Abstract
Background
We assembled a prospective cohort of 3144 children less than 15 years of age initiating ART in Dar es Salaam, Tanzania.
Methods
The relationships of nutritional status and other baseline characteristics in relation to mortality were examined using Cox proportional hazards model.
Results
Compared with children with weight for age (WAZ) >−1, those with WAZ ≤−2 to <−3 had a nearly double risk of death (RR, 1.85 (95% CI, 1.10–3.11), and among those with WAZ ≤−3, the risk more than tripled (RR, 3.36 (95% CI, 2.12–5.32). Other baseline risk factors for overall mortality included severe anemia (P<0.001), severe immune suppression (P=0.02), history of tuberculosis (P=0.01), opportunistic infections (P<0.001), living in the poorest district (P<0.001), and advanced WHO stage (P=0.003).
Conclusions
To sustain the obtained benefit of ART in this setting, interventions to improve nutritional status may be used as adjunct to ART.
INTRODUCTION
Human immunodeficiency virus (HIV) infection is a major contributor of the global disease burden among children and adults. Globally, it is estimate that 2.5% of all child deaths are associated with HIV infection [1]. Africa remains the region most heavily affected, with 5% of all child deaths associated with HIV infection [1]. Introduction of antiretroviral therapy (ART) has reduced the mortality rates globally [2] through suppression of viral replication and HIV disease progression among patients receiving ART. ART access, particularly among children however, is still lagging. In addition, many of the low– and middle–income countries still face significant challenges, including higher mortality in the first few months following the initiation of ART [3,4]. There is scarcity of information on factors contributing to high mortality among Tanzanian children on ART despite the existence of the free ART program by the government since 2004.
Furthermore, mortality experience from studies of adults are not necessarily applicable to children for several reasons including possible in utero exposure to antiretroviral drugs (ARVs), differences in immunologic markers and levels among children of different age groups, and the continuing development and maturation of organ systems involved in immunity. However, a few studies have identified predictors of mortality among children infected with HIV who are initiating ART in Africa [4,5,6,7]. Some factors that have been related to the increased risk of death in adults and children include immune reconstitution inflammatory syndrome [8], undernutrition [4,5,6], anemia [7,9], severe immune suppression [10,11], and opportunistic infections including TB [12,13].
Identification of predictors of mortality among children initiating ART is critical in efforts to improve pediatric patients' outcomes. This study examined child undernutrition and other characteristics in relation to mortality among children < 15 years, initiating ART in the Management and Development for Health (MDH) program in Dar es Salaam, Tanzania from October 2004 through December 2010.
METHODS
Study Design, Setting and Participants
We performed a prospective cohort study among HIV–infected children initiating ART at MDH between October 2004 and December 2010. MDH is a Tanzanian–based non-governmental organization supporting high quality HIV/AIDS care and treatment services in Dar es Salaam. The program was funded under the President's Emergency Plan for AIDS Relief (PEPFAR) as part of a Harvard School of Public Health initiative to provide ART access, management and care in Botswana, Nigeria and Tanzania. The program provides infrastructure, laboratory and technical support to HIV/AIDS Care and Treatment Centers and health facilities providing PMTCT and tuberculosis (TB) services in Dar es Salaam city and its suburbs.
All patients in this program received free routine care and treatment for HIV as per WHO–approved guidelines designed by the Tanzania National AIDS Control Program (NACP) of the Ministry of Health and Social Welfare. Accordingly, Children were eligible for ART when presented with a WHO clinical stage 4 or 3, irrespective of the absolute CD4+ cells count or percentage, or WHO stage 1 or 2 and severe immunodeficiency. In general, eligible children were initiated on one of the recommended first line ARV regimens which included at least two nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) and one non-nucleoside reverse transcriptase inhibitor (NNRTI) or two NRTIs and one protease inhibitor (PI). Patients also received supportive care which included prophylaxis and treatment of opportunistic infections and other conditions. Eligible patients for this study were children age <15 years initiating ART.
Clinical procedures
Following enrollment, patients were evaluated monthly at the outpatient clinics. At each visit, they were examined by a physician, underwent adherence and nutrition counseling, and received ARV refills. Laboratory tests (complete blood count, liver function tests, serum creatinine and a lipid panel, and CD4+ cells) were performed every four months. Measurements of CD4+ cells count and percentage were obtained by standard flow cytometric methods with FacsCount or FacsCalibur machines. All children were screened for TB by a chest X-ray and sputum analysis at enrollment, and those found to have TB were treated according to the standard of care according to the Tanzania National TB and Leprosy control program guidelines.
Weight, height and mid-upper arm circumference (MUAC) were measured by trained nurses. Weight was measured to a nearest 0.1 Kg using electronic Seca scales (Seca 334 for children aged ≤5 years, and Seca 700 for children aged >5 years) with children wearing minimal clothing. Child height (recumbent length if less than 2 years of age) was measured using wooden height/length boards. MUAC was measured by tape measures.
