Abstract
PLAC1 is a recently described X-linked gene with expression restricted primarily to cells derived from trophoblast lineage during embryonic development. PLAC1 localizes to a region of the X-chromosome thought to be important in placental development although its role in this process has not been defined. This review summarizes our current understanding of its expression, regulation, and function. PLAC1 is expressed throughout human pregnancy by the differentiated trophoblast and localizes to membranous structures in the syncytiotrophoblast, including the microvillous plasma membrane surface. Recent studies have demonstrated that PLAC1 is also expressed by a wide variety of human cancers. Studies of the PLAC1 promoter regions indicate that its expression in both normal placenta and cancer cells is driven by specific interactions involving a combination of transcription factors. While functional insight into PLAC1 in the normal trophoblast is lacking, preliminary studies suggest that cancer-derived PLAC1 has the potential to promote tumor growth and function. Additionally, it also appears to elicit a specific immunologic response that may influence survival in some cancer patients, suggesting that it may provide a therapeutic target for the treatment of some cancers. We also discuss a potential role for PLAC1 as a biomarker predictive of specific pregnancy complications, such as preeclampsia.
Keywords: PLAC1, Placenta-specific 1, placenta, cancer
Identification of the PLAC1 Gene
The PLAC1 gene is a recently described X-linked gene (Cocchia et al, 2000). Its expression is highly restricted in normal tissue and limited primarily to the placenta. Based on several independent studies, the region to which PLAC1 maps is thought to be important for normal placental and fetal development. First, large deletions of the mouse X chromosome spanning 200–700 Kb near the Hprt locus (Lesch-Nyhan syndrome) have been shown to result in fetal growth retardation and neonatal death (Kushi et al, 1998). Deletion of the Hprt gene itself does not result in this phenotype (Searle et al, 1994) suggesting that this phenotype is due to the existence of additional gene(s) near the Hprt locus. Second, independent evidence indicating that this region of the X chromosome is important for normal placental development has been provided. (Zechner et al, 1996; Hemberger et al, 1999). The Ihpd locus (interspecific hybrid placental dysplasia) in the region is associated with abnormal placental development in some mouse hybrids, (though later analysis suggested that several X-linked genes may be involved). It is characterized by male sterility, abnormal placental development, and fetal growth retardation. Additional evidence implicating a role for Plac1 in placental growth was provided by Suemizu et al. (2003). Nuclear transplantation of mouse ES cells is associated with placentomegaly. Differentially expressed genes of these large placentae were identified by microarray analysis. The Plac1 gene was one of a few genes consistently expressed at very significantly higher levels in the cloned placentae, suggesting its overexpression may contribute to the observed phenotype.
The sequence of the human X chromosome was obtained in the region of the HPRT gene in an attempt to characterize the gene content that may be relevant to these observations (Cocchia et al, 2000). Approximately 1 Mb of human Xq26 DNA from the glypican – 3 (GPC-3) gene to about 200 Kb telomeric of HPRT was sequenced and found to contain only a few genes. This was suggested by several criteria. First, the content of repetitive sequences is very high and GRAIL detected only two of the CpG islands that are “markers” for approximately half of all genes (Antequera and Bird, 1993). One of these is associated with glypican-3 (GPC3) and the other is with HPRT. This led to the identification of PLAC1, the only other gene in this region suggested by computer-assisted gene prediction and the absence of other mouse ESTs (Figure 1). Thus, PLAC1 is the prime candidate in this region of the X chromosome to contain the genetic information required for normal placental development. The latest version of the annotations includes additional genes, PHF6, MGC16121, and CR59647 in the human. Likewise, several ESTs and Phf6 have been identified in the mouse. However, none have been demonstrated to be placental specific, to date. The human PLAC1 gene maps 65 kb telomeric to the HPRT gene on Xq26. PLAC1 is the only gene identified in the region spanning approximately 700 kb and 300 kb on either side of the HPRT gene. A mouse cDNA 5′ EST, clone C0008F04 (GenBank Assession-AA409600) was independently mapped as an anonymous cDNA on the Jackson Laboratory backcross mouse DNA panel and designated DXWsu72e (Ko et al, 1998). In the composite genetic map compiled by the Jackson Laboratory Plac1 (DXWsu72e) is placed 16.0cM from the centromere and Hprt localizes 17.0 cM from the centromere, a locus found to be syntenically equivalent to the human ortholog (http://www.informatics.jax.org/).
