Abstract
The maintenance of immune homeostasis requires regulatory T cells (Tregs). Given their intrinsic self-reactivity, Tregs must stably maintain a suppressive phenotype to avoid autoimmunity. We report that impaired expression of the transcription factor (TF) Helios by FoxP3+ CD4 and Qa-1-restricted CD8 Tregs results in defective regulatory activity and autoimmunity in mice. Helios-deficient Treg develop an unstable phenotype during inflammatory responses characterized by reduced FoxP3 expression and increased effector cytokine expression secondary to diminished activation of the STAT5 pathway. CD8 Treg also require Helios-dependent STAT5 activation for survival and to prevent terminal T cell differentiation. Definition of Helios as a key transcription factor that stabilizes regulatory T-cells in the face of inflammatory responses provides a genetic explanation for a core property of regulatory T-cells.
Regulatory T cells (CD4 and CD8 Treg) dampen excessive immune responses and prevent or ameliorate autoimmune tissue damage, while immune suppression exerted by Treg can impede anti-tumor immune responses. In contrast to effector T cells, which rely on robust activation and differentiative plasticity, Treg depend on preservation of a stable, anergic and suppressive phenotype to maintain immune homeostasis (1, 2). Although FoxP3+ CD4 Treg are remarkably stable (1, 2), the genetic mechanisms that ensure phenotypic stability after expansion during inflammation, infection or autoimmunity, i.e., conditions that most require maintenance of an anergic and inhibitory Treg phenotype, are poorly understood.
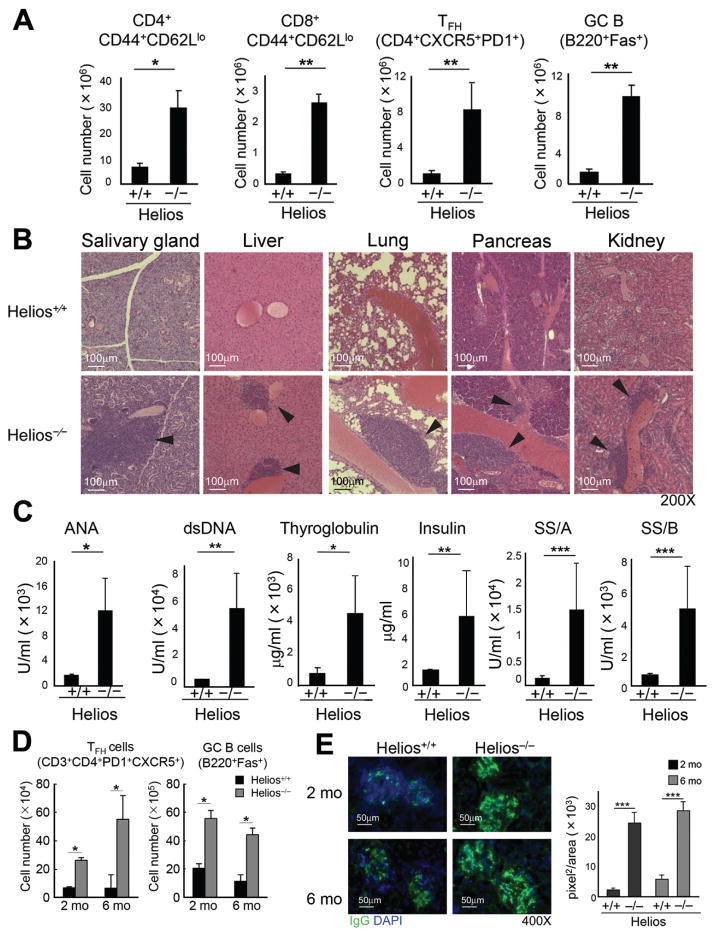
The Helios (Ikzf2) transcription factor (TF) is expressed by two regulatory T cell lineages– FoxP3+CD4+ and Ly49+CD8+ Treg (Fig. S1) (3–6). To determine the contribution of Helios to the regulatory phenotype, we analyzed mice deficient in Izkf2 (Helios−/−), the gene that encodes Helios (5). Helios−/− mice (6–8 wks old) displayed reduced numbers of CD8 but not CD4 Treg (Fig. S2) and no obvious signs of autoimmune disorder. However, 5 mo-old Helios-deficient mice exhibited increased numbers of activated CD4 and CD8 T cells, T follicular helper (TFH) cells and germinal center (GC) B cells compared to WT mice (Fig. 1A, S3A). Autoimmune disease was apparent by 6–8 m of age accompanied by infiltration of immune cells into non-lymphoid tissues (Fig. 1B), production of autoantibodies (Fig. 1C) and glomerular nephritis (Fig. S3B). Rag2−/− mice reconstituted with bone marrow (BM) from Helios−/− donors also developed autoimmunity (Fig. S4), indicating a lymphocyte intrinsic effect.
