Abstract
Aims/Introduction
Asian patients represent a large portion of the global population with type 2 diabetes mellitus, but are underrepresented in trials of glucose-lowering therapies. The present randomized, phase III, placebo-controlled, double-blind, 24-week study evaluated the dipeptidyl peptidase-4 inhibitor, linagliptin, as monotherapy in Asian patients with inadequately controlled type 2 diabetes mellitus.
Materials and Methods
Patients who were treatment naïve or had been treated with one oral antidiabetes drug were randomized to either linagliptin 5 mg daily or a placebo after washout. The primary end-point was a change from baseline in glycated hemoglobin after 24 weeks.
Results
A total of 300 Asian (87% Chinese) patients with type 2 diabetes mellitus were randomized to linagliptin or placebo at a 2:1 ratio. After 24 weeks of treatment, adjusted mean (standard error) glycated hemoglobin decreased by a placebo-corrected −0.50 ± 0.11 (P < 0.0001). In patients with baseline glycated hemoglobin ≥8.5%, the placebo-corrected decrease in glycated hemoglobin was −0.91 ± 0.20% (P < 0.0001). Adverse events occurred in 28.0 and 28.3% of linagliptin and placebo patients, respectively, but few were drug-related (3.0 and 2.0%, respectively). Hypoglycemia was reported by one linagliptin patient and no placebo patients. Treatment with linagliptin was weight neutral.
Conclusions
In Asian patients with inadequately controlled type 2 diabetes mellitus, linagliptin 5 mg as monotherapy was efficacious and well tolerated over 24 weeks.
Keywords: Asians, Linagliptin, Monotherapy
Introduction
Asia is home to more than 200 million patients with diabetes1, predominantly type 2 diabetes mellitus. In Asian patients, type 2 diabetes occurs at a younger age with relatively low body mass index (BMI), and also with a lower risk threshold for complications than in Western populations2–4. In East Asia, these factors are compounded by an increased risk for renal complications4.
Several treatment options are available to manage hyperglycemia in patients with type 2 diabetes5. However, some of the most common glucose-lowering drugs have important contraindications for patients with moderate or severe renal impairment (e.g., metformin and sulfonylureas), carry risk for hypoglycemia and weight gain (e.g., sulfonylureas, thiazolidinediones and insulin), or have the potential for gastrointestinal side-effects (e.g., metformin and α-glucosidase inhibitors).
Dipeptidyl peptidase-4 (DPP-4) inhibitors promote insulin secretion in a glucose-dependent manner by prolonging the half-life of the incretin hormone glucagon-like peptide-1 (GLP-1). In addition, DPP-4 inhibitors might improve regulation of glucagon secretion from pancreatic α-cells. Linagliptin is a potent, highly reversible binding to protein and selective DPP-4 inhibitor with a predominantly non-renal elimination, enabling it to be prescribed in a single 5-mg once-daily dose to patients with type 2 diabetes without dose change regardless of renal and liver function6–8.
In multinational phase III clinical trials that included Asian as well as Western patients, linagliptin reduced hyperglycemia without showing an increased propensity to cause weight gain or hypoglycemic events when used as a monotherapy or with other oral glucose-lowering drugs9–12. In a pooled subgroup analysis of these studies, linagliptin was shown to be an efficacious and well-tolerated treatment option for South and East Asian patients with inadequately controlled type 2 diabetes13. A subgroup analysis of a phase III trial of linagliptin added to metformin and a sulfonylurea showed that the combination was efficacious and well tolerated by Chinese patients14.
The present trial was undertaken to evaluate the efficacy and safety of linagliptin 5 mg given once daily as monotherapy in Asian (mainly Chinese) patients with inadequately controlled type 2 diabetes.
Materials and Methods
Study Overview
The present study was a randomized, double-blind, placebo-controlled, phase III clinical trial (ClinicalTrials.gov, no. NCT01214239). Monotherapy with linagliptin was evaluated over 24 weeks after a 4-week washout of any prior antidiabetes drugs and a 2-week placebo run-in period.
The trial was carried out in compliance with the principles laid down in the Declaration of Helsinki, in accordance with the International Conference on Harmonisation Harmonised Tripartite Guideline for Good Clinical Practice, and in accordance with applicable regulatory requirements. All participants gave written consent.