Definition of outcomes
The primary endpoint for this study was all-cause mortality. Child deaths were either recorded following notification by family members, friends or the patient tracking team. Date and place of death were recorded, and if the date of death was unknown, the date of the last encounter with the patient was used as the day of death. Early mortality was defined as death occurring within the first 90 days following ARV initiation, and overall mortality was defined as death occurring at any point in time since ARV initiation.
Statistical Analysis
Standardized weight-for-age (WAZ), height/length-for-age (HAZ), weight-for-height/length (WHZ) Z-scores (for children 2 years or younger) and body mass index (BMIZ) Z-scores (for children above 2 years) were calculated using the WHO Child Growth Standard reference data [14]. Z scores were categorized into the following categories, >−1, −2< and ≤−1, −3< and ≤−2, and ≤−3. A child was considered to be underweight, stunted, or wasted when WAZ, HAZ, or WHZ/BMIZ Z-score respectively was below −2 of the reference population. MUAC was conveniently categorized into >13.5 cm, 12.5 to 13.5cm, ≤11.5cm to <12.5cm, and <11.5cm. Using the WHO guidelines, children up to five years of age were classified as having low MUAC if they had MUAC <11.5cm. However, for children older than 5 years, a cut-off of 16.4cm (10th percentile) was adopted from NHANES (National Health and Nutritional examination Survey) 2003–06 conducted by Centers for Disease Control (CDC), National Centre for Health Statistics (NCHS) for children older than 5 years [15].
Kaplan-Meier curves were used to estimate time to death in strata of child nutritional status. The association between baseline nutritional status, change in nutritional status within the first 90 days, and other baseline characteristics with mortality were examined using the Cox proportional hazards model for time to death with age of the child in years as the time scale. Children were censored at the end of study in December 2010 if they were event-free, or at their last visit. Cox proportional hazards models were run for the entire period of study, and for the first 90 days after ART initiation. Relative risks (hazard ratios), 95% confidence intervals, and corresponding P values were obtained from the models adjusting for potential confounders. Variables were included in the multivariate models if the estimated RR for their association with mortality was statistically significant at P ≤0.20 in the univariate analyses, or if they were a priori believed to be risk factors for mortality. Accordingly, these variables were adjusted for in the models; nutritional status, calendar year, level of immune suppression, WHO stage, history of TB, opportunistic infections, ARV regimen, district, cotrimoxazole use, season of ART initiation, and child's sex. The missing indicator method was used for missing data in categorical covariates. The criterion for significance for all the analyses was a P value significant at level of α = 0.05. All P values were 2 tailed. All statistical analyses were performed with the statistical software package SAS (version 9.2, SAS Institute Inc., Cary, NC).
The study was approved by the Institutional Review Board of the Harvard School of Public Health.
RESULTS
Between October 2004, and December 2010, 3180 children were initiated on ART. After excluding 36 children who had invalid or missing information on age, 3144 remained for this analysis, contributing a median of 17.2 months (interquartile range [IQR], 4.6–31.4 months) of follow-up. Table 1 presents characteristics of the study participants at ART initiation. Briefly, the prevalence of child undernutrition at ART initiation was high, with 53% of the children underweight, 33% wasted, 56% stunted and 39% had low MUAC. All the children were initiated on the recommended first line ARV regimens, with the majority of children (52%) put on a zidovudine plus lamuivudine plus nevirapine regimen.
Table 1.
Demographic and clinical characteristics of HIV-infected children at the time of antiretroviral therapy (ART) initiation in Dar es Salaam, Tanzania between 2004–2010 (n = 3144)
| Characteristic | No. of subjects1 (%) |
|---|---|
| Age (years) | |
| 0 – 2 | 927 (29) |
| >2 – 5 | 687 (22) |
| >5 | 1530 (49) |
| Sex | |
| Male | 1562 (50) |
| Female | 1582 (50) |
| District of residence | |
| Ilala | 1372 (44) |
| Kinondoni | 888 (29) |
| Temeke | 824 (27) |
| Year of ART initiation | |
| 2004 – 2005 | 204 (6) |
| 2006 | 222 (7) |
| 2007 | 617 (20) |
| 2008 | 841 (27) |
| 2009 | 736 (23) |
| 2010 | 524 (17) |
| Underweight (weight-for-age z score <−2SD) | |
| Yes | 1275 (53) |
| No | 1145 (47) |
| Wasting (weight-for-height z score <−2SD if ≤2 years; body-mass index z score <−2 SD if >2 years) | |
| Yes | 990 (33) |
| No | 1976 (67) |
| Stunting (height-for-age z score <−2 SD) | |
| Yes | 1673 (56) |
| No | 1323 (44) |
| Low mid-upper arm circumference (11.5cm if ≤5 years; 16.4 if >5years)2 | |
| Yes | 1222 (39) |
| No | 1880 (61) |
| Severe anemia (Hemoglobin <8.5g/dL) | |
| Yes | 502 (23) |
| No | 1684 (77) |
| Severe immune suppression (CD4+ T-cells<15% if ≤5 years; <200 cells/μL if >5 years)3 | |
| Yes | 1035 (47) |
| No | 1160 (53) |
| WHO disease stage | |
| I | 266 (11) |
| II | 481 (19) |
| III | 1540 (61) |
| IV | 225 (9) |
| History of tuberculosis | |
| Yes | 612 (20) |
| No | 2472 (80) |
| Opportunistic infections4 | |
| Yes | 360 (11) |
| No | 2772 (89) |
| Use of Cotrimoxazole | |
| Yes | 1580 (53) |
| No | 1390 (47) |
| ARV regimen5 | |
| AZT/3TC/NVP | 1075 (52) |
| d4T/3TC/NVP | 615 (30) |
| AZT3TC/EFV | 210 (10) |
| d4T/3TC/EFV | 146 (7) |
| ABC/3TC/LPV | 1 (0.05) |
| ABC/3TC/NFV | 1 (0.05) |
| ARV regimen | |
| Contains stavudine | 761 (37) |
| No stavudine | 1287 (63) |
| ARV regimen | |
| Contains efavirenz | 356 (17) |
| No efavirenz | 1692 (83) |
Numbers may not add up to 3144 due to missing values
There are no established mid-upper arm circumference cut-offs for children >5 years. The 16.4cm reference (10th percentile) was adopted from NHANES (National Health and Nutritional examination Survey) 2003–06 conducted by Centres for Disease Control (CDC), National Centre for Health Statistics (NCHS) (McDowell MA).
