Abstract
Treatment of type 1 diabetes mellitus could be greatly improved by applying a closed-loop control strategy to insulin delivery, also known as an artificial pancreas (AP). In this work, we outline the design of a fully implantable AP using intraperitoneal (IP) insulin delivery and glucose sensing. The design process utilizes the rapid glucose sensing and insulin action offered by the IP space to tune a PID controller with insulin feedback to provide safe and effective insulin delivery. The controller was tuned to meet robust performance and stability specifications. An anti-reset windup strategy was introduced to prevent dangerous undershoot toward hypoglycemia after a large meal disturbance. The final controller design achieved 78% of time within the tight glycemic range of 80–140 mg/dL, with no time spent in hypoglycemia. The next step is to test this controller design in an animal model to evaluate the in vivo performance.
1. Introduction
Type 1 diabetes mellitus (T1DM) is a chronic disease that occurs when the pancreatic beta cells are destroyed, leaving the body unable to produce sufficient insulin to maintain glycemic homeostasis. To manage this condition, people with T1DM need to self-administer exogenous insulin based on measurements of their blood glucose concentration (BG) and an estimation of carbohydrate (CHO) content in their meals. This procedure requires the person to measure their blood glucose concentration several times per day using a fingerstick method to access capillary blood. The goal is to keep the BG in equilibrium, avoiding values that are too high (hyperglycemia, BG > 180 mg/dL) and too low (hypoglycemia, BG < 70 mg/dL). Both hyper- and hypoglycemia lead to health complications, although the effects of hypoglycemia are more sudden and can quickly escalate to become life-threatening. Untreated hyperglycemia can also be life-threatening if diabetic ketoacidosis occurs, although most of the health problems caused by chronic hyperglycemia, such as retinopathy, nephropathy, neuropathy, and cardiovascular disease, become more pronounced as more time is spent in hyperglycemia throughout a person’s life.1
While diabetes management can be an onerous task, technological advances have begun to reduce the difficulty of treatment and provide better health outcomes overall. The introduction of insulin pumps to provide continuous subcutaneous (SC) insulin infusion has allowed many people to achieve better glucose control than they could using multiple daily injections of insulin.2,3 These pumps provide basal insulin, which is a low background dose of insulin needed throughout the day, as well as larger insulin boluses to compensate for meal consumption or correct for high BG. Another important technological advance was the development of the continuous glucose monitor (CGM), a device that uses a subcutaneous electrode to measure the glucose concentration in the interstitial fluid.4 These sensors provide an estimate of the BG based on the subcutaneous glucose concentration every 5 min. In combination, insulin pumps and sensors allow people with diabetes to exert much more influence over their health than what was previously possible.5
Even with the use of insulin pumps and glucose sensors, the treatment process is ultimately an open-loop one, with the patient manually observing the glucose concentration, calculating an insulin dose, and using the pump to command that dose. While control engineering is a well-developed field, its use is relatively new in medical applications. The ability to close the loop between glucose sensor and insulin pump is an exciting development that will bring a new era to diabetes management. The artificial pancreas (AP) will advance the state-of-the-art technology of diabetes treatment by using a control algorithm to close the loop between the insulin pump and the CGM, providing automated insulin dosing. The system will use feedback and potentially feedforward control to maintain glucose concentrations near a desired set point or within a desired zone.6
Many variations of the AP have already been tested in clinical studies, with some even taking place in an outpatient environment.6 The AP designs used in these studies show promising results, but their performance is limited by the use of commercially available external insulin pumps and glucose sensors that operate in the subcutaneous space, introducing severe delays into the control loop. In this work, we present a design process for a controller that will work with implantable insulin pumps and glucose sensors, greatly reducing the delays and resulting in overall better glycemic control.
2. Implantable Artificial Pancreas Design
2.1. Control Objective, Challenges, and Constraints
The objective of the artificial pancreas is to provide safe and effective glycemic control for people with T1DM. Quantitatively, the goal is to maintain the blood glucose concentration within the tight range of 80–140 mg/dL for as much time as possible by delivering doses of insulin. In addition, the controller must prevent hypoglycemic episodes. Since safety must remain the top priority in any medical device system, some AP designs introduce glucagon as a second manipulated variable.7 This hormone stimulates the conversion of glycogen stored in the liver to glucose and may be used as a rescue treatment when a person’s BG approaches hypoglycemia. However, there are practical difficulties with using glucagon in a closed-loop system, and the effects of long-term glucagon use are unknown.8 In addition, a clinical study designed to compare an AP with and without glucagon did not find any significant improvement made by including glucagon in the system.7 For these reasons, we focus on the design of an insulin-only system. An important constraint in this system is that insulin cannot be removed once it has been delivered, so the AP must be tuned accordingly to avoid a potentially dangerous situation.
There are several disturbance challenges that the AP must face to successfully control BG. The most difficult disturbances to control occur following the ingestion of a meal, when the BG concentration increases rapidly. Other challenges include periods of exercise, which can result in unpredictable BG changes, and overnight periods, during which the AP user is asleep and therefore dependent on the AP to maintain the BG within a safe range.6 Periods of illness and stress, along with hormonal changes, affect the way the body responds to insulin.9 The AP must be able to adapt to changing insulin sensitivity to maintain glycemic control.
2.2. An Implantable System
To effectively reject glycemic disturbances, the AP controller must have access to rapid sensing and actuation. The majority of AP designs tested thus far rely on commercially available insulin pumps and glucose sensors that operate in the subcutaneous space.6 These devices have several advantages: they are minimally invasive, already approved by the United States Food and Drug Administration, and easy to use. Unfortunately, diffusion lags between the interstitial fluid and the blood introduce severe delays in both glucose sensing and insulin action, making fully automated closed-loop control much more difficult.10−12 To overcome these delays and achieve good results, most iterations of the AP have incorporated meal announcement, a type of feedforward action initiated by the user to trigger a bolus of insulin before the meal is consumed. While the addition of the meal announcement improves the resulting BG profile following a meal, it also poses a safety risk by requiring the user to accurately and reliably perform an action.13 The best solution would be to reduce delays in the system so that fully automated control is possible. The reduction of delays may be accomplished with the use of alternate insulin delivery and glucose sensing methods.
The intraperitoneal (IP) space was first introduced as an alternative insulin delivery route in the 1970s.14 Insulin delivered through the intraperitoneal route has faster pharmacokinetic and pharmacodynamic characteristics than insulin delivered through the subcutaneous route: when insulin is delivered through the SC route, the absorption peak occurs 50–60 min later,15 as opposed to 20–25 min when using the IP route.16 The insulin is also cleared more quickly: insulin delivered through the SC route has a residence time of 6–8 h,15 while IP insulin has a much shorter residence time of 1–2 h.16 A further advantage of IP insulin delivery is that it mimics healthy pancreatic activity by allowing a high uptake of insulin by the liver and producing a positive portal-systemic insulin gradient.17 The use of implanted insulin pumps can also lead to improved quality of life: a randomized crossover study showed that continuous intraperitoneal insulin infusion resulted in improved health-related quality of life and treatment satisfaction over continuous subcutaneous insulin infusion.18 The main obstacle barring adoption of IP insulin delivery is that it requires either a pump to be surgically implanted, as in Logtenberg et al.,19 or a percutaneous port to be created, as in Liebl et al.20 There is no IP insulin delivery system currently approved for use in the United States, so this hurdle would need to be passed before the implantable AP could be tested in human clinical trials in the US.