Patients
The trial recruited patients in China, Malaysia and the Philippines. Men and women aged 18–80 years with insufficiently controlled type 2 diabetes (glycated hemoglobin [HbA1c] between 7.0 and 10.0%) and BMI ≤45 kg/mg2, who had received no prior antidiabetes drugs or who had been treated with one antidiabetes drug, were eligible for participation in the present trial. Antidiabetes therapy had to be unchanged for 6 weeks before informed consent. Patients were excluded from the trial if they had experienced a myocardial infarction, stroke or transient ischemic attack ≤6 months before informed consent; if they had unstable or acute congestive heart failure; if they had impaired hepatic function (liver function tests >3 times the upper limit of normal); if they had confirmed hyperglycemia >240 mg/dL after an overnight fast during the wash-out/placebo run-in period; if they had been treated with a thiazolidinedione, GLP-1 analog, DPP-4 inhibitor, insulin or anti-obesity drug ≤3 months before informed consent; or were treated with systemic steroids at the date of informed consent.
Treatments
Eligible patients were randomly assigned in a two-to-one ratio to oral once-daily treatment with either linagliptin 5 mg daily or placebo. The randomization was stratified by HbA1c at the beginning of the placebo run-in period (HbA1c <8.5% or ≥8.5%) and by prior use of antidiabetes drugs (none or monotherapy). Patient assignments were determined by a computer-generated, pseudo-random sequence using an interactive voice response system. Access to the randomization code was restricted to dedicated randomization personnel, and the study was blinded until after the database was locked.
The use of metformin as rescue medication was allowed during the randomized period of this trial. During the first 12 weeks of treatment, rescue medication could be initiated with a confirmed glucose level >240 mg/dL after an overnight fast. During the last 12 weeks of treatment, rescue medication could be initiated with a confirmed glucose level >200 mg/dL after an overnight fast or a random >400 mg/dL. If a patient's fasted glucose levels remained >240 mg/dL during the first 12 weeks of treatment or >200 mg/dL during the last 12 weeks of treatment despite metformin rescue, he or she was withdrawn from the study.
End-Points
Efficacy was primarily assessed by change from baseline in mean HbA1c after 24 weeks. The secondary end-points were change from baseline in HbA1c in the subset of Chinese patients, occurrence of target efficacy responses (<7.0% or <6.5%) and a relative efficacy response (−0.5%), HbA1c reduction from baseline over time, change from baseline in fasting plasma glucose (FPG) and change from baseline in FPG over time. In addition, HbA1c change was compared in patients with baseline HbA1c <8.5% vs ≥8.5%, and changes in lipid profiles were evaluated as efficacy end-points.
Safety was evaluated by the incidence and intensity of adverse events (AEs) including hypoglycemia. Serious AEs were defined as events that resulted in death or were immediately life-threatening, resulted in persistent or significant disability, required or prolonged patient hospitalization, led to congenital anomalies or birth defect or were considered to be an important medical event that might have jeopardized the patient and required medical or surgical intervention to prevent one of the other outcomes.
Hypoglycemia was classified by investigators as asymptomatic with glucose concentration ≤70 mg/dL, documented symptomatic with glucose concentration of 54–70 mg/dL, documented symptomatic with glucose concentration <54 mg/dL but no need for external assistance, and severe hypoglycemia requiring the assistance of another person to actively administer carbohydrate, glucagon or other resuscitative actions.
An independent external clinical event committee (CEC) reviewed in a blinded fashion all fatal events and events suspected of being either stroke or cardiac ischemia. The CEC evaluated whether prespecified criteria for the adjudication end-points (non-fatal myocardial infarction, other myocardial ischemia, stroke, transient ischemic attack, cardiovascular death) were met.
Statistical Analysis
A total of 231 patients were determined to be sufficient to detect a between-group difference of 0.5% in change from baseline in HbA1c with 90% power (two-sided, α = 0.05; standard deviation assumed 1.1%).
