretion, culture medium was collected from cells following treatment and used for analysis of IL-6 and Ccl2 protein by ELISA. IL-6 secretion in control, untreated cells was 6.25 ± 1.3 pg/mL (Fig. 4A). This baseline value increased to 145.8 ± 23.6 pg/mL in the presence of TNF-α, which corresponds to a 23-fold increase. The TNF-α–induced increase in IL-6 secretion was attenuated significantly in the presence of NA (83% inhibition) and β-HB (82% inhibition). Exposure of cells to TNF-α increased Ccl2 protein secretion significantly (2.8-fold) (Fig. 4B). The increase in Ccl2 protein secretion induced by TNF-α was significantly reduced in the presence of NA (approximately 60% inhibition) and β-HB (approximately 60% inhibition).
Figure 4.
Suppression of TNF-α–induced proinflammatory cytokine production by nicotinate (NA) and β-HB at protein level. ARPE-19 cells were exposed to TNF-α (10 ng/mL; 24-hour incubation) in the presence or absence of NA (1 mM) or β-HB (5 mM). Cell culture medium was then collected and used for ELISA analysis of (A) IL-6 and (B) Ccl2 protein. *P < 0.01 compared with control cells; **P < 0.01 compared with TNF-α–treated cells.
Inhibition of G-Protein–Coupled Receptor Signaling by PTX Abolishes the Inhibitory Effects of Nicotinate and β-HB on IL-6 and Ccl2 Secretion
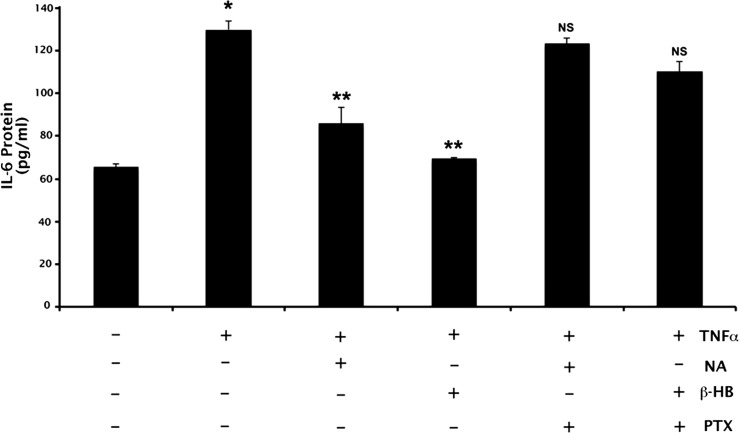
PTX is a well-characterized, specific, and irreversible inhibitor of the signaling of G-protein–coupled receptors that are coupled to Gi. Nicotinate and β-HB are agonists for GPR109A, a Gi-coupled receptor. Therefore, to assess whether the effects of NA and β-HB on the secretion of IL-6 and Ccl2 are mediated via GPR109A signaling, additional experiments were performed in which ARPE-19 cells were incubated with PTX (100 ng/mL) for 18 hours followed by 4-hour treatment with TNF-α (10 ng/mL) in the presence or absence of NA (1 mM) or β-HB (5 mM). As expected, exposure of cells to TNF-α increased IL-6 secretion significantly (approximately 2-fold), an effect that was blocked significantly by NA or β-HB (approximately 70% and approximately 90% inhibition, respectively) (Fig. 5). Treatment of cells with PTX markedly attenuated the abilities of NA and β-HB to block the TNF-α–induced secretion of IL-6, however. Analysis of Ccl2 secretion under identical treatment conditions produced results very similar to those shown for IL-6 (data not shown).
Figure 5.
Blockade of NA- and β-HB–induced suppression of IL-6 production by PTX. ELISA analysis of IL-6 production in ARPE-19 cells treated with PTX (100 ng/mL) before exposure to TNF-α (10 ng/mL) and/or NA (1 mM), β-HB (5 mM). *P < 0.01 compared with control cells; **P < 0.01 compared with TNF-α–treated cells; NS, not significantly different from cells treated with TNF-α alone.