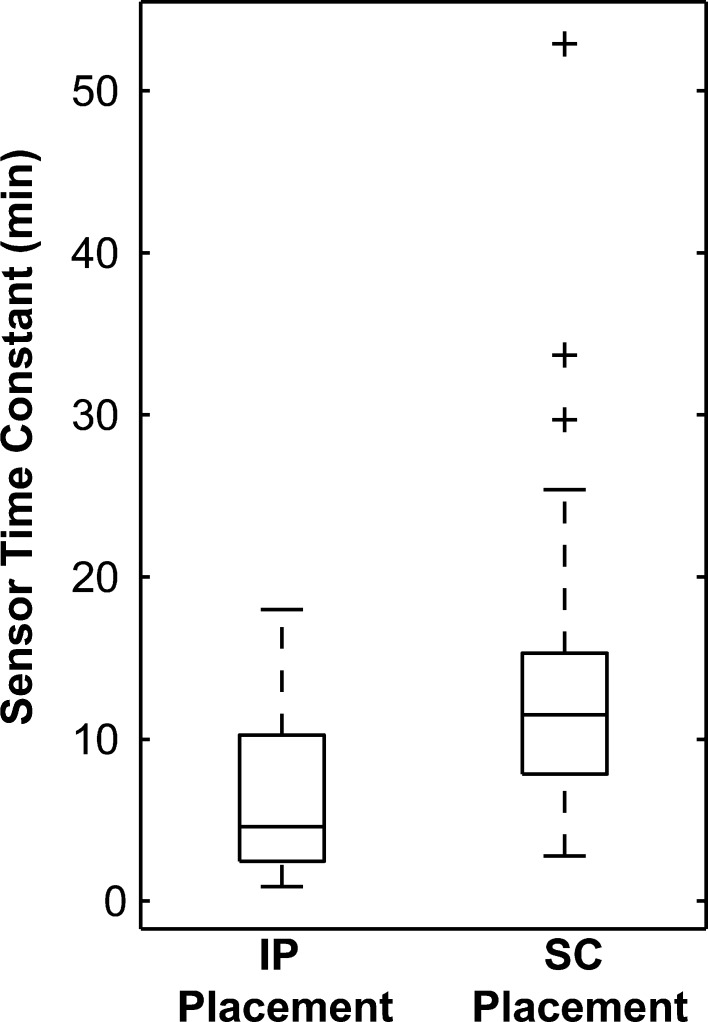
The improvements gained by faster actuation through IP insulin delivery will be limited without the implementation of fast glucose sensing. In initial clinical studies, an AP using intraperitoneal insulin delivery did not perform as well as expected because the sensor introduced a lag to the glucose measurement.21 Several studies have shown that there is a diffusion lag between the blood and the interstitial fluid, resulting in measurements that lag behind the blood glucose concentration.10,11,22 Preliminary animal studies have demonstrated that sensors placed in the IP space provide a more rapid measurement of blood glucose than sensors placed in the SC space due to the proximity to a highly vascularized area.23,24 The diffusion process can be modeled as a first-order transfer function with time constant τS (min). The time constants identified from experimental data in a swine model for sensors placed in the intraperitoneal and subcutaneous space are shown in Figure 1. The IP sensor time constants were lower and had a tighter distribution than the SC time constants. This evidence suggests that a glucose sensor implanted within the IP space will provide a more useful estimation of the blood glucose concentration by reducing the diffusion lag.
Figure 1.
Box plot showing the statistical properties of the fitted time constants for sensors placed in the IP space or the SC space of swine, demonstrating that the IP sensors had a lower mean time constant and a tighter distribution than the SC sensors (data from experimental study presented in Burnett et al.23).
The primary differences between IP and SC devices are summarized in Table 1. A fully implanted AP will make use of both intraperitoneal insulin delivery and glucose sensing. The pump, sensor, and controller will all be implanted, and the system will be operated using a hand-held remote. This approach will eliminate the need to remove and apply new sensors and insulin infusion sets, as must be done with subcutaneous devices. Externally worn devices can be cumbersome, so this approach may also increase patient compliance. We hypothesize that the glycemic control provided by a fully implantable system will be superior to that which is possible with a subcutaneous system. Since the sensing time constant is up to two times faster, the controller can react promptly to impending hypo- and hyperglycemia.23 Additionally, pump suspension will have an almost immediate effect on the BG, while with the SC system the insulin depot in the SC space may delay the effect by up to 60 min.25 The faster insulin action and clearance will lead to more predictable dynamics, making closed-loop control more successful.
Table 1. Summary of Differences between Subcutaneous and Intraperitoneal Insulin Pumps and Glucose Sensors.
| subcutaneous space | intraperitoneal space | |
|---|---|---|
| insulin absorption peak | 50–60 min15 | 20–25 min16 |
| insulin residence time | 6–8 h15 | 1–2 h16 |
| sensor measurement time constant | 12.4 min23 | 5.6 min23 |
| device placement | external, placed on skin with adhesive patches and tubing3,4 | implanted, no components attached to skin19,23 |
| device lifetime | replace sensor every 7 days and pump infusion set every 2–3 days3,4 | implanted pumps last years, with transcutaneous insulin refills every few months26 |
| device invasiveness | minimally invasive3,4 | requires surgery23,26 |
| device availability | commercially available3,4 | in development21,23 |
2.3. Controller Design and Tuning
Several control strategies have been evaluated for AP applications, including proportional-integral-derivative control (PID), model predictive control (MPC), and fuzzy logic.6 Records of information related to clinical trials using each type of controller are available in the searchable database located at www.thedoylegroup.org/apdatabase. Model predictive control has been proposed as a suitable strategy for AP designs using subcutaneous insulin delivery and sensing because of the large delays in these systems.27 When using intraperitoneal insulin delivery and glucose sensing, the system lags are highly reduced, and we are left with a standard single-input, single-output control problem. In this case, we anticipate that the advanced control capability of MPC may no longer needed, and that a PID controller will provide satisfactory performance. Because the insulin will act quickly and glucose changes will be sensed rapidly, the system can operate well without the predictive power offered by MPC.
The use of model based tuning is recommended for the AP because online tuning through trial and error is not acceptable for a medical application; however, we need to find a balance between a general and personalized model. Completing time-consuming model identification procedures for individual subjects is not feasible, especially if the AP is to be adopted on a large scale. Still, individual subjects have widely varying insulin sensitivities.28 In a previous study, a third-order discrete-time model structure was identified that adequately captures the behavior of insulin action on the blood glucose concentration.29,30 The poles of the model were found to be consistent between subjects, while a personalization factor was added in the model gain. The model that was identified for intraperitoneal insulin action on blood glucose concentration is
| 1 |
where TDI is the total daily insulin dose of the patient (U), G is the blood glucose concentration (mg/dL), UD is the insulin delivered through the IP route (U/h), and the sampling time is 5 min. The inclusion of the TDI allows the model gain to be tailored to an individual subject’s insulin sensitivity.
Internal model control (IMC) is a comprehensive tuning method that allows PID parameters to be calculated directly from the process model. This method leaves a single tuning parameter,τC, which is used to set the closed-loop time constant.31 Internal model control tuning has been used successfully in AP designs for SC insulin delivery.32,33 To make the model easier to work with for controller tuning and robustness analysis, the model MD is converted to continuous time. This conversion can be done using several methods, but the zero-pole matching method was determined to best preserve the model characteristics.34 It should be noted, however, that the final tuning parameters obtained using other methods of conversion are the same within choice of τC. Therefore, the final tuning parameters are robust to the conversion method.
The model resulting from the conversion from discrete to continuous time is
| 2 |
where the time constant units are minutes. Internal model control tuning rules require a second-order model to obtain a PID controller. Skogestad’s half rule was developed as a method to reduce higher-order models to the first- or second-order model required to use IMC PID tuning rules.35 Using this method, the reduced-order model parameters are determined by the following relations:
| 3 |
The final model obtained is
| 4 |
Using this model, the tuning parameters are determined using IMC tuning relations:
| 5 |
The digital PID controller is implemented using the velocity form, with
| 6 |
where
| 7 |
| 8 |
| 9 |
| 10 |
In this set of equations, u (U/h) is the insulin delivery calculated by the controller, P, I, and D (U/h) represent the proportional, integral, and derivative action terms respectively, Δt is the time step (5 min), Gsp is the set point, Gm is the measured glucose concentration (mg/dL) and the integer k denotes the sample number. An important feature of the velocity PID form is that it must include the use of integral action. If it is desired to exclude integral action, the position form should be used instead.31
A derivative filter can be implemented with this controller. The derivative filter prevents excessive controller action in the presence of measurement noise. In this case, the derivative term becomes
| 11 |
The parameter β determines the level of filtering of the derivative term, with a larger value indicating a higher filtering effect. After preliminary testing we selected β as 0.1, which is a commonly used value.31 The derivative filter was used when sensor noise was added during simulation studies.
The tuning parameters obtained using the procedure outlined above are shown in Table 2, along with parameters determined for a PID controller using SC insulin in Laxminarayan et al.36
Table 2. Parameters for PID Control Using IMC Tuning for Intraperitoneal Insulin Compared to Parameters Previously Identified for PID Control Using Subcutaneous Insulin.
| parameter | IMC for intraperitoneal insulin | previously suggested for subcutaneous insulin36 |
|---|---|---|
| KC ([U/h]/[mg/dL])a | 0.023(TDI)(τC + 11)−1 | 0.0026(TDI)/(body weight) |
| τI (min) | 273 | 450 (day), 150 (night) |
| τD (min) | 23.5 | 98 |
The units on the variables in this row are body weight (kg), TDI (U), and τC (min).
The remaining parameter τC will be selected using robust stability and performance considerations.