Adopted from CDC. Guidelines for Preventing Opportunistic Infections Among HIV-Infected Persons – 2002. MMWR June 14, 2002 (CDC)
Patient having at least one or more of the following; herpes zoster, fungal infection, oral candidiasis, oral hairy leukoplakia, recurrent severe bacterial infection, pneumocystis jirovecii pneumonia, toxoplasmosis, cryptosporidiosis with diarrhea, cryptococcal meningitis, extrapulmonary TB, lymphoma, kaposis sarcoma, HIV encephalopathy
AZT = Zidovudine, d4t = Stavudine, 3TC = Lamivudine, ABC = Abacavir, NVP = nevirapine, EFV = Efavirenz, NFV = Nelfinavir, LPV = lopinavir, Trimu = Triomune (d4t, 3TC, NVP)
Overall, 268 children died during the six years of follow up (six years cumulative mortality rate=12.4%). Of these, 151 (56.3%) children died within the first 90 days of ART initiation. The estimated cumulative mortality rates were 4.8%, 7.3%, 8.2%, and 8.5% at 3, 12, 24, and 60 months following ART initiation respectively. The cumulative loss to follow-up rates at 3, 12, 36, and 60 months after ART initiation were respectively, 10.6, 21.1, 30.8, and 58.2. Figure 1 presents the cumulative incidence of deaths by child WAZ and age at ART initiation. Death rates were highest among children with WAZ ≤−3 compared with those with WAZ >−1 (P<0.0001).
Figure 1.
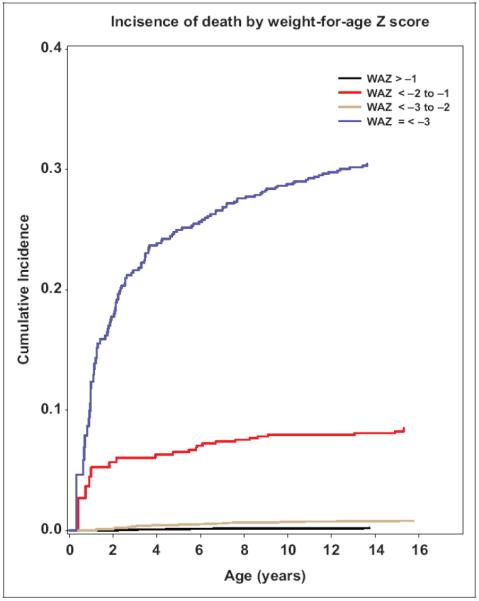
Cumulative incidence of death by WAZ and age at antiretroviral therapy initiation. WAZ indicates weight-for-age Z score.
The relationships between mortality and nutritional status after ART initiation are shown in Table 2. In the overall mortality, compared with children with WAZ score >−1, children with WAZ score from ≤−2 to <−3 had a nearly double risk of death (RR, 1.85 (95% CI, 1.10–3.11), and those with severe underweight (WAZ ≤−3), the risk was more than tripled (RR, 3.36 (95% CI, 2.12–5.32). For deaths that occurred in the first 90 days after ART initiation, compared with children with WAZ score >−1, those with WAZ score from ≤−2 to <−3 had more than doubled risk of death (RR, 2.51 (95% CI, 1.05–5.95), and those with severe underweight (WAZ ≤−3), the risk more than 7-folds (RR, 7.12 (95% CI, 3.33–15.22). Similarly, WHZ and BMIZ scores of −2 or lower were associated with a significantly increased risk of mortality, more than double for the overall mortality and up to five-fold for mortality in the first 90 days of ART initiation. HAZ score of −1 or less on the other hand was associated with about two- to three-fold increased risk of early mortality and overall mortality. A MUAC of 13.5cm or less was associated with a two- to six-fold increased risk of early mortality as well as a two- to four-fold overall mortality.