Overexpression of GPR109A Enhances the Suppressive Effects of Nicotinate and β-HB on IL-6 and Ccl2 Expression
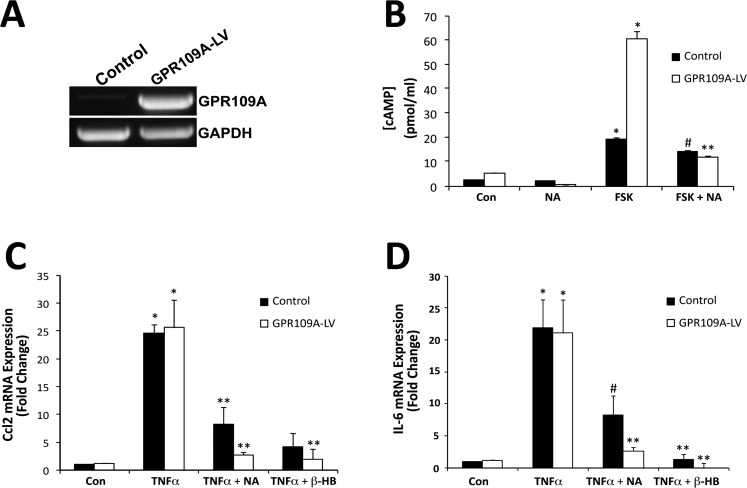
Given that PTX is not a specific blocker of GPR109A alone, but rather blocks all Gi-coupled receptors, to demonstrate that GPR109A is the key molecule responsible for the anti-inflammatory effects observed in RPE cells exposed to TNF-α in the presence of NA or β-HB, additional experiments were performed using ARPE-19 cells in which GPR109A was overexpressed (GPR109A-LV) using a lentiviral vector expression system (Fig. 6). RT-PCR was used to compare GPR109A expression in GPR109A-LV versus control ARPE-19 cells (Fig. 6A). At only 25 cycles, robust expression of mRNA transcripts encoding GPR109A was detected in GPR109A-LV cells. To verify that GPR109A expressed in GPR109A-LV cells was functional, GPR109A-LV and control ARPE-19 cells were treated with forskolin (FSK) (10 μM) in the presence or absence of NA (1 mM), and cAMP assay was performed (Fig. 6B). The increase in intracellular cAMP concentration observed in the presence of FSK was significantly greater in GPR109A-LV cells than in control ARPE-19 cells. Likewise, the ability of cells to suppress FSK-induced increases in cAMP in the presence of NA or β-HB was enhanced in GPR109A-LV cells. Suppression of TNF-α–induced increases in IL-6 and Ccl2 expression in GPR109A-LV cells in the presence of NA or β-HB was similarly enhanced (Figs. 6C, 6D, respectively).
Figure 6.
Enhanced suppression of TNF-α–induced IL-6 and Ccl2 expression by NA and β-HB in GPR109A-overexpressing ARPE-19 cells. GPR109A was overexpressed in ARPE-19 cells using a lentiviral vector expression system. (A) RT-PCR was used to evaluate GPR109A expression in GPR109A-overexpressing (GPR109A-LV) and control ARPE-19 cells. (B) cAMP assay of GPR109A-LV and control ARPE-19 cells treated with FSK (10 μM) and/or NA (1 mM). qPCR was used to evaluate (C) Ccl2 and (D) IL-6 mRNA expression in GPR109A-LV and control ARPE-19 cells following exposure to TNF-α in the presence or absence of NA (1 mM) or β-HB (5 mM). *P < 0.01 compared with control cells; **P < 0.01 compared with FSK- or TNF-α–treated cells; #P < 0.05 compared with FSK- or TNF-α–treated control ARPE-19 cells.
