Abstract
Aims/Introduction
Previous studies have reported osteoporosis measured by dual-energy X-ray absorptiometry in younger patients with type 1 diabetes. Limitations of 2-D imaging, however, limit the precision of dual-energy X-ray absorptiometry for the measurement of bone mineral density and bone strength.
Materials and Methods
Three-dimensional quantitative computed tomography was used to calculate volumetric-bone mineral density (vBMD) and strength in femoral bone subfractions. A total of 17 male type 1 diabetes patients and 18 sex-matched healthy controls aged from 18 to 49 years were investigated in the present cross-sectional study. Patients with overt nephropathy were excluded.
Results
Type 1 diabetes patients had significantly lower cortical vBMD in the femoral neck, and significantly lower total vBMD, cortical thickness and cortical cross-sectional area (cortical CSA) in the intertrochanter. Bone strength estimated by the buckling ratio (an index of cortical instability) of the intertrochanter was significantly higher in type 1 diabetes patients. The following serum bone markers were comparable between the two groups: bone-specific alkaline phosphatase, N-terminal propeptide of type 1 procollagen, osteocalcin, pentosidine and homocysteine. Serum insulin-like growth factor-1 values were significantly lower in the type 1 diabetes patients than in controls. Serum insulin-like growth factor-1values were positively correlated with serum bone formation markers, and the total vBMD of the femoral neck and lumbar spine in type 1 diabetes patients.
Conclusions
The present study is the first investigation by quantitative computed tomography measurement to show cortical instability and lower vBMD in the intertrochanter of young and middle-aged type 1 diabetes patients. Low insulin-like growth factor-1 might be a causative factor for osteoporosis in type 1 diabetes.
Keywords: Insulin-like growth factor-1, Quantitative computed tomography, Type 1 diabetes
Introduction
Many studies by dual-energy X-ray absorptiometry (DXA) have shown an elevated risk of decreased bone mineral density (BMD) in children with type 1 diabetes1–4. Other studies, meanwhile, have found no significant differences in BMD at the lumbar spine or proximal femur between patients with type 1 diabetes and controls5,6.
DXA is a two-dimensional imaging modality with limited precision in the measurement of BMD. DXA scans are also of limited use for longitudinal studies, as the planar technique is unsuitable for evaluating volumetric-BMD (vBMD) or tracking changes in body and skeletal size during growth7,8. DXA also fails to differentiate between cortical and trabecular BMD, or to measure cross-sectional geometry or biomechanical parameters, such as the cross-sectional moment of inertia (CSMI), section modulus (SM; a geometric index of bending stress) or buckling ratio (BR; an index of cortical instability).
Quantitative computed tomography (QCT), in contrast, can take 3-D measurements of the vBMD independently in cortical and trabecular bone compartments, and assess both cross-sectional geometry and biomechanical parameters. Researchers are taking advantage of these capabilities of QCT in a growing number of clinical studies on topics such as the effects of drug therapy on hip structure9–13, mechanical loading in the proximal femur, and the association of hip fractures with femoral strength and bone structure14–18.
Peripheral QCT studies of the radius or tibia have shown a lower vBMD in pediatric and adolescent patients with type 1 diabetes19–21. Yet to our knowledge, QCT has not been previously used to investigate the vBMD, cross-sectional geometry, or biomechanical parameters of femoral bone subfractions or the vBMD of the lumbar spine in young and middle-aged patients with type 1 diabetes.
Insulin-like growth factor-1 (IGF-1) is a growth-promoting polypeptide essential for normal growth and development. IGF-1 administration increases bone turnover and BMD in both humans and experimental animals22–24. Dysregulation of the growth hormone/IGF-1 axis is well-recognized in patients with type 1 diabetes. Many studies have shown that the low serum IGF-1 values in pediatric and adult patients with type 1 diabetes might contribute to the pathogenesis of reduced BMD25,26.
The aims of the present study were to determine the vBMD, cross-sectional geometry, and biomechanical parameters of femoral bone subfractions and the vBMD of the lumbar spine by QCT, and to elucidate the relationships between bone measurements and IGF-1values, in young and middle-aged patients with type 1 diabetes.
Materials and Methods
Materials
A total of 17 consecutive male patients with type 1 diabetes and 18 sex-matched controls were enrolled in the present cross-sectional study. All of the patients were observed regularly at the Showa University Hospital (Tokyo, Japan), and met the following criteria: (i) aged 18–49 years; (ii) duration of diabetes of at least 3 months; (iii) no evidence of overt nephropathy (urine albumin-to-creatinine ratio >300 mg/g creatinine or eGFR <60 mL/min/1.73 m2); (iv) no evidence of pre-proliferative diabetic retinopathy or proliferative diabetic retinopathy; (v) no chronic disease apart from positive thyroid antibodies with euthyroid status; and (vi) no restriction of physical activity. None of the participants were taking calcium preparations or medications or hormones known to affect bone metabolism. Diabetic retinopathy was graded as simple, pre-proliferative or proliferative retinopathy by ophthalmologists.
Healthy controls were recruited to serve as controls for patient groups from our medical staff who were interested in the status of their bone and accepted our study design. Possible side-effects of X-ray exposure with the radiation dose of approximately equal to abdomen and pelvic computed tomography (CT) were explained. All participants provided written informed consent, and the study was approved by the ethics committee of the Showa University School of Medicine.
Background
Bodyweight was measured with digital scales. Body mass index (BMI) was calculated as bodyweight (kg) / height2 (m2). Physical activity (1. exercise every day; 2. exercise two or three times a week; 3. never exercise) and calcium intake were evaluated by questionnaire. Smoking history and alcohol intake were evaluated in each group.
