SUMMARY
An activity-dependent form of intermediate memory (AD-ITM) for sensitization is induced in Aplysia by a single tail shock that gives rise to plastic changes (AD-ITF) in tail sensory neurons (SNs) via the interaction of action potential firing in the SN coupled with the release of serotonin in the CNS. Activity-dependent long-term facilitation (AD-LTF, lasting >24hr) requires protein synthesis dependent persistent mitogen-activated protein kinase (MAPK) activation and translocation to the SN nucleus. We now show that the induction of the earlier temporal phase (AD-ITM and AD-ITF), which is translation and transcription independent, requires the activation of a compartmentally distinct novel signaling cascade that links second messengers, MAPK and PKC into a unified pathway within tail SNs. Since both AD-ITM and AD-LTM require MAPK activity, these collective findings suggest that presynaptic SNs route the flow of molecular information to distinct subcellular compartments during the induction of activity-dependent long-lasting memories.
Keywords: Aplysia, MAPK, PKC, kinase, memory, facilitation, compartment, routing
INTRODUCTION
Memories can last from a few seconds to a lifetime. The synaptic plasticity that underlies memory over this vast temporal range is subserved by a wide variety of temporally and spatially specific molecular cascades. One intriguing hypothesis is that the induction of a lasting associative memory requires the coordination between two qualitatively distinct sets of mechanisms: an activity-dependent (or Hebbian) process that induces transient synaptic plasticity that primarily relies on post-translational signaling cascades, and a neuromodulatory signal that stabilizes plasticity by engaging synaptic, as well as somatic, translational and transcriptional molecular machinery (Bailey et al., 2000).
Despite their qualitative differences, both types of mechanisms share common signaling components including kinase cascades. Hebbian activity triggers multiple kinases at the synapse, both presynaptically (Arancio et al., 1995; Casey et al., 2002; Ghirardi et al., 1992; Wierda et al., 2007) and postsynaptically (Biou et al., 2008; Boehm et al., 2006; Kauer and Kopec et al., 2007). Modulatory factors also recruit kinase activity but often with different spatial and temporal dynamics. For instance, in several systems BDNF and serotonin have been shown to regulate MAPK translocation to the nucleus where it can alter gene expression and promote synaptic growth (Martin et al., 1997; Ormond et al., 2004; Patterson et al., 2001; Thompson et al., 2004). Finally, second messengers that mediate kinase activity such as Ca2+ (acting through adenylate cyclase) have also been directly implicated in memory (Wong et al., 1999)
The fact that the stimuli that produce distinct temporal domains of memory trigger common signaling cascades that diverge into qualitatively distinct downstream molecular pathways raises two critical questions. Do these molecular cascades occur in the same set of neurons? If so, to what extent does the interaction between their shared elements affect compartment-specific downstream signaling properties and ultimately synaptic plasticity and memory? To address these questions we used the model system Aplysia californica whose simplicity makes it possible to forge direct links between intracellular signaling cascades, changes in synaptic efficacy between identified sensory and motor neurons (SNs and MNs), and the formation and expression of memory.
We focused on activity-dependent memory for sensitization and its cellular analog, activity-dependent facilitation, because: (i) their induction requires the pairing of serotonin (5HT) and SN neuronal activity, (ii) plastic changes occur within the same population of SNs and (iii) this pairing produces two temporally and mechanistically distinct forms of long-lasting memory, AD-ITM and AD-LTM. Behavioral training (a single tail shock) produces AD-ITM, a translation-independent intermediate term memory that lasts for several hours, followed by AD-LTM, a translation- and transcription- dependent form of long-term memory that is evident the next day (Sutton et al., 2004; Sutton and Carew, 2000; Sutton et al., 2002; Sutton et al., 2001).
Early work revealed that associative training, activity (via Ca2+) and modulation (via 5HT), synergistically activate the dually-regulated adenylate cyclase to generate maximal levels of cAMP (Abrams, 1985; Abrams and Kandel, 1988; Abrams et al., 1991; Abrams et al., 1998; Braha et al., 1990; Eliot et al., 1989; Ocorr et al., 1986; Ocorr et al., 1985; Yovell et al., 1992). A similar synergistic relationship has been described in mammalian cells (Wayman et al., 1994). More recently, we found that plasma membrane translocation and activation of calcium-dependent PKC (Apl I) are required for AD-ITM and for AD-ITF (Sutton et al., 2004; Sutton and Carew, 2000; Zhao et al., 2006), suggesting that PKC redistribution plays an important role in the intermediate temporal domain (Zhao et al., 2006). A recent study also demonstrates that associative training induces delayed translocation of MAPK to the SN nucleus, presumably to upregulate genes necessary for activity-dependent long-term facilitation (AD-LTF) (Hu et al., 2007). This requires persistent PKA- and PKC- signaling and subsequent release of the peptide neurotransmitter sensorin. Thus both intermediate-term and long-term processes are initiated by the same stimuli.
These collective findings led us to hypothesize that associative training triggers distinct signaling cascades that are compartmentally routed with temporal specificity to produce two mechanistically distinct processes: a synaptically mediated intermediate-term plasticity and a somatically mediated long-term plasticity. We further postulated that MAPK was well positioned to serve as a key element in this molecular routing due to its requirement for AD-LTF and its ability to integrate and direct a variety of downstream signal transduction cascades (Reissner et al., 2006). We first tested whether AD-ITM requires MAPK activity, and then examined its relationship with other signaling pathways. We found that MAPK activation (via a cascade of identified upstream molecular steps) is essential for both AD-ITM and AD-ITF. Moreover, we discovered a novel pathway in which MAPK activates PKC, inducing its translocation to the plasma membrane. Taken together, these results provide a unifying molecular cascade that integrates activity and neuromodulatory signals during the formation of AD-ITM and further suggests that MAPK can route the molecular flow of information within a neuron to regulate two temporally distinct phases of plasticity.
RESULTS
AD-ITM induction requires MAPK
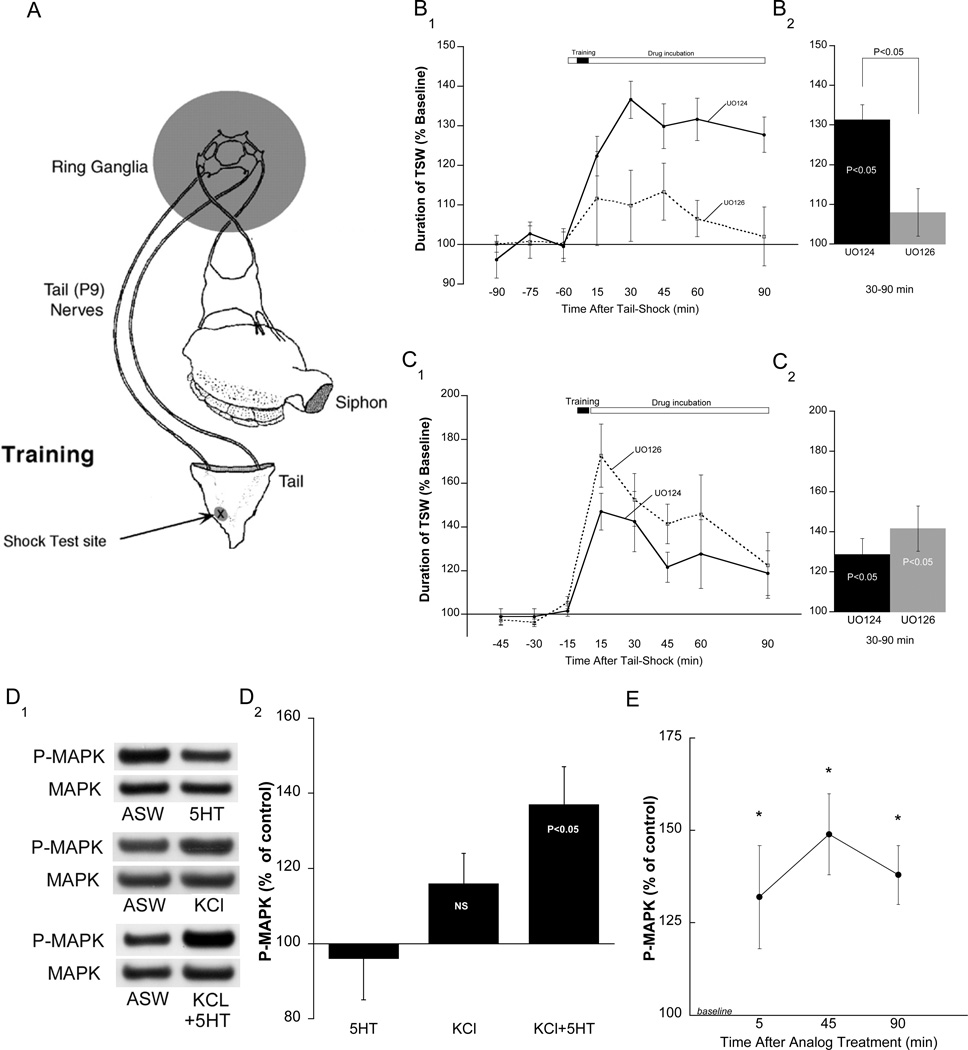
We previously demonstrated that a single tail-shock (TS) produces intermediate-term memory for sensitization (AD-ITM) if testing and TS occur at the same site (Sutton et al., 2004; see also Walters, 1987) (Figure 1A). The hallmark of AD-ITM is that the sensitizing stimulus induces plasticity from the temporal combination of global 5HT release (Marinesco and Carew, 2002) and the depolarization of tail SNs within the receptive field of the TS (Sutton et al., 2004). We first examined the requirement for MAPK in AD-ITM using a reduced preparation in which pharmacological agents were selectively applied to the ring ganglia while simultaneously monitoring the behavioral response, tail-elicited siphon withdrawal ([T-SWR] Figure 1A, see Sutton et al., 2004). Preparations were trained using a single strong TS to the test site and memory was tested with a subsequent weak electrical stimulus to the same site. Since short- term memory (STM) peaks at 15 min, tests ranging from the 30 to 90 min were pooled to generate summary data for ITM (Figure 1B2). As expected, preparations that received the inactive analog of the MEK inhibitor (U0124) displayed significant AD-ITM that lasted at least 90 minutes (mean ± SEM: 131.0 ± 3.8%, p<0.05). In contrast, the MEK inhibitor U0126 significantly reduced the magnitude of ITM (108 ± 6.0%, NS). A between-group analysis comparing the active and inactive MEK inhibitor revealed a significant difference (Figure 1B2, p<0.05). Consistent with previous observations (Sharma et al., 2003), U0126 did not reduce T-SWR responses of non-shock preparations, indicating that the observed difference is not due to an effect on baseline.
Figure 1. Induction but not expression of AD-ITM requires MAPK activity, and combined KCl depolarization and 5HT application activates MAPK.