Nicotinate and β-HB Suppress IL-6 and Ccl2 Expression and Secretion in Gpr109a+/+ but Not in Gpr109a−/− mRPE Cells
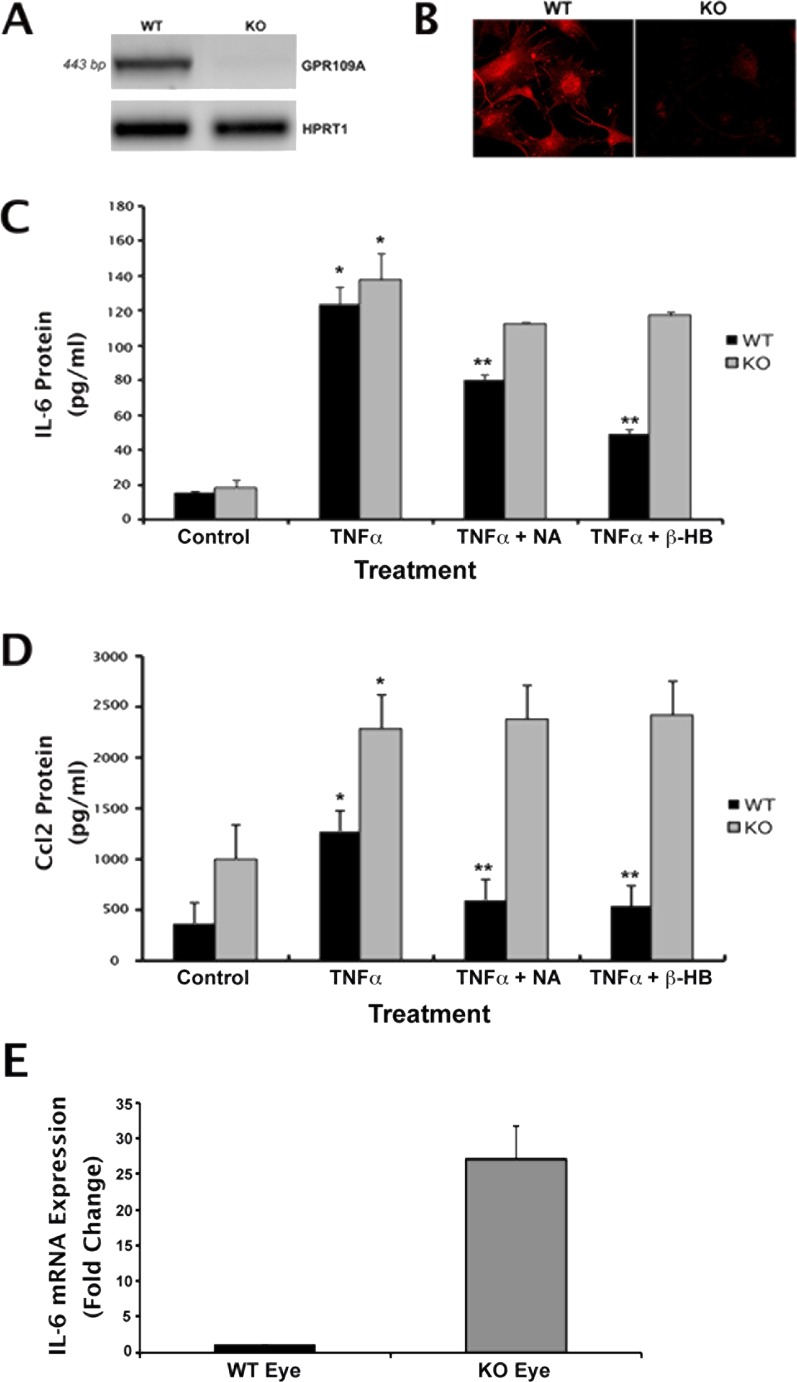
Overexpression of GPR109A in ARPE-19 cells enhanced the suppression of FSK-induced elevations in cAMP and TNF-α–induced increases in IL-6 and Ccl2 expression observed in the presence of NA or β-HB, supporting the critical involvement of GPR109A in these processes. To establish definitively that it is GPR109A specifically, however, not its ligands (NA or β-HB) or any other Gi-linked protein that mediates the observed anti-inflammatory effects, additional experiments were performed using primary RPE cells isolated from wild-type (Gpr109a+/+) and Gpr109a−/− mouse retinas. Following isolation, total RNA was collected from cells and used for RT-PCR analysis to confirm the presence or absence of receptor expression. Gpr109a-specific mRNA transcripts were detected in mRPE cells obtained from wild-type mouse eyes, but not in those obtained from Gpr109a−/− mouse eyes (Fig. 7A). Immunocytochemical analysis of the cells confirmed the presence of Gpr109a protein in wild-type cells and its absence in the knockout cells (Fig. 7B).
Figure 7.