Biochemical Measurements
Blood samples were obtained from all participants in a non-fasting state and quickly stored at −80°C. Serum levels of total protein, albumin, alkaline phosphatase, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, calcium, magnesium and phosphorus were measured in all participants with an autoanalyzer in our hospital. Plasma levels of intact parathyroid hormone and serum IGF-1 were measured by chemiluminescent enzyme immunoassay and immunoradiometric assay, respectively. The Z-score of the serum IGF-1 measurement was calculated from the LMS method, using values appropriate for the age and sex27.
Serum levels of the following bone metabolic and quality markers were measured: bone-specific alkaline phosphatase, osteocalcin, N-propeptide of type I collagen, tartrate-resistant acid phosphatase type 5 protein, cross-linked N-telopeptide of type I collagen, pentosidine and homocysteine. The following methods were used to carry out the measurements: bone-specific alkaline phosphatase, by chemiluminescent enzyme immunoassay; osteocalcin, by immunoradiometric assay; intact N-propeptide of type I collagen, by double antibody-radio immunoassay; tartrate-resistant acid phosphatase type 5 protein and serum-N-telopeptide of type I collagen, by enzyme immunoassay; pentosidine, by enzyme-linked immunosorbent assay; and homocysteine, by high-performance liquid chromatography. The value for glycated hemoglobin (HbA1c; %) in the present study was estimated as a National Glycohemoglobin Standardization Program-equivalent value (%) calculated by the following formula: HbA1c (%) = HbA1c (Japan Diabetes Society [JDS]) (%) + 0.4%28.
CT Data Acquisition
CT data were acquired with a SOMATOM Definition AS+ multidetector-row CT scanner (Siemens, AG, Forchheim, Germany) using predefined scanning conditions (X-ray energy, 120 kV; X-ray current, 200 mA; rotation speed, 1.0 s/rot; beam pitch, 0.9) and predefined reconstruction parameters. The CT values were converted to a scale of bone mineral density using a calibration phantom (Mindways, Austin, TX, USA) placed underneath the patients during the scans.
Patient Position for CT Scanning
The participants were scanned in a supine position. The calibration phantom was placed directly beneath the participant in a position covering the region from the top of the lumbar spine (L1) to 2 cm below the bottom of the lesser trochanter.
Analysis of vBMD and bone geometry obtained by CT
The vBMD of the lumbar spine (L2, 3, 4 and L2-4), and the vBMD and bone geometry of the proximal femur were analyzed using QCT-Pro software v4.1.3 with the QCT-Pro Bone Investigational Toolkit v2.0 (BIT; Mindways Software, Austin, TX, USA). The cross-sectional femoral neck data were based on the geometrical axis. The total vBMD, cortical vBMD, cortical perimeter, cortical thickness, total cross-sectional area (CSA) and cortical CSA were also calculated for the femoral bone subfractions (femoral neck, intertrochanter and femoral shaft). These parameters could not be calculated for the femoral shaft in some of the cases (2 patients with type 1 diabetes and 8 controls), however, as a 2-cm distance could not be maintained below the bottom of the lesser trochanter when these participants were scanned.
Biomechanical Parameters of the Femoral Neck, Intertrochanter and Femoral Shaft
The CSMI, SM, and BR of the femoral neck, intertrochanter and femoral shaft were measured. The CSMI is defined as an integration of the product of the incremental cross-sectional area and the square of its distance from the center of mass (centroid). The SM, the ratio of CSMI to the maximal distance of the material from the centroid, is directly related to the strength with respect to a corresponding bending stress. BR, the maximal distance from the centroid divided by the average cortical thickness, is used to estimate the instability of the cortex in thin-walled regions subject to local compressive stress12.
Quality Control Procedures
To adjust for longitudinal changes of the detector, quality assurance scans were carried out with a calibration phantom every 3 months. Quality assurance measurements were evaluated according to the QCT-Pro quality assurance guide from Mindways. The reproducibility (% coefficient of variation) of the analysis by the QCT PRO program was calculated using five repeated analyses, each with visual matching, from the CT data sets of 10 healthy participants without visual artifacts. The QCT parameter values were as follows: vBMD 1.74%, cortical vBMD 1.70%, cortical thickness 2.29%, cortical perimeter 3.19%, total CSA 5.49%, cortical CSA 5.54%, CSMI 7.02%, SM 8.07%, BR 5.89% and total vBMD (spine) 0.42%.
Statistical Analysis
Data were analyzed using Stat Flex Ver.6 (Artech, Tokyo, Japan). The Student's t-test was used to determine the significance of differences between the groups. The Mann–Whitney U-test and χ2-tests were used to determine differences between patients with type 1 diabetes and controls with respect to physical activity and current smoking, respectively. Correlations were based on Pearson's correlation coefficient. All values calculated in the analysis were two-sided, and none were adjusted for multiple testing. P-values of <0.05 were considered statistically significant.
Results
Table1 shows the clinical characteristics of the study participants. The mean age of patients with type 1 diabetes was 38.2 ± 7.2 years (range 19–48 years). The mean age at onset and the duration of diabetes were 22.6 ± 9.9 years (range 1–39 years) and 15.6 ± 8.6 years (range 0–35 years), respectively. The bodyweight, height and BMI were similar between the two groups. Metabolic control was moderate to acceptable in most patients, with a mean HbA1c of 7.4% (range 6.4–10.3%). A total of 16 patients were receiving multiple injections (4–5 daily) of rapid-acting insulin-analogs and long-acting basal insulin, and one patient was receiving a continuous subcutaneous infusion of insulin. The mean daily insulin dose was 0.83 ± 0.17 U/kg/day. Two patients (12%) had simple retinopathy. The mean urine albumin-to-creatinine ratio was 11.2 ± 13.8 mg/g (range 2.5–60.9 mg/g) creatinine. A total of 16 patients (94%) had normoalbuminuria and one patient (6%) had microalbuminuria.