Table 2.
Nutritional status in relation to mortality at different time periods after antiretroviral therapy initiation1,2
| Anthropometric indices | Mortality within the first 90 days | Overall mortality | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| No. deaths/No. of children | RR (95% CI) | P for trend | No. deaths/No. of children | RR (95% CI) | P for trend | |
| Weight-for-age z score (WAZ) | <0.001 | <0.001 | ||||
| WAZ > −1 | 8/528 | 1.00 | 24/528 | 1.00 | ||
| −2< WAZ ≤ −1 | 11/612 | 1.39 (0.55, 3.49) | 27/612 | 1.13 (0.65, 1.97) | ||
| −3 < WAZ ≤ −2 | 16/548 | 2.51 (1.05, 5.95) | 39/548 | 1.85 (1.10, 3.11) | ||
| WAZ≤ −3 | 116/1456 | 7.12 (3.33, 15.22) | 178/1456 | 3.36 (2.12, 5.32) | ||
| Weight-for-height (WHZ) or body-mass index (BMIZ) z score3 | <0.001 | <0.001 | ||||
| WHZ > −1 | 29/1789 | 1.00 | 81/1789 | 1.00 | ||
| −2< WHZ ≤ −1 | 8/181 | 0.90 (0.40, 2.04) | 12/181 | 0.58 (0.31, 1.09) | ||
| −3 < WHZ ≤ −2 | 21/457 | 2.25 (1.27, 3.99) | 51/457 | 2.09 (1.46, 3.00) | ||
| WHZ≤ −3 | 93/717 | 5.80 (3.65, 9.22) | 124/717 | 2.94 (2.14, 4.03) | ||
| Height-for-age z score (HAZ) | <0.001 | <0.001 | ||||
| HAZ > −1 | 18/598 | 1.00 | 32/598 | 1.00 | ||
| −2< HAZ ≤ −1 | 27/722 | 1.90 (1.03, 3.50) | 54/722 | 1.92 (1.23, 3.00) | ||
| −3 < HAZ ≤ −2 | 32/756 | 2.09 (1.14, 3.82) | 54/756 | 1.80 (1.14, 2.82) | ||
| HAZ ≤ −3 | 74/1068 | 3.69 (2.11, 6.45) | 128/1068 | 3.03 (2.00, 4.60) | ||
| Mid-Upper Arm Circumference (MUAC) | <0.001 | <0.001 | ||||
| MUAC>13.5 | 36/1962 | 1.00 | 85/1962 | 1.00 | ||
| 12.5–13.5 | 25/484 | 2.48 (1.41, 4.36) | 48/484 | 1.97 (1.34, 2.89) | ||
| 27/292 | 3.55 (1.99, 6.31) | 40/292 | 2.28 (1.49, 3.49) | |||
| 11.5≤MUAC<12.5 | 63/406 | 6.29 (3.62, 10.96) | 95/406 | 3.87 (2.63, 5.70) | ||
| MUAC<11.5 | ||||||
Cox proportional hazards model with age as time scale
Adjusted for calendar year (categorized as 2004–2005, 2006, 2007, 2008, 2009, 2010), hemoglobin level (categorized as HB>11, 8.5–11, <8.5g/dL), immune suppression (categorized as severe, CD4+ T-cells<15% if ≤5 years; <200 cells/μL if >5 years and non severe), HIV disease stage (categorized as I, II, II and IV), history of TB (yes vs. no), opportunistic infections (yes vs. no), ARV regimen containing EFV (yes vs. no), district (Ilala, Kinondoni and Temeke), cotrimoxazole use (yes vs. no) and season at ART initiation (categorized as long dry, short dry, long rainy, short rainy) and sex
WHZ for children ≤2 years and BMIZ for children >2 years
The effects of other independent baseline risk factors for death are shown in table 3. The risk factors for death included severe anemia (RR, 1.91 (95% CI, 1.21–2.99); severe immune suppression (RR, 1.46 (95% CI, 1.07–2.00); history of TB (RR, 1.55 (95% CI, 1.09–2.18); having opportunistic infections (RR, 2.23 (95% CI, 1.61–3.09); living in the Temeke district of Dar es Salaam (RR, 1.58 (95% CI, 1.18–2.11), and advanced HIV disease stage (III and IV) at ART initiation [(RR, 1.85 (95% CI, 1.05–3.27) and (RR, 2.43 (95% CI, 1.33–4.42)] respectively. Child's sex, initial ART regimen, season of ART initiation and use of cotrimoxazole were not significantly associated with risk of mortality.
Table 3.