The primary evaluation of change in HbA1c from baseline to week 24 used an analysis of covariance (ancova) with baseline HbA1c as a linear covariate, and prior use of antidiabetes drugs and treatment as fixed classification effects. The primary evaluation was carried out on the full analysis set, which included all randomized patients who received one or more doses of the study drug, and had a baseline and one or more on-treatment HbA1c measurement. Missing values were imputed using last observation carried forward. Categorical HbA1c responses were assessed without imputation of missing data by assuming that patients with missing values had failed to achieve the response. Changes in FPG were assessed for the full analysis set using ancova with last observation carried forward (the model included continuous baseline HbA1c, continuous baseline FPG, prior antidiabetes drugs and treatment). Efficacy was additionally assessed by changes in lipid profiles, and bodyweight and composition. Safety data were analyzed descriptively for the treated set, which consisted of all randomized patients who received one or more doses of the study drug.
Results
The trial was carried out between 11 November 2010 and 24 May 2012. The investigators randomized 300 patients (China 261; Malaysia 22; the Philippines 17). One randomized patient in the linagliptin group was not treated. Of the treated patients, 182 in the linagliptin group and 87 in the placebo group completed the trial (Figure S1). Baseline demographics and clinical characteristics were well matched between groups. Mean ± standard deviation HbA1c at baseline was 7.95 ± 0.89% in the linagliptin group and 8.09 ± 0.91% in the placebo group (Table1). In the overall population, 47.5% of patients had a BMI <25 kg/m2.
Table 1.
Baseline demographics and clinical characteristics
| Linagliptin 5 mg | Placebo | |
|---|---|---|
| Patients (treated set), n | 200 | 99 |
| Age, years (mean ± SD) | 54.6 (10.1) | 54.1 (9.3) |
| Sex, n (%) | ||
| Male | 116 (58.0) | 59 (59.6) |
| Female | 84 (42.0) | 40 (40.4) |
| Country, n (%) | ||
| China | 172 (86.0) | 88 (88.9) |
| Malaysia | 15 (7.5) | 7 (7.1) |
| Philippines | 13 (6.5) | 4 (4.0) |
| Bodyweight, kg (mean ± SD) | 69.0 (11.6) | 68.2 (10.4) |
| BMI, kg/m2 (mean ± SD) | 25.5 (3.3) | 25.1 (3.4) |
| eGFR, n (%) | ||
| ≥90 mL/min/1.73 m2 | 118 (59.0) | 66 (66.7) |
| 60 to <90 mL/min/1.73 m2 | 79 (39.5) | 31 (31.3) |
| 30 to <60 mL/min/1.73 m2 | 3 (1.5) | 2 (2.0) |
| <30 mL/min/1.73 m2 | 0 (0.0) | 0 (0.0) |
| Patients, FAS, n | 196 | 94 |
| HbA1c, % (mean ± SD) | 7.95 (0.89) | 8.09 (0.91) |
| Mean FPG, mg/dL (mean ± SD) | 151.4 (32.4) | 161.4 (39.9) |
| Time since diagnosis, n (%) | ||
| ≤1 year | 98 (50.0) | 49 (52.1) |
| >1–5 years | 62 (31.6) | 30 (31.9) |
| >5 years | 36 (18.4) | 15 (16.0) |
| Prior OADs, n (%) | ||
| 0 | 157 (80.1) | 74 (78.7) |
| 1 | 39 (19.9) | 20 (21.3) |
BMI, body mass index; eGFR, estimated glomerular filtration rate; FAS, full analysis set; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; OAD, oral antidiabetes drug; SD, standard deviation.
Efficacy
After 24 weeks of treatment, the effectiveness of linagliptin monotherapy at reducing hyperglycemia in the study population was superior to the placebo. At 24 weeks, the adjusted mean ± standard error HbA1c decreased by −0.68 ± 0.07% in the linagliptin group and −0.18 ± 0.10% in the placebo group (placebo-corrected difference, −0.50 ± 0.11; 95% confidence interval [CI] −0.71, −0.28; P < 0.0001; Figure1). Results for the predefined Chinese subgroup were similar to the overall Asian population, with linagliptin reducing HbA1c by −0.71 ± 0.08% compared with −0.25 ± 0.11% in the placebo group. Reductions in HbA1c with linagliptin were statistically significant compared with the placebo in patients with baseline HbA1c <8.5% or ≥8.5% (Figure2).