Table 1.
Clinical characteristics of type 1 diabetes patients and controls
| Type 1 diabetes patients (n = 17) | Controls (n = 18) | P | |
|---|---|---|---|
| Age (years) | 38.2 ± 7.2 | 35.7 ± 5.6 | 0.27 |
| Age at onset (years) | 22.6 ± 9.9 | – | – |
| Duration of diabetes (years) | 15.6 ± 8.6 | – | – |
| Bodyweight (kg) | 67.7 ± 7.9 | 71.3 ± 7.6 | 0.18 |
| Height (cm) | 171.6 ± 6.2 | 174.1 ± 5.0 | 0.19 |
| BMI (kg/m2) | 23.0 ± 2.6 | 23.6 ± 2.2 | 0.49 |
| HbA1c (%) | 7.4 ± 0.9 | – | – |
| Insulin dose (units/kg/day) | 0.83 ± 0.17 | – | – |
| Retinopathy | – | – | – |
| No retinopathy | 15 cases (88%) | – | – |
| Simple retinopathy | 2 cases (12%) | – | – |
| Urine albumin-to-creatinine ratio (mg/g creatinine) | 11.2 ± 13.8 | – | – |
| Physical activity† | Grade I: 2, grade II: 8, grade III: 7 | Grade I: 1, grade II: 15, grade III: 2 | 0.07 |
| Current smokers | 6 cases (35.3%) | 9 cases (50.0%) | 0.38 |
| Alcohol intake (g/day) | 21.5 ± 9.0 | 21.5 ± 8.3 | 1 |
Data are shown as mean ± standard deviation or n (%). †Grade I: exercise every day; grade II: exercise twice or thrice a week; grade III: no exercise. BMI, body mass index; HbA1c, glycated hemoglobin.
Physical activity, current smoking and alcohol intake were similar between the two groups.
Table2 shows comparative data on biochemical markers in patients with type 1 diabetes and controls. Serum levels of total protein, albumin, calcium and magnesium were significantly lower in patients with type 1 diabetes than in controls. Serum high-density lipoprotein cholesterol levels were significantly higher in patients with type 1 diabetes than in controls. Serum low-density lipoprotein cholesterol levels tended to be lower in patients with type 1 diabetes than in controls. The plasma level of intact parathyroid hormone and serum levels of alkaline phosphatase, total cholesterol and phosphorous did not significantly differ between the two groups. Serum IGF-1 and the IGF-1 Z-score were significantly lower in patients with type 1 diabetes than in controls (130.4 ± 34.0 ng/mL vs 158.0 ± 35.0 ng/mL, −1.2 ± 0.74 vs −0.53 ± 0.65, P < 0.05 and P < 0.01, respectively). Serum levels of bone metabolic markers were comparable between the two groups.
Table 2.
Serum biochemical markers and bone metabolic/quality markers of type 1 diabetes patients and controls
| Type 1 diabetes patients (n = 17) | Controls (n = 18) | P | |
|---|---|---|---|
| Total protein (g/dL) | 7.2 ± 0.4 | 7.5 ± 0.4 | <0.05 |
| Albumin (g/dL) | 4.5 ± 0.2 | 4.9 ± 0.30 | <0.01 |
| ALP (U/L) | 264.5 ± 92.5 | 225.8 ± 58.6 | 0.15 |
| TC (mg/dL) | 201.2 ± 34.5 | 207.7 ± 27.6 | 0.54 |
| HDL-C (mg/dL) | 67.7 ± 18.3 | 53.6 ± 9.3 | <0.01 |
| LDL-C (mg/dL) | 108.8 ± 22.0 | 123.0 ± 24.0 | 0.08 |
| Intact PTH (pg/mL) | 35.9 ± 14.7 | 35.5 ± 11.0 | 0.93 |
| Ca (mg/dL) | 9.3 ± 0.3 | 9.6 ± 0.3 | <0.01 |
| Mg (mg/dL) | 2.0 ± 0.2 | 3.3 ± 4.9 | 0.25 |
| P (mg/dL) | 3.5 ± 0.8 | 3.5 ± 0.6 | 0.94 |
| Serum IGF-1 (ng/mL) | 130.4 ± 34.0 | 158.0 ± 35.0 | <0.05 |
| IGF-1 Z-score | −1.2 ± 0.74 | −0.53 ± 0.65 | <0.01 |
| BAP (ug/L) | 16.7 ± 11.4 | 14.0 ± 5.7 | 0.39 |
| OC (ng/mL) | 5.9 ± 2.6 | 5.7 ± 2.9 | 0.80 |
| P1NP (ug/L) | 42.7 ± 37.0 | 47.1 ± 19.2 | 0.67 |
| TRACP-5b (mU/dL) | 316.6 ± 121.7 | 293.4 ± 96.1 | 0.54 |
| NTX (nmol BCE/L) | 16.1 ± 3.2 | 16.2 ± 4.3 | 0.92 |
| Pentosidine (ug/L) | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.58 |
| Homocysteine (nmol/mL) | 11.9 ± 3.0 | 10.8 ± 2.0 | 0.24 |
Data are shown as mean ± standard deviation. ALP, alkaline phosphatase; BAP, bone specific alkaline phosphatase; HDL-C, high-density lipoprotein cholesterol; IGF-1, insulin-like growth factor-1; LDL-C, low-density lipoprotein cholesterol; OC, osteocalcin; NTX, cross-linked N-telopeptide of type 1 collagen; P1PN, N-propeptide of type 1 collagen; PTH, parathyroid hormone; TC, total cholesterol; TRACP-5P, tartrate-resistant acid phosphatase type5 protein.