2.4. In Silico Artificial Pancreas Evaluation
The safety and efficacy of an AP device need to be demonstrated in human clinical trials before it can be considered for widespread use. Prior to these clinical studies, the controller must first show promise in simulation studies. In the case of the implantable AP, there is a further requirement to be evaluated in an animal model because the system involves novel pump and sensor devices that are not already approved for use by the United States Food and Drug Administration. Researchers at the Universities of Virginia (UVA) and Padova developed a metabolic simulator to facilitate the design of AP algorithms.37,38 This platform allows the algorithm to be evaluated on 10 in silico T1DM subjects.
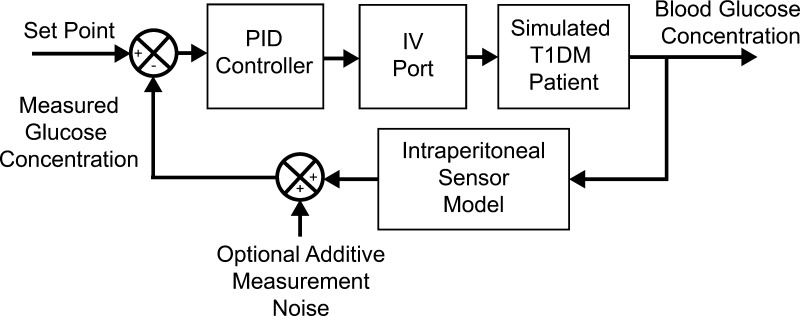
In this study, the metabolic simulator was used to determine the optimal tuning parameters and evaluate the controller performance. The setup that was used in this work is shown in Figure 2.
Figure 2.
Block diagram representation of the configuration of the UVA/Padova metabolic simulator used in this work to test a fully implantable artificial pancreas.
To evaluate the intraperitoneal insulin and intraperitoneal sensing (IP-IP) design we used the intravenous (IV) insulin port and a simulated IP sensor. The IV port was used to approximate the delivery of IP insulin, as was done in Lee et al.30 The IP sensor was implemented by a first-order diffusion model from the IV glucose input with a time constant of 5 min. This value was chosen based on the data presented in Burnett et al.23
The four clinical scenarios shown below were used to evaluate the controllers.
Scenario 1: A large meal of 100 g of carbohydrates (CHO) was administered to evaluate the meal response and the set point undershoot.
Scenario 2: A 30% decrease in insulin sensitivity was tested. The change was simulated by multiplying the insulin delivered by 0.7.
Scenario 3: A 30% increase in insulin sensitivity was tested by multiplying the insulin delivered by 1.3.
Scenario 4: A 27 h clinical protocol was simulated to evaluate the controller performance for a typical real-life scenario. Closed-loop control was initiated at 14:00, followed by a 70 g-CHO meal at 19:00. This meal was followed by an overnight period from 24:00 to 08:00. A breakfast of 40 g-CHO occurred at 08:00, and then a lunch of 70 g-CHO followed at 13:00. Closed-loop control was ended at 17:00.
Scenarios 1–3 were previously tested in Laxminarayan et al.36 for an AP using subcutaneous insulin. The scenarios were repeated here to allow for direct comparison to show the improvement gained by using IP insulin and the design procedure implemented in this paper. The best controller design was selected using Scenarios 1–3. The final controller was tested in Scenario 4, including simulated sensor noise to demonstrate a true-to-life protocol with potential measurement errors. Scenario 4 was used in Lee et al.30 to test a zone-MPC controller using IP insulin delivery and SC glucose sensing. We repeated this protocol to show that we achieved comparable results with our IP-IP PID approach.
2.5. Introduction of Anti-Reset Windup
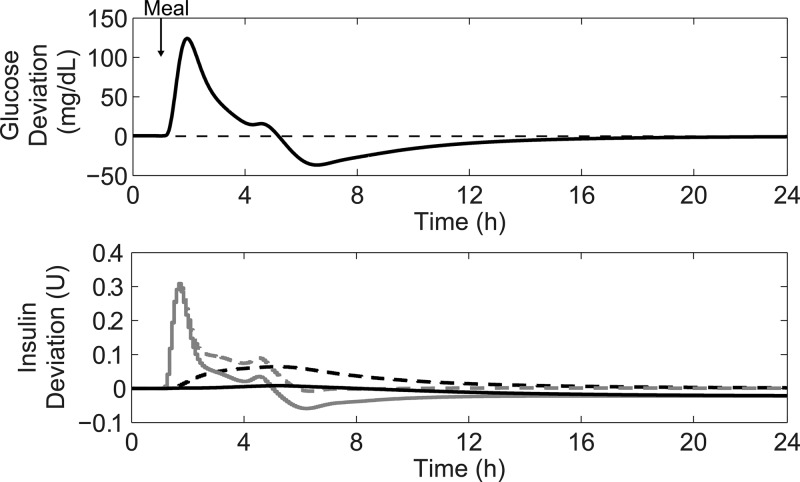
The PID controller may cause the BG to undershoot the set point after a large meal, as shown in Figure 3. In this figure, PID control was used on subject 1 in the UVA/Padova metabolic simulator to control a 100 g-CHO meal disturbance. The bottom panel shows the buildup of the integral term that occurs during the large meal disturbance, leading to the set point undershoot.
Figure 3.
Demonstration of set point undershoot encountered when using integral action after a 100 g-CHO meal. The top panel shows the glucose deviation from the set point after the meal for subject 1 under PID control. The bottom panel shows the insulin trace for PID control (dashed gray line) with the integral component plotted separately (dashed black line). Also on the bottom panel are the advisory mode calculations for PID with anti-reset windup protection (solid lines) with the gray line showing the total insulin and the black line showing the integral component.
This undershoot is highly undesirable because it indicates insulin overdelivery and increases the risk of hypoglycemia. Several approaches have been used to circumvent this effect. One option, applied in several clinical studies9,39−43 and the in silico study presented by Laxminarayan et al.,36 is to remove the integral component and use a proportional-derivative controller. However, the use of PD control is not ideal because set point tracking is sacrificed. Without set point tracking, the controller will not be able to react to changes in insulin sensitivity. Other clinical studies have detuned the integral component to prevent insulin overdelivery. For example, in Steil et al.44 and Laxminarayan et al.36 the integral time constant was set to 150 min at night and increased to 450 min during the day when meals are expected to occur. Nearly all clinical studies using PID control for the AP have placed an upper limit on the integral term as an additional safety feature. For example, in Steil et al. the integral term was constrained to be less than three times the 06:00 basal rate when BG > 60 mg/dL and was restricted to KC(GSP – 60) U/h when BG < 60 mg/dL.44 In Laxminarayan et al. the integral limits were set to 1.4 times the basal rate when BG > 80 mg/dL, 0.7 times the basal rate when BG < 60 mg/dL, and a linear interpolation between those two limits for 60 < BG < 80 mg/dL.
During initial testing, we found that placing an upper limit on the integral term to reduce the undershoot also negatively affected the set point tracking ability of the controller. We found that the best option is to instead implement an anti-reset windup strategy. The relevant approach here is to use conditional integration, which involves increasing or decreasing the amount of integration depending on specified conditions. A key feature of the AP is that the controller will frequently encounter large output disturbances. Even with IP insulin delivery it is anticipated that BG will be elevated for approximately 3 h following a meal. The ideal AP would exhibit the characteristics of a PD controller during large but temporary disturbances, while retaining the characteristics of integral action during smaller but persistent disturbances.
The method of anti-reset windup described in Hansson et al. can be used to meet these requirements.45 The idea behind the method is to attenuate the rate of change of the integral term, I(k), based on the size of the error term, e(k). When the error is large, the rate of change of the integral term should approach zero. When the error is small, the rate of change should be unmodified. To accomplish this goal, the authors introduced a fuzzy logic scheme with two rules: when error is small, KI = KC(Δt/τI), and when error is large, KI = 0.
By using the membership functions defined in Hanssen et al. and applying the min-max inference rule, the equation for the integral term in (8) is adjusted to
| 12 |
| 13 |
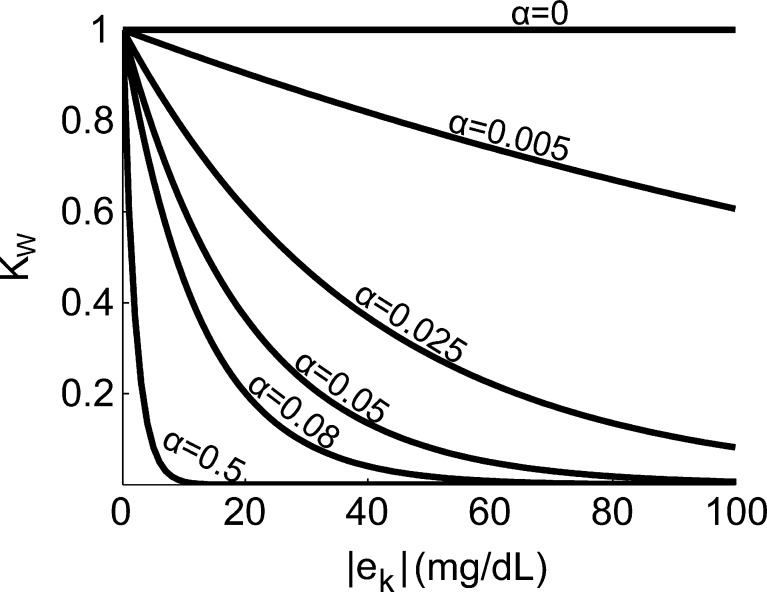
This method introduces a single tuning parameter, α, which sets the degree of attenuation for the integral term. Figure 4 shows a plot of KW versus |e(k)| for increasing values of α.