Fig 1. Helios−/− mice develop an autoimmune phenotype.
A) Activated CD4 and CD8 T cells (CD44+CD62Llo), GC B (B220+Fas+) and TFH (CD4+PD-1+CXCR5+) cells in spleens from (5 mo old) Helios+/+ and Helios−/− mice were compared (n=3–6). Representative data from three independent experiments. The mean ± SEM is indicated. *P < 0.05 and **P < 0.01 (Mann-Whitney test). B) Microscopy (200X) of representative hematoxylin and eosin staining of salivary gland, liver, lung, pancreas, kidney sections from (7 mo old) Helios+/+ and Helios−/− mice (n=4). Representative data from two independent experiments. C) Generation of autoantibodies was compared in sera from 7 mo old Helios+/+ and Helios−/− mice (n=7–10). Representative data from three independent experiments. The mean ± SEM is indicated. *P < 0.05, **P < 0.01 and ***P < 0.001 (Mann-Whitney test). D) Viral infection of Helios-deficient mice. 2 mo and 6 mo old Helios+/+ and Helios−/− mice infected i.p. with 2×105 plaque forming units (PFU) LCMV-Armstrong. 30 days later, spleen cells analyzed for GC B and TFH cells (n=4). Representative data from two independent experiments.The mean ± SEM is indicated. *P < 0.05 (Mann-Whitney test). E) Kidney sections from virus-infected mice were analyzed for IgG deposition and IgG+ areas in glomeruli (n=4). Representative data from two independent experiments are shown. The mean ± SEM is indicated. ***P < 0.001 (Mann-Whitney test).
Although Helios−/− mice did not develop overt signs of autoimmunity until 5–6m of age, upon challenge with viral infection (LCMV-Armstrong), both young (2m) and older (6m) Helios−/− mice but not Helios+/+ mice developed inflammatory and autoimmune changes characterized by increased levels of TFH and GC B cells (Fig. 1D) and IgG deposition in kidney (Fig. 1E), although Helios+/+ and Helios−/− mice cleared virus with equal efficiency (Fig. S5).
Since autoimmunity in Helios−/− mice did not result from defective negative selection (Figs. S6–S8). we asked whether it instead reflected defective Treg activity. Analyses of BM chimeras that express a selective Helios deficiency in either CD4 or CD8 T cells revealed that mice with either Helios-deficient CD4 or CD8 T cells develop autoimmune disease with similar features (Fig. S9). Moreover, tolerance was dominant, since Rag2−/− mice given Helios−/− BM + Helios+/+ BM did not develop autoimmunity (Fig. S10).