NA- and β-HB–induced suppression of IL-6 and Ccl2 production in RPE cells is Gpr109a-dependent. Primary RPE cells were isolated from Gpr109a+/+ (wild-type [WT]) and Gpr109a−/– (knockout [KO]) mouse eyes. The presence or absence of Gpr109a mRNA and protein in these cells was confirmed by (A) RT-PCR and (B) immunofluorescence. WT and KO cells were exposed to TNF-α (10 ng/mL) in the presence or absence of NA (1 mM) or β-HB (5 mM) for 24 hours. Cell culture medium was collected and used for ELISA analysis of (C) IL-6 and (D) Ccl2 protein. (E) qPCR analysis of GPR109A mRNA expression in whole eyes obtained from WT and Gpr109a−/− mice. *P < 0.05 compared with control cells; **P < 0.01 compared with TNF-α–treated cells.
mRPE cells isolated from Gpr109a+/+ and Gpr109a−/− mice were exposed to TNF-α in the presence or absence of NA or β-HB in a fashion identical to that described above for treatment of ARPE-19 cells. Exposure of wild-type mRPE cells to TNF-α was associated with robust increases in IL-6 (8-fold) and Ccl2 (4-fold) secretion (Figs. 7C, 7D). The increases in IL-6 and Ccl2 secretion observed in the presence of TNF-α were inhibited significantly in the presence of NA (approximately 40% and approximately 70% inhibition, respectively) and β-HB (approximately 70% and approximately 80% inhibition, respectively). These results were similar to what was observed in ARPE-19 cells. We then repeated the same experiments with Gpr109a−/− mRPE cells. Exposure of these cells to TNF-α was also associated with robust increases in IL-6 (8-fold) and Ccl2 (2.5-fold) secretion; however, NA and β-HB were unable to block the TNF-α–induced increase in the secretion of these cytokines, indicating that the effects of NA and β-HB are mediated via Gpr109a. It is also of interest to note that levels of proinflammatory cytokine secretion were consistently higher in Gpr109a−/− cells than in wild-type cells under basal conditions. This suggests that Gpr109a elicits a negative effect on the secretion of these molecules in RPE in vitro even in the absence of TNF-α. To determine whether the same is true under in vivo conditions in intact eye tissues, whole eyes were obtained from Gpr109a−/− and wild-type littermates and used for RNA preparation/qPCR analysis of IL-6 and Ccl2 expression. Ocular levels of IL-6 mRNA expression were 20- to 25-fold higher in Gpr109a−/− eyes than in wild-type eyes (Fig. 7E). Similar increases in Ccl2 expression were observed in Gpr109a−/− eyes (data not shown). These data suggest an enhanced inflammatory reaction/increased susceptibility to inflammation-induced damage in Gpr109a-null ocular tissues.
Gpr109a-Mediated Suppression of NF-κB Activity
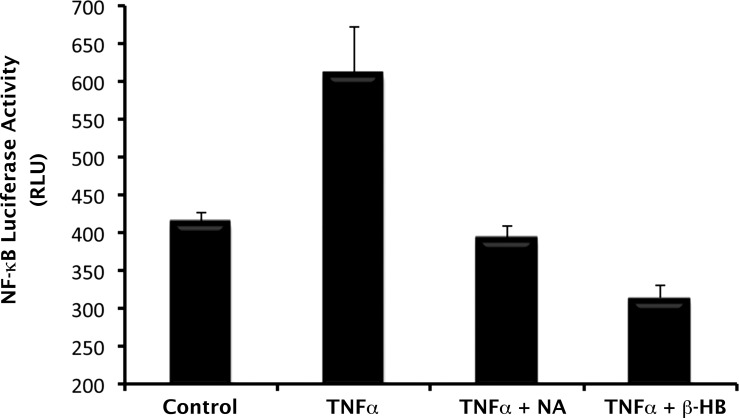
In a previous article, we showed that interaction of NA and other GPR109A-specific ligands with GPR109A is associated with blockade of NF-κB activation in colon cells.7 Given that NF-κB signaling pathways play a critical role in the regulation of cytokine/chemokine production by RPE cells in health and disease, we investigated the relevance of Gpr109a to TNF-α–induced NF-κB activation in mRPE cells. For this, we prepared mRPE cells from a transgenic mouse that carries the NF-κB–luciferase reporter gene under the control of the β-actin promoter. Because normal mouse RPE cells constitutively express Gpr109a, we directly tested the effects of NA and β-HB on TNF-α–induced activation of NF-κB in these cells. Treatment of the cells with TNF-α stimulated the activity of the NF-κB–luciferase reporter. This effect of TNF-α was blocked completely by the Gpr109a agonists NA and β-HB (Fig. 8). In fact, these agonists appeared to inhibit even the basal activity of the NF-κB–luciferase reporter because the activity of the reporter was even lower than the control value in the presence of these agonists.