Figure 1.
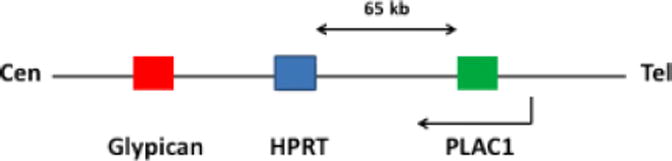
X-chromosomal locus containing PLAC1
The original report of the PLAC1 gene by Cocchia et al (2000) indicated that it contained 3 exons. The first two exons were relatively small, 152 and 72 bp. The third and larger exon (885 bp) contained the entire open reading frame of 636 bp. The final mRNA represented about 2% of the primary PLAC1 transcript (92,700 bp). The mouse cDNA C0008F04 was completely sequenced and the mouse and human genes were found to be 75% identical at the DNA level and 60% identical at the amino acid level. There are now indications from both mouse and human EST databases that the gene is alternatively spliced and there are potentially up to 3 additional 5′ exons.
Placenta-restricted Expression of PLAC1 mRNA
Northern analysis and in situ hybridization revealed PLAC1 mRNA expression is restricted to cells of trophoblastic lineage (Cocchia et al, 2000; Fant et al, 2002; Massabbal et al, 2005). No detectable expression of PLAC1 is observed in adult or fetal tissues by Northern analysis although recent studies utilizing quantitative RT-PCR demonstrated expression at much lower levels in testis and cerebellum (Yin et al, 2006; Silva et al, 2007). Within the mouse placenta, in situ hybridization revealed Plac1 expression in the ectoplacental cone and trophoblastic giant cells at day 7.5 dpc. At day 11.5 Plac1 expression occurs primarily in the labyrinthine layer with some faint expression in the GC’s. By day 14.5 dpc, decreased expression of Plac1 is observed and persists throughout the remainder of gestation. These data clearly point to a trophoblast-specific pattern of Plac1 gene expression at the maternal-fetal interface. Human PLAC1 expression, like its murine counterpart, is also relatively restricted to the trophoblast (Fant et al, 2002; Massabbal et al 2005). Unlike the mouse, however, It is expressed at relatively constant levels from 22–40 weeks gestation.
In the human trophoblast, PLAC1 expression is tightly linked to cell differentiation (Massabbal et al, 2005). When placed in culture, normal cytotrophoblasts aggregate and fuse over 72–96 hours to form fully differentiated syncytiotrophoblasts and provides a useful model to study trophoblast differentiation. PLAC1 mRNA expression is markedly upregulated during this process (Figure 2). Additionally, PLAC1 is positively regulated by peptide growth factors known to be important in trophoblast differentiation. KGF, a peptide growth factor known to be relevant to trophoblast differentiation, stimulates PLAC1 mRNA expression in the BeWo choriocarcinoma cell. Its effect is potentiated by epidermal growth factor (EGF), another growth factor important in trophoblast differentiation. However, EGF alone has no effect on PLAC1 expression.
Figure 2.