Figure 1.
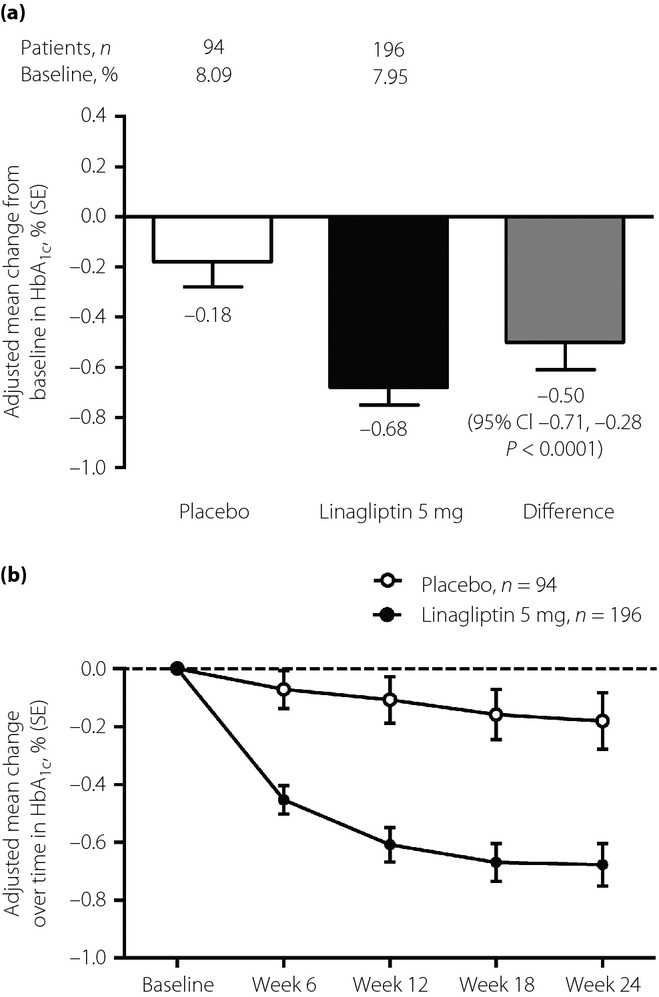
(a) Adjusted mean change in glycated hemoglobin (HbA1c) from baseline at week 24 with linagliptin or placebo as monotherapy (full analysis set – last observation carried forward). (b) Adjusted mean change from baseline in HbA1c over time (full analysis set – last observation carried forward). Model includes treatment, baseline HbA1c and previous antidiabetes medication.
Figure 2.
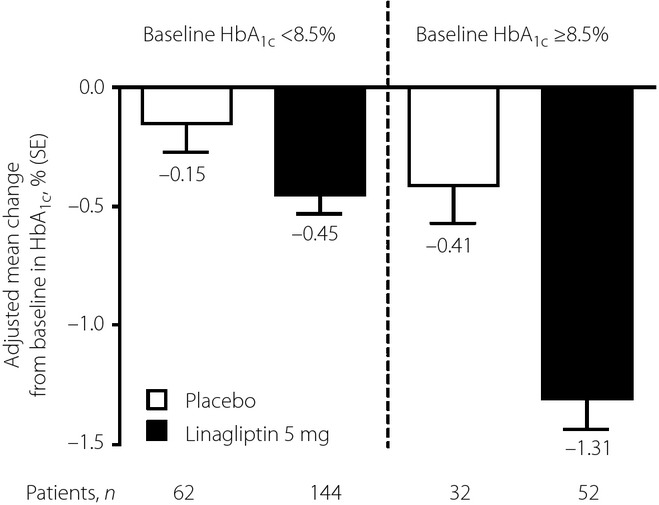
Adjusted mean change in glycated hemoglobin (HbA1c) from baseline at week 24 in patients with baseline HbA1c <8.5 and ≥8.5% (full analysis set – last observation carried forward). Model includes categorical baseline HbA1c, number of previous antidiabetes drugs, treatment group and treatment by baseline HbA1c (categorical) interaction. SE, standard error.