Figure 8.
Blockade of TNF-α–induced activation of NF-κB by GPR109A ligands in normal mouse RPE cells. Primary RPE cells were isolated from transgenic mice carrying the NF-κB–luciferase reporter construct under control of β-actin promoter. Cells were pretreated with or without NA (1 mM) or β-HB (5 mM) for 30 minutes, followed by exposure to TNF-α (10 ng/mL). NA and β-HB were present also during TNF-α treatment. The cells were then collected and the lysates used for measurement of luciferase activity.
Discussion
The purpose of the present study was to investigate the effect of diabetes on GPR109A expression and the functional role of the receptor in RPE. Here we show upregulated expression of the receptor under diabetic conditions in human and mouse retina. Given that others have demonstrated a role for GPR109A in modulating inflammation in various tissues/cell types, we examined whether the same is true in RPE also. Specifically, we investigated the potential anti-inflammatory actions of NA and β-HB, two well-characterized agonists for GPR109A, in cultured human and mouse RPE cells. Our studies demonstrate that in RPE, NA and β-HB potently suppress TNF-α–induced expression and release of the proinflammatory cytokine IL-6 and the chemokine Ccl2. The reduction in IL-6 and Ccl2 release observed when cells were cultured with TNF-α in the presence of NA or β-HB was abolished by treatment of cells with PTX, indicating that the anti-inflammatory effects observed in the presence of these two compounds are mediated via a receptor that is coupled to PTX-sensitive Gi. This corroborates with the findings that GPR109A is a Gi-coupled receptor.1 Additional studies performed with GPR109A-overexpressing ARPE-19 cells and primary RPE cells isolated from Gpr109a+/+, Gpr109a−/−, and NF-κB–luciferase transgenic mouse retinas confirmed not only that Gpr109a was indeed the receptor responsible for mediating the anti-inflammatory effects of NA and β-HB, but also that the process occurred via blockade of NF-κB activation. Thus, based on our present findings, we conclude that in RPE, activation of GPR109A is associated with anti-inflammatory signaling. This represents the first report on a functional role for GPR109A in RPE.
In the present study, we focused specifically on the effects of GPR109A activation on IL-6 and Ccl2 production in RPE. Whether additional cytokines/chemokines are affected by activation of the receptor is a topic that warrants further investigation, as IL-6 and Ccl2 represent only two of a plethora of molecules known to be involved in inflammation; however, the fact that GPR109A may suppress the secretion of IL-6 and Ccl2 has clinical significance. This is supported by a recent report demonstrating that in patients with vitreoretinal diseases, including diabetic macular edema and proliferative diabetic retinopathy, IL-6 and Ccl2 are two of only three inflammatory molecules identified to be strongly correlated with development and progression of vitreoretinal disease in human patients, even though a long list of other inflammatory-related molecules were also evaluated.23 IL-6 is a potent proinflammatory cytokine; levels of IL-6 have been shown to be elevated in the eyes of human patients with diabetic retinopathy, particularly in the more advanced, proliferative stages of the disease, and in the eyes of patients with age-related macular degeneration.23–25 Ccl2, formerly called monocyte chemotactic protein-1, also plays a critical role in the inflammatory process in retina and other tissues, by inducing leukocyte recruitment and activation, and regulating the expression/secretion of additional cytokines by leukocytes on their activation. Hence, regulating IL-6 and Ccl2 expression in retina is of critical importance and could have a large impact on the treatment of vitreoretinal diseases such as diabetic retinopathy.
Retinal inflammation is a common feature of blinding diseases such as age-related macular degeneration, diabetic retinopathy, retinopathy of prematurity, and uveitis.23,24 RPE is known to play a major role in regulating immunity/inflammation in retina.13–15 As such, identification of a receptor (GPR109A) capable of stimulating anti-inflammatory pathways in RPE may have broad implications in terms of limiting retinal inflammation. This makes GPR109A a new and attractive drug target for treatment of a broad spectrum of retinal diseases. The fact that β-HB, a metabolite that is elevated in the circulation of patients with diabetes, is the only identified endogenous ligand for GPR109A in noncolonic tissues, including the retina, greatly enhances the biologic relevance and therapeutic significance of our findings in the clinical management of diabetic retinopathy. Even though it has long been known that uncontrolled diabetes, particularly type 1 diabetes, is associated with ketoacidosis (i.e., elevated circulating levels of β-HB), the biologic significance of this metabolic feature is not known except that insulin insufficiency/insulin resistance, as occurs in uncontrolled diabetes, enhances the metabolism of fatty acids via β-oxidation, thus increasing the generation of β-HB. Because this metabolite is now known to be an agonist for GPR109A, this raises a question as to whether the elevated levels of β-HB in diabetes have any biologic significance in the progression of the disease. Currently, ketoacidosis in diabetes is viewed only as a signal of the increased severity of the disease, caused simply by the shift in the metabolic use of free fatty acids in favor of glucose as the energy source. Could there be something more to it?