Figure 4.
Plot of KW versus |e(k)| (mg/dL) for increasing values of α, for error sizes typically encountered after a large meal.
This strategy is ideal for the AP because it is a flexible and dynamic method characterized by a simple algebraic expression. Instead of placing fixed limitations on the integral term that apply for all BG levels, it instead applies a weighting factor appropriate for the current situation. This method is equivalent to using an increasing value for τI as the error becomes larger. The flexibility provided by this method allows for the minimization of undershoot after large meals, while still offering set point tracking to react to changes in insulin sensitivity. In addition, no information about meal timing needs to be supplied for the algorithm to function well. The bottom panel of Figure 3 shows an advisory mode calculation of insulin action that includes anti-reset windup protection. The buildup of the integral term that was observed when using PID control was prevented, leading to a lower recommended insulin dose during the meal.
2.6. Insulin Feedback
When designing the artificial pancreas, it is prudent to draw inspiration from nature by examining how the pancreas is able to achieve glycemic control in people without T1DM. A key feature of physiological glycemic control that is missing from a single-input single-output PID design is that insulin in the blood suppresses further insulin production.46 Most studies using PID control with subcutaneous insulin have incorporated this feature by using an insulin feedback algorithm.44,47 Since it is currently not possible to measure plasma insulin concentration in real time, this method relies on a model of insulin pharmacokinetics to estimate the plasma insulin concentration based on past insulin delivery. The model has been represented as a second-order continuous-time transfer function between insulin delivered and plasma insulin concentration, with gain KPI ([μU/mL]/[U/h]) and time constants τ1 and τ2 (min) determined from experimental data.47 This model can then be discretized to match the sampling period of the controller, giving the following equation:
| 14 |
Here, UD (U/h) is the closed-loop insulin delivery profile, and ĈP(k) is the estimated plasma insulin concentration. The final insulin dose is then calculated as
| 15 |
where u(k) is the insulin dose that was calculated in eq 6. Typically, the insulin plasma concentration units are normalized so that the gain KPI is equal to one.44,47 The parameter γ determines the degree to which the presence of plasma insulin suppresses insulin delivery. The factor (1+(γ/KPI)) is needed so that the insulin delivery UD(k) is equal to the basal rate when the system is at steady-state. In subcutaneous insulin applications, the parameter γ is selected to be 0.5 to achieve good performance.44,47
There is limited information available in the literature to supply a pharmacokinetic model of IP insulin. For SC insulin, the second-order continuous-time model was identified to have time constants of 70 and 55 min.47 One study that was completed to identify corresponding parameters for IP insulin delivery found time constants of 60 ± 8.7 min and 27.2 ± 9.3 min,48 while an earlier study by the same authors found parameters to be 34.6 ± 5.9 min and 17.4 ± 4.7 min.49 In the absence of further modeling data, we chose the more recently identified model parameters to use in the implementation of insulin feedback for our system. Once further experimental data is obtained for the pharmacokinetics of the specific insulin to be used, the model can be updated to provide a more accurate estimation.
3. Controller Optimization and Evaluation
The controller design procedure outlined above leaves several design parameters to be determined: τC, α, and γ. First, candidate values for τC were selected using robust stability and performance analysis. The other two parameters were selected using simulation studies with Scenarios 1–3. The best value for α was determined without IFB by examining the trade-off between the amount of postprandial undershoot and offset after a change in insulin sensitivity. Next, the best value for γ was chosen without anti-reset windup protection (AWP) by examining the minimum and maximum postprandial BG values. Lastly, the controller was tested with both IFB and AWP implemented.
3.1. Robust Stability and Performance
In order to determine whether the system will be stable for a specified model uncertainty, the robust stability condition can be evaluated. In order to use this method, we must first represent a suitable family of possible plants ΠI, in this case using multiplicative uncertainty
| 16 |
where GP is a possible process model, G is the nominal process model, and the uncertainty weight satisfies the inequality |wI(jω)| ≥ lI(ω), ∀ω where
| 17 |
The stability criterion is then given as
| 18 |
where T is the complementary sensitivity function, and wI is the multiplicative uncertainty weight. To represent the parametric uncertainty in the gain and delay of the nominal model, we use
| 19 |
where rk = ((Kmax – Kmin)/(Kmax + Kmin)), and θmax is the maximum delay considered.50
Robust performance analysis allows us to determine whether certain specified performance measures will be met even in the presence of model uncertainty. The necessary relation to show robust performance is given by
| 20 |
where S is the sensitivity function, and wP is the performance weight
| 21 |
where M is the maximum peak of the sensitivity function, A is the steady state tracking error, and ωB* is the bandwidth frequency where the sensitivity function crosses the magnitude of 0.707. In this study, A ≈ 0, ωB= 5 × 10–5 hz, and M = 2, as recommended in Skogestad et al.50
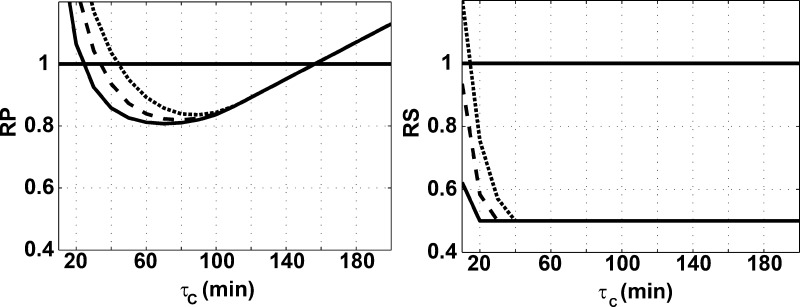
We can use the robust stability and performance analyses to inform our choice of τC. Figure 5 shows whether the RP and RS conditions were met under a specified model uncertainty for varying values of τC. In order to be able to retain RP and RS for a delay uncertainty of 10 min and a gain uncertainty of 0.5, we should choose a τC between 40 and 150 min. The lower value will result in faster, more aggressive control, while the higher value will result in slower, more conservative control.
Figure 5.
Robust performance (left) and robust stability (right) for varying values of τC. The analysis was done for three values of delay uncertainty: 5 min (solid line), 10 min (dashed line), and 15 min (dotted line). The gain uncertainty was kept constant at 0.5.
Setting τC to 40 min to obtain the fastest response, the controller designs in Table 3 were evaluated.
Table 3. Variations on the PID Controller Design Tested in This Work.
| controller | integral action | anti-reset windup (AWP) | insulin feedback (IFB) |
|---|---|---|---|
| PD | |||
| PID | √ | ||
| PID+AWP | √ | √ | |
| PID+IFB | √ | √ | |
| PID+AWP+IFB | √ | √ | √ |
To evaluate the controller with no integral action, the position form was used
| 22 |
where
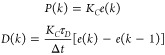 |
23 |
and u̅ is the basal rate needed to maintain a fasting glucose concentration of 110 mg/dL.
3.2. Evaluation of the Anti-Reset Windup Protection
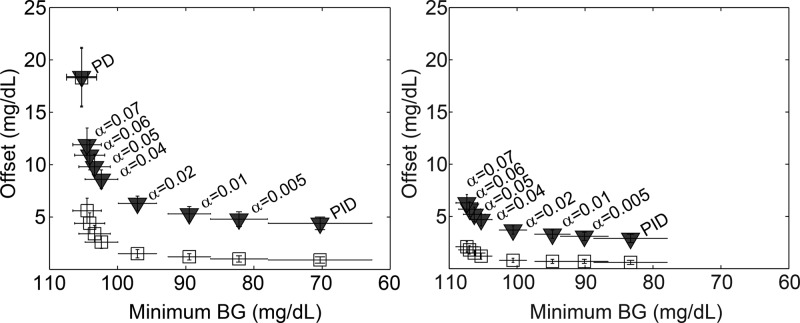
To determine the best parameter α to use for the anti-reset windup algorithm, we examined the trade-off between undershoot mitigation and set point tracking using Scenarios 1 and 2. The undershoot was characterized by the minimum blood glucose concentration during the postprandial period after a large meal. The set point tracking was evaluated by examining the offset remaining at two time points following a change in insulin sensitivity for the different AWP tunings as compared to the PID controller with no AWP. The PID controller with no AWP represents the ideal tracking case at each time point since it has full integral action. The first time point, 11 h, was chosen because after this amount of time the PID controller had made partial progress toward the set point. The 20 h time point was chosen because after this amount of time, the PID controller had nearly returned the BG to the set point. By examining the offset at these two time points, we compared the asymptotic set point tracking of the PID+AWP controllers to the ideal PID tracking on both a short- and long-term time scale. We then plotted the offset versus the minimum BG for various values of α, as shown in the left panel of Figure 6.