Table3 shows the vBMD and cross-sectional geometry of the femoral neck, intertrochanter, and femoral shaft and the vBMD of the lumbar spine in patients with type 1 diabetes and controls. In the femoral neck, patients with type 1 diabetes had a lower total vBMD (266.4 mg/cm3 vs 292.1 mg/cm3, P = 0.09), lower cortical thickness (3.0 mm vs 3.4 mm, P = 0.12) and significantly lower cortical vBMD compared with controls (557.1 mg/cm3 vs 581.5 mg/cm3, P < 0.05). In the intertrochanter, patients with type 1 diabetes had a significantly lower total vBMD, cortical thickness and cortical CSA compared with controls (222.7 mg/cm3 vs 258.5 mg/cm3, 2.8 mm vs 3.3 mm, 4.2 cm2 vs 5.2 cm2, respectively, all P < 0.01). In the femoral shaft, patients with type 1 diabetes had a significantly lower cortical perimeter and cortical CSA compared to controls. The vBMD of the lumbar spine (L2, 3, 4 and L2-4) was comparable between the two groups.
Table 3.
Quantitative computed tomography measurements data of type 1 diabetes patients and controls
| Type 1 diabetes patients | Controls | P | |
|---|---|---|---|
| Femoral neck | n = 17 | n = 18 | |
| Total vBMD (mg/cm3) | 266.4 ± 33.5 | 292.1 ± 51.1 | 0.09 |
| Cortical vBMD (mg/cm3) | 557.1 ± 31.8 | 581.5 ± 27.6 | <0.05 |
| Cortical perimeter (cm) | 10.3 ± 1.5 | 10.9 ± 1.4 | 0.23 |
| Cortical thickness (mm) | 3.0 ± 0.6 | 3.4 ± 0.9 | 0.12 |
| Total CSA (cm2) | 9.9 ± 1.2 | 9.7 ± 1.1 | 0.57 |
| Cortical CSA (cm2) | 2.7 ± 0.5 | 3.1 ± 0.8 | 0.11 |
| Inter trochanter | n = 17 | n = 18 | |
|---|---|---|---|
| Total vBMD (mg/cm3) | 222.7 ± 28.3 | 258.5 ± 42.8 | <0.01 |
| Cortical vBMD (mg/cm3) | 654.9 ± 32.6 | 668.3 ± 23.5 | 0.18 |
| Cortical perimeter (cm) | 16.0 ± 0.64 | 16.4 ± 1.0 | 0.18 |
| Cortical thickness (mm) | 2.8 ± 0.4 | 3.3 ± 0.6 | <0.01 |
| Total CSA (cm2) | 19.8 ± 1.5 | 20.5 ± 2.1 | 0.25 |
| Cortical CSA (cm2) | 4.2 ± 0.6 | 5.2 ± 1.1 | <0.01 |
| Femoral shaft | n = 15 | n = 10 | |
|---|---|---|---|
| Total vBMD (mg/cm3) | 582.9 ± 66.4 | 629.4 ± 82.5 | 0.13 |
| Cortical vBMD (mg/cm3) | 965.2 ± 65.6 | 981.0 ± 57.3 | 0.54 |
| Cortical perimeter (cm) | 9.9 ± 0.6 | 10.4 ± 0.5 | <0.05 |
| Cortical thickness (mm) | 5.8 ± 0.5 | 6.5 ± 1.1 | 0.08 |
| Total CSA (cm2) | 8.9 ± 3.2 | 8.8 ± 0.8 | 0.86 |
| Cortical CSA (cm2) | 4.6 ± 0.4 | 5.4 ± 0.9 | <0.05 |
| Lumbar spine | n = 17 | n = 18 | |
|---|---|---|---|
| L2 vBMD (mg/cm3) | 134.7 ± 22.8 | 145 ± 32.7 | 0.29 |
| L3 vBMD (mg/cm3) | 131.9 ± 23.4 | 139.5 ± 30.5 | 0.41 |
| L4 vBMD (mg/cm3) | 138.1 ± 23.8 | 142.5 ± 28.8 | 0.65 |
| Total (L2-4) vBMD (mg/cm3) | 134.9 ± 23.0 | 142.2 ± 30.4 | 0.42 |
Data are shown as mean ± standard deviation. CSA, cross-sectional area; vBMD, volumetric bone mineral density.
Long disease duration (≥20 years) was not associated with vBMD or cross-sectional geometry in the femoral bone subfractions or vBMD in the lumbar spine (Table S1). Type 1 diabetes patients with low BMI (≤22.5 kg/m2) had significantly lower total BMD and higher BR in the intertrochanter than type 1 diabetes patients with high BMI (Table S2).
Figure1 shows biomechanical parameters of the femoral neck, intertrochanter and femoral shaft in patients with type 1 diabetes and controls. In the femoral neck and femoral shaft, the CMSI, SM and BR were comparable between the two groups. In the intertrochanter, patients with type 1 diabetes had a significantly higher BR compared with controls (12.9 ± 1.9 vs 10.8 ± 1.9, P < 0.01), but no significant difference was found between the two groups in the CMSI or SM.
Figure 1.
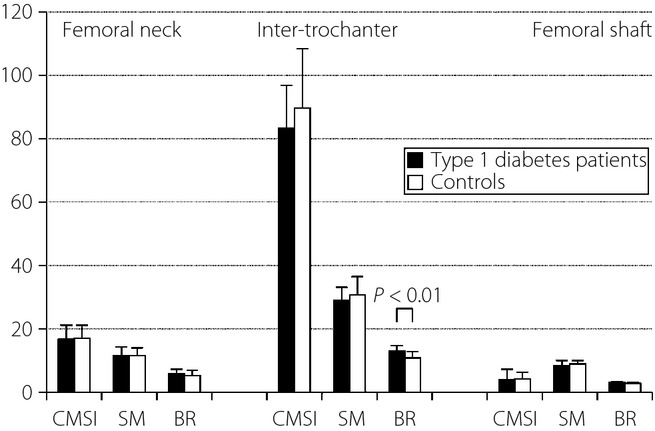
Biomechanical parameters of the femoral neck, intertrochanter, and femoral shaft of type 1 diabetes patients and controls. BR, buckling ratio; CMSI, cross-sectional moment of inertia; SM, section modulus.