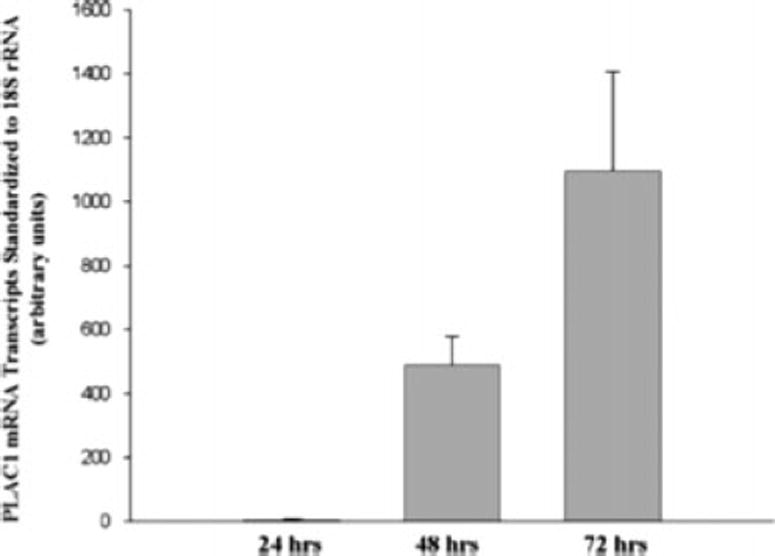
PLAC1 mRNA expression during normal human trophoblast differentiation
The PLAC1 Protein is also Restricted to the Placenta
The PLAC1 protein localizes primarily to the syncytiotrophoblast (Massabbal et al, 2005; Fant et al, 2007). As shown in Figure 3, immunohistochemical analysis of human placental tissue at 8 weeks gestation demonstrated PLAC1 localizes primarily to the differentiated syncytiotrophoblast (black arrows) whereas the underlying cytotrophoblast exhibits little or no protein (panel A). At 23 weeks, however, specific immunostaining of an occasional cytotrophoblast was observed (red arrows). Moreover, the syncytial staining appeared more intense along the microvillous brush border membranes (black arrow, panel B). Deconvolution fluorescence microscopy further indicated that PLAC1 localizes to cytosolic sites with close proximity to the apical plasma membrane (Figure 4, red fluorescence). Integration into the plasma membrane by a fraction of the PLAC1 pool is also suggested.
Figure 3.
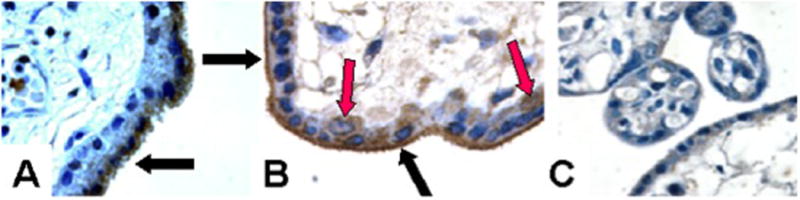
Immunohistochemical localization of the PLAC1 protein in human placenta. A. 8 weeks gestation. B. 23 weeks gestation. C. Non-immune serum, 23 weeks gestation
Figure 4.
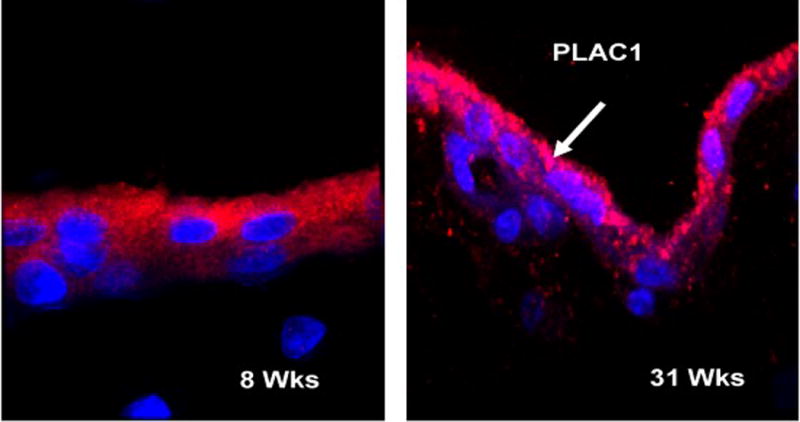
Immunofluorescence microscopy of PLAC1 protein in human placenta.
A variety of biochemical methods were applied to further define the subcellular location of PLAC1 in normal placental tissue. Subcellular fractionation followed by differential centrifugation was utilized initially to assess PLAC1 distribution among the major intracellular compartments. Additionally, plasma membranes enriched specifically from the maternal-facing, microvillous membrane surface of the syncytiotrophoblast (MVM) and the fetal-facing, basal surface of the syncytiotrophoblast membrane (BM) were also examined. As shown in Figure 5 (Panel A), a 27–30 kD band was observed in the microsomal fraction (mic), but not the soluble (sol) or mitochondrial (mit) fractions. The microsomal fraction includes the plasma membrane as well as the endoplasmic reticulum and golgi apparatus, Further analysis of the basal and apical surfaces of the syncytiotrophoblast plasma membrane (Panel B) revealed PLAC1 immunoreactivity exclusively associated with the MVM (M1-M4) but not the BM (B1-B4) (Each lane represents a different placenta). These observations indicate that the PLAC1 polypeptide is a membrane-associated protein and associates, in part, with the microvillous brush border membrane. It is not clear from these studies, however, if PLAC1 faces extracellularly, intracellularly, or is, in fact, an integral membrane component that becomes internalized. Analysis of human amniotic fluid (16–18 weeks gestation) and pregnant, maternal serum (25–33 weeks) failed to identify soluble PLAC1 protein in these compartments suggesting that the PLAC1 polypeptide does not circulate but acts locally at the level of the trophoblast.