Glycemic targets of HbA1c <7.0% or <6.5% and reductions of ≥0.5% were observed in a greater proportion of the linagliptin group than the placebo group. Of the linagliptin and placebo patients who had baseline HbA1c ≥7.0%, target HbA1c <7.0% was attained by 45.5 and 25.8%, respectively. Of the linagliptin and placebo patients whose baseline values were ≥6.5%, target HbA1c <6.5% was attained by 18.8 and 11.8%, respectively. Additionally, a higher percentage of all patients treated with linagliptin (65.3%) achieved a ≥ 0.5% reduction in HbA1c than those treated with the placebo (45.7%).
Linagliptin was associated with a significantly greater reduction in FPG compared with the placebo. The placebo-corrected adjusted mean ± standard error change in FPG at week 24 with linagliptin was −9.6 ± 3.8 mg/dL (95% CI −17.1, −2.2; P = 0.0113; Figure S2).
Lipid profiles remained mostly within normal ranges in either treatment group from baseline to week 24 (Appendix S1). No clinically relevant changes were observed in the laboratory parameters or vital signs. Mean ± standard deviation bodyweight slightly decreased in both the linagliptin and placebo groups (−0.51 ± 2.67 and −0.99 ± 2.67 kg, respectively), with no significant difference between the groups. Neither treatment caused increases in BMI nor waist circumference, and no statistically significant differences between groups were observed for either parameter (data not shown).
Subgroup analyses of HbA1c reductions from baseline indicated that the effectiveness of linagliptin vs the placebo was largely unaffected by factors such as age, sex, BMI, baseline bodyweight or estimated glomerular filtration rate (Appendix S2). However, in several subgroups (i.e., age groups 65–74 years and ≥75 years, baseline BMI ≥30 kg/m2, baseline bodyweight >80–90 kg and >90 kg, and baseline estimated glomerular filtration rate 30 to <60 mL/min) patient numbers were too small to allow meaningful comparisons.
Safety
Adverse events occurred in similar proportions of patients in the linagliptin and placebo groups (28.0 and 28.3%, respectively), and drug-related AEs occurred in 3.0% of the linagliptin patients and 2.0% of the placebo patients (Table2). Small proportions of patients in both groups withdrew from the study because of AEs (3.0 and 4.0% of linagliptin and placebo patients, respectively). There were no cases of hypersensitivity in linagliptin-treated patients, and only one case (1.0%) in the placebo group. There was one case of urticaria (0.5%) reported in the linagliptin group and none in the placebo group. No episodes of worsening of heart failure or hospitalization with heart failure were reported in either group.
Table 2.
Incidence of adverse events over 24 weeks – treated set
| Linagliptin 5 mg (n = 200) | Placebo (n = 99) | |
|---|---|---|
| Any AEs*, n (%) | 56 (28.0) | 28 (28.3) |
| AEs by MedDRA-preferred term with an incidence >2.0%, n (%) | ||
| Metabolism and nutrition disorders | 21 (10.5) | 10 (10.1) |
| Hyperglycemia | 4 (2.0) | 4 (4.0) |
| Hyperlipidemia | 11 (5.5) | 3 (3.0) |
| Drug-related AEs*, n (%) | 6 (3.0) | 2 (2.0) |
| Gastrointestinal disorders | 0 (0.0) | 1 (1.0) |
| General disorders and administration-site conditions | 1 (0.5) | 0 (0.0) |
| Hepatobiliary disorders | 1 (0.5) | 0 (0.0) |
| Investigations | 1 (0.5) | 1 (1.0) |
| Metabolism and nutrition disorders | 3 (1.5) | 0 (0.0) |
| Psychiatric disorders | 0 (0.0) | 1 (1.0) |
| Respiratory, thoracic and mediastinal disorders | 1 (0.5) | 0 (0.0) |
| AEs leading to discontinuation, n (%) | 6 (3.0) | 4 (4.0) |
| SAEs, n (%) | 1 (0.5) | 2 (2.0) |
| Requiring hospitalization | 1 (0.5) | 2 (2.0) |
| Investigator-defined hypoglycemia, n (%) | 1 (0.5) | 0 (0.0) |
| Confirmed adjudicated cardiovascular events, n (%) | 0 (0.0) | 1 (1.0) |
| Acute MI | 0 (0.0) | 1 (1.0) |
Listed by system organ class in alphabetical order; individual patients could have had more than one adverse event (AE). MedDRA, Medical Dictionary for Regulatory Activities version 15.0; MI, myocardial infarction; SAE, serious adverse event.