Table4 shows simple correlations of serum IGF-1 values with the QCT measurements in the femoral neck, intertrochanter and femoral shaft, and with the vBMD of the lumbar spine. The serum IGF-1 values in patients with type 1 diabetes correlated positively with the vBMD of the femoral neck and lumbar spine, and tended to correlate with the cortical CSA of the femoral neck and intertrochanter, but the relationships did not reach statistical significance. Serum IGF-1 values in patients with type 1 diabetes tended to correlate negatively with the BR of the femoral neck and intertrochanter.
Table 4.
Simple correlations between serum insulin-like growth factor-1 levels and quantitative computed tomography measurements
| Type 1 diabetes patients | Controls | |||
|---|---|---|---|---|
| r | P | r | P | |
| Femoral neck | n = 17 | n = 18 | ||
| Total vBMD (mg/cm3) | 0.49 | <0.05 | 0.13 | 0.59 |
| Cortical vBMD (mg/cm3) | −0.13 | 0.63 | 0.32 | 0.19 |
| Cortical perimeter (cm) | 0.06 | 0.82 | −0.38 | 0.12 |
| Cortical thickness (mm) | 0.39 | 0.12 | 0.03 | 0.92 |
| Total CSA (cm2) | 0.08 | 0.77 | −0.09 | 0.72 |
| Cortical CSA (cm2) | 0.44 | 0.07 | 0.01 | 0.98 |
| CSMI (cm4) | −0.04 | 0.89 | −0.13 | 0.62 |
| SM (cm3) | −0.03 | 0.9 | −0.04 | 0.86 |
| BR | −0.44 | 0.08 | −0.11 | 0.66 |
| Intertrochanter | n = 17 | n = 18 | ||
|---|---|---|---|---|
| Total vBMD (mg/cm3) | 0.28 | 0.28 | 0.26 | 0.3 |
| Cortical vBMD (mg/cm3) | −0.25 | 0.33 | 0.34 | 0.16 |
| Cortical perimeter (cm) | −0.04 | 0.88 | −0.16 | 0.53 |
| Cortical thickness (mm) | 0.39 | 0.12 | 0.19 | 0.46 |
| Total CSA (cm2) | 0.04 | 0.88 | −0.14 | 0.58 |
| Cortical CSA (cm2) | 0.36 | 0.16 | 0.05 | 0.83 |
| CSMI (cm4) | −0.07 | 0.78 | −0.20 | 0.43 |
| SM (cm3) | −0.04 | 0.88 | −0.17 | 0.51 |
| BR | −0.42 | 0.09 | −0.21 | 0.39 |
| Femoral shaft | n = 15 | n = 10 | ||
|---|---|---|---|---|
| Total vBMD (mg/cm3) | 0.26 | 0.34 | −0.08 | 0.82 |
| Cortical vBMD (mg/cm3) | 0.13 | 0.65 | −0.19 | 0.61 |
| Cortical perimeter (cm) | −0.04 | 0.89 | 0.35 | 0.32 |
| Cortical thickness (mm) | 0.35 | 0.2 | 0.03 | 0.93 |
| Total CSA (cm2) | 0.39 | 0.15 | 0.34 | 0.46 |
| Cortical CSA (cm2) | 0.18 | 0.52 | 0.15 | 0.68 |
| CSMI (cm4) | −0.19 | 0.5 | 0.34 | 0.34 |
| SM (cm3) | −0.19 | 0.51 | 0.35 | 0.32 |
| BR | −0.28 | 0.32 | 0.02 | 0.95 |
| Lumbar spine total (L2-4) | n = 17 | n = 18 | ||
|---|---|---|---|---|
| Total vBMD (mg/cm3) | 0.60 | <0.05 | 0.08 | 0.76 |
BR, buckling ratio; CSA, cross-sectional area; CSMI, cross-sectional moment of inertia; SM, section modulus; vBMD, volumetric bone mineral density.
We also measured 2-D BMD using QCT (CTXA-hip). The 2-D BMD values measured by QCT did not significantly differ between the patients with type 1 diabetes and controls in the total hip and femoral neck (0.85 ± 0.10 g/cm2 vs 0.97 ± 0.17 g/cm2 and 0.74 ± 0.10 g/cm2 vs 0.80 ± 0.15 g/cm2, P = 0.07, P = 0.23, respectively).
Discussion
The QCT findings in this cross-sectional case–control study demonstrated cortical instability and lower vBMD, cortical thickness, and cortical CSA in the intertrochanter of young and middle-aged patients with type 1 diabetes.
Peripheral QCT has been the chief imaging modality for the assessment of the vBMD at appendicular sites in pediatric and adolescent patients with type 1 diabetes for several years19,20. Peripheral QCT is preferred to central QCT, as the higher radiation dose of the latter poses greater risk, especially to pediatric patients21,29. The present report is the first to determine bone measurements of the lumbar spine and proximal femur by central QCT in young and middle-aged patients with type 1 diabetes.