Baseline characteristics in relation to overall mortality after antiretroviral therapy initiation1
| Predictor | No. deaths/No. of children | Univariate | Multivariate2 | ||
|---|---|---|---|---|---|
|
| |||||
| RR (95% CI) | P for trend | RR (95% CI) | P for trend | ||
| Sex | 0.03 | 0.10 | |||
| Female | 116/1582 | 1.00 | 1.00 | ||
| Male | 152/1562 | 1.31 (1.03, 1.67) | 1.23 (0.96, 1.57) | ||
| District of ART initiation | <0.001 | <0.001 | |||
| Ilala | 103/1372 | 1.00 | 1.00 | ||
| Kinondoni | 58/888 | 0.84 (0.61, 1.16) | 0.81 (0.58, 1.12) | ||
| Temeke | 107/884 | 1.56 (1.19, 2.05) | 1.58 (1.18, 2.11) | ||
| ARV regimen | 0.01 | 0.11 | |||
| Contains stavudine | 68/761 | 1.58 (1.14, 2.19) | 1.32 (0.94, 1.84) | ||
| No stavudine | 95/1287 | 1.00 | 1.00 | ||
| Use of Cotrimoxazole | 0.43 | 0.59 | |||
| No | 138/1564 | 1.00 | 1.00 | ||
| Yes | 130/1580 | 1.09 (0.85, 1.40) | 0.93 (0.71, 1.21) | ||
| Hemoglobin at ART initiation | <0.001 | <0.001 | |||
| HB>11 | 23/403 | 1.00 | 1.00 | ||
| 8.5–11 | 89/1281 | 1.06 (0.67, 1.68) | 0.98 (0.61, 1.56) | ||
| HB<8.5 | 156/1460 | 2.09 (1.34, 3.26) | 1.91 (1.21, 2.99) | ||
| Severe immune | 0.01 | 0.02 | |||
| suppression3 | 1.00 | 1.00 | |||
| No | 178/2109 | 1.67 (1.23, 2.28) | 1.46 (1.07, 2.00) | ||
| Yes | 90/1035 | ||||
| HIV disease stage | <0.001 | 0.003 | |||
| I | 16/266 | 1.00 | 1.00 | ||
| II | 27/481 | 2.30 (1.22, 4.35) | 1.63 (0.85, 3.12) | ||
| III | 126/1540 | 3.70 (2.15, 6.35) | 1.85 (1.05, 3.27) | ||
| IV | 99/857 | 3.71 (2.14, 6.45) | 2.43 (1.33, 4.42) | ||
| History of TB | 0.01 | 0.01 | |||
| No | 213/2532 | 1.00 | 1.00 | ||
| Yes | 55/612 | 1.61 (1.17, 2.22) | 1.55 (1.09, 2.18) | ||
| Opportunistic infections4 | <0.001 | <0.001 | |||
| No | 213/2532 | 1.00 | 1.00 | ||
| Yes | 55/612 | 2.24 (1.66, 3.02) | 2.23 (1.61, 3.09) | ||
Cox proportional hazards model with age as time scale
Adjusted for weight-for-age Z score (categorized as WAZ > −1, −2< WAZ ≤−1, −3 < WAZ ≤ −2, WAZ≤ −3), calendar year (categorized as 2004–2005, 2006, 2007, 2008, 2009, 2010), hemoglobin level (categorized as HB>11, 8.5–11, <8.5g/dL), immune suppression (categorized as severe, CD4+ T-cells<15% if ≤5 years; <200 cells/μL if >5 years and non severe), HIV disease stage (categorized as I, II, II and IV), history of TB (yes vs. no), opportunistic infections (yes vs. no), ARV regimen containing EFV (yes vs. no), district (Ilala, Kinondoni and Temeke), cotnmoxazole use (yes vs. no) and season at ART initiation (categorized as long dry, short dry, long rainy, short rainy) and sex
CD4+ T-cells<15% if ≤5 years; <200 cells/μL if >5 years
Patient having at least one or more of the following; herpes zoster, fungal infection, oral candidiasis, oral hairy leukoplakia, recurrent severe bacterial infection, pneumocystis jirovecii pneumonia, toxoplasmosis, cryptosporidiosis with diarrhea, cryptococcal meningitis, extrapulmonary TB, lymphoma, kaposis sarcoma, HIV encephalopathy
We next examined the associations of change in nutritional status by 3 months after ART initiation and mortality 3 months after ART initiation (Table 4). This analysis included the subset of 2993 children who survived past the first 90 days of ART initiation. There were 117 deaths in total (mortality rate, 7.5%) after the first 90 days of ART initiation. Decline in WHZ or BMIZ score by more than 2SD was associated with a more than 3-fold increased risk of death (95% CI 1.63, 5.76). Similarly, a decline in HAZ score of more than 2SD was associated with a more than 2-fold increased risk of death (95% CI 1.09, 7.07). Decline in WAZ was not associated with a statistically significant increase in risk of death. A decline in MUAC of 1 cm or more was associated with an 84% increased risk of death (95% CI 1.24, 2.75).
Table 4.