A. The semi-intact preparation used to monitor T-SWR in the presence of pharmacological agents (shaded area). B1. The MEK inhibitor, U0126 (20µM; n=5) or its inactive analog, U0124 (20uM; n=6) were applied to the ring ganglia 1 hour before 1xTS and were present throughout the remainder of the experiment. B2. Summary data of 30–90 min time points combined. Within-group analysis revealed significant AD-ITM in controls, but not in groups treated with UO126, and a between group test revealed a significance difference. In this and subsequent figures, data are expressed as means ±SEM. C1. The MEK inhibitor, U0126 (20µM; n=6) or its inactive analog, U0124 (20uM; n=8) were applied to the ring ganglia immediately after 1xTS and were present throughout the remainder of the experiment. C2. Summary data in which intermediate time points 30–90 min were combined. Both groups exhibited significant AD-ITM; there was no significant difference between groups. D1. The treated pleural-pedal pair received i) a single pulse of 5HT (50µM, 5min; n=8), ii) a single pulse of high KCl ASW (100mM, 5 min; n=10), or iii) a combination of KCl and 5HT (KCl first, 2 min overlap with 5HT; n=11). Control side remained in ASW to establish a within-animal baseline (see Methods). Quantification of MAPK activation is represented as the ratio of phospho-MAPK (P-MAPK)/ total MAPK (MAPK) between the two sides (see Methods). D2. Summary data showing that only the combination of KCl+5HT produced significant MAPK activation. E. Pleural-Pedal pairs were divided and one side was stimulated with the analog (KCl+5HT). Sensory clusters were harvested at the indicated times. Within sample t-tests revealed that MAPK was significantly activated by the associative analog at each time point (p<0.05).
A recent study found that AD-LTF (24 hr) requires persistent MAPK activation (Hu et al., 2007). In contrast, we found that application of the MEK inhibitor immediately following training did not affect AD-ITM, indicating that sustained MAPK activity is not required for the maintenance of AD-ITM (Figure 1C, 142 ± 11%, p<0.05). This result is not attributable to a slow onset of inhibition because in separate experiments we found that the MEK inhibitor caused a significant reduction in MAPK activity within an hour of its application (Supplementary Figure S1). The fact that AD-ITM did not require persistent MAPK activity was the first indication that MAPK signaling differentially regulates intermediate-term versus long-term activity dependent plasticity.
An associative analog of AD-ITM activates MAPK
Since AD-ITM required MAPK activity, we predicted that associative training (activity with modulation) would induce MAPK activation in SNs. To test this we treated pleural-pedal ganglion pairs with a single 5-minute pulse of high KCl-ASW ([KCl]-100mM) overlapped with a 5 min pulse of 5HT (50µM) to mimic a single bout of activity experienced by SNs during behaviorally relevant training. We found that 100mM KCl depolarized SNs by approximately 20mV (Supplementary Figure S2); a sufficient change to open voltage-gated Ca2+ channels (Walters and Byrne, 1983).
A single 5 min pulse of KCl did not significantly activate MAPK (116 ± 8%, p=0.08, NS) in SNs. However, the combination of a 5 min pulse of KCl with a 5 min pulse of 5HT (analog) produced robust MAPK activity (137 ± 10%, p<0.05) that was larger than that generated by the single pulse of KCl (Figure 1D2). This enhancement was not due to the actions of 5HT alone because, consistent with previous observations (Martin et al., 1997; Michael et al., 1998), a single pulse of 5HT did not induce MAPK activity (96 ± 11%, NS).
To further validate the associative analog we examined the temporal profile of MAPK activity in SNs following analog treatment. Since the induction of AD-LTF with activity and 5HT requires sustained MAPK activity, we predicted that the associative analog would also produce persistent MAPK activation (as is the case for AD-LTF, Hu et al 2007). Following analog treatment, we excised SN clusters at 5, 45 min or 90 min time points (Figure 1E) and found that MAPK activity remained significantly activated at each time point (5min, 132 ± 14%; 45min, 149.2 ± 11%; 90min, 138.0 ± 8%; p<0.05).
Mechanisms that mediate MAPK activity
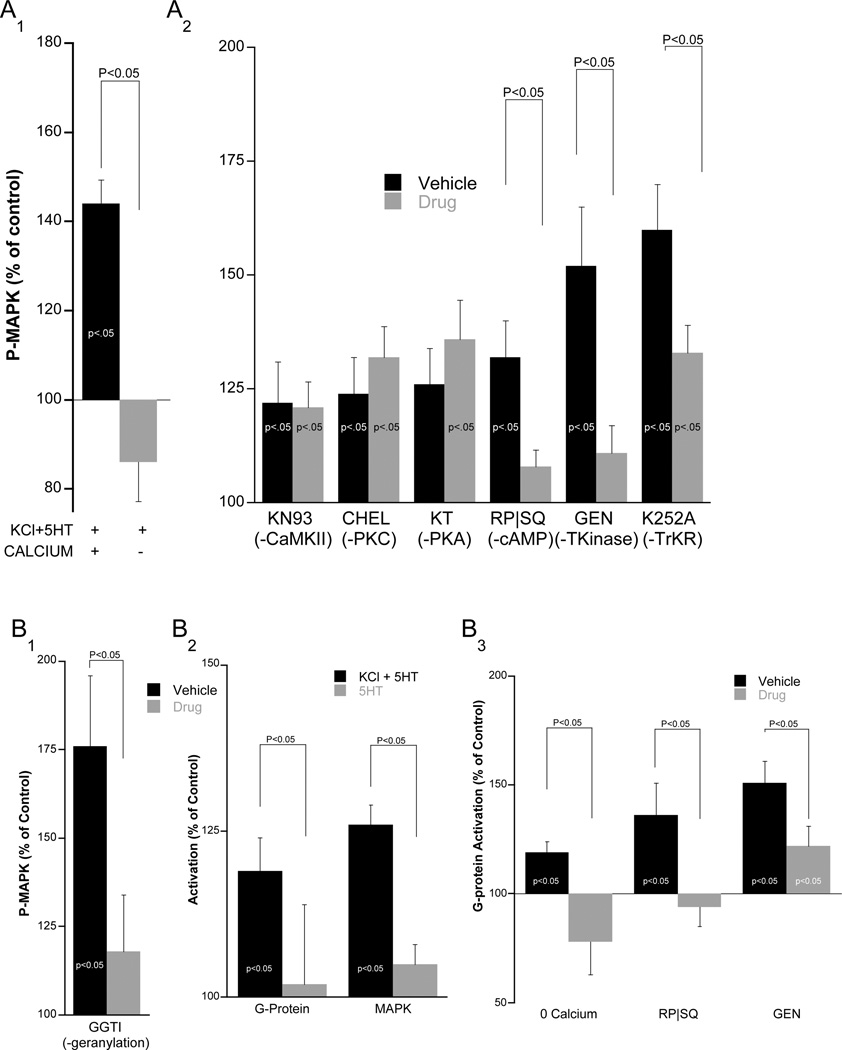
To elucidate the specific MAPK cascade involved in AD-ITM, we first examined the role of Ca2+ influx during activity-dependent depolarization. To test the requirement for extracellular Ca2+, we conducted the analog stimulation in Ca2+-free ASW and found that this completely prevented MAPK activation (p<0.05, Figure 2A1).
Figure 2. MAPK activation requires G-protein activity, and analog-induced MAPK activation funnels through G-proteins.
A1. When extracellular Ca2+ is replaced with equimolar Co2+, the analog (KCl+5HT) no longer activates MAPK. A2. Pleural-Pedal ganglia were treated with either i) CaMKII inhibitor, KN-93 (50µM, n=11), ii) PKC inhibitor, Chelerythrine (50µM, n=9), iii) cAMP blocker, Rp-cAMP (500µM) + SQ22536 (1.5mM, n=11), iv) PKA inhibitor, KT5720 (10uM, n=7); Genistein (100µM; n=10) or K252A (1µM; n=17). Control groups were treated with vehicle (n=8, 6, 12, 7, 10 from left to right). In each case ganglia were subjected to a 1-hour incubation with compounds before analog stimulation and SN clusters were harvested immediately after stimulation and subjected to Western blot analysis. Indicated groups (*) were all significantly above their own baseline (within group t-test, p<0.05) and brackets indicate a between-group difference (unpaired t-test). B1 Excised pleural-pedal pairs were preincubated overnight with the geranylgeranylation inhibitor (GGTI, 30µM, n=7;VEH, n=7). The next day the pleural-pedal pair received analog stimulation and the SN cluster was lysed and assayed for MAPK activity. Only GGTI inhibited MAPK activation (paired t-test indicated with brackets). B2. Pleural-pedal pairs were stimulated with the analog (n=17) or with a single 5 min pulse of 5HT (n=10) and both ganglia were immediately lysed. Only the associative analog induced significant activation of G-proteins and MAPK. B3. Pleural-pedal pairs were stimulated with the analog either in the absence of extracellular Ca2+ (n=9) or in the presence of the combined blockers to inhibit cAMP signaling [Rp-cAMP (500µM) + SQ22536 (1.5mM), n=5] or TK inhibitor, [Genistein (100µM; n=16)]. G-protein activity was assessed immediately following analog stimulation
We next examined the intracellular mechanisms that function to integrate Ca2+ and 5HT signals. Importantly, all inhibitors used have previously been shown in Aplysia to effectively inhibit their respective targets (Goldsmith and Abrams, 1991; Purcell et al., 2003; Sharma et al., 2003; Sutton et al., 2004; Sutton and Carew, 2000). We first tested the role of Ca2+-sensitive kinases upstream of MAPK such as PKC and CaMKII. The PKC inhibitor, chelerythrine, did not block MAPK activity induced by the analog (124 ± 8% [VEH] vs. 132 ± 6.7% [CHEL], NS, Figure 2A2). This observation is consistent with previous findings that phorbol ester and 5HT-induced activation of MAPK do not require PKC activity (Dyer et al., 2003). We also found that the CaMKII inhibitor, KN-93, did not affect MAPK activity induced by the associative analog (122 ± 9% [VEH] vs. 121 ± 5.6% [KN], NS, Figure 2A2).
Stimulation of the dually-activated adenylate cyclase by the combination of Ca2+ and 5HT generates maximal levels of cAMP (Abrams, 1985). To test whether cAMP signaling was required for MAPK activation we inhibited adenylate cyclase enzymatic activity with SQ22535, and out competed endogenous cAMP with the inactive isomer, Rp-cAMP. In the presence of both inhibitors, analog stimulation failed to activate MAPK (132 ± 8% [VEH] vs. 108 ± 3.6% [RPSQ], p<0.05, Figure 2A2). Consistent with our previous observations (Sutton et al. 2000, 2004), however, the PKA inhibitor, KT5720, did not affect MAPK activation (126 ± 8% [VEH] vs. 136 ± 8.5% [KT], NS, Figure 3B) suggesting that cAMP activates MAPK in a PKA-independent manner.