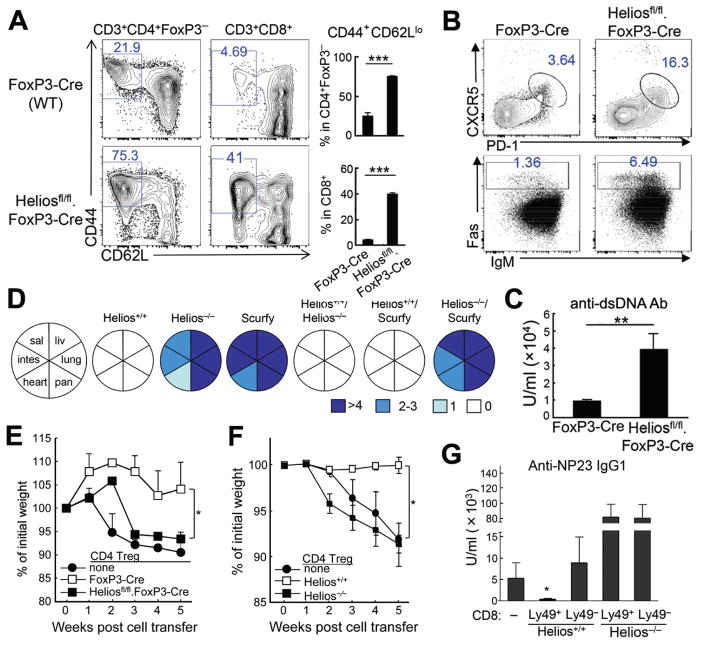
Direct evidence for the contribution of Helios to CD4 Treg activity and prevention of autoimmune disease came from analysis of Heliosfl/fl.FoxP3YFP-Cre mice, which develop autoimmune disease at >5m of age characterized by increased numbers of activated CD4 and CD8 T cells, increased numbers of TFH and GC B cells (Fig. 2A,B), autoantibody production (Fig. 2C), and immune cell infiltration (Fig. S11). Moreover, BM chimeras from Heliosfl/fl.FoxP3-Cre donors developed this disorder within 6 wks (Fig. S12).
Fig 2. Helios-deficient CD4 and CD8 Treg contribute to autoimmune disease.
A) Phenotype of Heliosfl/fl.FoxP3-Cre mice at 6 mo age. The percentage and numbers of activated CD4 T cells (CD44+CD62Llo) in spleen are shown (n=4–5). Data representative of two independent experiments. The mean ± SEM is indicated. ***P < 0.001 (Mann-Whitney test). B) FACS profiles for TFH and GC B cells from spleens of FoxP3-Cre and Heliosfl/fl.FoxP3-Cre mice (n=4–5). Data representative of two independent experiments. C) Levels of anti-dsDNA Ab in sera of FoxP3-Cre and Heliosfl/fl.FoxP3-Cre mice at 6 mo age (n=4–5). Data representative of two independent experiments. The mean ± SEM is indicated. **P < 0.01 (Mann-Whitney test). D) Lethally irradiated Rag2−/− mice were reconstituted with hematopoietic progenitors from Helios+/+, Helios−/−, Scurfy, Helios+/+/Helios−/− (1:1), Helios+/+/Scurfy (1;1), and Helios−/−/Scurfy (1;1) mice (n=4–6). Intensity of immune cell infiltration was quantified by scoring immune cell infiltration 7 weeks after reconstitution: >4 (most severe), 2–3 (severe), 1 (mild) and 0 (none). Data representative of two independent experiments. E) Defective inhibitory activity of Helios-deficient CD4 and CD8 Treg. Rag2−/− hosts received sort-purified Teff cells (CD25−CD44loCD62Lhi, CD45.1) and CD4 Treg (CD3+CD4+YFP+) from spleens of FoxP3-Cre or Heliosfl/fl.FoxP3-Cre mice. Recipients were examined for changes in weight, microscopy of intestine pathology and number of CD11b+Gr-1+ cells in spleen (n=4). Representative data from two independent experiments. The mean ± SEM is indicated. *P < 0.05 and ***P < 0.001 (Kruskal-Wallis test). F) Rag2−/− hosts received CD4 T cells (Teff: CD25−CD44loCD62Lhi, CD4 Treg: CD3+CD4+CD25+) from spleens of defined donor mouse strains. Recipients were examined for changes in weight (n=4). Representative data from three independent experiments. The mean ± SEM is indicated. *P < 0.05 (Kruskal-Wallis test). G) WT B and CD25-depleted CD4 T cells were transferred into Rag2−/− hosts along with Ly49+ or Ly49− CD8 T cells from spleens of Helios+/+ or Helios−/− mice. Rag2−/− adoptive hosts were immunized with NP19-KLH in CFA at day 0 and reimmunized with NP19-KLH in IFA at day 10. NP specific IgG1 responses were measured at day 15 (n=3). Data representative of three independent experiments. The mean ± SEM is indicated. *P < 0.05 (Kruskal-Wallis test).