Figure 5.
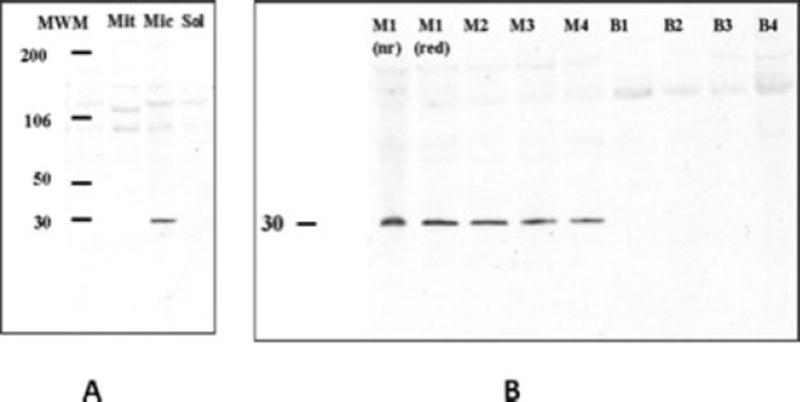
Subcellular distribution of the PLAC1 protein by immunoblot analysis.
Panel A: Mit = mitochodria, Mic = microsomal, Sol = soluble.
Panel B: M = Microvillous membrane surface of syncytiotrophoblast. B = basal membrane surface of syncytiotrophoblast
Putative PLAC1/Plac1 Protein
While nothing is known of its functional properties, certain insights can be inferred from cDNA sequence analysis of PLAC1. The human ORF encodes a putative protein of 212 amino acids whereas the mouse gene encodes a 173 amino acid product (Cocchia et al, 2000). They share approximately 60% homology. Figure 6 shows alignment of PLAC1 from multiple species as indicated, based on the currently available genomic sequences.
Figure 6.

Sequence alignment of PLAC1 from multiple species (inferred from genomic sequence).
Additionally, both mouse and human PLAC1 are predicted to target the secretory pathway and exist as extracellular peptides. The PSORT program (psort.ims.u-tokyo.ac.jp) suggests that the polypeptide products of both PLAC1 and Plac1 are extracellular, 56% and 66.7% respectively. Each contains a cleavable signal peptide (SP) of 23 aa (Figure 7). Both the mouse and the human proteins end with methionine. The significance of this is unknown. There is also a transmembrane (TM) domain predicted by aa 23 – 40 of the N-terminus (immediately downstream of the signal peptide), suggesting PLAC1 is targeted to a membranous domain. Finally, there are 4 possible glycosylation sites suggesting it is O-glycosylated in its mature form, and 13 potential phosphorylation sites. The conserved 4 cysteine residues are suggested to be involved in forming 1–4, 2–3 disulfide bridges, similar to the zona pellucida-N (ZP-N) subdomain (Jovine et al, 2006)
Figure 7.

Schematic model of PLAC1 protein domains
Significant sequence homology (approximately 30%) between the ZP3 (zona pellucida 3) protein of several species of eutherian mammals and PLAC1 was demonstrated. ZP3 is a sperm-binding glycoprotein in the zona pellucida and thought to confer species specificity during fertilization. The presence of the ZP3 motif has been also been recognized in a variety of extracellular receptor-like glycoproteins, i.e. TGF-beta receptor type III (betaglycan) and uromodulin (Bork and Sander, 1992). Recently an oocyte-specific protein, Oosp1, has been identified that exhibits significant homology to the Plac1 (Yan et al, 2001). It also appears to code for an extracellular protein. However, its function has not been defined. The ZP3 motif has been shown to be essential for polymerization of the ZP3 protein and other ZP3 motif-containing proteins, i.e. uromudulin, to form filamentous structures (Jovine et al, 2002; Jovine et al, 2004). Its conserved presence in PLAC1 is therefore likely to be important for protein-protein interactions relevant to its function, i.e. polymerization or interactions with heterologous protein partners.