Change in nutritional status within the first three months of antiretroviral therapy initiation in relation to mortality after 3 months1
| Change in Anthropometric indices | No. deaths/No. of children | Univariate | Multivariate2 | ||
|---|---|---|---|---|---|
|
| |||||
| RR (95% CI) | P for trend | RR (95% CI) | P for trend | ||
| Weight-for-age z score Change | 0.11 | 0.07 | |||
| Decline by >2 SD | 16/520 | 2.00 (0.62, 6.42) | 1.87 (0.56, 6.18) | ||
| Decline by >1 to 2 SD | 16/601 | 1.54 (0.66, 3.57) | 1.84 (0.78, 4.34) | ||
| Decline by 0 to 1 SD | 23/532 | 1.21 (0.77, 1.90) | 1.27 (0.80, 2.02) | ||
| Any increase | 62/1340 | 1.00 | 1.00 | ||
| Weight-for-height (WHZ) or Body-Mass Index (BMIZ) z score change3 | 0.01 | 0.003 | |||
| Decline >2 SD | 52/1760 | 2.93 (1.58, 5.45) | 3.06 (1.63, 5.76) | ||
| Decline by >1 to 2 SD | 4/173 | 0.78 (0.32, 1.94) | 0.90 (0.36, 2.24) | ||
| Decline by 0 to 1 SD | 30/436 | 1.43 (0.91, 2.23) | 1.57 (0.99, 2.50) | ||
| Any increase | 31/624 | 1.00 | 1.00 | ||
| Height-for-age Z-score change | |||||
| Decline by >2 SD | 14/580 | 2.33 (0.93, 5.84) | 0.46 | 2.78 (1.09, 7.07) | 0.21 |
| Decline by >1 to 2 SD | 27/695 | 0.56 (0.20, 1.55) | 0.62 (0.22, 1.74) | ||
| Decline by 0 to 1SD | 22/724 | 1.18 (0.80, 1.73) | 1.29 (0.87, 1.93) | ||
| Any increase | 54/994 | 1.00 | 1.00 | ||
| Mid-Upper Arm Circumference change | 0.02 | 0.02 | |||
| Decline by >2 cm | 49/1926 | 2.05 (0.83, 5.10) | 1.94 (0.76, 4.96) | ||
| Decline by >1 to 2 cm | 23/459 | 1.01 (0.37, 2.77) | 1.05 (0.38, 2.92) | ||
| Decline by 0 to 1 cm | 13/265 | 1.71 (1.16, 2.52) | 1.84 (1.24, 2.75) | ||
| Any increase | 32/343 | 1.00 | 1.00 | ||
Cox proportional hazards model with age as time scale
Adjusted for calendar year (categorized as 2004–2005, 2006, 2007, 2008, 2009, 2010), hemoglobin level (categorized as HB>11, 8.5–11, <8.5g/dL), immune suppression (categorized as severe, CD4+ T-cells<15% if ≤5 years; <200 cells/μL if >5 years and non severe), HIV disease stage (categorized as I, II, II and IV), history of TB (yes vs. no), opportunistic infections (yes vs. no), ARV regimen containing EFV (yes vs. no), district (Ilala, Kinondoni and Temeke), cotrimoxazole use (yes vs. no) and season at ART initiation (categorized as long dry, short dry, long rainy, short rainy) and sex
WHZ for children ≤2 years and BMIZ for children >2 years
DISCUSSION
In this prospective study of children initiating ART in Tanzania, we found a mortality rate of 12.4% over a median follow up time of 17.2 months. Mortality within the first 90 days of starting ART was found to be high in this study with more than 56% of deaths occurring in the first 90 days of ART initiation. These findings in the trend of mortality was consistent with findings from other studies in both low- and middle-income countries [3,4,9,16] although the mortality rate was slightly higher than the mortality rate of 8.3% reported over three years of follow up by Bolton-Moore and colleagues among 2938 children receiving ART in Zambia [4]. In this Zambian study, 1130 (38.5%) children were aged 59 months or younger while 1808 (61.3%) were 5 years or older. In contrast, 51% of the children in our study were 5 years or younger which could partly explain the higher mortality rate we observed. Similar to our findings, of those who died in the Zambian cohort, 56.6% died within the first 90 days of starting ART. A study in Haiti, though of smaller sample size (n=236) and shorter duration of follow up (0–36 months) compared to our study, reported similar findings; overall mortality of 9% and 61% of deaths occurred within the first six months of ART [16]. In this study from Haiti, 13% of the children were younger than 18 months old, 73% were between 1.5 and 12 years and only 13% were 13 years old or older.
A similar trend has also been reported in adults [9,17]. In a study among Tanzanian adult patients starting ART in the Manyara region, 62.1% of the patients who died during the follow up period, died within 3 months of starting ART [9]. Among patients receiving ART within the MDH program in Dar es Salam, Tanzania, Liu and colleagues reported that 79% of deaths occurred in the first 3 months after ART initiation [17].
We found that undernutrition at the time of ART initiation was associated with increased risk of mortality in this population of HIV-infected children. Children with low WAZ, HAZ and WHZ or BMIZ scores had increased risk of death. Even though there are some difficulties in comparing results from different studies due to differences in the definition of undernutrition in children, these results are similar to those reported previously by studies from similar resource limited settings [4,5,6,18]. In a large cohort of children in Zambia, mortality was associated with lower WAZ score among children younger than 5 years [4]. Another study in Uganda showed that children with lower WAZ score in the first year of life had increased risk of mortality by 25 months [5]. Similar associations between undernutrition and mortality have been reported in Malawi [6], South Africa [11], and Ethiopia [18].