Helios sufficient but not Helios-deficient FoxP3+ CD4 Treg exerted dominant, lymphocyte-intrinsic inhibition that prevented autoimmune disease in the presence of highly-activated self-reactive T cells from scurfy mice, which have no FoxP3 forkhead domain. BM chimeras reconstituted with Helios−/−/Scurfy BM but not Helios+/+/Scurfy BM cells rapidly developed autoimmunity (Fig. 2D; Fig. S13A,B).
Impaired suppressive activity of Helios-deficient FoxP3+ CD4 Treg was observed when FoxP3+ CD4 cells (YFP+) from Heliosfl/fl.FoxP3YFP-Cre were co-transferred into Rag2−/− hosts with naive CD4+ T cells, resulting in wasting disease (Fig. 2E, S14A). Analysis of CD4 Treg from Helios−/− mice with a global Helios deletion (5) showed that mice given naive CD4 cells developed colitis that could be prevented by Helios+/+ but not Helios−/− FoxP3+ CD4 Treg (Fig. 2F, S14B).
Helios deficiency also resulted in defective CD8 Treg function. CD8 Treg (CD44+CD122+Ly49+) recognize target TH cells through a Qa-1/peptide–TCR interaction that prevents autoantibody-mediated autoimmune disease (3). Helios-deficient CD8 Treg failed to inhibit the adoptive helper response of TFH cells (Fig. 2G). Defective suppressive activity of Helios−/− CD8 Treg was lymphocyte intrinsic, since Ly49+ CD8 cells isolated from Rag2−/− recipients reconstituted with Helios+/+ but not Helios−/− BM cells mediated inhibitory activity (Fig. S14C), consistent with impaired suppressive activity by CD8 treg from Heliosfl/fl.CD4-Cre mice (Fig. S14D). These data indicate that Helios-dependent suppressive activity exerted by both CD4 and CD8 Treg is required to maintain self-tolerance.
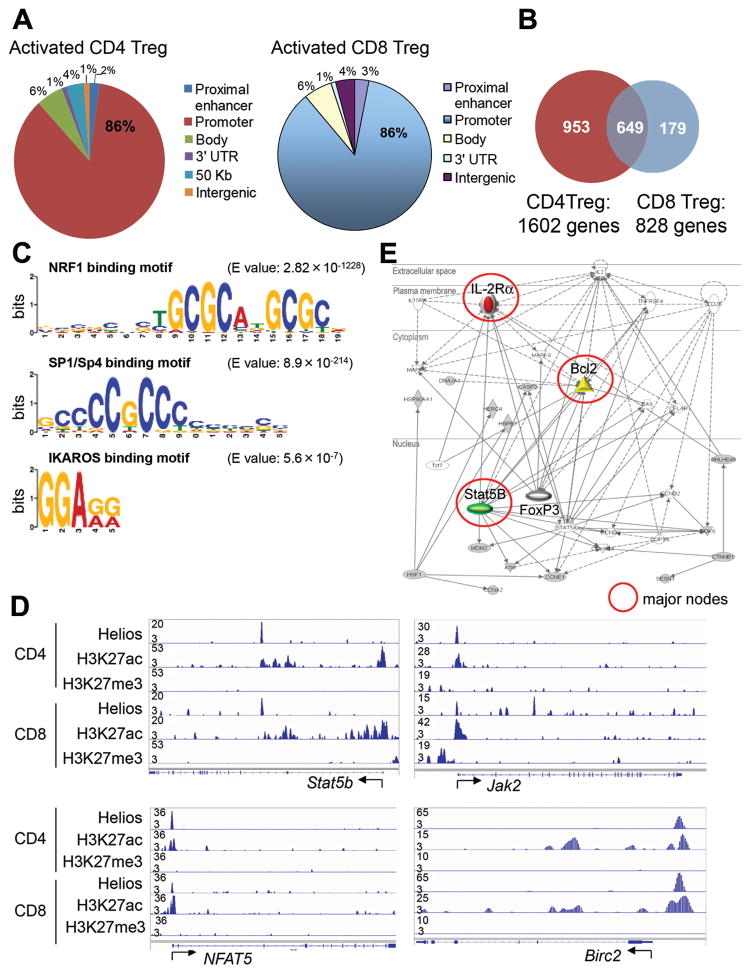
These findings suggested that Helios may control common genetic pathway(s) in CD4 and CD8 Treg. To address this issue we defined the genome-wide distribution of Helios binding sites in these cells by chromatin immunoprecipitation followed by DNA sequencing (ChIP-Seq) (7). The chromatin state of Helios-bound regions was determined according to acetylation of histone H3 at Lys27 (H3K27ac) for active regulatory regions and trimethylation of histone H3 at Lys27 for polycomb-repressed regions (H3K27me3) (8).