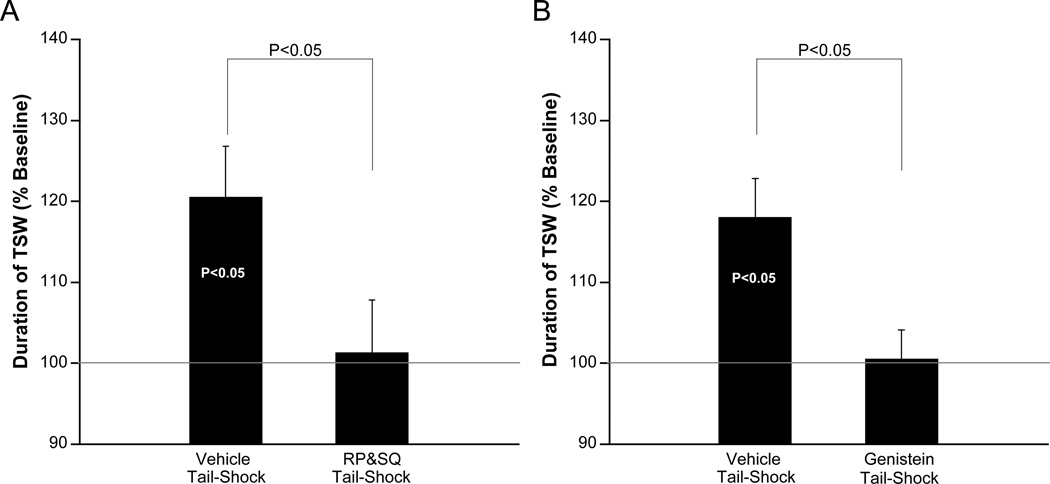
Figure 3. AD-ITM requires cAMP and TK signaling.
A) The cAMP blocker [Rp-cAMP (500µM) + SQ22536 (1.5mM, n=9), or vehicle (n=11)] was applied to the ring ganglia 1 hr before 1xTS and maintained throughout the experiment or washed off after 1xTS. Since both experimental protocols produced similar results, data have been pooled. B) Genistein (100µM, n=6), or vehicle (n=6) were applied to the ring ganglia immediately before 1xTS and washed off immediately after 1xTS. In all cases, bars are the means of pooled 30, 60, and 90 min time points.
Finally we examined whether tyrosine kinases (TKs) are required for the analog activation of MAPK (Purcell et al., 2003). The broad spectrum TK inhibitor genistein significantly reduced analog activation of MAPK (152 ± 11% [VEH] vs. 111 ± 6% [GEN], p<0.05, Figure 3B). To identify the class of TK responsible for MAPK activation we performed an additional experiment with the TrK inhibitor, K252a, and found that this partially but significantly inhibited MAPK activation (Figure 2A2), suggesting that several parallel TK pathways activate MAPK.
Transduction signals funnel through G-proteins to activate MAPK
To investigate the mechanisms whereby second messengers such as cAMP and Ca2+ activate MAPK independent of PKA or PKC activity, we examined two small G-proteins, Ras and Rap. We focused on these G-proteins because: (i) they are activated by a diverse family of guanine exchange factors (GEFs), which can be directly activated by cAMP, Ca2+, and/or TKs (Garcia-Mata and Burridge, 2007; Mitin et al., 2005; Siderovski and Willard, 2005; Springett et al., 2004), (ii) kinase-independent activation of MAPK typically funnels through Ras and/or Rap (Garcia-Mata and Burridge, 2007; Overbeck et al., 1995; Siderovski and Willard, 2005; Springett et al., 2004; Zwartkruis and Bos, 1999), and (iii) Aplysia Ras and Rap share the same consensus sequence (Ye et al., 2005) for geranylgeranylation, a posttranslational modification required for downstream signaling. This last feature allowed us to simultaneously block the activity of both GTPases by manipulating geranylgeranylation.
We incubated pleural-pedal ganglia for 24 hours with a geranylgeranyltransferase inhibitor, 30µM GGTI (the long incubation is necessary because only newly synthesized protein will lack the irreversible lipid modification) and found that this treatment significantly inhibited MAPK activation by the associative analog in the SN cluster (Figure 2B1; 176 ± 20% [VEH] vs. 118 ± 16% [GGTI], p<0.05). Inhibition of a similar lipid modification process, farnesylation, had no effect on MAPK activation (data not shown).
To further determine if G-proteins are engaged by the associative analog, we performed double pull-down assays with pleural-pedal ganglia and simultaneously monitored the activation of the G-proteins, Rap and Ras. As expected, we found that a single pulse of 5HT alone did not significantly activate G-proteins, whereas stimulation with the associative analog induced robust MAPK activation (p<0.05), and significant G-protein activation (p<0.05; Figure 2B2).
How could Ca2+, cAMP, and TKs activate MAPK without signaling through PKC, PKA, or CaMKII? We reasoned that these pathways could operate through guanine exchange factors (GEFs) to directly activate G-proteins. To examine this possibility we searched the recently released Aplysia EST database and found that the CNS contains many GEFs that are activated by cAMP, RTKs, and Ca 2+. This includes (but is not limited to) homologs to human EPACs, PDZ-GEFs, CDC25, RasGRPs and SOS (Moroz et al., 2006).
In testing the mechanistic requirements for Rap and Ras activation, we found that G-protein activation was blocked by removing extracellular Ca2+ (Figure 4C), and by inhibiting cAMP (136 ± 15% [VEH] vs 95±9 [RP&SQ], p<0.05) or TK signaling (151 ± 10 [VEH] vs 110 ± 9 [GEN]; p<0.05) ; Figure 2B3). These findings indicate that the same mechanisms activate G-proteins and MAPK and suggest that Ca2+, cAMP, and TKs activate MAPK via G-proteins.
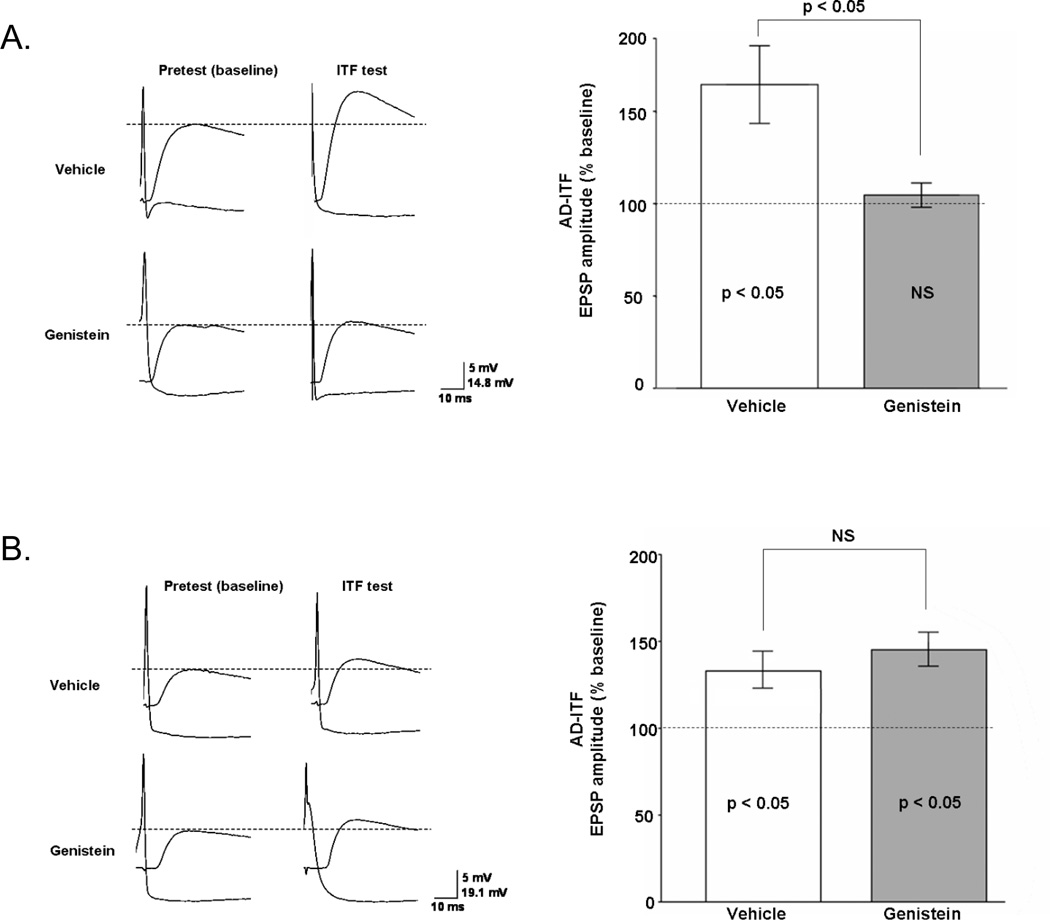
Figure 4. Tyrosine kinase activity is required for AD-ITF.
Representative traces (A1) and summary data (A2) show that the induction of AD-ITF by SN activation in the presence of a single 5HT pulse is blocked in ganglia that were treated with 100µM genistein or vehicle for 30 min prior to and during training (Vehicle: n=8; Genistein: n=6). (B1 & B2) AD-ITF was induced as described above, but genistein/vehicle was applied to the preparation 10 min after training and remained present throughout testing to specifically assay effects on expression as opposed to induction of AD-ITF. This treatment did not affect AD-ITF expression (vehicle: n=6; genistein: n=5). Data are presented as mean of three normalized ITF tests.
Activity-dependent ITM requires cAMP signaling and TK activity
The fact that the associative activation of MAPK required TK and cAMP signaling generated a clear behavioral prediction: induction of AD-ITM should also require TK activity and cAMP signaling. We confirmed both of these predictions. Treatment with the TK inhibitor, genistein, or the cAMP signaling blocker, RP&SQ, before training, completely blocked the induction of AD-ITM (100.5 ± 3.6% [GEN]; 101.3 ± 6.5% [RP&SQ]; p<0.05 vs. [VEH]; Figure 3). Thus, both cAMP and TK pathways are required for the induction of AD-ITM, as directly predicted by our molecular findings.