Figure 6.
Offset 11 h (black triangles) and 20 h (white squares) after a decrease in insulin sensitivity plotted versus minimum BG after a 100 g-CHO meal for varying values of anti-reset windup parameter α. The left panel shows the offset versus minimum BG for PID+AWP, while the right shows the results for PID+AWP+IFB (γ = 0.5). The data points represent the 10-subject mean and the error bars show standard deviation.
From this analysis, we determined that a good choice for α is 0.04. This option keeps the undershoot above 100 mg/dL but also reduces the offset after a change in insulin sensitivity. Note that the offset will be eliminated over time for all values of α. The larger α is, the longer it takes to reach the set point again after a change in insulin sensitivity.
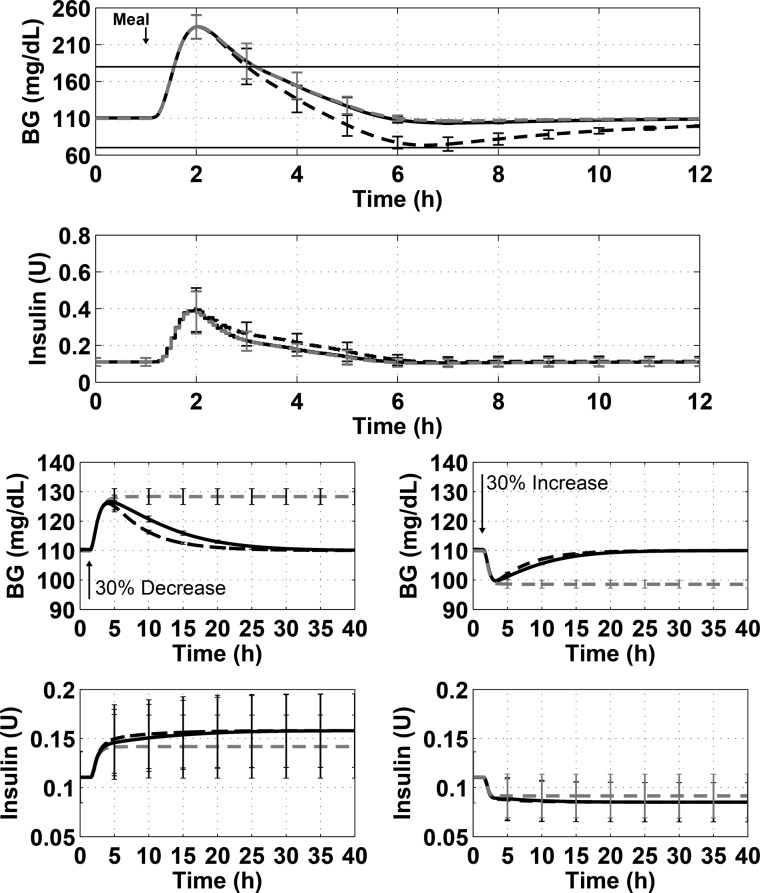
Figure 7 shows the simulation results for Scenarios 1–3 for the optimal value of α, PID control with no anti-reset windup, and PD control.
Figure 7.
Demonstration of the best anti-reset windup tuning (solid black line) compared to PID (dashed black line) and PD (dashed gray line) control. The top panel of each plot shows the blood glucose concentration over time, while the bottom panels show insulin delivered over time. The figures show the results from Scenario 1 (100 g-CHO meal, top), Scenario 2 (30% decrease in insulin sensitivity, bottom left), and Scenario 3 (30% increase in insulin sensitivity, bottom right). The lines show the mean of the 10 subjects, and the error bars show standard deviation.
3.3. Tuning the Insulin Feedback Algorithm
The insulin feedback strategy was tested using Scenario 1 for several values of γ with no anti-reset windup protection. Values of γ were tested from 0 to 0.5. The value of 0.5, which has been used previously for SC insulin, gave the best performance. When IFB was added to PID control, the minimum BG was raised by an average of 13.3 ± 2.4 mg/dL, and the maximum BG was lowered by an average of 9.8 ± 3.8 mg/dL. When using a paired-sample t test to compare the minimum BG for each subject with and without IFB, the difference is significant with a p-value of 3 × 10–8. The same statistical test for the maximum BG for each subject with and without IFB showed significant difference with a p-value of 1.8 × 10–5. The results of the simulation are shown in Figure 8.
Figure 8.
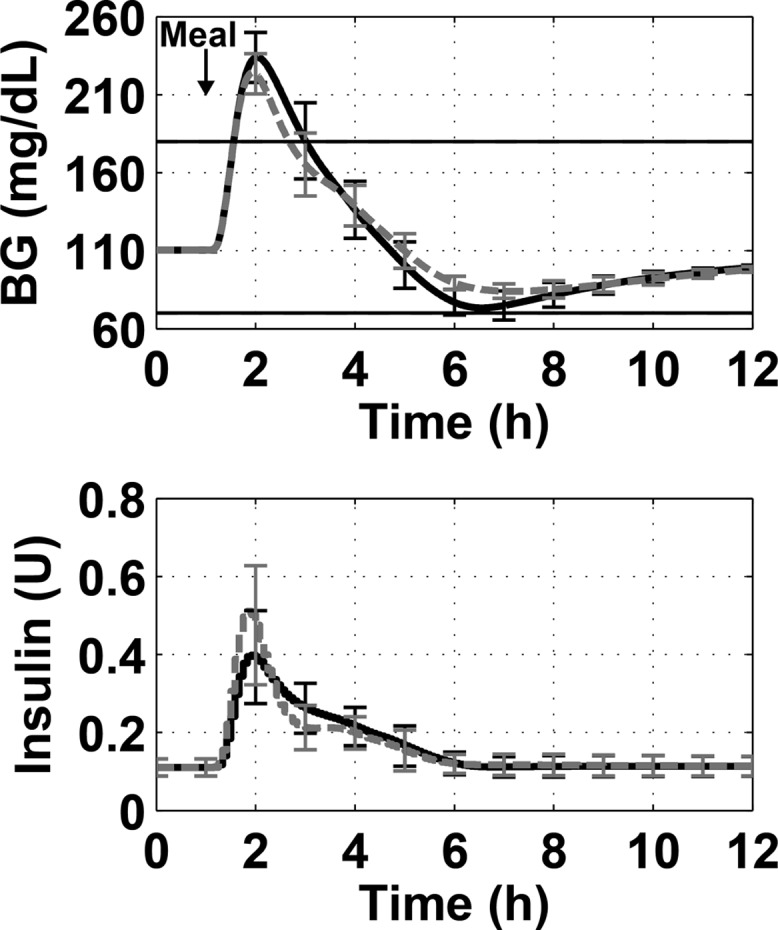
Demonstration of best insulin feedback tuning (dashed gray line) compared to unmodified PID control (solid black line) for a 100 g-CHO meal. The top panel shows the blood glucose concentration over time and the bottom panel shows the insulin delivered. The lines show the mean of the 10 subjects, and the error bars show standard deviation.
To determine whether adding IFB to the controller affects the choice of anti-reset windup parameter α, we repeated the anti-reset windup evaluation with IFB added (γ = 0.5). The results are presented in the right panel of Figure 6. As seen in the figure, the shape of the data curve and optimal value of α = 0.04 remain the same when IFB is added. For all values of α, the performance is better with IFB than without it.