Serious AEs were rare; in the linagliptin group, a case of intervertebral disc protrusion and lumbar stenosis that required hospitalization was characterized as serious, but not drug related. Two serious AEs in the placebo group (acute coronary syndrome and gastrointestinal hemorrhage) required hospitalization, but were not considered drug related. No cases of pancreatitis or pancreatic cancer were reported in either treatment group. The CEC adjudicated cardiac or cerebrovascular events for one patient each in the linagliptin and placebo groups; in the linagliptin group, the patient had a non-fatal event that was not assessable. The patient in the placebo group had confirmed non-fatal acute myocardial infarction and a coronary revascularization procedure. No cases of worsening heart failure or hospitalization for heart failure were confirmed.
Investigator-defined hypoglycemia occurred in one patient (0.5%) receiving linagliptin, but the event was not severe. No patients receiving placebo experienced a hypoglycemic event.
Discussion
In the present randomized clinical trial, monotherapy with linagliptin 5 mg was superior to the placebo at reducing hyperglycemia over 24 weeks in Asian patients with uncontrolled type 2 diabetes. Linagliptin achieved clinically relevant reductions in HbA1c with a low incidence of hypoglycemia and without weight increase. The drug was well tolerated with no instances of pancreatitis, pancreatic cancer or congestive heart failure (CHF). After the publication of the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus – Thrombolysis in Myocardial Infarction 53 (SAVOR-TIMI 53)15 and Examination of Cardiovascular Outcomes with Alogliptin vs Standard of Care (EXAMINE)16 trials, concerns regarding CHF have been raised due to the finding of a statistically significant increase in risk of hospitalization for CHF associated with saxagliptin therapy in SAVOR-TIMI 53, and a non-significant hazard ratio for alogliptin of above 1.0 in EXAMINE. Thus, it is reassuring to note that no cases of CHF were reported in the present study, although patient numbers were small. A more definitive answer on the CV safety profile of linagliptin will be provided by the CARdiovascular Outcome Study of LINAgliptin vs Glimerpiride in Patients with Type 2 Diabetes (CAROLINA®)17, expected to report between 2017 and 2018.
The results of the present trial are in accordance with those of another international phase III trial with linagliptin monotherapy in a mixed population of Asian and White patients9. In that trial, patients treated with linagliptin monotherapy achieved a placebo-corrected mean ± standard error change in HbA1c of −0.69 ± 0.08% (95% CI −0.85, −0.53; P < 0.0001). In addition, a pooled analysis of Asian patient data from the present study and other studies using linagliptin added to other treatments showed a placebo-corrected reduction in HbA1c of −0.79 ± 0.06% (95% CI −0.92, −0.67; P < 0.0001) with linagliptin and a safety profile similar to placebo13.
In the present trial, HbA1c reduction ≥0.5% was observed in nearly one half of the patients in the placebo group, which represents a strong placebo effect. Similar placebo effects in treatment-naïve Asian (predominantly Chinese) patients have been reported18,19. Thus, for Asian patients with inadequately controlled type 2 diabetes, initial monotherapy with linagliptin could provide an alternative to initial metformin, which is recommended as first-line therapy in international treatment guidelines5,20. However, metformin has multiple contraindications, including for some stages of chronic kidney disease depending on creatinine clearance. Although the present study enrolled patients who were in the early stages of type 2 diabetes, nearly 40% of the participants had some degree of renal impairment. This finding is consistent with observations of early-stage renal complications in Asian type 2 diabetes populations4, and could underscore the need for alternatives to metformin as first-line therapy. Linagliptin can be used without dose adjustment in patients with reduced renal function8. Among Asian populations, metformin might also be under-dosed to offset gastrointestinal tolerability issues. In a study carried out in Japanese patients comparing linagliptin once daily with metformin (once to three times daily) as add-on to a sulfonylurea or an α-glucosidase inhibitor, comparable or lower incidence of AEs was observed with linagliptin21. The mean doses of metformin were 653.23 mg/day and 709.02 mg/day in the sulfonylurea and α-glucosidase groups, respectively.