Activity-dependent synaptic facilitation requires TK and MAPK activity
The synaptic correlate of AD-ITM, AD-ITF, is induced at individual SN-MN synapses by direct activation of the SN paired with a pulse of 5HT (Eliot et al., 1994; Ghirardi et al., 1995; Schacher et al., 1997; Sutton et al., 2004; Sutton and Carew, 2000; Zhao et al., 2006). Previous studies indicated that AD-ITF has the same molecular signature as its behavioral counterpart: both AD-ITF and AD-ITM require PKC, but not PKA activity during expression (Sutton et al., 2004). To address whether the MAPK cascade identified in this study governs AD-ITF, we initially focused on TKs because, unlike the cAMP/PKA pathway, TKs are not required for STF (Purcell et al., 2003). Thus any observable block of facilitation would not be attributable to an effect on STF. In agreement with our behavioral observations (Figure 3B), we found that genistein applied before training blocked AD-ITF (Figure 4A, 164 ± 21 VEH, 104 ± 7 GEN, p<0.05). Moreover, if the inhibitor was applied after training, AD-ITF remained stable, showing that TK activity is required for the induction but not the expression of AD-ITF (Figure 4B; 132 ± 11 VEH, 144 ± 10 GEN, NS). These synaptic observations are consistent with the role of TKs as upstream activators of MAPK, since we also found that MAPK is required for the induction but not the expression of AD-ITM (Figure 1B and C).
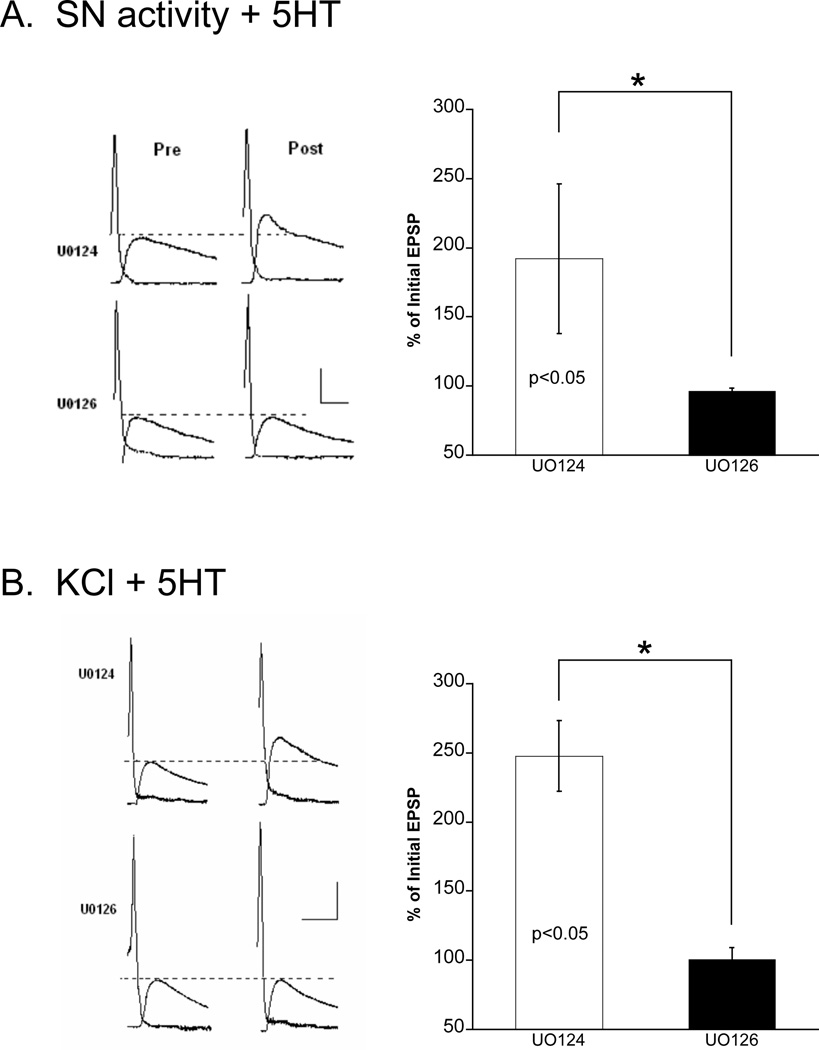
In SN-MN co-cultures, two different types of PKC are involved in unique forms of facilitation: 5HT-activated PKC, Apl II, is required for short-lasting facilitation (5 min) of a depressed synapse (activity-independent), whereas Ca2+-sensitive PKC, Apl I, is required for longer lasting AD-ITF (Manseau et al., 2001; Zhao et al., 2006). To begin to examine the relationship between MAPK and PKC, we tested the role of MAPK in the two forms of PKC-mediated facilitation. We reasoned that only AD-ITF should require MAPK activity and indeed found that MAPK activity is required for Apl I-dependent AD-ITF, but not Apl II-dependent activity-independent facilitation (Figure 5 and S4). In Figure 5A, activity was induced by a tetanus delivered to the SN. However, to establish that KCl- induced depolarization shares the same mechanistic properties as bona fide activity, we substituted a pulse of KCl for tetanic stimulation of the SN, as in the associative analog used in all the previous studies. We found that the associative analog produced robust ITF that was still completely blocked by the MEK inhibitor, UO126 (Figure 5B), further validating our use of KCl as a proxy for activity in the induction of activity-dependent plasticity. These findings, together with earlier studies, demonstrate that both MAPK and PKC activity are required for AD-ITM and its synaptic counterpart AD-ITF (Sutton et al., 2004; Sutton and Carew, 2000).
Figure 5. MAP kinase is required for AD-ITF in SN-MN culture.
AD-ITF induced by either (A) SN activity (4 trains 10Hz, 2 sec) with 5 min 5HT (10 µM) or (B) 5 min 100mM KCl-ASW + 10µM 5HT was blocked by the MEK inhibitor U0126. (A) Sample traces of EPSPs before and 30 min after AD-ITF induction in the presence of U0124 (inactive analog) or U0126. Scale bar: 20mV, 25ms. Histogram of group data shows that U0126 significantly blocked AD-ITF (t-test, p<0.05: n=4 for each group). (B) U0126 specifically blocked KCl+5HT induced ITF. Sample traces of EPSPs before and after KCl+5HT stimulation with U0126 or U0124. Scale bar: 20mV, 25ms. Histogram of group data shows that U0126 significantly blocks AD-ITF induced by KCl+5HT (t-test, p<0.05; n=20 for U0124 and 12 for U0126).
Associative stimulation induces MAPK activation and PKC translocation
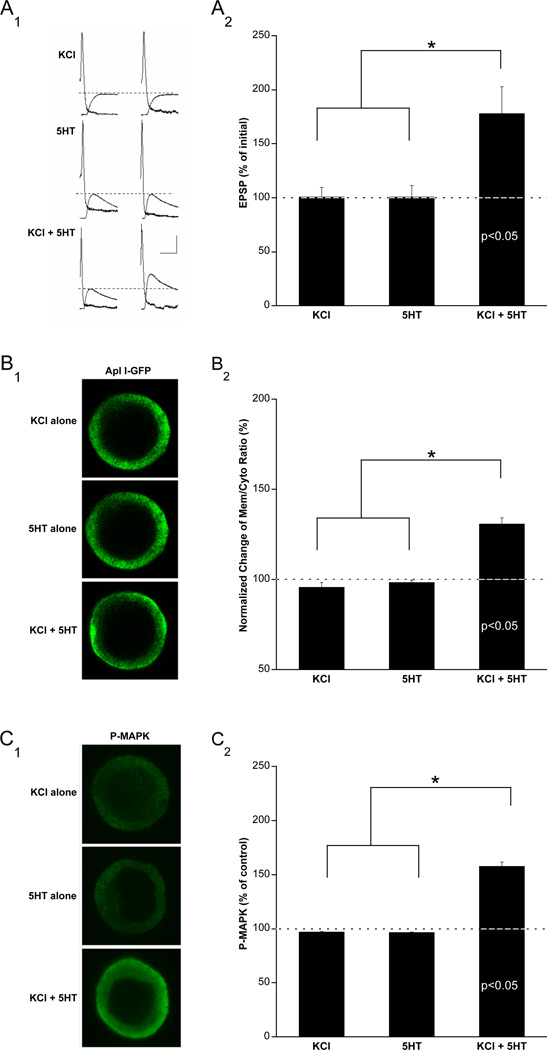
Associative stimulation induces two critical molecular events in SNs: (i) MAPK activation and (ii) translocation of Ca2+-sensitive PKC (Apl I) to the plasma membrane (Kruger et al., 1991; Lim and Sossin, 2006; Manseau et al., 2001; Pepio et al., 2002; Sossin, 1997; Sossin et al., 1994; Sossin and Schwartz, 1992; Zhao et al., 2006). To directly explore the relationship between these sets of events in cultured SNs, we examined the effects of: (i) KCl alone (ii) 5HT alone or (iii) KCl + 5HT.
To measure PKC translocation, we microinjected Apl I – GFP constructs into SNs (Supplementary Figure S3). We monitored P-MAPK levels using immunocytochemistry with phospho-specific MAPK antibodies. We found that the same analog stimulation that produces robust AD-ITF (Figure 6A) induced both Apl I translocation (Figure 6B) and MAPK activation (Figure 6C). Moreover, consistent with our hypothesis, a single pulse of KCl or 5HT alone did not produce any AD-ITF, Apl I translocation, or MAPK activation (Figure 6) demonstrating that the temporal pairing of activity and 5HT is critical to AD-ITF.
Figure 6. AD-ITF training induces MAPK activation and PKC translocation.
Cultured sensory neurons stimulated with 100mM KCl-ASW + 5HT (10µM) exhibit robust Apl I translocation as well as MAPK activation. (A) AD-ITF induced by pairing KCl depolarization with 5HT. (A1) sample traces showing increased EPSP amplitude 1hr after paired KCl (100mM, 5 min) and 5HT(10µM, 5 min) stimulation, but no change in EPSP amplitude 1 hr after stimulation with either KCl (100mM, 5 min) or 5HT (10µM, 5 min) alone. Scale bar: 20mV, 25ms. (A2) Histogram of group data (n=6 for KCl alone, n=8 for 5HT alone and n=10 for KCl+5HT, *p<0.05). One way ANOVA F(2,21) = 5.76; n<0.001. (B) Pairing of KCl depolarization with 5HT stimulation triggers PKC Apl I translocation in sensory neurons. (B1) left panel: Apl I-GFP translocates from cytosol to membrane following paired KCl+5HT stimulation only. Scale bar: 20µm. (B2) Histogram of group data showing mean pixel intensity ratio of membrane to cytosol for Apl I ± SEM (n=5 for KCl, n=4 for 5HT and n=5 for KCl+5HT, *p<0.05) One way ANOVA F(2,11) = 50.5; n<0.001. (C) Pairing of KCl depolarization with 5HT stimulation triggers MAP kinase activation in sensory neurons. (C1) left panel: immunocytochemistry with phospho-specific MAPK (PMAPK) antibodies shows increased MAPK activation in sensory neurons only with paired KCl+5HT stimulation. Scale bar: 20µm. (C2) Histogram of group data showing mean pixel intensity for PMAPK ± SEM (n=4 for KCl, n=4 for 5HT and n=6 for KCl+5HT, *p<0.05) One way ANOVA F(2,11) = 22.8; n<0.001. Post-hoc tests following ANOVA (p<0.05) using LSD found that *=p<0.05. Values listed in bars indicate significance above baseline (with respect to ASW alone group) using a between group t-test (p<0.05).