3.4. Evaluation of Finalized Design
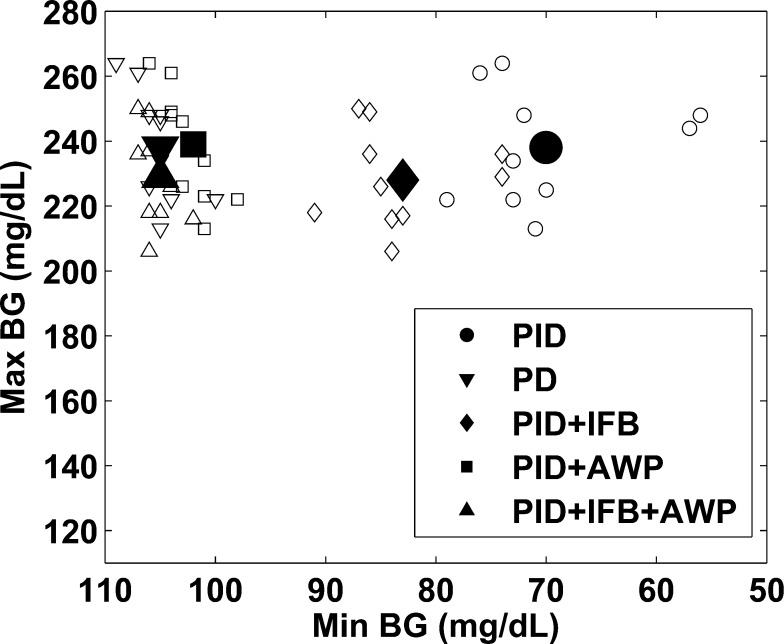
Figure 9 shows a plot of the maximum versus minimum BG achieved by the 5 controller designs tested in this work following a 100 g-CHO meal. The insulin feedback algorithm is able to raise the minimum BG but not to the same degree that anti-reset windup does. Insulin feedback has the added benefit of lowering the maximum BG peak. Overall, PID plus insulin feedback and anti-reset windup provides better control than either strategy alone and both provide great improvements over PID alone. The PD, PID+AWP, and PID+AWP+IFB controllers have some overlap on the plot in Figure 9; however, the PID iterations have a clear advantage over the PD approach since they include set point tracking while PD does not. The most important comparison to make is to determine whether adding IFB to the PID+AWP controller results in significant improvement. These two cases were compared using a paired-sample t test to compare the maximum BG and the minimum BG following the 100 g-CHO meal. The maximum BG was decreased by an average of 10 ± 3.8 mg/dL when IFB was added to the PID+AWP controller. This difference is significant with a p-value of 1.5 × 10–5. The minimum BG was raised by an average of 2.9 ± 1.5 mg/dL when IFB was added. While the difference in the minimum BG is relatively small and not likely of clinical significance, it is still statistically significant with a p-value of 2 × 10–4. The benefit of adding IFB in addition to AWP is the more aggressive initial action that is taken when there is little insulin already in the body. Additionally, including the insulin feedback mechanism is superior clinically because it adds a safety layer to prevent insulin overdelivery. This type of mechanism is a must for clinical application since preventing hypoglycemia is the first priority.
Figure 9.
Plot of the maximum BG versus the minimum BG following a 100 g-CHO meal. The large icon shows the mean, and the small icons show the individual 10 subjects for each case. The PID with IFB and anti-reset windup strategy was able to raise the minimum BG while also lowering the maximum BG, leading to better and safer control than using either strategy alone.
The results achieved with IP insulin using IFB+AWP in this work are compared to those achieved for Scenarios 1–3 with SC insulin in Laxminarayan et al.36 in Table 4. The IP approach resulted in a much lower peak BG than the SC approach. In addition, the IP system did not drive the BG as low as the SC system following the meal, resulting in an overall safer scenario. The time to return to set point after a change in insulin sensitivity was also much faster using IP insulin with the anti-reset windup strategy presented in this work.
Table 4. Comparison of Results with the Intraperitoneal System to Those Achieved with the Subcutaneous System in a Previous Study.
| intraperitoneal system | subcutaneous system36 | |
|---|---|---|
| Scenario 1 max BG (mg/dL) | 229 (15) | 279 (14) |
| Scenario 1 min BG (mg/dL) | 105 (1.6) | 92 (3) |
| Scenario 2 return to set point (h) | 20–30 | ∼80 |
| Scenario 3 return to set point (h) | 20–30 | ∼80 |
The final controller design was evaluated for Scenario 4 with sensor measurement noise to create a realistic test. The measurement noise included in the metabolic simulator was designed to emulate an SC sensor. There is currently no IP sensor model available due to the paucity of data. The SC sensor noise model included in the simulator is described in Breton et al.51 The results are shown in Figure 10.
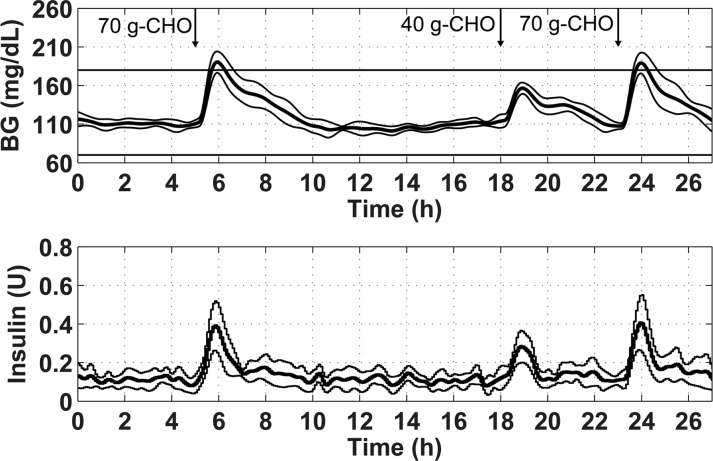
Figure 10.
Blood glucose and insulin trace for the final controller design evaluated on 10 in silico subjects using the 27 h protocol from Scenario 4. The acceptable glycemic zone of 70–180 mg/dL is shown by the black horizontal lines on the top panel. The thick line shows the mean of the 10 subjects, and the thin lines show plus and minus one standard deviation.
A summary of the numerical results from the simulation study displayed in Figure 10 is shown in Table 5.
Table 5. Numerical Results for the Simulation of the Final Controller Settings.
| max BG (mg/dL) | min BG (mg/dL) | % time BG 80–140 mg/dL | % time BG < 70 mg/dL | % time BG > 180 mg/dL |
|---|---|---|---|---|
| 196 ± 14 | 93 ± 7.3 | 78 ± 6 | 0 ± 0 | 5 ± 4 |
The controller was able to maintain the BG within the tight glycemic range of 80–140 mg/dL for 78% of the time, even in the presence of measurement noise. The added noise did cause a lower minimum BG to occur during the simulation, but hypoglycemia was still avoided. These results are comparable to those achieved in Lee et al. using a zone-MPC control strategy with IP insulin and SC sensing.30
4. Discussion
An artificial pancreas that uses IP insulin combined with IP sensing has the potential to greatly improve closed-loop glycemic control. Since IP insulin has faster pharmacokinetic and pharmacodynamic characteristics than SC insulin, the AP will be able to bring BG back to the desired set point faster after glycemic disturbances occur. Also, since the insulin is cleared more quickly, there is less risk of hypoglycemia20 due to delayed insulin action.
In this study, the tuning of the PID controller was informed using robust stability and performance analysis. The robustness of the controller is of great importance, due to inter- and intrapatient variability in the response to insulin. The controller was designed to be able to maintain robust performance and stability even in the presence of 50% gain uncertainty and 10 min delay uncertainty. These estimations of uncertainty were based on Lee et al.32 and are intended to capture changes in insulin sensitivity that can occur throughout the day, as well as unexpected delays due to measurement dropouts, temporary pump failures, or other problems.
The addition of the anti-reset windup strategy used in this work decreases the risk of hypoglycemia after meals, without increasing time spent in hyperglycemia. In addition, set point tracking is maintained following changes in insulin sensitivity. The anti-reset windup strategy used in this paper can also be applied when SC insulin is used, although the tuning factor may need to be adjusted. This method is recommended because it dynamically adjusts the amount of integration based on the situation, leading to better control for both large, temporary disturbances and smaller but persistent disturbances.
Insulin feedback is an important addition to an AP controller because it imitates the physiology of the human body. Increased plasma insulin concentration inhibits the delivery of more insulin, meaning there is less chance for insulin stacking and hypoglycemia. Insulin feedback was initially introduced after the first clinical study of PID control with SC insulin resulted in postprandial undershoot leading to hypoglycemia. A following clinical study applying IFB showed that the postprandial hypoglycemia was reduced, but there were still episodes requiring rescue CHO to be delivered.52 Our study shows that IFB alone is not enough to attenuate postprandial undershoot and that an anti-reset windup strategy in combination with IFB provides the best results. A more accurate model of insulin pharmacokinetics may lead to improved performance of the IFB algorithm. We recommend that such a model be identified before in vivo studies using IFB with IP insulin are conducted.