Low MUAC is another measure of undernutrition that was significantly associated with an increased risk of mortality in this study. Similar findings have been reported in both children [19,20] and adult patients initiating ART in Tanzania [17].
Apart from food insecurity, symptoms of HIV disease such as anorexia, pain, nausea, vomiting, malabsorption, disease pathology, and diarrhea contribute to undernutrition in HIV-infected children. Furthermore, patients with opportunistic infections may have limited food intake and also increased resting energy expenditure leading to undernutrition. The result of all of these is often inadequate nutritional intake leading to the inability to maintain weight and lean tissue mass, and to micronutrient deficiency.
A history of TB and infections with other opportunistic infections were found to be associated with increased risk of mortality in this study. A few other studies have reported that TB and other opportunistic infections are the major causes of morbidity and mortality among HIV-infected patients even in the presence of ART. A study among patients receiving ART in Uganda found that baseline TB infection and non-TB opportunistic infections were significantly associated with increased risk of early as well as late mortality [12]. A recent study in South Africa reported that TB infection was associated with a 76% increased risk of death or loss to follow up [13]. Similarly, opportunistic infections increased the risk of death among patients receiving ART by up to 60% in a study in Uganda [21]. Furthermore, TB or other opportunistic infections may increase the risk of death in the first few months after initiating ART due to immune reconstitution inflammatory syndrome (IRIS), a paradoxical worsening or recurrence of opportunistic infections symptoms as a result of rapid immunological recovery [8,22].
Anemia was a strong predictor of mortality in this cohort of children initiating ART. Children with severe anemia (hemoglobin<8.5g/dL) had an 87% increased risk of overall death compared to children who were not severely anemic. Several studies have shown that anemia is an independent risk factor for death in children and adults [4,6,7,9]. A study in Ethiopia [18] found that survival among children on ART was higher among children who had higher hemoglobin. Anemia may be multifactorial, and for this particular population, causes other than iron deficiency, such as malaria infection and hookworm infestation, may have stronger influence due to the endemicity of these conditions although we have no data to support this claim.
The association between socioeconomic status and mortality is a long recognized phenomenon. We found that living in the Temeke district of Dar es Salaam was associated with a 60% increased risk of death compared to living in Ilala. The district in which the patient lives is a proxy for the socioeconomic status of the patient since direct measurement of socioeconomic status was not undertaken in this study. The Temeke district is considered the poorest of the three districts of Dar es Salaam city.
We also found, as expected, that, severe immune suppression as evidenced by advanced HIV disease stage particularly stage III and IV or depleted CD4+ T-cells was associated with increased risk of death in this study. These findings are in line with those reported both in developed [18] and developing countries [4,11].
Decline in HAZ, WHZ and BMIZ scores in the first 3 months of ART was associated with increased risk of death after the first 90 days of ART initiation. These findings were consistent with those reported in adults [3,17]. However; it was not clear why only decrease in MUAC by 1cm or no change were associated with increased risk of death and not greater decreases in MUAC.
The major strengths of this study included the large sample size (3144 children) and long follow-up time (up to 6 years). Furthermore, since the study was population-based, the findings may be generalizable to HIV-infected children initiating ART in Tanzania as well as other countries in Sub-Saharan Africa. There are a number of limitations to this study however. We were unable to adjust for some potential confounders including maternal factors and viral load. In addition, there was high loss to follow up; 10.6%, 14.6%, 21.1% and 58.2% respectively at 3, 6, and 12 months, and overall. We looked at predictors of the combined endpoint of death or loss to follow and results were similar to findings for the overall mortality endpoint with only a few exceptions. It could therefore be argued that perhaps most of those who were lost to follow up did actually die, although we have no way to confirm that. Furthermore, we did not have data on causes of death.
In conclusion, undernutrition at the time of ART initiation was associated with increased risk of death, particularly during the first months after ART initiation. To sustain the obtained benefit of ART in this setting, interventions to improve nutritional status should be considered as an adjunct to ART.
Acknowledgements
We thank the nursing and technical staff in Dar es Salaam, Tanzania; and the various scientists at Harvard School of Public Health and Muhimbili University of Health and Allied Sciences for their collaboration and assistance in this study.