Translocation of Ca2+-dependent PKC requires the MAPK cascade
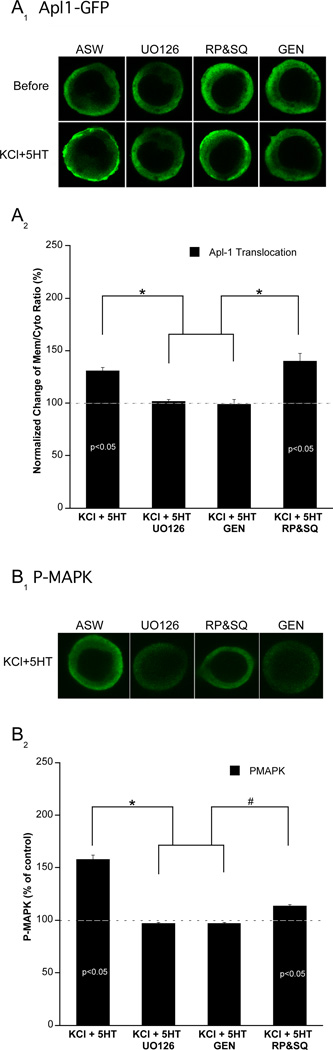
Based on these observations, we hypothesized that MAPK and PKC might be in series. To determine if the MAPK signaling is upstream of PKC, we examined PKC membrane translocation while blocking various elements of the MAPK cascade. We found that PKC translocation was significantly attenuated by the MAPK inhibitor, U0126 (Figure 7A), whereas, the inactive isomer, U0124, had no effect (data not shown). This effect was specific for the Ca2+-sensitive PKC Apl I because in separate experiments we observed that 5HT-activated PKC (Apl II - a distinct isoform of PKC) displayed normal translocation to the plasma membrane in the presence of UO126 (Supplementary Figure S4). However, we found that cAMP signaling was not required for Apl I translocation (Figure 7A; see below). Finally, as predicted by our model, we confirmed that TK activity is required for Apl I translocation (Figure 7A).
Figure 7. PKC translocation requires tyrosine kinase and MAPK signaling.
Video confocal microscopy of PKC Apl IGFP in cultured sensory neurons reveals that membrane translocation of PKC induced by KCl+5HT is blocked by MAPK and tyrosine kinase inhibitors. Shown are representative confocal optical sections before and immediately after paired KCl+5HT stimulation (5 min). (A1) 1 hr preincubation with the MAPK inhibitor (U0126, 20 µM) or the tyrosine kinase inhibitor (genistein 100 µM) blocks KCl+5HT induced PKC Apl I membrane translocation. In contrast, KCl+5HT induced Apl I membrane translocation is not blocked by a 1 hr preincubation with cAMP inhibitors (SQ22536 1.5mM+RP isomer 500 µM). (A2) Histogram of group data for normalized changes in the ratio of Apl I –GFP mean intensity on membrane to cytosol before and after KCl+5HT stimulation in sensory neurons preincubated with MAPK (U0126, n=5), tyrosine kinase (genistein, n=7), and cAMP (RP&SQ, n=5) inhibitors. ANOVA F (3,17)=13.3; n<0.001. (B1) KCl+5HT induced MAPK phosphorylation in sensory neurons is blocked by MAPK and tyrosine kinase inhibitors, but only partially blocked by cAMP inhibitors. Shown are representative confocal optical sections of sensory neurons stained with phospho-specific MAPK (PMAPK) antibodies. (B2): Histogram of mean pixel intensity ± SEM of P-MAPK immunoreactivity after stimulation with KCl+5HT (n=6) in sensory neurons preincubated with MAPK (U0126, n=4), tyrosine kinase (genistein, n=4), and cAMP (RP&SQ, n=7) inhibitors. ANOVA F (3,17)=20.1; n<0.001. Post-hoc tests following ANOVA using LSD found that *=p<0.05 whereas #=p<0.1. Values listed in bars indicate significance above baseline (with respect to ASW alone group) using a between group t-test (p<0.05).
To determine if these inhibitors completely blocked MAPK activation we monitored P-MAPK activity using the same conditions. As expected, UO126 and GEN completely blocked MAPK activation (Figure 7B). However in the presence of the cAMP signaling inhibitors (RP&SQ) we detected a modest (but significant) amount of residual MAPK activity. This result could explain why the cAMP inhibitors did not inhibit Apl I translocation (see Discussion). Nonetheless, the fact that we do observe a significant block of MAPK activity in culture with RP&SQ is consistent with our earlier findings that cAMP signaling is required for MAPK activation in the SN cluster (Figure 2A). Taken collectively our findings suggest that, during associative training, at least three distinct upstream signals, Ca2+, TKs, and cAMP contribute to MAPK activation, which in turn leads to Ca2+-sensitive PKC translocation and ultimately AD-ITF.
DISCUSSION
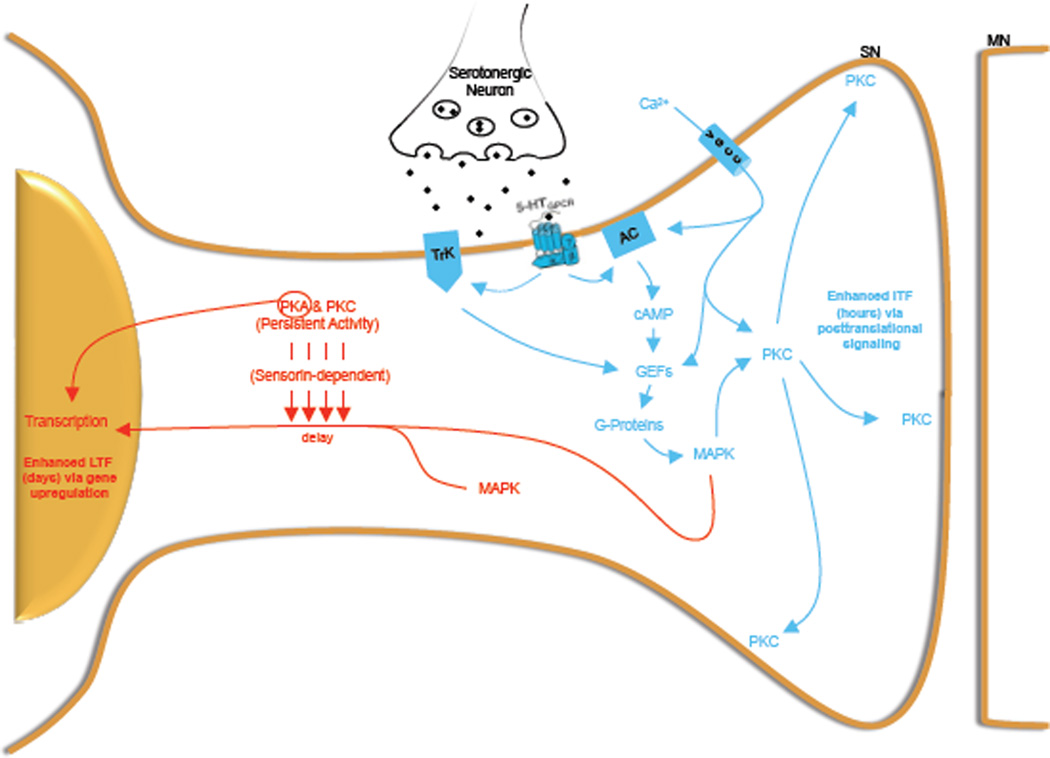
A fundamental question in the neurobiology of memory concerns the mechanistic relationship between distinct temporal phases of enduring memory. Here we combine behavioral, synaptic and molecular levels of analysis to elucidate a novel MAPK-PKC signaling pathway that requires the association of activity and serotonin for its induction. We demonstrate that this novel cascade operates presynaptically (in SNs) to induce an enduring form of activity-dependent intermediate-term plasticity (AD-ITF) and memory (AD-ITM) that is independent of translation and transcription. Importantly, within the same SN population, Hu and colleagues (2007) found that activity and 5HT induces a form of presynaptically-mediated activity-dependent long-term plasticity (AD-LTF) that, in contrast to our present data, requires MAPK translocation to the nucleus of SNs for the eventual up-regulation of genes that promote synaptic growth. Together these findings provide the first demonstration that, within a presynaptic neuronal population, MAPK can serve as a divergent molecular node that compartmentally routes molecular information to mediate distinct temporal phases of synaptic plasticity and memory (see model in Figure 8).
Figure 8. An integrated model depicting how the MAPK cascade can direct intermediate and long-term phases of plasticity by differential routing of downstream signals.
Behavioral training releases endogenous 5HT and activates SNs, which initiates two distinct but MAPK –dependent signaling cascades. Initially, rapid Ca2+ entry, TK receptor activation and the increase in cAMP levels funnels through G-proteins to activate MAPK. This MAPK then mediates the translocation of Ca2+-dependent PKC to the plasma membrane placing it in the proper compartment to activate those substrates necessary for the induction of activity-dependent intermediate-term plasticity and memory (blue). This is followed by a delayed kinase-dependent translocation of MAPK to the nucleus, which enables MAPK to phosphorylate transcription factors and upregulate genes necessary for AD-LTF (red).
Activity and neuromodulatory signals converge on the MAPK cascade
The novel MAPK-PKC cascade we have identified clarifies the relationship between previously identified activity-dependent signaling cascades in the Aplysia CNS. Early studies indicated that Ca2+ and 5HT could generate high levels of cAMP via the dually-regulated adenylate cyclase, suggesting that downstream signaling occurred through PKA. However, to our surprise we found that AD-ITF and AD-ITM did not require PKA activity but rather were critically dependent on PKC activity (Sutton et al., 2004; Sutton and Carew, 2000). In a subsequent study, we determined that the Ca2+ dependent Apl I PKC isoform translocated to the plasma membrane to induce AD-ITF (Zhao et al., 2006).
Our present findings support the hypothesis that MAPK activity links cAMP signaling to downstream PKC function. First, we show that within SNs, the associative analog produces a robust activation of MAPK that is dependent on TK, Ca2+ and cAMP. However, consistent with our earlier behavioral observations, this MAPK activation does not require PKA activity. Rather, MAPK is activated by the convergence of these upstream pathways that funnel through the small G-proteins (collective Ras and Rap activity). Initially, we expected that PKC would activate MAPK (as is the case in many systems). However, we found the converse: MAPK is upstream of PKC and regulates its activation by inducing translocation to the plasma membrane, consistent with the established role of Ca2+-sensitive PKC (Apl I) in AD-ITF (Zhao et al., 2006).