There are other benefits to using intraperitoneal insulin delivery beyond faster insulin action. This route better mimics the natural insulin production process by the pancreas. When the insulin is delivered into the intraperitoneal space, it introduces a positive portal-systemic insulin gradient throughout the body. This gradient is expected to lead to better overall health. Other hormones involved in the metabolism are also affected by the use of IP insulin, and there is some evidence to suggest that the benefits of IP insulin use extend beyond improved glycemia. A thorough explanation of these benefits is presented in Van Dijk et al.53
5. Conclusions and Future Work
A fully implanted artificial pancreas operating in the IP space allows many of the challenges associated with subcutaneous insulin delivery to be overcome. Faster insulin transport and action, along with more rapid glucose sensing, allow the controller to maintain excellent glycemic control. In addition, IP insulin delivery has the potential to lead to better metabolic health. In this work, a model-based tuning strategy was introduced to develop a PID controller for a fully implantable AP. Furthermore, a dynamic anti-reset windup strategy was applied to minimize undershoot of the set point after meals while still maintaining set point tracking. Insulin feedback was also added to improve the controller response. This design may be further refined with the development of more accurate models based on experimental data. Once this data has been collected and analyzed, the updated controller will be evaluated in an animal model to quantify the improved performance offered by this controller in vivo.
Acknowledgments
We thank our funding sources for supporting this work. We also thank Dr. Brett Mensh and Dr. Dan Burnett for allowing us to present the IP sensor time constant data in a new figure format in this paper. Access to the University of Virginia/Padova metabolic simulator was provided by an agreement with Prof. C. Cobelli (University of Padova) and Prof. B. P. Kovatchev (University of Virginia) for research purposes.
Glossary
ABBREVIATIONS
- AP
artificial pancreas
- IP
intraperitoneal
- SC
subcutaneous
- PID
proportional-integral-derivative
- MPC
model predictive control
- AWP
anti-reset windup protection
- IFB
insulin feedback
- T1DM
type 1 diabetes mellitus
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This work was supported by the National Science Foundation Graduate Research Fellowship Program and the National Institutes of Health grant DP3DK101068.
The authors declare no competing financial interest.
References
- The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1993, 329 ( (14), ), 977. [DOI] [PubMed] [Google Scholar]
- Pickup J.; Keen H. Continuous Subcutaneous Insulin Infusion at 25 Years Evidence Base for the Expanding Use of Insulin Pump Therapy in Type 1 Diabetes. Diabetes Care 2002, 25 (3), 593. [DOI] [PubMed] [Google Scholar]
- Walsh J. T.Pumping Insulin: Everything You Need for Success with an Insulin Pump, 3rd ed.; Schreiner B., Ed.; Torrey Pines Press: San Diego, CA, 2000. [Google Scholar]
- Cunningham D. D.; Stenken J. A.. In Vivo Glucose Sensing; John Wiley & Sons: Hoboken, NJ, 2010. [Google Scholar]
- Bergenstal R. M.; Tamborlane W. V.; Ahmann A.; Buse J. B.; Dailey G.; Davis S. N.; Joyce C.; Peoples T.; Perkins B. A.; Welsh J. B.; et al. Effectiveness of Sensor-Augmented Insulin-Pump Therapy in Type 1 Diabetes. N. Engl. J. Med. 2010, 363 (4), 311. [DOI] [PubMed] [Google Scholar]
- Doyle F. J. III; Huyett L. M.; Lee J. B.; Zisser H. C.; Dassau E. Closed-Loop Artificial Pancreas Systems: Engineering the Algorithms. Diabetes Care 2014, 37 (5), 1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidar A.; Legault L.; Messier V.; Mitre T. M.; Leroux C.; Rabasa-Lhoret R. Comparison of Dual-Hormone Artificial Pancreas, Single-Hormone Artificial Pancreas, and Conventional Insulin Pump Therapy for Glycaemic Control in Patients with Type 1 Diabetes: An Open-Label Randomised Controlled Crossover Trial. Lancet Diabetes Endocrinol. 2015, 3 (1), 17. [DOI] [PubMed] [Google Scholar]
- Russell S. J.; El-Khatib F. H.; Sinha M.; Magyar K. L.; McKeon K.; Goergen L. G.; Balliro C.; Hillard M. A.; Nathan D. M.; Damiano E. R. Outpatient Glycemic Control with a Bionic Pancreas in Type 1 Diabetes. N. Engl. J. Med. 2014, 371 (4), 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Youssef J.; Castle J. R.; Branigan D. L.; Massoud R. G.; Breen M. E.; Jacobs P. G.; Bequette B. W.; Ward W. K. A Controlled Study of the Effectiveness of an Adaptive Closed-Loop Algorithm to Minimize Corticosteroid-Induced Stress Hyperglycemia in Type 1 Diabetes. J. Diabetes Sci. Technol. 2011, 5 (6), 1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A.; Dube S.; Veettil S.; Slama M.; Kudva Y. C.; Peyser T.; Carter R. E.; Cobelli C.; Basu R. Time Lag of Glucose from Intravascular to Interstitial Compartment in Type 1 Diabetes. J. Diabetes Sci. Technol. 2015, 9 (1), 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A.; Dube S.; Slama M.; Errazuriz I.; Amezcua J. C.; Kudva Y. C.; Peyser T.; Carter R. E.; Cobelli C.; Basu R. Time Lag of Glucose from Intravascular to Interstitial Compartment in Humans. Diabetes 2013, 62 (12), 4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steil G. M.; Rebrin K.; Darwin C.; Hariri F.; Saad M. F. Feasibility of Automating Insulin Delivery for the Treatment of Type 1 Diabetes. Diabetes 2006, 55 (12), 3344. [DOI] [PubMed] [Google Scholar]
- Chase H. P.; Doyle F. J. III; Zisser H.; Renard E.; Nimri R.; Cobelli C.; Buckingham B. A.; Maahs D. M.; Anderson S.; Magni L.; et al. Multicenter Closed-Loop/Hybrid Meal Bolus Insulin Delivery with Type 1 Diabetes. Diabetes Technol. Ther. 2014, 16 (10), 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botz C. K.; Leibel B. S.; Zingg W.; Gander R. E.; Albisser A. M. Comparison of Peripheral and Portal Routes of Insulin Infusion by a Computer-Controlled Insulin Infusion System (Artificial Endocrine Pancreas). Diabetes 1976, 25 (8), 691. [DOI] [PubMed] [Google Scholar]
- Homko C.; Deluzio A.; Jimenez C.; Kolaczynski J. W.; Boden G. Comparison of Insulin Aspart and Lispro: Pharmacokinetic and Metabolic Effects. Diabetes Care 2003, 26 (7), 2027. [DOI] [PubMed] [Google Scholar]
- Schaepelynck Belicar P.; Vague P.; Lassmann-Vague V. Reproducibility of Plasma Insulin Kinetics during Intraperitoneal Insulin Treatment by Programmable Pumps. Diabetes Metab. 2003, 29 (4), 344. [DOI] [PubMed] [Google Scholar]
- Nelson J. A.; Stephen R.; Landau S. T.; Wilson D. E.; Tyler F. H. Intraperitoneal Insulin Administration Produces a Positive Portal-Systemic Blood Insulin Gradient in Unanesthetized, Unrestrained Swine. Metabolism 1982, 31 (10), 969. [DOI] [PubMed] [Google Scholar]
- Logtenberg S. J.; Kleefstra N.; Houweling S. T.; Groenier K. H.; Gans R. O.; Bilo H. J. Health-Related Quality of Life, Treatment Satisfaction, and Costs Associated With Intraperitoneal Versus Subcutaneous Insulin Administration in Type 1 Diabetes: A Randomized Controlled Trial. Diabetes Care 2010, 33 (6), 1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logtenberg S. J.; Kleefstra N.; Houweling S. T.; Groenier K. H.; Gans R. O.; Ballegooie E.; van Bilo H. J. Improved Glycemic Control With Intraperitoneal Versus Subcutaneous Insulin in Type 1 Diabetes A Randomized Controlled Trial. Diabetes Care 2009, 32 (8), 1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl A.; Hoogma R.; Renard E.; Geelhoed-Duijvestijn P. H. L. M.; Klein E.; Diglas J.; Kessler L.; Melki V.; Diem P.; Brun J.-M.; et al. A Reduction in Severe Hypoglycaemia in Type 1 Diabetes in a Randomized Crossover Study of Continuous Intraperitoneal Compared with Subcutaneous Insulin Infusion. Diabetes, Obes. Metab. 2009, 11 (11), 1001. [DOI] [PubMed] [Google Scholar]