This analysis revealed that Helios bound mainly to promoter regions of (~85%) target genes in both CD4 and CD8 Treg (Fig. 3A); 1602 and 828 genes, respectively, with 649 shared target genes (Fig. 3B). Analysis of DNA regions bound by Helios showed significant enrichment of NRF1, Sp1/Sp4 and IKAROS binding motifs (Fig. 3C), as well as genes that regulate cell cycle progression and apoptosis/cell survival (Table S1A), including STAT5b, Jak2, NFAT5 and Birc2, and autophagy genes, whose loci showed activated chromatin marks, as evidenced by expression of H3K27ac (Fig. 3D and Table S1B). Pathway analysis of Helios target genes in CD4 and CD8 Treg revealed STAT5b/interleukin-2 receptor α (IL-2Rα as a central node within a Helios target gene network, suggesting that Helios might regulate genes involved in IL-2 signaling and sustained survival (Fig. 3E).
Fig 3. Helios-dependent STAT5 activation and stabilization of CD4 Treg.
A) Distribution of genome-wide Helios binding sites in activated FoxP3+ CD4 and Ly49+ CD8 Treg, B) Number of Helios target genes and overlapping Helios binding sites in CD4 and CD8 Treg, C) DNA motif analysis of Helios bound regions, D) ChiP-Seq analysis of Helios binding and modified histones at STAT5b, Jak2, NFAT5, and Birc2 in CD4 and CD8 Treg. Start sites of each gene locus are indicated. Vertical lines in gene diagrams (bottom) indicate exons. E) Pathway analysis of genes targeted by Helios in CD4 and CD8 Treg. Solid lines represent genes known to have a direct connection. Dotted lines represent a presumptive interaction based on reported studies. Major nodes of genes are marked by red circles. Filled symbols indicate Helios target genes and white symbols indicate neighboring genes that are functionally associated but not included in Helios target genes.