We then demonstrated the relevance of this novel-signaling cascade to synaptic plasticity and memory. Specifically, we showed that (i) AD-ITM requires MAPK and TK activity, and that (ii) MAPK activation and AD-ITM requires PKA-independent cAMP signaling (consistent with previous behavioral observations). Taken together with our earlier findings that AD-ITM requires PKC activity but not PKA activity, we are now able to track the flow of molecular information induced by behavioral training. Our data support the model shown in Figure 10: training activates 5HT receptors and induces Ca2+ entry, which stimulates cAMP generation and MAPK-dependent Apl I translocation to the plasma membrane causing the eventual phosphorylation of membrane localized PKC substrates that are required for the induction of AD-ITF and AD-ITM (shown in blue in Figure 10). A second route for MAPK mediated signaling is temporally delayed and gives rise to transcription-dependent AD-LTM (shown in red in Figure 10, see below).
The MAPK cascade and PKC translocation to the plasma membrane
Consistent with our model (Figure 8), we find that PKC translocation (and MAPK activation) in cultured SNs requires TK and MAPK activity. However, our results also suggest that PKC translocation is differentially sensitive to cAMP signaling in cultured SNs as compared to SNs in intact ganglia. In both cases, we found that MAPK activation was significantly attenuated by cAMP inhibitors. However, in cultured SNs, MAPK, although reduced, was significantly above baseline levels (by about 14%). The residual MAPK activation in cultured SNs could explain the difference in the cAMP dependence of PKC translocation. Specifically, it raises the possibility that there may be a threshold level of cAMP required for PKC translocation. From this perspective, even though cAMP was reduced in ganglia and in culture, in the ganglion the blockers reduced cAMP levels below the translocation threshold, whereas in cultured SNs they did not. This indicates that the absolute concentration of cAMP may function together with its spatial and temporal profile to differentially regulate downstream signaling cascades. Since we cannot exclude the possibility that the cAMP pathway also regulates parallel, MAPK-independent processes that are required for activity-dependent intermediate-term plasticity, these questions pave the way for important future studies.
How does MAPK regulate PKC translocation? There are a number of possibilities. First, MAPK could gate additional Ca2+ entry (via positive feedback) or modulate DAG binding to PKC, both of which are required for translocation. Second, MAPK could, either directly or indirectly, phosphorylate PKC, although phosphorylation of PKC is considered unrelated to its translocation. A third, and more likely possibility, is that MAPK activity regulates another factor that in turn is required for PKC translocation. Indeed, a recent study in cerebellar Purkinje cells suggests that MAPK acts through arachidonic acid to activate and translocate PKC to the plasma membrane (Tanaka and Augustine, 2008). One clue that constrains the number of possible intervening molecules in our system is the fact that MAPK is required for Apl I, but not Apl II, translocation, suggesting that Apl I possesses unique structural features that regulate its binding to the plasma membrane. In fact, PKC Apl I has two distinct regions that are not present in Apl II, a PDZ binding site at the C-terminus and its C2 domain (Pepio et al., 1998; Pepio and Sossin, 1998, 2001). Further investigation will focus on the possibility that these regions bind specifically to factors that are regulated by MAPK activity.
A central question is the identity of the downstream effectors of PKC. Although this is beyond the scope of the present study, the fact that PKC translocates to the plasma membrane suggests that some of its effectors are membrane-associated. In fact, activation of PKC by phorbol esters increases transmitter release at both naïve and depressed SN-MN synapses (Braha et al., 1990; Ghirardi et al., 1992; Nakhost et al., 2003), suggesting that PKC may regulate components of the release machinery, such as SNAP25, which was recently identified as a PKC substrate (Houeland et al., 2007). Evidence also suggests that PKC promotes spike broadening via inactivation of voltage-gated K channels (Sugita et al., 1994). Moreover, after membrane translocation, PKC may be cleaved by calpain into the constitutively active form, PKM (Sutton et al. 2004).
Is the novel MAPK-PKC cascade conserved?
The present paper, together with the results of Tanaka and Augustine (2008), provide the first evidence of MAPK-dependent PKC translocation in neurons. Our study suggests a presynaptic locus, and in complementary fashion, Tanaka and Augustine (2008) provide evidence for a postsynaptic locus. Taken together, these two studies encourage a reconsideration of the relationship between PKC and MAPK in a wide range of neural systems. Interestingly, both MAPK and PKC activity are required for hippocampal CA1 pyramidal cell LTP and insertion of the same AMPAR subunit, GluR1 (Boehm et al., 2006; Correia et al., 2003; Gomes et al., 2007; Ling et al., 2006; McDonald et al., 2001; Tomita et al., 2005). Moreover, PKC (specifically PKMζ) is necessary and sufficient for AMPAR insertion (Ling et al 2006) and PKMϒ translocates to the plasma membrane of primary hippocampal neurons in response to glutamate or NMDA stimulation (Codazzi et al., 2006; Craske et al., 2005). Together with our present findings, these studies suggest that MAPK may regulate PKC translocation and subsequent GluR1 insertion associated with LTP. Finally, in addition to its role in PKC regulation, a complementary role of MAPK has been provided by Sindreu et al. 2007 who found that, following fear conditioning, MAPK signaling to CREB depends on Ca2+ stimulated adenylate cyclase activity, which could require the G-protein activation by cAMP that we observe.
MAPK routes signals to regulate IT and LT temporal domains of plasticity
Our current findings, together with the results of Hu and colleagues (2007) suggest that MAPK activated in an associative, activity-dependent manner can serve as a significant molecular node (point of convergence and divergence, see Reissner et al., 2006). Despite the fact that both AD-ITF and AD-LTF require MAPK activity, each of these MAPK cascades involves a distinct set of upstream activators and compartment-specific downstream effectors. Interestingly, Hu et al (2007) find that secretion of the sensorin peptide requires both PKC and PKA activity. Moreover, they show that sensorin secretion does not occur immediately after associative training but is necessary for the activation and translocation of MAPK to the nucleus when measured 1hr after associative training. Taken together, their findings suggest that sustained (but not immediate) MAPK function is downstream of PKA and PKC signaling.
In contrast, we find that the immediate MAPK activity requires neither PKA nor PKC activity but rather funnels through the small G-proteins Ras and Rap. Also, we do not observe MAPK translocation to the SN nucleus when examined immediately following associative training (Figure 6C1). All these findings lead to a model in which early MAPK is quickly activated via the G-protein pathway to rapidly redistribute PKC to the plasma membrane so that membrane localized PKC substrates are phosphorylated for AD-ITF (Figure 8). However since this plasticity is transient, the same neuron must upregulate a more sustained kinase-dependent sensorin pathway that translocates MAPK to nucleus in order to upregulate the genes necessary for AD-LTF. How does MAPK manage to effectively orchestrate both temporal domains of plasticity? One possibility is that same activated MAPK first directs PKC translocation to the membrane and only then translocates to the nucleus. Another is that separate intracellular pools of MAPK are activated and therefore linked to distinct downstream functions.
In conclusion, our overall results, in combination with recent work in the field, emphasizes the importance of not only asking whether a particular cascade, such as MAPK, is involved in a particular form of plasticity, but also asking two more refined sets of questions. First, where and how is it activated? and second, where and how does it exert its effects? Our data provide an example of how the activation of MAPK by activity-dependent behavioral training gives rise to subsequent MAPK-dependent compartmental routing that ultimately determines the nature and mechanisms of specific forms of memory.
METHODS
Behavioral Procedures
A reduced behavioral preparation was used in all behavioral experiments (see supplemental data and Sutton et al., 2001). All drug treatments were restricted to the ring ganglia sub-chamber. Following pre-tests, drugs were applied from 30–60 min before training and were present throughout the experiment (see figure legends for details). Control and experimental groups were always performed in parallel. A blind observer measured the duration of TSW.
Analysis of MAPK and G-protein activation
To examine P-MAPK levels in ganglia, desheathed left and right pleural-pedal ganglia (from a single animal) were randomly assigned to treated or control group. The treated side received an 8 min treatment, first 5 min with 50µM 5HT and last 5 min with 100mM KCl ASW (3 min overlap). SN clusters were immediately lysed and processed for western analysis (see supplemental). In time course experiments (Figure 2B), ganglia were maintained in ASW before lysing.
To examine P-MAPK levels in culture, SNs were fixed in 4% paraformaldehyde in PBS/30% sucrose for 15 min, permeabilized in 0.1% Triton X-100 for 5 min, quenched in 50 mM NH4Cl for 15 min, blocked in 10% goat serum in PBS for 30 min, incubated with anti-active MAPK antibody (Promega) overnight at 4°C, and with Alexafluor 488 goat anti-rabbit secondary antibodies (Invitrogen) for one hour at RT. Samples were imaged on a Zeiss Pascal Laser Scanning Microscope using a 63X Apochromat objective.
Ras and Rap activation were measured in whole pleural-pedal extracts (Stressgen). Ras and Rap (from a single sample) were trapped on the same column with appropriate binding domains. Levels of active Ras and Rap were assessed by immunoblotting and the active G-proteins were normalized to total G-proteins (input). The magnitude of Ras, Rap activation was calculated as the ratio of the signal on the treated side (derived from the bound fraction) normalized to the control side (derived from the input signal) from the same animal. Activation of MAPK was assessed on the same blot.
Physiology in intact ganglia
For AD-ITF physiology on SN-MN synapses see supplemental data and Sutton and Carew, 2000). The general TK inhibitor genistein (100 µM) (Sigma, St. Louis, MO) or vehicle (0.33% DMSO/ 0.43% ETOH) was applied to the ganglia following the second pretest and perfused constantly until 5HT offset to test the requirement for TK activity during induction of AD-ITF. To test if ongoing TK activity was required for expression of AD-ITF, genistein was applied beginning 10 minutes after 5HT offset and throughout the experiment.
Physiology in cultured cells
After recording a baseline EPSP, AD-ITF was induced with 5min of 5HT (10 µM) in combination with either four trains of action potentials (10 Hz for 2 s with 1 min ITI) delivered to the SNs or KCl (100mM). The EPSP was recorded 30 min later. Facilitation was expressed as the percent change in EPSP amplitude. Recovery from homosynaptic depression was induced by low frequency stimulation (0.05Hz, 20 stimuli), followed by a 5 min application of 5HT (10 µM) and measured by calculating the percent change in EPSP amplitude between the EPSP recorded after the 5HT stimulation (PSP21) and the EPSP recorded at the end of the low frequency train (PSP20). In experiments using MEK inhibitors, cultures were preincubated with the active inhibitor U0126 (20 µM) or its inactive analog U0124 (20µM) for 30 min, and inhibitors were present throughout the experiment.