- Renard E. Insulin Delivery Route for the Artificial Pancreas: Subcutaneous, Intraperitoneal, or Intravenous? Pros and Cons. J. Diabetes Sci. Technol. 2008, 2 (4), 735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan D. B.; Mastrototaro J. J.; Voskanyan G.; Steil G. M. Delays in Minimally Invasive Continuous Glucose Monitoring Devices: A Review of Current Technology. J. Diabetes Sci. Technol. 2009, 3 (5), 1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett D. R.; Huyett L. M.; Zisser H. C.; Doyle F. J. III; Mensh B. D. Glucose Sensing in the Peritoneal Space Offers Faster Kinetics Than Sensing in the Subcutaneous Space. Diabetes 2014, 63 (7), 2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fougner A. L.; Kolle K.; Skjaervold N. K.; Elvemo N. A.; Ellingsen R.; Stavdahl O.; Carlsen S. M. Intraperitoneal Glucose Sensing - Rapid and Accurate. Diabetes Technol. Ther. 2015, 17, A38. [Google Scholar]
- Ly T. T.; Nicholas J. A.; Retterath A.; Davis E. A.; Jones T. W. Analysis of Glucose Responses to Automated Insulin Suspension With Sensor-Augmented Pump Therapy. Diabetes Care 2012, 35 (7), 1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt H. A.; Saudek C. D.; Zacur H. A. Long-Term Intraperitoneal Insulin Delivery. Ann. Surg. 1992, 216 (4), 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosman B.; Dassau E.; Zisser H. C.; Jovanoviĉ L.; Doyle F. J. III Zone Model Predictive Control: A Strategy to Minimize Hyper- and Hypoglycemic Events. J. Diabetes Sci. Technol. 2010, 4 (4), 961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G.; Chen X.; Urbain J. L. Evidence for a Circadian Rhythm of Insulin Sensitivity in Patients with NIDDM Caused by Cyclic Changes in Hepatic Glucose Production. Diabetes 1996, 45 (8), 1044. [DOI] [PubMed] [Google Scholar]
- Van Heusden K.; Dassau E.; Zisser H. C.; Seborg D. E.; Doyle F. J. III Control-Relevant Models for Glucose Control Using A Priori Patient Characteristics. IEEE Trans. Biomed. Eng. 2012, 59 (7), 1839. [DOI] [PubMed] [Google Scholar]
- Lee J. J.; Dassau E.; Zisser H.; Doyle F. J. III Design and in Silico Evaluation of an Intraperitoneal–subcutaneous (IP–SC) Artificial Pancreas. Comput. Chem. Eng. 2014, 70, 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seborg D. E.; Mellichamp D. A.; Edgar T. F.; Doyle F. J. III. Process Dynamics and Control, 3rd ed.; John Wiley & Sons: Hoboken, NJ, 2011. [Google Scholar]
- Lee J. J.; Dassau E.; Zisser H.; Tamborlane W.; Weinzimer S.; Doyle F. J. III The Impact of Insulin Pharmacokinetics and Pharmacodynamics on the Closed-Loop Artificial Pancreas. IEEE 52nd Annual Conference on Decision and Control 2013, 127–132. [Google Scholar]
- Lee J. B.; Dassau E.; Seborg D. E.; Doyle F. J. III Model-Based Personalization Scheme of an Artificial Pancreas for Type 1 Diabetes Applications. American Control Conference; IEEE 2013, 2911–2916. [Google Scholar]
- Franklin G. F.; Powell J. D.; Workman M. L.. Digital Control of Dynamic Systems, 3rd ed.; Addison-Wesley: Menlo Park, CA, 1997. [Google Scholar]
- Skogestad S. Simple Analytic Rules for Model Reduction and PID Controller Tuning. Model. Identif. Control 2004, 25 (2), 85. [Google Scholar]
- Laxminarayan S.; Reifman J.; Steil G. M. Use of a Food and Drug Administration-Approved Type 1 Diabetes Mellitus Simulator to Evaluate and Optimize a Proportional-Integral-Derivative Controller. J. Diabetes Sci. Technol. 2012, 6 (6), 1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patek S. D.; Bequette B. W.; Breton M.; Buckingham B. A.; Dassau E.; Doyle F. J. III; Lum J.; Magni L.; Zisser H. In Silico Preclinical Trials: Methodology and Engineering Guide to Closed-Loop Control in Type 1 Diabetes Mellitus. J. Diabetes Sci. Technol. 2009, 3 (2), 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovatchev B. P.; Breton M.; Man C. D.; Cobelli C. In Silico Preclinical Trials: A Proof of Concept in Closed-Loop Control of Type 1 Diabetes. J. Diabetes Sci. Technol. 2009, 3 (1), 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs P. G.; El Youssef J.; Castle J.; Bakhtiani P.; Branigan D.; Breen M.; Bauer D.; Preiser N.; Leonard G.; Stonex T.; et al. Automated Control of an Adaptive Bihormonal, Dual-Sensor Artificial Pancreas and Evaluation During Inpatient Studies. IEEE Trans. Biomed. Eng. 2014, 61 (10), 2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bon A. C.; Jonker L. D.; Koebrugge R.; Koops R.; Hoekstra J. B.; DeVries J. H. Feasibility of a Bihormonal Closed-Loop System to Control Postexercise and Postprandial Glucose Excursions. J. Diabetes Sci. Technol. 2012, 6 (5), 1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bon A. C.; Luijf Y. M.; Koebrugge R.; Koops R.; Hoekstra J. B. L.; DeVries J. H. Feasibility of a Portable Bihormonal Closed-Loop System to Control Glucose Excursions at Home under Free-Living Conditions for 48 h. Diabetes Technol. Ther. 2014, 16 (3), 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle J. R.; Engle J. M.; Youssef J. E.; Massoud R. G.; Yuen K. C. J.; Kagan R.; Ward W. K. Novel Use of Glucagon in a Closed-Loop System for Prevention of Hypoglycemia in Type 1 Diabetes. Diabetes Care 2010, 33 (6), 1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bon A. C.; Hermanides J.; Koops R.; Hoekstra J. B.; DeVries J. H. Postprandial Glycemic Excursions with the Use of a Closed-Loop Platform in Subjects with Type 1 Diabetes: A Pilot Study. J. Diabetes Sci. Technol. 2010, 4 (4), 923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steil G. M.; Palerm C. C.; Kurtz N.; Voskanyan G.; Roy A.; Paz S.; Kandeel F. R. The Effect of Insulin Feedback on Closed Loop Glucose Control. J. Clin. Endocrinol. Metab. 2011, 96 (5), 1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson A.; Gruber P.; Tödtli J. Fuzzy Anti-Reset Windup for PID Controllers. Control Eng. Pract. 1994, 2 (3), 389. [Google Scholar]
- Argoud G. M.; Schade D. S.; Eaton R. P. Insulin Suppresses Its Own Secretion in Vivo. Diabetes 1987, 36 (8), 959. [DOI] [PubMed] [Google Scholar]
- Palerm C. C. Physiologic Insulin Delivery with Insulin Feedback: A Control Systems Perspective. Comput. Methods Programs Biomed. 2011, 102 (2), 130. [DOI] [PubMed] [Google Scholar]
- Panteleon A. E.; Renard E.; Miller M. E.; Steil G. M. Evaluation of IV Glucose Sensor Performance and the Pharmacokinetics of IP Insulin Delivery during Standard Meals. Diabetes 2006, 55, A95. [Google Scholar]
- Panteleon A.; Renard E.; Han J.; Leong P.; Rebrin K.; Kolopp M.; Steil G.. Quantification of Delays Associated with Intraperitoneal Insulin Delivery and IV Glucose Sensing Aiming at Closed Loop Insulin Delivery. In American Diabetes Association Annual Meeting, 2004; Vol. 53, pp A105–A105.
- Skogestad S.; Postlethwaite I.. Multivariable Feedback Control: Analysis and Design, 2nd ed.; Wiley-Interscience: Hoboken, NJ, 2005. [Google Scholar]
- Breton M.; Kovatchev B. Analysis, Modeling, and Simulation of the Accuracy of Continuous Glucose Sensors. J. Diabetes Sci. Technol. 2008, 2 (5), 853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyser T.; Dassau E.; Breton M.; Skyler J. S. The Artificial Pancreas: Current Status and Future Prospects in the Management of Diabetes. Ann. N.Y. Acad. Sci. 2014, 1311 (1), 102. [DOI] [PubMed] [Google Scholar]
- Van Dijk P. R.; Logtenberg S. J. J.; Gans R. O. B.; Bilo H. J. G.; Kleefstra N. Intraperitoneal Insulin Infusion: Treatment Option for Type 1 Diabetes Resulting in Beneficial Endocrine Effects beyond Glycaemia. Clin. Endocrinol. (Oxford, U. K.) 2014, 81 (4), 488. [DOI] [PubMed] [Google Scholar]