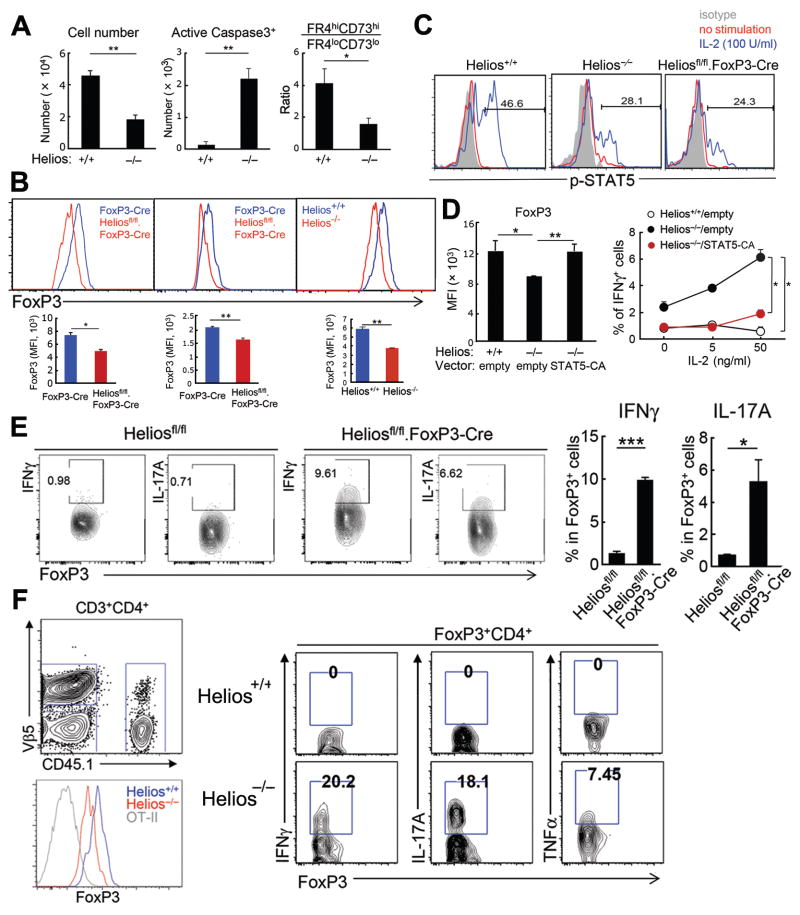
The IL-2Rα–STAT5 pathway is essential for CD4 Treg survival while maintenance of FoxP3 expression reflects binding of activated STAT5 to the Foxp3 promoter and CNS2 regions (9). Identification of the IL-2Rα–STAT5 pathway as a major Helios gene network opened the possibility that Helios contributed to Treg survival and/or FoxP3 stability through increased STAT5 activation and enhanced IL-2 responsiveness (10, 11). We noted reduced survival of Helios−/− CD4 Treg after transfer into lymphopenic hosts (Fig. 4A, left & middle), consistent with an essential contribution of the IL-2Rα–STAT5 signaling pathway to Treg viability. Moreover, Helios-deficient CD4 Treg displayed reduced co-expression of FR4 and CD73 (Fig. 4A, right), which indicate anergy by self-reactive CD4 cells (12). Indeed, a significant portion of Helios-deficient CD4 Treg developed into non-anergic cells under inflammatory conditions, including autoimmunity and infection (12) (Fig. S12C, S15). Since cytokine signaling can induce binding of activated STAT6 to the Foxp3 locus and competition with p-STAT5 (10), diminished STAT5 activation in Helios-deficient CD4 Treg may allow increased STAT6 binding to the Foxp3 locus and diminished FoxP3 expression. Reduced STAT5 activation by Helios-deficient Treg was accompanied by reduced expression of FoxP3 under conditions including progressive autoimmune disease (6 mo old Heliosfl/fl.FoxP3-Cre mice) and colitis (Fig. 4B), consistent with the contribution of STAT5 to stable FoxP3 expression (10).
Fig 4. Helios-dependent STAT5 activation and stabilization of CD4 Treg.