The vBMD of the proximal femur measured by QCT is strongly predictive of hip fracture in both postmenopausal women17,18,30 and older men15. Few reports, however, have described the associations of vBMD with hip fracture in diabetic patients. The bone geometry of the proximal femur measured by QCT is thought to be strongly linked to bone strength. According to a recent report from Ito et al.18, a lower cortical CSA measured by QCT in the proximal femur is predictive of intertrochanteric fracture in elderly Japanese women. CSA has proven to be a reliable estimator of compression strength18. Several studies have shown associations linking femoral neck fracture to a lower CSMI and SM, and higher BR of the proximal femur18,30,32. The relationship of hip fractures with the hip axis length and neck-shaft angle measured by DXA scan has been investigated for years. A longer hip axis length9,18 and wider neck-shaft angle33 were found to be associated with hip fracture independently of BMD in postmenopausal women. Hip axis length and neck-shaft angle were comparable between patients with type 1 diabetes and controls in the present study (data not shown). Consensus has yet to be reached on which structural features of the proximal femur are most important as risk factors for fracture. Ample evidence has shown, however, that proximal femurs with thinner walls tend to buckle, regardless of the BMD9,12,13,18. Given this finding, our present results suggest that a young or middle-aged patient with type 1 diabetes with a lower vBMD, lower cortical CSA, lower cortical thickness and higher BR of the intertrochanter could face an elevated risk of intertrochanteric fracture in the future.
The BR in patients with type 1 diabetes was significantly higher only in the intertrochanter compared with controls. This stands to reason, as the cortical thickness differed between the groups in the intertrochanter, but not in the femoral neck or femoral shaft (see Table3).
The total vBMD of the lumbar spine did not differ between patients with type 1 diabetes and controls in the present study. The lumbar spine is mainly composed of metabolically active trabecular bone, and decreased BMD of the lumbar spine is associated with poor metabolic control4,34,35. The relatively good metabolic control in our participants (mean HbA1c 7.4%) could explain the absence of any decrease in the total vBMD of the lumbar spine in the present results.
Although the underlying mechanisms contributing to skeletal deficits in type 1 diabetes are poorly understood and probably multifactorial, a consistent feature of type 1 diabetes, both in humans and rodents, is a deficiency of insulin within the hepatic portal circulation36. A lack of portal insulin is associated with systemic deficiencies of IGF-1. Accumulating evidence suggests that disturbed IGF-1 homeostasis in type 1 diabetes might be responsible for the skeletal deficits observed. Indeed, the replacement of IGF-1 in rodent models of type 1 diabetes results in significant improvement in bone healing despite persistent hyperglycemia37. In the present study, patients with type 1 diabetes had significantly lower IGF-1 values and IGF-1 Z-scores than controls. Furthermore, the serum IGF-1 values were positively correlated with the total vBMD of the femoral neck and lumbar spine in patients with type 1 diabetes, but not in controls.
Previous studies of children and adolescents with type 1 diabetes have similarly shown positive associations between serum IGF-1 values and bone mineral content measured by DXA20,25,38.
A transgenic mouse model with specific IGF-1 gene inactivation in the liver manifested a reduction of the cortical CSA associated with decreased periosteal circumference and weaker bone determined by mechanical testing39. Meanwhile, Jiang et al.40 observed a longer cortical width and increased cortical CSA in transgenic mice with osteoblast-targeted IGF-1. Similarly, the serum IGF-1 values in patients with type 1 diabetes showed a weak positive association with the cortical CSA, and a negative, less-than-significant association with the BR in both the femoral neck and intertrochanter (see Table4). Serum IGF-1 values could be indispensable for maintaining the cortical CSA of the proximal femur in patients with type 1 diabetes. Although both our results and previous studies37 show that IGF-1 is more important in cortical bone than in trabecular bone, serum IGF-1 values in patients with type 1 diabetes were positively correlated with the total vBMD of the lumbar spine containing a relatively higher proportion of trabecular bone in the present study. Further studies will be required to show the relationship between IGF-1 and cortical or trabecular bone distribution.
Bone formation markers (bone-specific alkaline phosphatase, N-propeptide of type I collagen and osteocalcin) were comparable between patients with type 1 diabetes and controls, whereas serum IGF-1 values were positively correlated with the serum bone formation markers in patients with type 1 diabetes, but not in controls (data not shown). Several earlier studies have also found that IGF-1 values correlate positively with serum markers of bone formation in patients with type 1 diabetes20,25,41.
The present study had several limitations requiring further consideration. First, the study was a preliminary clinical study with a cross-sectional design that precluded any revelations on cause–effect relationships. Second, the relatively small sample in the present study (n = 35 total) limited the precision of our results. Larger studies will be required to more precisely characterize the proximal femur geometry measured by QCT in patients with type 1 diabetes. Third, we were unable to count the numbers of patients with osteopenia and osteoporosis in the present study, as no consensus standards have been developed in Japan for assigning diagnostic categories for osteopenia and osteoporosis based on measurements of spine BMD by QCT.
We conclude that QCT might be a useful tool for investigating the proximal femur geometry in patients with type 1 diabetes. The present study is the first investigation by QCT to show cortical instability and lower vBMD in the intertrochanter of young and middle-aged patients with type 1 diabetes. IGF-1 markedly influences the vBMD of the femoral neck and bone formation in patients with type 1 diabetes.
Disclosure
The authors declare no conflict of interest.
Supporting Information
Comparison of quantitative computed tomography measurements data between diabetes duration <20 years groups and diabetes duration ≥20 years groups in type 1 diabetes patients.
Table S2 Comparison of quantitative computed tomography measurements data between body mass index <22.5 kg/m2 groups and body mass index ≥22.5 kg/m2 groups in type 1 diabetes patients.