Based on our present findings, we speculate that induction of GPR109A in diabetic retina, coupled with elevation of its agonist β-HB, represents a mechanism by which the tissue tries to stop the progression of the damage caused by the disease; a speculation well-supported by our present in vitro data. Additional studies are needed to, however, confirm the truth of this speculation under in vivo conditions. Although we observed significant increases in GPR109A expression in the diabetic animals used in the present study, interaction of β-HB with GPR109A was not a factor, as diabetes in mice is not associated with significant elevations in serum β-HB levels in the absence of a high-fat diet or other experimental manipulations.26,27 Hence, β-HB levels in our animals likely remained too low to significantly affect receptor activity and thereby protect retina from diabetes-induced inflammation. The same would be true also in diabetic human patients treated with insulin or other blood glucose–regulating drugs, as they would prevent increases in circulating levels of β-HB.21 As such, further studies are needed to gain a better understanding of the exact molecular mechanisms involved in GPR109A-dependent regulation of retinal inflammation and how to control these processes. This is particularly true given the fact that the effects elicited in response to GPR109A activation are known to be cell type/tissue dependent. For example, activation of GPR109A in some cell types produces desirable effects (i.e., antilipolytic and anti-inflammatory effects in adipocytes,4 colonocytes,8 and in the present study, RPE) whereas activation of the same receptor in other cell types (Langerhans cells and keratinocytes) is associated with unwanted effects (i.e., flushing and other proinflammatory responses).28,29 Nonetheless, our discovery of the anti-inflammatory function of GPR109A in RPE and the increase in the expression of the receptor in diabetes suggest that this receptor has potential as a therapeutic drug target in the clinical management of diabetic retinopathy. Pharmacological approaches to activate the receptor may provide an additional therapeutic avenue for effective management of the progression of the disease process in retina as well as in other tissues.
Footnotes
Supported by a Pilot Project Award from the Georgia Health Sciences University Vision Discovery Institute, Augusta, Georgia.
Disclosure: D. Gambhir, None; S. Ananth, None; R. Veeranan-Karmegam, None; S. Elangovan, None; S. Hester, None; E. Jennings, None; S. Offermanns, None; J.J. Nussbaum, None; S. B. Smith, None; M. Thangaraju, None; V. Ganapathy, None; P.M. Martin, None
References
- 1. Ahmed K, Tunaru S, Offermans S. GPR109A, GPR109B, and GPR81, a family of hydroxy-carboxylic acid receptors. Trends Pharmacol Sci. 2009;30:557–562. [DOI] [PubMed] [Google Scholar]
- 2. Taggert AK, Kero J, Gan X, et al. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem. 2005;280:26649–26652. [DOI] [PubMed] [Google Scholar]
- 3. Soga T, Kamohara M, Takasak J, et al. Molecular identification of the nicotinic acid receptor. Biochem Biophys Res Commun. 2003;303:364–369. [DOI] [PubMed] [Google Scholar]
- 4. Tunaru S, Kero J, Schaub A, et al. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med. 2006;9:352–355. [DOI] [PubMed] [Google Scholar]
- 5. Wise A, Foord S, Raser N, et al. Molecular identification of high and low affinity receptors for nicotinic acid. J Biol Chem. 2003;278:9869–9874. [DOI] [PubMed] [Google Scholar]
- 6. Li X, Millar JS, Brownell N, et al. Modulation of HDL metabolism by the niacin receptor GPR109A in mouse hepatocytes. Biochem Pharmacol. 2010;80:1450–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thangaraju M, Cresci GA, Lui K, et al. GPR109A is a G-protein coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009;69:2826–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cresci GA, Thangaraju M, Mellinger JD, et al. Colonic gene expression in conventional and germ-free mice with a focus on the butyrate receptor GPR109A and the butyrate transporter SLC5A8. J Gastrointest Surg. 2010;14:449–461. [DOI] [PubMed] [Google Scholar]