A) CD4 Treg from spleens of Helios+/+ and Helios−/− mice were transferred into Rag2−/− γc−/− mice and numbers, apoptosis and the anergic phenotype of recovered CD4 Treg from spleens were analyzed 5 days after transfer (n=4). Representative data from three independent experiments. The mean ± SEM is indicated. *P < 0.05 and **P < 0.01 (Mann-Whitney test). B) Levels of FoxP3 expression were compared between splenic CD4 Treg from 6 mo old FoxP3-Cre and Heliosfl/fl.FoxP3-Cre mice (left panel) and from indicated hosts that were induced for colitis (n=4) (middle, right panels). Representative plots > 3 different experiments. The mean ± SEM is indicated. *P < 0.05 and **P < 0.01 (Mann-Whitney test). C) BM chimeras were generated by reconstituting Rag2−/− mice with hematopoietic progenitors from Helios+/+, Helios−/−, or Heliosfl/fl/FoxP3-Cre mice. After six-eight weeks, IL-2 responsiveness of FoxP3+ CD4 cells from spleens was tested as described in Methods. Representative histograms for expression of p-STAT5 from two independent experiments. D) CD4 Treg from Helios+/+ and Helios−/− mice were transduced with retrovirus expressing GFP or STAT5-CA/GFP, before stimulation with anti-CD3/CD28 Abs in the presence of IL-2 (0–50 ng/ml) and IL-4 (20 ng/ml). Levels of FoxP3 expression after 5 days at 5 ng/ml IL-2 are shown (left panel). IFNγ production by FoxP3+ cells is shown (right panel). Representative data from two independent experiments. The mean ± SEM is indicated. *P < 0.05 and **P < 0.01 (Mann-Whitney test). E) 2 mo old Heliosfl/fl and Heliosfl/fl.FoxP3-Cre mice were immunized i.p. with 8 × 108 SRBC. After 7 days, FoxP3+ cells from spleen were analyzed for IFNγ and IL-17A expression (n=4–5). Representative data from two independent experiments. The mean ± SEM is indicated. *P < 0.05 and ***P < 0.001 (Mann-Whitney test). F) OT-II cells (1×106) were transferred into Rag2−/− hosts along with CD25+CD4+ T cells (2×105) from spleens of CD45.1+ Helios+/+ or CD45.2+ Helios−/− mice followed by immunization with OT-II peptide (10 μg) in CFA. After 5 days spleen cells from Rag2−/− hosts were analyzed for FoxP3 expression and effector cytokine expression by CD4 Treg. (n=4). Representative data from three independent experiments. Data are mean ± SEM (error bars). *P < 0.05, **P < 0.01 and ***P < 0.001 (Mann-Whitney test).
Examination of STAT5-dependent IL-2 responsiveness revealed decreased STAT5 activation in FoxP3+ CD4 cells from Helios−/− and Heliosfl/fl.FoxP3-Cre mice compared to Helios+/+ mice (Fig. 4C). Enforced expression of constitutively-active STAT5 (STAT5-CA) (13) in Helios-deficient CD4 Treg restored FoxP3 expression to levels similar to WT CD4 Treg and prevented expression of interferon-γ, an effector cytokine (Fig. 4D; Fig. S16).
Decreased FoxP3 expression may result in phenotypic instability including derepression of effector T cell programs (10, 11). Analysis of the CD4 Treg phenotype after immunization of mice with sheep red blood cells revealed that Helios-deficient (Heliosfl/fl.FoxP3-Cre) but not Helios sufficient (Heliosfl/fl) CD4 Treg express effector cytokines, including IFNγ and IL-17. (Fig. 4E). Acquisition of an effector T cell phenotype under inflammatory conditions by Helios-deficient CD4 Treg was a cell intrinsic phenotype. Rag2−/− hosts were injected with CD4 T cells that transgenically express the OT-II T cell receptor (Helios WT), Helios+/+ (CD45.1+) and Helios−/− (CD45.2+) Treg and immunized with OT-II peptide. Helios-deficient but not Helios sufficient CD4 Treg displayed reduced FoxP3 expression and produced effector cytokines, including IFNγ, IL-17 and tumor necrosis factor (TNF)α (Fig. 4F). Decreased FoxP3 expression by Helios-deficient CD4 Treg during colitis progression was also accompanied by expression of effector cytokines, including IFNγ and IL-17 and the FR4loCD73lo phenotype (Fig. S17).
Defective suppressive activity by Helios-deficient CD8 Treg was associated with a similar phenotypic defect under inflammatory conditions. In adoptive Rag2−/−Prf1−/− hosts Helios-deficient CD8 Treg exhibited increased apoptosis and reduced cell recovery compared to Helios WT CD8 Treg, as observed for Helios-deficient FoxP3+ CD4 Treg (Fig. S18A).