References
- Levin ME, Boisseau VC, Avioli LV. Effects of diabetes mellitus on bone mass in juvenile and adult-onset diabetes. N Engl J Med. 1976;294:241–245. doi: 10.1056/NEJM197601292940502. [DOI] [PubMed] [Google Scholar]
- Hui SL, Epstein S, Johnston CC., Jr A prospective study of bone mass in patients with type I diabetes. J Clin Endocrinol Metab. 1985;60:74–80. doi: 10.1210/jcem-60-1-74. [DOI] [PubMed] [Google Scholar]
- Heap J, Murray MA, Miller SC, et al. Alterations in bone characteristics associated with glycemic control in adolescents with type 1 diabetes mellitus. J Pediatr. 2004;144:56–62. doi: 10.1016/j.jpeds.2003.10.066. [DOI] [PubMed] [Google Scholar]
- Valerio G, Del Puente A, Esposito-del Puente A, et al. The lumbar bone mineral density is affected by long-term poor metabolic control in adolescents with type 1 diabetes mellitus. Horm Res. 2002;58:266–272. doi: 10.1159/000066441. [DOI] [PubMed] [Google Scholar]
- Pascual J, Argente J, Lopez MB, et al. Bone mineral density in children and adolescents with diabetes mellitus type 1 of recent onset. Calcif Tissue Int. 1998;62:31–35. doi: 10.1007/s002239900390. [DOI] [PubMed] [Google Scholar]
- Liu EY, Wactawski-Wende J, Donahue RP, et al. Does low bone mineral density start in post-teenage years in women with type 1 diabetes? Diabetes Care. 2003;26:2365–2369. doi: 10.2337/diacare.26.8.2365. [DOI] [PubMed] [Google Scholar]
- Lang TF, Guglielmi G, van Kuijk C, et al. Measurement of bone mineral density at the spine and proximal femur by volumetric quantitative computed tomography and dual-energy X-ray absorptiometry in elderly women with and without vertebral fractures. Bone. 2002;30:247–250. doi: 10.1016/s8756-3282(01)00647-0. [DOI] [PubMed] [Google Scholar]
- Blake GM, Fogelman I. Technical principles of dual energy X-ray absorptiometry. Semin Nucl Med. 1997;27:210–228. doi: 10.1016/s0001-2998(97)80025-6. [DOI] [PubMed] [Google Scholar]
- Ito M, Oishi R, Fukunaga M, et al. The effects of once-weekly teriparatide on hip structure and biomechanical properties assessed by CT. Osteoporos Int. 2014;25:1163–1172. doi: 10.1007/s00198-013-2596-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DM, Greenspan SL, Ensrud KE, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–1215. doi: 10.1056/NEJMoa031975. [DOI] [PubMed] [Google Scholar]
- Eastell R, Lang T, Boonen S, et al. Effect of once-yearly zoledronic acid on the spine and hip as measured by quantitative computed tomography: results of the HORIZON Pivotal Fracture Trial. Osteoporos Int. 2010;21:1277–1285. doi: 10.1007/s00198-009-1077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Nakamura T, Fukunaga M, et al. Effect of eldecalcitol, an active vitamin D analog, on hip structure and biomechanical properties: 3D assessment by clinical CT. Bone. 2011;49:328–334. doi: 10.1016/j.bone.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Borggrefe J, Graeff C, Nickelsen TN, et al. Quantitative computed tomography assessment of the effects of 24 months of teriparatide treatment on 3-D femoral neck bone distribution, geometry and bone strength: results from the EUROFORS. J Bone Miner Res. 2010;25:472–481. doi: 10.1359/jbmr.090820. [DOI] [PubMed] [Google Scholar]
- Amin S, Kopperdhal DL, Melton LJ, III, et al. Association of hip strength estimates by finite element analysis with fractures in women and men. J Bone Miner Res. 2011;26:1593–1600. doi: 10.1002/jbmr.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DM, Bouxsein ML, Marshall LM, et al. Proximal femoral structure and the prediction of hip fracture in men: a large prospective study using QCT. J Bone Miner Res. 2008;23:1326–1333. doi: 10.1359/JBMR.080316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousson VD, Adams J, Engelke K, et al. In vivo discrimination of hip fracture with quantitative computed tomography: results from the prospective European Femur Fracture Study (EFFECT) J Bone Miner Res. 2011;26:881–893. doi: 10.1002/jbmr.270. [DOI] [PubMed] [Google Scholar]
- Cheng X, Li J, Lu Y, et al. Proximal femoral density and geometry measurements by quantitative computed tomography: association with hip fracture. Bone. 2007;40:169–174. doi: 10.1016/j.bone.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Ito M, Wakao N, Hida T, et al. Analysis of hip geometry by clinical CT for the assessment of hip fracture risk in elderly Japanese women. Bone. 2010;46:453–457. doi: 10.1016/j.bone.2009.08.059. [DOI] [PubMed] [Google Scholar]
- Lettgen B, Hauffa B, Möhlmann C, et al. Bone mineral density in children and adolescents with juvenile diabetes: selective measurement of bone mineral density of trabecular and cortical bone using peripheral quantitative computed tomography. Horm Res. 1995;43:173–175. doi: 10.1159/000184273. [DOI] [PubMed] [Google Scholar]
- Moyer-Mileur LJ, Slater H, Jordan KC, et al. IGF-1 and IGF-binding proteins and bone mass, geometry, and strength: relation to metabolic control in adolescent girls with type 1 diabetes. J Bone Miner Res. 2008;23:1884–1891. doi: 10.1359/jbmr.080713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggen I, Gies I, Vanbesien J, et al. Trabecular bone mineral density and bone geometry of the distal radius at completion of pubertal growth in childhood type 1 diabetes. Horm Res Paediatr. 2013;79:68–74. doi: 10.1159/000346686. [DOI] [PubMed] [Google Scholar]