- 9. Martin PM, Ananth S, Cresci G, et al. Expression and localization of GPR109A (PUMA-G/HM74A) mRNA and protein in mammalian retinal pigment epithelium. Mol Vis. 2009;15:362–372. [PMC free article] [PubMed] [Google Scholar]
- 10. Digby JE, McNeill E, Dyar OJ, et al. Anti-inflammatory effects of nicotinic acid in adipocytes demonstrated by suppression of fractalkine, RANTES, and MCP-1 and upregulation of adiponectin. Atherosclerosis. 2010;209:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Montecucco F, Quercioli A, Dallegri F, et al. New evidence for nicotinic acid treatment to reduce atherosclerosis. Expert Rev Cardiovasc Ther. 2010;8:1457–1467. [DOI] [PubMed] [Google Scholar]
- 12. Lukasova M, Malaval C, Gille A, et al. Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J Clin Invest. 2011;121:1163–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sparrow JR, Hicks D, Haunel CP. The retinal pigment epithelium in health and disease. Curr Mol Med. 2010;10:802–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Detrick B, Hooks JJ. Immune regulation in the retina. Immunol Res. 2010;47:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Simó R, Villarroel M, Corraliza L, et al. The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier – implications for the pathogenesis of diabetic retinopathy. J Biomed Biotechnol. 2010;2010:190724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Penfold PL, Madigan MC, Gillies MC, Provis JM. Immunological and aetiological aspects of macular degeneration. Prog Retin Eye Res. 2001;20:385–414. [DOI] [PubMed] [Google Scholar]
- 17. Singh N, Thangaraju M, Prasad PD, et al. Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. J Biol Chem. 2010;285:27601–27608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martin PM, Roon P, Van Ells TK, et al. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci. 2004;45:3330–3336. [DOI] [PubMed] [Google Scholar]
- 19. Smith SB, Duplantier J, Dun Y, et al. In vivo protection against retinal neurodegeneration by sigma receptor 1 ligand (+)-pentazocine. Invest Ophthalmol Vis Sci. 2008;49:4154–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barber AJ, Lieth E, Khin SA, et al. Neural apoptosis in retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998;102:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eledrisi JM, Alshanti MS, Shah F, et al. Overview of the diagnosis and management of diabetic ketoacidosis. Am J Med Sci. 2006;331:243–251. [DOI] [PubMed] [Google Scholar]
- 22. Umpierrez GE, Smiley D, Kitabchi AE. Narrative review: Ketosis-prone type 2 diabetes mellitus. Ann Intern Med. 2006;144:350–357. [DOI] [PubMed] [Google Scholar]
- 23. Yoshimura T, Sonoda KH, Sugahara M, et al. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS One. 2009;4:e8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boehm MR, Oellers P, Thanos S. Inflammation and immunology of the vitreoretinal compartment. Inflamm Allergy Drug Targets. 2011;10:283–309. [DOI] [PubMed] [Google Scholar]
- 25. Swernarchuk LE, Whetter LE, Adamis AP. The role of inflammation in the pathophysiology of diabetic retinopathy. Semin Immunopathol. 2008;30:65–84. [DOI] [PubMed] [Google Scholar]
- 26. Rose KL, Pin CL, Wang R, Fraser DD. Combined insulin and bicarbonate therapy elicits cerebral edema in a juvenile mouse model of diabetic ketoacidosis. Ped Res. 2007;61:301–306. [DOI] [PubMed] [Google Scholar]
- 27. Polotsky VY, Wilson JA, Haines AS, et al. The impact of insulin-dependent diabetes on ventilatory control in the mouse. Am J Resp Crit Care Med. 2001;163:624–632. [DOI] [PubMed] [Google Scholar]
- 28. Walters RW, Shukla AK, Kovacs JJ, et al. β-arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. J Clin Invest. 2009;119:1312–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hanson J, Gille A, Zwykiel S, et al. Nicotinic acid- and monomethylfumarate-induced flushing involves GPR109A expressed by keratinocytes and COX-2-dependent prostanoid formation in mice. J Clin Invest. 2010;120:2910–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]