PLAC1 in Human Cancer
Recently, PLAC1 expression has been demonstrated in a variety of human cancers. PLAC1 expression, as detected by differential microarray analysis, was noted to be altered in EBV-transformed lymphoid cell lines where loss of EBV expression was enforced (Yin et al, 2006). This led to further analyses demonstrating PLAC1 expression in a variety of human cancers, including breast and prostate cancer, as well as low expression in testis and cerebellum. This identified PLAC1 as a new member of the cancer-testis group of antigens. Subsequent studies failed to confirm a direct relationship between EBV and PLAC1 expression but suggested a role for PLAC1 expression in cancer biology. During the same time period, Chen, et al (Chen et al, 2006) reported the expression of (cancer-placenta antigen 1) in gastric cancer patients using subtractive hybridization. CP-1 was later determined to be identical to PLAC1. They further demonstrated that some of these patients developed circulating anti-PLAC1 antibodies, implicating PLAC1 as a tumor antigen and potential therapeutic target. This speculation was supported by subsequent studies examining patients with hepatocellular and colorectal cancers (Dong et al, 2008; Liu et al, 2008). These patients exhibited a high rate of PLAC1 expression that was associated with PLAC1 auto-antibodies in some patients. Preliminary analysis of a small sample of the colorectal cancer patients further suggested the ability of these patients to mount a PLAC1-specific immune response conveyed a survival advantage. At about the same time Silva et al, (2007) also reported the expression of PLAC1 in a variety of human cancers as well as the presence of circulating anti-PLAC1 antibodies, supporting the initial observation reported by Chen et al (2006). These observations support the therapeutic potential of targeting the PLAC1 antigen and indicate a significant role for PLAC1 in cancer cell biology. A subsequent study by Tchabo et al (2009), however, did not detect anti-PLAC1 antibodies in a relatively small cohort of patients (n = 21) with epithelial ovarian cancer. Furthermore PLAC1 expression was not significantly associated with survival.
In vitro evidence consistent with a functionally important role for PLAC1 in some tumor cells was provided by Koslowski et al (2007). They reported the expression of PLAC1 in breast and ovarian cancer and demonstrated that PLAC1 localized to the plasma membrane in the MCF-7 breast cancer cell line. Neutralizing the PLAC1 antigen by gene silencing or neutralizing antibodies resulted in decreased proliferation and motility, processes essential to cancer survival. Collectively, these studies indicate PLAC1 is a new member of the cancer-testis-placenta group of antigens and likely to have a significant role in modulating processes essential to cancer cell biology, including proliferation, invasion, and survival. It is not surprising that these processes are relevant to some aspects of trophoblast function, as well. The fact that PLAC1 elicits an antibody response, that appears to confer a survival advantage in some cancer patients who express it, further suggests its functional relevance. Interestingly, data reported by Silva et al (2007) also indicated a small number of female control patients, not exposed to the cancer-derived PLAC1 antigen, may develop anti-PLAC1 antibodies by prior exposure to PLAC1 during pregnancy, raising the intriguing possibility that circulating anti-PLAC1 antibodies may have a deleterious effect on trophoblast function and pregnancy outcome.
Koslowski et al (2009) have recently reported a feature of promoter action that is responsible for the ectopic expression of PLAC1 in breast cancer cells. Their studies indicate that the ubiquitous transcription factors SP1 and C/EBPβ-2 account for the full expression of PLAC1 in these cells. Additionally, ERα also appears to be involved via non-classical mechanisms. Interestingly, C/EBPβ-2 exhibits a placenta-specific expression pattern suggesting it may be relevant to PLAC1 expression in the placenta as well. Moreover, it drives the expression of Dlx3, a transcription factor critical for normal placental development (Holland et al, 2004). Finally, SP1 and its homologue have also been shown to be important to placental development in mouse knockout models (Kruger et al, 2007). The possibility that this promoter region acts in concert with other promoter regions to regulate PLAC1 in the normal trophoblast remains to be determined.