The mechanism of action of DPP-4 inhibitors, such as linagliptin, addresses challenges endemic to Asian type 2 diabetes populations. Specifically, carbohydrate-rich diets causing elevation of post-prandial glucose (PPG) and glycemic variability are seen as a key factor in Asians, and DPP-4 inhibitors primarily affect control of PPG excursions. Although this trial did not include meal tolerance testing, statistically significant reductions in 2-h PPG with linagliptin have been shown in a population of Asian patients pooled from four phase III trials. In that analysis, linagliptin reduced PPG by a placebo-corrected –56.9 mg/dL (95% CI –85.17, –28.52)13.
This trial has some important limitations. The evaluation period of 24 weeks is relatively short to assess the safety of long-term use of linagliptin, and the trial was not powered to evaluate cardiovascular outcomes in this population. Homeostatic model assessments of β-cell function and insulin resistance were not carried out in this study, although they were included in previous trials9–12. These evaluations showed enhanced β-cell function with linagliptin. The extent to which the studied population is representative of patients in Asia as a whole might also be considered a potential limitation. Nevertheless, the data reported here are consistent with previous studies in Asian populations, which have shown the safety and efficacy of linagliptin as monotherapy in Japanese patients22 and when added to metformin plus sulfonylurea in Chinese patients with type 2 diabetes14.
In summary, these results show that linagliptin monotherapy was efficacious and well tolerated over 24 weeks in Asian patients with type 2 diabetes who were treatment naïve or had been previously treated with one oral antidiabetes drug.
Acknowledgments
This work was supported by Boehringer Ingelheim, Pharma GmbH & Co. KG. Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Mark Poirier of Envision Scientific Solutions during the preparation of this manuscript.
Disclosure
YC, GN, CW and WW have no financial interests to disclose. YG, SP, CZ, TI and HJW are employees of Boehringer Ingelheim. CZ was an employee of Boehringer Ingelheim at the time of the study, but is now an employee of AstraZeneca China.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Appendix S1| Changes in lipid levels from baseline at week 24 (full analysis set – observed cases).
Appendix S2 | Adjusted mean glycated hemoglobin change from baseline by subgroup at week 24 (full analysis set – last observation carried forward).
Figure S1 | Disposition of randomized patients.
Figure S2 | (a) Adjusted mean change in fasting plasma glucose (FPG) from baseline at week 24 with linagliptin or placebo as monotherapy (full analysis set – last observation carried forward). (b) Adjusted mean change from baseline in FPG over time (full analysis set – last observation carried forward). Model includes categorical baseline glycated hemoglobin, categorical baseline FPG, number of previous antidiabetes drugs, treatment group, and treatment by baseline glycated hemoglobin (categorical) interaction. SE, standard error.
References
- International Diabetes Federation. IDF Diabetes Atlas. 2011. Available at: www.idf.org/diabetesatlas/ (accessed April 18 2014)
- Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Snehalatha C, Vijay V. Low risk threshold for acquired diabetogenic factors in Asian Indians. Diabetes Res Clin Pract. 2004;65:189–195. doi: 10.1016/j.diabres.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281:64–91. doi: 10.1111/nyas.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graefe-Mody U, Retlich S, Friedrich C. Clinical pharmacokinetics and pharmacodynamics of linagliptin. Clin Pharmacokinet. 2012;51:411–427. doi: 10.2165/11630900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Graefe-Mody U, Rose P, Retlich S, et al. Pharmacokinetics of linagliptin in subjects with hepatic impairment. Br J Clin Pharmacol. 2012;74:75–85. doi: 10.1111/j.1365-2125.2012.04173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehringer Ingelheim Ltd. 2013. Trajenta Summary of Product Characteristics. Available at: http://www.medicines.org.uk/emc/medicine/25000/spc (accessed March 20 2013)