Supplementary Material
Acknowledgements
We thank Wayne Sossin for helpful discussions and members of the Carew lab as well as Besim Uzgil for providing suggestions on an earlier version of the manuscript. This work was supported by NIH grant 2R01MH041083-19 and NSF grant IOB-0444762j to TJC; a grant from the W.M. Keck Foundation to KCM; and NRSA grant 5F31MH70065-2 to JLS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abrams TW. Activity-dependent presynaptic facilitation: an associative mechanism in Aplysia. Cellular and molecular neurobiology. 1985;5:123–145. doi: 10.1007/BF00711089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams TW, Kandel ER. Is contiguity detection in classical conditioning a system or a cellular property? Learning in Aplysia suggests a possible molecular site. Trends in neurosciences. 1988;11:128–135. doi: 10.1016/0166-2236(88)90137-3. [DOI] [PubMed] [Google Scholar]
- Abrams TW, Karl KA, Kandel ER. Biochemical studies of stimulus convergence during classical conditioning in Aplysia: dual regulation of adenylate cyclase by Ca2+/calmodulin and transmitter. J Neurosci. 1991;11:2655–2665. doi: 10.1523/JNEUROSCI.11-09-02655.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams TW, Yovell Y, Onyike CU, Cohen JE, Jarrard HE. Learning & memory. Vol. 4. Cold Spring Harbor, N.Y: 1998. Analysis of sequence-dependent interactions between transient calcium and transmitter stimuli in activating adenylyl cyclase in Aplysia: possible contribution to CS--US sequence requirement during conditioning; pp. 496–509. [DOI] [PubMed] [Google Scholar]
- Arancio O, Kandel ER, Hawkins RD. Activity-dependent long-term enhancement of transmitter release by presynaptic 3',5'-cyclic GMP in cultured hippocampal neurons. Nature. 1995;376:74–80. doi: 10.1038/376074a0. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Giustetto M, Huang YY, Hawkins RD, Kandel ER. Is heterosynaptic modulation essential for stabilizing Hebbian plasticity and memory? Nature reviews. 2000;1:11–20. doi: 10.1038/35036191. [DOI] [PubMed] [Google Scholar]
- Biou V, Bhattacharyya S, Malenka RC. Endocytosis and recycling of AMPA receptors lacking GluR2/3. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1038–1043. doi: 10.1073/pnas.0711412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Braha O, Dale N, Hochner B, Klein M, Abrams TW, Kandel ER. Second messengers involved in the two processes of presynaptic facilitation that contribute to sensitization and dishabituation in Aplysia sensory neurons. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:2040–2044. doi: 10.1073/pnas.87.5.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne JH, Kandel ER. Presynaptic facilitation revisited: state and time dependence. J Neurosci. 1996;16:425–435. doi: 10.1523/JNEUROSCI.16-02-00425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll RC, Nicoll RA, Malenka RC. Effects of PKA and PKC on miniature excitatory postsynaptic currents in CA1 pyramidal cells. Journal of neurophysiology. 1998;80:2797–2800. doi: 10.1152/jn.1998.80.5.2797. [DOI] [PubMed] [Google Scholar]
- Casey M, Maguire C, Kelly A, Gooney MA, Lynch MA. Analysis of the presynaptic signaling mechanisms underlying the inhibition of LTP in rat dentate gyrus by the tyrosine kinase inhibitor, genistein. Hippocampus. 2002;12:377–385. doi: 10.1002/hipo.10036. [DOI] [PubMed] [Google Scholar]
- Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- Codazzi F, Di Cesare A, Chiulli N, Albanese A, Meyer T, Zacchetti D, Grohovaz F. Synergistic control of protein kinase Cgamma activity by ionotropic and metabotropic glutamate receptor inputs in hippocampal neurons. J Neurosci. 2006;26:3404–3411. doi: 10.1523/JNEUROSCI.0478-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia SS, Duarte CB, Faro CJ, Pires EV, Carvalho AL. Protein kinase C gamma associates directly with the GluR4 alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor subunit. Effect on receptor phosphorylation. The Journal of biological chemistry. 2003;278:6307–6313. doi: 10.1074/jbc.M205587200. [DOI] [PubMed] [Google Scholar]
- Craske ML, Fivaz M, Batada NN, Meyer T. Spines and neurite branches function as geometric attractors that enhance protein kinase C action. The Journal of cell biology. 2005;170:1147–1158. doi: 10.1083/jcb.200503118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer JR, Manseau F, Castellucci VF, Sossin WS. Serotonin persistently activates the extracellular signal-related kinase in sensory neurons of Aplysia independently of cAMP or protein kinase C. Neuroscience. 2003;116:13–17. doi: 10.1016/s0306-4522(02)00566-3. [DOI] [PubMed] [Google Scholar]
- Eliot LS, Dudai Y, Kandel ER, Abrams TW. Ca2+/calmodulin sensitivity may be common to all forms of neural adenylate cyclase. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:9564–9568. doi: 10.1073/pnas.86.23.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliot LS, Hawkins RD, Kandel ER, Schacher S. Pairing-specific, activity-dependent presynaptic facilitation at Aplysia sensory-motor neuron synapses in isolated cell culture. J Neurosci. 1994;14:368–383. doi: 10.1523/JNEUROSCI.14-01-00368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Schroeder H, Matthies H. Dopaminergic antagonists prevent long-term maintenance of posttetanic LTP in the CA1 region of rat hippocampal slices. Brain research. 1990;522:69–75. doi: 10.1016/0006-8993(90)91578-5. [DOI] [PubMed] [Google Scholar]
- Garcia-Mata R, Burridge K. Catching a GEF by its tail. Trends Cell Biol. 2007;17:36–43. doi: 10.1016/j.tcb.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Ghirardi M, Braha O, Hochner B, Montarolo PG, Kandel ER, Dale N. Roles of PKA and PKC in facilitation of evoked and spontaneous transmitter release at depressed and nondepressed synapses in Aplysia sensory neurons. Neuron. 1992;9:479–489. doi: 10.1016/0896-6273(92)90185-g. [DOI] [PubMed] [Google Scholar]
- Ghirardi M, Montarolo PG, Kandel ER. A novel intermediate stage in the transition between short- and long-term facilitation in the sensory to motor neuron synapse of aplysia. Neuron. 1995;14:413–420. doi: 10.1016/0896-6273(95)90297-x. [DOI] [PubMed] [Google Scholar]
- Goldsmith BA, Abrams TW. Reversal of synaptic depression by serotonin at Aplysia sensory neuron synapses involves activation of adenylyl cyclase. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:9021–9025. doi: 10.1073/pnas.88.20.9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AR, Correia SS, Esteban JA, Duarte CB, Carvalho AL. PKC anchoring to GluR4 AMPA receptor subunit modulates PKC-driven receptor phosphorylation and surface expression. Traffic (Copenhagen, Denmark) 2007;8:259–269. doi: 10.1111/j.1600-0854.2006.00521.x. [DOI] [PubMed] [Google Scholar]
- Houeland G, Nakhost A, Sossin WS, Castellucci VF. PKC modulation of transmitter release by SNAP-25 at sensory-to-motor synapses in aplysia. Journal of neurophysiology. 2007;97:134–143. doi: 10.1152/jn.00122.2006. [DOI] [PubMed] [Google Scholar]
- Hu JY, Chen Y, Schacher S. Protein kinase C regulates local synthesis and secretion of a neuropeptide required for activity-dependent long-term synaptic plasticity. J Neurosci. 2007;27:8927–8939. doi: 10.1523/JNEUROSCI.2322-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:2446–2450. doi: 10.1073/pnas.92.7.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nature reviews. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kentros CG, Agnihotri NT, Streater S, Hawkins RD, Kandel ER. Increased attention to spatial context increases both place field stability and spatial memory. Neuron. 2004;42:283–295. doi: 10.1016/s0896-6273(04)00192-8. [DOI] [PubMed] [Google Scholar]
- Kopec CD, Real E, Kessels HW, Malinow R. GluR1 links structural and functional plasticity at excitatory synapses. J Neurosci. 2007;27:13706–13718. doi: 10.1523/JNEUROSCI.3503-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger KE, Sossin WS, Sacktor TC, Bergold PJ, Beushausen S, Schwartz JH. Cloning and characterization of Ca(2+)-dependent and Ca(2+)-independent PKCs expressed in Aplysia sensory cells. J Neurosci. 1991;11:2303–2313. doi: 10.1523/JNEUROSCI.11-08-02303.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner SA, Elgersma Y, Murphy GG, Jaarsma D, van Woerden GM, Hojjati MR, Cui Y, LeBoutillier JC, Marrone DF, Choi ES, De Zeeuw CI, Petit TL, PozzoMiller L, Silva AJ. Modulation of presynaptic plasticity and learning by the H-ras/extracellular signal-regulated kinase/synapsin I signaling pathway. J Neurosci. 2005;25:9721–9734. doi: 10.1523/JNEUROSCI.2836-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan ES. Signaling for vesicle mobilization and synaptic plasticity. Molecular neurobiology. 2008;37:39–43. doi: 10.1007/s12035-008-8014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim T, Sossin WS. Phosphorylation at the hydrophobic site of protein kinase C Apl II is increased during intermediate term facilitation. Neuroscience. 2006;141:277–285. doi: 10.1016/j.neuroscience.2006.03.063. [DOI] [PubMed] [Google Scholar]
- Ling DS, Benardo LS, Sacktor TC. Protein kinase Mzeta enhances excitatory synaptic transmission by increasing the number of active postsynaptic AMPA receptors. Hippocampus. 2006;16:443–452. doi: 10.1002/hipo.20171. [DOI] [PubMed] [Google Scholar]
- Manseau F, Fan X, Hueftlein T, Sossin W, Castellucci VF. Ca2+-independent protein kinase C Apl II mediates the serotonin-induced facilitation at depressed aplysia sensorimotor synapses. J Neurosci. 2001;21:1247–1256. doi: 10.1523/JNEUROSCI.21-04-01247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinesco S, Carew TJ. Serotonin release evoked by tail nerve stimulation in the CNS of aplysia: characterization and relationship to heterosynaptic plasticity. J Neurosci. 2002;22:2299–2312. doi: 10.1523/JNEUROSCI.22-06-02299.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KC, Michael D, Rose JC, Barad M, Casadio A, Zhu H, Kandel ER. MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia. Neuron. 1997;18:899–912. doi: 10.1016/s0896-6273(00)80330-x. [DOI] [PubMed] [Google Scholar]
- McDonald BJ, Chung HJ, Huganir RL. Identification of protein kinase C phosphorylation sites within the AMPA receptor GluR2 subunit. Neuropharmacology. 2001;41:672–679. doi: 10.1016/s0028-3908(01)00129-0. [DOI] [PubMed] [Google Scholar]