PLAC1 as a Marker for Threatened Pregnancies
Every since the detection of fetal-specific RNA transcripts in maternal plasma was reported by Poon, et al (2000), their utility as biomarkers useful in screening for genetic disorders and as biomarkers for gestational disorders has been of great interest. The restricted nature of PLAC1 expression suggests it might serve as a useful biomarker indicative of functional disruptions at the maternal-fetal interface. This notion was supported when Concu et al (2005) demonstrated that PLAC1 mRNA can be detected in maternal serum as early as 8 weeks gestation and throughout pregnancy. Based on this work, Farina et al (2005) were able to demonstrate that circulating PLAC1 mRNA was markedly diminished in pregnancies associated with threatened abortion prior to 20 weeks gestation but not after this time point. Subsequent studies demonstrated elevated levels of circulating PLAC1 mRNA in preeclampsia that were directly related to disease severity (Fujito et al, 2006; Purwosunu et al, 2007). Several possibilities to explain the observed changes in circulating PLAC1 mRNA with preeclampsia were offered by Ng et al (2003). First, increased apoptosis occurring within villous trophoblasts during preeclampsia (DeFrederico et al, 1999) may be associated with increased release of trophoblast-specific mRNA into maternal plasma. Similarly, the shedding of membrane particles derived from the syncytiotrophoblast is also increased during preeclampsia (Redman and Sargent, 2008). These particles contain antigens that appear capable of interacting with and modulating the maternal immune system. Second, the circulating half-life of circulating fetal DNA is increased four-fold in patients with preeclampsia (Lau et al, 2002). Similar mechanisms might contribute to the higher circulating concentrations of PLAC1 mRNA observed in preeclampsia. The fact that PLAC1 mRNA levels was shown to be positively and negatively associated with distinct gestational pathologies suggests that circulating PLAC1 mRNA can serve as a clinically useful marker predictive of gestational disorders.
Summary
Recent studies have identified PLAC1 as a novel, X-linked gene whose expression is restricted to the placenta in healthy individuals. Circulating maternal PLAC1 mRNA levels are significantly associated with distinct gestational disorders suggesting it can serve as a clinically useful biomarker for specific pregnancy complications, i.e. miscarriage and preeclampsia. Additionally, its ectopic expression in a variety of human cancers identifies it as a new member of the cancer-placenta-testis antigens. Its tissue and cell-specific expression appear to be due to the combination of ubiquitous transcription factors that interact in specific ways to drive its placental or tumor-specific expression. While its role in placental development and function has yet to be defined, available evidence suggests it is important to tumor cell growth that, in some cases, may negatively impact patient survival. Speculatively, it has been intriguingly suggested that PLAC1 may serve an analogous role in the invasion of the placenta into the uterine wall and the invasive spread of cancers.
Acknowledgments
The research presented in this review was supported, in part, by the Intramural Research Program of the National Institute on Aging, NIH (RN, DS) and NIH grants HD41404 and 048862 (MEF)
Footnotes
Conflict of Interest
The authors certify that there are no conflicts of interest to declare in regards to the work communicated in this review.