- Mueller K, Cortesi R, Modrowski D, et al. Stimulation of trabecular bone formation by insulin-like growth factor I in adult ovariectomized rats. Am J Physiol. 1994;267:E1–E6. doi: 10.1152/ajpendo.1994.267.1.E1. [DOI] [PubMed] [Google Scholar]
- Wakisaka A, Tanaka H, Barnes J, et al. Effect of locally infused IGF-I on femoral gene expression and bone turnover activity in old rats. J Bone Miner Res. 1998;13:13–19. doi: 10.1359/jbmr.1998.13.1.13. [DOI] [PubMed] [Google Scholar]
- Zhao G, Monier-Faugere MC, Langub MC, et al. Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology. 2000;141:2674–2682. doi: 10.1210/endo.141.7.7585. [DOI] [PubMed] [Google Scholar]
- Kemink SA, Hermus AR, Swinkels LM, et al. Osteopenia in insulin-dependent diabetes mellitus; prevalence and aspects of pathophysiology. J Endocrinol Invest. 2000;23:295–303. doi: 10.1007/BF03343726. [DOI] [PubMed] [Google Scholar]
- Mohan S, Baylink DJ. IGF-binding proteins are multifunctional and act via IGF-dependent and –independent mechanisms. J Endocrinol. 2002;175:19–31. doi: 10.1677/joe.0.1750019. [DOI] [PubMed] [Google Scholar]
- Isojima T, Shimatsu A, Yokoya S, et al. Standardized centile curves and reference intervals of serum insulin-like growth factor-I (IGF-I) levels in a normal Japanese population using the LMS method. Endocr J. 2012;59:771–780. doi: 10.1507/endocrj.ej12-0110. [DOI] [PubMed] [Google Scholar]
- The Committee of Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig. 2010;1:212–228. doi: 10.1111/j.2040-1124.2010.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows M, Liu D, McKay H. High-resolution peripheral QCT imaging of bone micro-structure in adolescents. Osteoporos Int. 2010;21:515–520. doi: 10.1007/s00198-009-0913-2. [DOI] [PubMed] [Google Scholar]
- Yang L, Udall WJM, McCloskey EV, et al. Distribution of bone density and cortical thickness in the proximal femur and their association with hip fracture in postmenopausal women: a quantitative computed tomography study. Osteoporos Int. 2014;25:251–263. doi: 10.1007/s00198-013-2401-y. [DOI] [PubMed] [Google Scholar]
- Rivadeneira F, Zillikens MC, De Laet CE, et al. Femoral neck BMD is a strong predictor of hip fracture susceptibility in elderly men and women because it detects cortical bone instability: the Rotterdam Study. J Bone Miner Res. 2007;22:1781–1790. doi: 10.1359/jbmr.070712. [DOI] [PubMed] [Google Scholar]
- Kaptoge S, Beck TJ, Reeve J, et al. Prediction of Incident Hip Fracture Risk by Femur Geometry Variables Measured by Hip Structural Analysis in the Study of Osteoporotic Fractures. J Bone Miner Res. 2008;23:1892–1904. doi: 10.1359/JBMR.080802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnudi S, Sitta E, Pignotti E. Prediction of incident hip fracture by femoral neck bone mineral density and neck–shaft angle: a 5-year longitudinal study in post-menopausal females. Br J Radiol. 2012;85:e467–e473. doi: 10.1259/bjr/57130600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer-Mileur LJ, Dixon SB, Quick JL, et al. Bone mineral acquisition in adolescents with type 1 diabetes. J Pediatr. 2004;145:662–669. doi: 10.1016/j.jpeds.2004.06.070. [DOI] [PubMed] [Google Scholar]
- Danielson KK, Elliott ME, LeCaire T, et al. Poor glycemic control is associated with low BMD detected in premenopausal women with type 1 diabetes. Osteoporos Int. 2009;20:923–933. doi: 10.1007/s00198-008-0763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedman CA, Frystyk J, Lindstrom T, et al. Residual beta-cell function more than glycemic control determines abnormalities of the insulin-like growth factor system in type 1 diabetes. J Clin Endocrinol Metab. 2004;89:6305–6309. doi: 10.1210/jc.2004-0572. [DOI] [PubMed] [Google Scholar]
- Fowlkes JL, Nyman JS, Bunn RC, et al. Osteo-promoting effects of insulin-like growth factor I (IGF-I) in a mouse model of type 1 diabetes. Bone. 2013;57:36–40. doi: 10.1016/j.bone.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léger J, Marinovic D, Alberti C, et al. Lower bone mineral content in children with type 1 diabetes mellitus is linked to female sex, low insulin-like growth factor type I levels, and high insulin requirement. J Clin Endocrinol Metab. 2006;91:3947–3953. doi: 10.1210/jc.2006-0711. [DOI] [PubMed] [Google Scholar]
- Sjogren K, Sheng M, Moverare S, et al. Effects of liver-derived insulin-like growth factor I on bone metabolism in mice. J Bone Miner Res. 2002;17:1977–1987. doi: 10.1359/jbmr.2002.17.11.1977. [DOI] [PubMed] [Google Scholar]
- Jiang J, Lichtler AC, Gronowicz GA, et al. Transgenic mice with osteoblast-targeted insulin-like growth factor-I show increased bone remodeling. Bone. 2006;39:494–504. doi: 10.1016/j.bone.2006.02.068. [DOI] [PubMed] [Google Scholar]
- Bouillon R, Bex M, Van Herck E, et al. Influence of age, sex, and insulin on osteoblast function: osteoblast dysfunction in diabetes mellitus. J Clin Endocrinol Metab. 1995;80:1194–1202. doi: 10.1210/jcem.80.4.7714089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of quantitative computed tomography measurements data between diabetes duration <20 years groups and diabetes duration ≥20 years groups in type 1 diabetes patients.
Table S2 Comparison of quantitative computed tomography measurements data between body mass index <22.5 kg/m2 groups and body mass index ≥22.5 kg/m2 groups in type 1 diabetes patients.