- Michael D, Martin KC, Seger R, Ning MM, Baston R, Kandel ER. Repeated pulses of serotonin required for long-term facilitation activate mitogen-activated protein kinase in sensory neurons of Aplysia. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:1864–1869. doi: 10.1073/pnas.95.4.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitin N, Rossman KL, Der CJ. Signaling interplay in Ras superfamily function. Curr Biol. 2005;15:R563–R574. doi: 10.1016/j.cub.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Moroz LL, Edwards JR, Puthanveettil SV, Kohn AB, Ha T, Heyland A, Knudsen B, Sahni A, Yu F, Liu L, et al. Neuronal transcriptome of aplysia: neuronal compartments and circuitry. Cell. 2006;127:1453–1467. doi: 10.1016/j.cell.2006.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhost A, Houeland G, Castellucci VF, Sossin WS. Differential regulation of transmitter release by alternatively spliced forms of synaptotagmin I. J Neurosci. 2003;23:6238–6244. doi: 10.1523/JNEUROSCI.23-15-06238.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocorr KA, Tabata M, Byrne JH. Stimuli that produce sensitization lead to elevation of cyclic AMP levels in tail sensory neurons of Aplysia. Brain research. 1986;371:190–192. doi: 10.1016/0006-8993(86)90828-0. [DOI] [PubMed] [Google Scholar]
- Ocorr KA, Walters ET, Byrne JH. Associative conditioning analog selectively increases cAMP levels of tail sensory neurons in Aplysia. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:2548–2552. doi: 10.1073/pnas.82.8.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormond J, Hislop J, Zhao Y, Webb N, Vaillaincourt F, Dyer JR, Ferraro G, Barker P, Martin KC, Sossin WS. ApTrkl, a Trk-like receptor, mediates serotonin-dependent ERK activation and long-term facilitation in Aplysia sensory neurons. Neuron. 2004;44:715–728. doi: 10.1016/j.neuron.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Overbeck AF, Brtva TR, Cox AD, Graham SM, Huff SY, Khosravi-Far R, Quilliam LA, Solski PA, Der CJ. Guanine nucleotide exchange factors: activators of Ras superfamily proteins. Mol Reprod Dev. 1995;42:468–476. doi: 10.1002/mrd.1080420415. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Pittenger C, Morozov A, Martin KC, Scanlin H, Drake C, Kandel ER. Some forms of cAMP-mediated long-lasting potentiation are associated with release of BDNF and nuclear translocation of phospho-MAP kinase. Neuron. 2001;32:123–140. doi: 10.1016/s0896-6273(01)00443-3. [DOI] [PubMed] [Google Scholar]
- Pepio AM, Fan X, Sossin WS. The role of C2 domains in Ca2+-activated and Ca2+-independent protein kinase Cs in aplysia. The Journal of biological chemistry. 1998;273:19040–19048. doi: 10.1074/jbc.273.30.19040. [DOI] [PubMed] [Google Scholar]
- Pepio AM, Sossin WS. The C2 domain of the Ca(2+)-independent protein kinase C Apl II inhibits phorbol ester binding to the C1 domain in a phosphatidic acid-sensitive manner. Biochemistry. 1998;37:1256–1263. doi: 10.1021/bi971841u. [DOI] [PubMed] [Google Scholar]
- Pepio AM, Sossin WS. Membrane translocation of novel protein kinase Cs is regulated by phosphorylation of the C2 domain. The Journal of biological chemistry. 2001;276:3846–3855. doi: 10.1074/jbc.M006339200. [DOI] [PubMed] [Google Scholar]
- Pepio AM, Thibault GL, Sossin WS. Phosphoinositide-dependent kinase phosphorylation of protein kinase C Apl II increases during intermediate facilitation in aplysia. The Journal of biological chemistry. 2002;277:37116–37123. doi: 10.1074/jbc.M202264200. [DOI] [PubMed] [Google Scholar]
- Purcell AL, Sharma SK, Bagnall MW, Sutton MA, Carew TJ. Activation of a tyrosine kinase-MAPK cascade enhances the induction of long-term synaptic facilitation and long-term memory in Aplysia. Neuron. 2003;37:473–484. doi: 10.1016/s0896-6273(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Reissner KJ, Shobe JL, Carew TJ. Molecular nodes in memory processing: insights from Aplysia. Cell Mol Life Sci. 2006;63:963–974. doi: 10.1007/s00018-006-6022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajikumar S, Frey JU. Late-associativity, synaptic tagging, and the role of dopamine during LTP and LTD. Neurobiology of learning and memory. 2004;82:12–25. doi: 10.1016/j.nlm.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Schacher S, Wu F, Sun ZY. Pathway-specific synaptic plasticity: activity- dependent enhancement and suppression of long-term heterosynaptic facilitation at converging inputs on a single target. J Neurosci. 1997;17:597–606. doi: 10.1523/JNEUROSCI.17-02-00597.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakiryanova D, Klose MK, Zhou Y, Gu T, Deitcher DL, Atwood HL, Hewes RS, Levitan ES. Presynaptic ryanodine receptor-activated calmodulin kinase II increases vesicle mobility and potentiates neuropeptide release. J Neurosci. 2007;27:7799–7806. doi: 10.1523/JNEUROSCI.1879-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, Sherff CM, Shobe J, Bagnall MW, Sutton MA, Carew TJ. Differential role of mitogen-activated protein kinase in three distinct phases of memory for sensitization in Aplysia. J Neurosci. 2003;23:3899–3907. doi: 10.1523/JNEUROSCI.23-09-03899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siderovski DP, Willard FS. The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits. Int J Biol Sci. 2005;1:51–66. doi: 10.7150/ijbs.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossin WS. Learning & memory. Vol. 3. Cold Spring Harbor, N.Y: 1997. An autonomous kinase generated during long-term facilitation in Aplysia is related to the Ca(2+)-independent protein kinase C Apl II; pp. 389–401. [DOI] [PubMed] [Google Scholar]
- Sossin WS, Sacktor TC, Schwartz JH. Learning & memory. Vol. 1. Cold Spring Harbor, N.Y: 1994. Persistent activation of protein kinase C during the development of long-term facilitation in Aplysia; pp. 189–202. [PubMed] [Google Scholar]
- Sossin WS, Schwartz JH. Selective activation of Ca(2+)-activated PKCs in Aplysia neurons by 5-HT. J Neurosci. 1992;12:1160–1168. doi: 10.1523/JNEUROSCI.12-04-01160.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springett GM, Kawasaki H, Spriggs DR. Non-kinase second-messenger signaling: new pathways with new promise. Bioessays. 2004;26:730–738. doi: 10.1002/bies.20057. [DOI] [PubMed] [Google Scholar]
- Sugita S, Baxter DA, Byrne JH. Activators of protein kinase C mimic serotonin-induced modulation of a voltage-dependent potassium current in pleural sensory neurons of Aplysia. Journal of neurophysiology. 1994;72:1240–1249. doi: 10.1152/jn.1994.72.3.1240. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Bagnall MW, Sharma SK, Shobe J, Carew TJ. Intermediate-term memory for site-specific sensitization in aplysia is maintained by persistent activation of protein kinase C. J Neurosci. 2004;24:3600–3609. doi: 10.1523/JNEUROSCI.1134-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Carew TJ. Parallel molecular pathways mediate expression of distinct forms of intermediate-term facilitation at tail sensory-motor synapses in Aplysia. Neuron. 2000;26:219–231. doi: 10.1016/s0896-6273(00)81152-6. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Ide J, Masters SE, Carew TJ. Learning & memory. Vol. 9. Cold Spring Harbor, N.Y: 2002. Interaction between amount and pattern of training in the induction of intermediate- and long-term memory for sensitization in aplysia; pp. 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Masters SE, Bagnall MW, Carew TJ. Molecular mechanisms underlying a unique intermediate phase of memory in aplysia. Neuron. 2001;31:143–154. doi: 10.1016/s0896-6273(01)00342-7. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Augustine GJ. A positive feedback signal transduction loop determines timing of cerebellar long-term depression. Neuron. 2008;59:608–620. doi: 10.1016/j.neuron.2008.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KR, Otis KO, Chen DY, Zhao Y, O'Dell TJ, Martin KC. Synapse to nucleus signaling during long-term synaptic plasticity; a role for the classical active nuclear import pathway. Neuron. 2004;44:997–1009. doi: 10.1016/j.neuron.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Tomita S, Stein V, Stocker TJ, Nicoll RA, Bredt DS. Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron. 2005;45:269–277. doi: 10.1016/j.neuron.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Walters ET. Site-specific sensitization of defensive reflexes in Aplysia: a simple model of long-term hyperalgesia. J Neurosci. 1987;7:400–407. doi: 10.1523/JNEUROSCI.07-02-00400.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters ET, Byrne JH. Slow depolarization produced by associative conditioning of Aplysia sensory neurons may enhance Ca2+ entry. Brain research. 1983;280:165–168. doi: 10.1016/0006-8993(83)91186-1. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Wu Z, Kindsvogel W, Prichard L, Storm DR. Synergistic activation of the type I adenylyl cyclase by Ca2+ and Gs-coupled receptors in vivo. The Journal of biological chemistry. 1994;269:25400–25405. [PubMed] [Google Scholar]
- Wierda KD, Toonen RF, de Wit H, Brussaard AB, Verhage M. Interdependence of PKC-dependent and PKC-independent pathways for presynaptic plasticity. Neuron. 2007;54:275–290. doi: 10.1016/j.neuron.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Wong ST, Athos J, Figueroa XA, Pineda VV, Schaefer ML, Chavkin CC, Muglia LJ, Storm DR. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron. 1999;23:787–798. doi: 10.1016/s0896-6273(01)80036-2. [DOI] [PubMed] [Google Scholar]
- Yamagata Y, Jovanovic JN, Czernik AJ, Greengard P, Obata K. Bidirectional changes in synapsin I phosphorylation at MAP kinase-dependent sites by acute neuronal excitation in vivo. Journal of neurochemistry. 2002;80:835–842. doi: 10.1046/j.0022-3042.2001.00753.x. [DOI] [PubMed] [Google Scholar]
- Ye X, Sharma SK, Shobe J, Carew T. Identification, cDNA cloning, and functional analysis of the small GTP binding proteins Ras and Rap in the CNS of Aplysia . SFN abstract. 2005 [Google Scholar]
- Yovell Y, Kandel ER, Dudai Y, Abrams TW. A quantitative study of the Ca2+/calmodulin sensitivity of adenylyl cyclase in Aplysia, Drosophila, and rat. Journal of neurochemistry. 1992;59:1736–1744. doi: 10.1111/j.1471-4159.1992.tb11005.x. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Leal K, Abi-Farah C, Martin KC, Sossin WS, Klein M. Isoform specificity of PKC translocation in living Aplysia sensory neurons and a role for Ca2+-dependent PKC APL I in the induction of intermediate-term facilitation. J Neurosci. 2006;26:8847–8856. doi: 10.1523/JNEUROSCI.1919-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]
- Zwartkruis FJ, Bos JL. Ras and Rap1: two highly related small GTPases with distinct function. Experimental cell research. 1999;253:157–165. doi: 10.1006/excr.1999.4695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.