References
- Antequera F, Bird A. Number of CpG islands and genes in human and mouse. Proc Natl Acad Sci USA. 1993;90:11995–11999. doi: 10.1073/pnas.90.24.11995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork P, Sander C. A large domain common to sperm receptors (ZP2 and ZP3) and TGF-beta type III receptor. FEBS Lett. 1992;300:237–240. doi: 10.1016/0014-5793(92)80853-9. [DOI] [PubMed] [Google Scholar]
- Chen J, Pang XW, Liu FF, Dong XY, et al. PLAC1/CP1 gene expression and autologous humoral immunity in gastric cancer patients. Beijing Da Xue Xue Bao. 2006;38:124–127. [PubMed] [Google Scholar]
- Cocchia M, Huber R, Pantano S, et al. PLAC1, an Xq26 gene with placenta- specific expression. Genomics. 2000;68:305–312. doi: 10.1006/geno.2000.6302. [DOI] [PubMed] [Google Scholar]
- Concu M, Banzola L, Farina A, et al. Rapid clearance of mRNA for PLAC1 gene in maternal blood after delivery. Fetal Diagn Ther. 2005;20:27–30. doi: 10.1159/000081365. [DOI] [PubMed] [Google Scholar]
- DiFederico E, Genbacev O, Fisher SJ. Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am J Pathol. 1999;155:293–301. doi: 10.1016/S0002-9440(10)65123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XY, Peng JR, Ye YJ, et al. Plac1 is a tumor-specific antigen capable of eliciting spontaneous antibody responses in human cancer patients. Int J Cancer. 2008;122:2038–43. doi: 10.1002/ijc.23341. [DOI] [PubMed] [Google Scholar]
- Fant M, Weisoly D, Cocchia M, et al. PLAC1, a trophoblast-specific gene is expressed throughout human pregnancy and regulated by ketatinocyte growth factor (KGF) Mol Reprod Develop. 2002;63:430–436. doi: 10.1002/mrd.10200. [DOI] [PubMed] [Google Scholar]
- Fant M, Barerra-Saldana H, Dubinsky W, et al. The PLAC1 protein localizes to membranous compartments in the apical region of the syncytiotrophoblast. Mol Reprod Dev. 2007;74:922–929. doi: 10.1002/mrd.20673. [DOI] [PubMed] [Google Scholar]
- Farina A, Rizzo N, Concu M, et al. Lower maternal PLAC1 mRNA in pregnancies complicated with vaginal bleeding (threatened abortion < 20 weeks) and a surviving fetus. Clin Chem. 2005;51:224–227. doi: 10.1373/clinchem.2004.041228. [DOI] [PubMed] [Google Scholar]
- Fujito N, Samura O, Miharu N, et al. Increased plasma mRNAs of placenta- specific 1 (PLAC1) and glial cells-missing 1 (GCM1) in mothers with pre-eclampsia. Hiroshima J Med Sci. 2006;55:9–15. [PubMed] [Google Scholar]
- Hemberger MC, Pearsall RS, Zechner U, et al. Genetic dissection of X-linked interspecific hybrid placental dysplasia in congenic mouse strains. Genetics. 1999;153:383–390. doi: 10.1093/genetics/153.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland M, Bliss S, Berghorn K, Roberson M. A role for CCAAT/Enhancer- binding protein b in the basal regulation of the distal-less gene promoter in placental cells. Endocrinology. 2004;145:1096–1105. doi: 10.1210/en.2003-0777. [DOI] [PubMed] [Google Scholar]
- Jovine L, Qi H, Williams Z, et al. The ZP domain is a conserved module for polymerization of extracellular proteins. Nat Cell Biol. 2002;4:457–61. doi: 10.1038/ncb802. [DOI] [PubMed] [Google Scholar]
- Jovine L, Qi H, Williams Z, et al. A duplicated motif controls assembly of zona pellucida domain proteins. PNAS (U S A) 2004;101:5922–7. doi: 10.1073/pnas.0401600101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovine L, Janssen WG, Litscher, et al. The PLAC1-homology region of the ZP domain is sufficient for protein polymerisation. BMC Biochem. 2006;7:11. doi: 10.1186/1471-2091-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MS, Threat TA, Wang X, et al. Genome-wide mapping of unselected transcripts from extraembryonic tissue of 7.5 day mouse embryos reveals enrichment in the t complex and under-representation on the X-chromosome. Hum Mol Genet. 1998;7:1967–1978. doi: 10.1093/hmg/7.12.1967. [DOI] [PubMed] [Google Scholar]
- Koslowski M, Sahin U, Mitnacht-Kraus R, et al. A placenta-specific gene ectopically activated in many human cancers is essentially involved in malignant cell processes. Cancer Res. 2007;67:9528–9534. doi: 10.1158/0008-5472.CAN-07-1350. [DOI] [PubMed] [Google Scholar]