Footnotes
Conflicts of Interests The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- 1.World Health Organization . The global burden of disease 2004 update. 2008. [Google Scholar]
- 2.UNAIDS . UNAIDS report on the global AIDS epidemic 2010. 2010. Global Report. [Google Scholar]
- 3.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 4.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, Chintu N, Stringer EM, Chi BH, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298:1888–1899. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- 5.Berhane R, Bagenda D, Marum L, Aceng E, Ndugwa C, Bosch RJ, et al. Growth failure as a prognostic indicator of mortality in pediatric HIV infection. Pediatrics. 1997;100:E7. doi: 10.1542/peds.100.1.e7. [DOI] [PubMed] [Google Scholar]
- 6.Laufer MK, van Oosterhout JJ, Perez MA, Kanyanganlika J, Taylor TE, Plowe CV, et al. Observational cohort study of HIV-infected African children. Pediatr Infect Dis J. 2006;25:623–627. doi: 10.1097/01.inf.0000220264.45692.a0. [DOI] [PubMed] [Google Scholar]
- 7.Wamalwa DC, Obimbo EM, Farquhar C, Richardson BA, Mbori-Ngacha DA, Inwani I, Benki-Nugent S, John-Stewart G. Predictors of mortality in HIV-1 infected children on antiretroviral therapy in Kenya: a prospective cohort. BMC Pediatr. 2010;10:33. doi: 10.1186/1471-2431-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Achenbach CJ, Harrington RD, Dhanireddy S, Crane HM, Casper C, Kitahata MM. Paradoxical Immune Reconstitution Inflammatory Syndrome in HIV-Infected Patients Treated With Combination Antiretroviral Therapy After AIDS-Defining Opportunistic Infection. Clin. Infect. Dis. 2011 doi: 10.1093/cid/cir802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johannessen A, Naman E, Ngowi BJ, Sandvik L, Matee MI, Aglen HE, et al. Predictors of mortality in HIV-infected patients starting antiretroviral therapy in a rural hospital in Tanzania. BMC Infect Dis. 2008;8:52. doi: 10.1186/1471-2334-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel K, Hernan MA, Williams PL, Seeger JD, McIntosh K, Van Dyke RB, et al. Long-term effectiveness of highly active antiretroviral therapy on the survival of children and adolescents with HIV infection: a 10-year follow-up study. Clin Infect Dis. 2008;46:507–515. doi: 10.1086/526524. [DOI] [PubMed] [Google Scholar]
- 11.Zanoni BC, Phungula T, Zanoni HM, France H, Feeney ME. Risk factors associated with increased mortality among HIV infected children initiating antiretroviral therapy (ART) in South Africa. PLoS One. 2011;6:e22706. doi: 10.1371/journal.pone.0022706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore DM, Yiannoutsos CT, Musick BS, Tappero J, Degerman R, Campbell J, Were W, Kaharuza F, Alexander LN, Downing R, Mermin J. Determinants of Early and Late Mortality Among HIV-Infected Individuals Receiving Home-Based Antiretroviral Therapy in Rural Uganda. J Acquir Immune Defic Syndr. 2011;58:289–296. doi: 10.1097/QAI.0b013e3182303716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bassett IV, Chetty S, Wang B, Mazibuko M, Giddy J, Lu Z, Walensky RP, Freedberg KA, Losina E. Loss to follow-up and mortality among HIV-infected people co-infected with TB at ART initiation in Durban, South Africa. J Acquir Immune Defic Syndr. 2011 doi: 10.1097/QAI.0b013e31823d3aba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO . The WHO Child Growth Standards. [Accessed 03/15, 2011]. [Google Scholar]
- 15.McDowell MA, Fryar CD, Ogden CL, Flegal KM. National health statistics reports; no 10. National Center for Health Statistics; Hyattsville, MD: 2008. Anthropometric reference data for children and adults: United States, 2003–2006. [PubMed] [Google Scholar]
- 16.George E, Noel F, Bois G, Cassagnol R, Estavien L, Rouzier Pde M, et al. Antiretroviral therapy for HIV-1-infected children in Haiti. J Infect Dis. 2007;195:1411–1418. doi: 10.1086/514823. [DOI] [PubMed] [Google Scholar]
- 17.Liu E, Spiegelman D, Semu H, Hawkins C, Chalamilla G, Aveika A, et al. Nutritional status and mortality among HIV-infected patients receiving antiretroviral therapy in Tanzania. J Infect Dis. 2011;204:282–290. doi: 10.1093/infdis/jir246. [DOI] [PubMed] [Google Scholar]
- 18.Taye B, Shiferaw S, Enquselassie F. The impact of malnutrition in survival of HIV infected children after initiation of antiretroviral treatment (ART) Ethiop Med J. 2010;48:1–10. [PubMed] [Google Scholar]
- 19.Sommer A, Loewenstein M. Nutritional status and mortality: a prospective evaluation of the QUAC stick. Am J ClinNutr. 1975;28:287–92. doi: 10.1093/ajcn/28.3.287. [DOI] [PubMed] [Google Scholar]
- 20.Berkley J, Mwangi I, Griffiths K, Ahmed I, Mithwani S, English M, Newton C, Maitland K. Assessment of Severe Malnutrition Among Hospitalized Children in Rural Kenya: Comparison of Weight for Height and Mid Upper Arm Circumference. JAMA. 2005;294:591–7. doi: 10.1001/jama.294.5.591. [DOI] [PubMed] [Google Scholar]
- 21.Moore, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS. 2007;21:713–9. doi: 10.1097/QAD.0b013e328013f632. [DOI] [PubMed] [Google Scholar]
- 22.French MA. HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin. Infect. Dis. 2009;48:101–107. doi: 10.1086/595006. [DOI] [PubMed] [Google Scholar]