- Del Prato S, Barnett AH, Huisman H, et al. Effect of linagliptin monotherapy on glycaemic control and markers of beta-cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. 2011;13:258–267. doi: 10.1111/j.1463-1326.2010.01350.x. [DOI] [PubMed] [Google Scholar]
- Gomis R, Espadero RM, Jones R, et al. Efficacy and safety of initial combination therapy with linagliptin and pioglitazone in patients with inadequately controlled type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2011;13:653–661. doi: 10.1111/j.1463-1326.2011.01391.x. [DOI] [PubMed] [Google Scholar]
- Taskinen MR, Rosenstock J, Tamminen I, et al. Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2011;13:65–74. doi: 10.1111/j.1463-1326.2010.01326.x. [DOI] [PubMed] [Google Scholar]
- Owens DR, Swallow R, Dugi KA, et al. Efficacy and safety of linagliptin in persons with type 2 diabetes inadequately controlled by a combination of metformin and sulphonylurea: a 24-week randomized study. Diabet Med. 2011;28:1352–1361. doi: 10.1111/j.1464-5491.2011.03387.x. [DOI] [PubMed] [Google Scholar]
- Zeng Z, Choi DS, Mohan V, et al. Linagliptin is efficacious and well tolerated in Asian patients with inadequately controlled type 2 diabetes. Diabetes. 2012;61(Suppl 1):A300. [Google Scholar]
- Zeng Z, Yang JK, Tong N, et al. Efficacy and safety of linagliptin added to metformin and sulphonylurea in Chinese patients with type 2 diabetes: a sub-analysis of data from a randomised clinical trial. Curr Med Res Opin. 2013;29:921–929. doi: 10.1185/03007995.2013.805123. [DOI] [PubMed] [Google Scholar]
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- White W. Results from EXAMINE. Oral presentation at EASD 2013. Available at: http://www.easdvirtualmeeting.org/resources/7109 [accessed 16 December 2014]
- Rosenstock J, Marx N, Kahn SE, et al. Cardiovascular outcome trials in type 2 diabetes and the sulphonylurea controversy: rationale for the active-comparator CAROLINA trial. Diab Vasc Dis Res. 2013;10:289–301. doi: 10.1177/1479164112475102. [DOI] [PubMed] [Google Scholar]
- Pan CY, Yang W, Tou C, et al. Efficacy and safety of saxagliptin in drug-naive Asian patients with type 2 diabetes mellitus: a randomized controlled trial. Diabetes Metab Res Rev. 2012;28:268–275. doi: 10.1002/dmrr.1306. [DOI] [PubMed] [Google Scholar]
- Mohan V, Yang W, Son HY, et al. Efficacy and safety of sitagliptin in the treatment of patients with type 2 diabetes in China, India, and Korea. Diabetes Res Clin Pract. 2009;83:106–116. doi: 10.1016/j.diabres.2008.10.009. [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation. 2012. Global guideline for type 2 diabetes. Available at: http://www.idf.org/global-guideline-type-2-diabetes-2012 (accessed October 2 2013)
- Inagaki N, Watada H, Murai M, et al. Linagliptin provides effective, well-tolerated add-on therapy to pre-existing oral antidiabetic therapy over 1 year in Japanese patients with type 2 diabetes. Diabetes Obes Metab. 2013;15:833–843. doi: 10.1111/dom.12110. [DOI] [PubMed] [Google Scholar]
- Kawamori R, Inagaki N, Araki E, et al. Linagliptin monotherapy provides superior glycaemic control versus placebo or voglibose with comparable safety in Japanese patients with type 2 diabetes: a randomized, placebo and active comparator-controlled, double-blind study. Diabetes Obes Metab. 2012;14:348–357. doi: 10.1111/j.1463-1326.2011.01545.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1| Changes in lipid levels from baseline at week 24 (full analysis set – observed cases).
Appendix S2 | Adjusted mean glycated hemoglobin change from baseline by subgroup at week 24 (full analysis set – last observation carried forward).
Figure S1 | Disposition of randomized patients.
Figure S2 | (a) Adjusted mean change in fasting plasma glucose (FPG) from baseline at week 24 with linagliptin or placebo as monotherapy (full analysis set – last observation carried forward). (b) Adjusted mean change from baseline in FPG over time (full analysis set – last observation carried forward). Model includes categorical baseline glycated hemoglobin, categorical baseline FPG, number of previous antidiabetes drugs, treatment group, and treatment by baseline glycated hemoglobin (categorical) interaction. SE, standard error.