- Koslowski M, Tuereci O, Biesterfeld S, et al. Selective activation of trophoblast-specific PLAC1 in breast cancer by CCAAT/enhancer binding protein β (C/EBPβ) isoform 2. JBC. 2009 Aug 3; doi: 10.1074/jbc.M109.031120. 2009. E published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger I, Vollmer M, Simmons D, et al. Sp1/Sp3 compound heterozygous mice are not viable: Impaired erythropoiesis and severe placental defects. Developmental Dynamics. 2007;236:2235–2244. doi: 10.1002/dvdy.21222. [DOI] [PubMed] [Google Scholar]
- Kushi A, Edamura K, Noguchi M, et al. Generation of mutant mice with large chromosomal deletion by use of irradiated ES cells—analysis of large deletion around hprt locus of ES cells. Mamm Genome. 1998;9:269–273. doi: 10.1007/s003359900747. [DOI] [PubMed] [Google Scholar]
- Lau TW, Leung TN, Chan LY, et al. Fetal DNA clearance from maternal plasma is impaired in preeclampsia. Clin Chem. 2002;48:2141–2146. [PubMed] [Google Scholar]
- Liu F-F, Dong XY, Pang XW, et al. The specific immune response to tumor antigen CP1 and its correlation with improved survival in colon cancer patients. Gastroenterology. 2008;134(4):998–1006. doi: 10.1053/j.gastro.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Massabbal E, Parveen S, Weisoly DL, et al. PLAC1 expression increases during trophoblast differentiation: evidence for regulatory interactions with the fibroblast growth factor-7 (FGF-7) axis. Mol Reprod Develop. 2005;71:229–304. doi: 10.1002/mrd.20272. [DOI] [PubMed] [Google Scholar]
- Ng EK, Leung TN, Tsui NB, et al. The concentration of circulating corticotropin-releasing hormone mRNA in maternal plasma is increased in preeclampsia. Clin Chem. 2003;49:727–731. doi: 10.1373/49.5.727. [DOI] [PubMed] [Google Scholar]
- Poon LL, Leung TN, Lau TK, Lo YM. Presence of fetal RNA in maternal plasma. Clin Chem. 2000;46:1832–1834. [PubMed] [Google Scholar]
- Purwosunu Y, Sekizawa A, Farina A, et al. T-Cell-free mRNA concentrations of CRH, PLAC1, and selectin-P are increased in the plasma of pregnant women with preeclampsia. Prenat Diagn. 2007;27:772–777. doi: 10.1002/pd.1780. [DOI] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Circulating microparticles in normal pregnancy and pre-eclampsia. Placenta. 2008;29(SupplA):S73–7. doi: 10.1016/j.placenta.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Sapin V, Dolle P, Hindelang C, et al. Defects of the chorioallantoic placenta in mouse RXRα null fetuses. Developmental Biology. 1997;191:29–41. doi: 10.1006/dbio.1997.8687. [DOI] [PubMed] [Google Scholar]
- Searle AG, Edwards JH, Hall JG. Mouse homologues of human hereditary disease. J Med Genet. 1994;31:1–19. doi: 10.1136/jmg.31.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva WA, Gnjatic S, Ritter E, et al. PLAC1, a trophoblast-specific cell surface protein, is expressed in a range of human tumors and elicits spontaneous antibody responses. Cancer Immun. 2007;7:18–25. [PMC free article] [PubMed] [Google Scholar]
- Suemizu H, Aiba k, Yoshikawa T, et al. Expression profiling of placentomegaly associated with nuclear transplantation of mouse ES cells. Developmental Biology. 2003;253:36–53. doi: 10.1006/dbio.2002.0870. [DOI] [PubMed] [Google Scholar]
- Tchabo NE, Mhawech-Fauceglia P, Caballero OL, et al. Expression and serum immunoreactivity of developmentally restricted differentiation antigens in epithelial ovarian cancer. Cancer Immun. 2009 Aug 26;9:6. 2009. [PMC free article] [PubMed] [Google Scholar]
- Yan C, Pendola FL, Jacob R, et al. Oosp1 encodes a novel mouse oocyte-secreted protein. Genesis. 2001;31:105–10. doi: 10.1002/gene.10010. [DOI] [PubMed] [Google Scholar]
- Yin Q, Fant M, Flemington E. The Placental Specific Gene, PLAC1, is induced by the Epstein Barr virus and is expressed in human tumor cells. American Association of Clinical Research. 2006 doi: 10.1186/1743-422X-11-107. April, 2006 (Abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner U, Reule M, Orth A, et al. An X-chromosome linked locus contributes to abnormal placental development in mouse interspecific hybrids. Nat Genet. 1996;12:398–403. doi: 10.1038/ng0496-398. [DOI] [PubMed] [Google Scholar]