In CD8 T-cells, cytokines IL-2 and IL-15 induce STAT5 activation, while sustained activation depends on IL-2 (14, 15). Helios-deficient CD8 Treg displayed reduced IL-2 responsiveness and diminished STAT5 activation (Figs. S18B), suggesting that Helios may serve an overlapping function in both regulatory cell types through promotion of STAT5-dependent IL-2 responsiveness and increased survival under inflammatory conditions. Indeed, in vitro stimulation of Ly49+ CD8 Treg vs. Ly49− CD8 Tcon in the presence of STAT5 inhibitor (AG490) revealed impaired survival of CD8 Treg but not conventional memory CD8 cells (Fig. S18C).
Analysis of CD8 Treg responses under inflammatory conditions revealed that Helios−/− CD8 Treg expressed high levels of PD-1, TIM-3 and Lag3 and low levels of CD127 (Fig. S18D, S19A). Transfer of Helios+/+ and Helios−/− CD8 Treg (>99% Ly49+ from 2 mo old mice) into Rag2−/− hosts along with OT-II cells and antigen also revealed that Helios−/− Ly49+ CD8 cells expressed high levels of PD-1 and TIM3 (Fig. S19B) and reduced survival (Fig. S19C), These findings suggest a Helios-dependent genetic program that enhances STAT5-dependent IL-2 responsiveness of Treg may promote survival and maintenance of an inhibitory CD8 Treg phenotype.
Earlier studies of Helios and FoxP3+ CD4 Treg suggesting no impact on Treg (4, 5) relied mainly on analysis in the steady state of non-immune mice. Although young Helios−/− mice do not develop signs of disease, they develop a dysregulated immune response at ~5 mo of age that is markedly accelerated by immune stimulation, including viral infection. Although Helios belongs to a set of TFs that regulate FoxP3+ Treg (16–18), Helios does not form protein complexes with FoxP3 (19) nor bind to the Foxp3 locus (Fig. S20). Downregulation of FoxP3 expression and expression of effector cytokines by Helios-deficient Treg may reflect reduced activation of the IL-2Rα–STAT5 pathway and diminished binding of STAT5b to CNS-2, resulting in Treg conversion into effector cells (Fig. S21)(10). The ability of Treg to sense IL-2 may also be particularly critical under inflammatory conditions, where small changes in proliferation rate or apoptosis can exert major changes in the niche-filling response by Treg and Treg insufficiency (20). Current views that thymic-derived Treg are phenotypically and functionally more stable than induced Treg may reflect, in part, Helios-dependent activation of the Treg IL-2Rα–STAT5 pathway, described here. Recently-developed surrogate surface markers may allow isolation of stable Helios+ CD4 Treg for autoimmune disease or after organ transplantation (21, 22).
Helios-dependent maintenance of CD8 Treg integrity also includes inhibition of terminal differentiation and maintenance of suppressive/cytolytic activity by activating the STAT5 signaling pathway (Fig. S21). Although Helios-dependent regulation of overlapping genetic pathways, including cell survival, may stabilize the suppressive phenotype of both FoxP3+ CD4 Treg and CD8+ Treg, much of the Helios+ phenotype may reflect subset-specific disparities in lineage commitment, development and distinct mechanism of suppression.
Identification of bona fide signaling pathways that induce and maintain Helios expression in FoxP3+ CD4 Treg may allow Helios inhibition and conversion of memory Treg into T effector cells that express self-reactive TCR with specificity for tumor antigens (23). Since Treg→Teff conversion may be confined to inflammatory intratumoral microenvironments, antibody- or small molecule-based approaches that target Helios may lead to improved Treg-dependent cancer immunotherapy.
Supplementary Material
Acknowledgments
We thank A. Thornton and E. Shevach (NIH) for provision of Heliosfl/fl.CD4-Cre mice,; S. Crotty (La Jolla Institute for Allergy and Immunology) for provision of retroviral vector STAT5-CA,; R. Bronson (DF/HCC Rodent Histopathology Core) for histology analysis,; and A. Angel for manuscript and figure preparation. The data reported in this manuscript are tabulated in the main paper and in the supplementary materials. Raw data are archived in the Gene Expression Omnibus under accession numbers GSE72997 (CHiP-Seq) and GSE73015 (microarray). These studies were supported in part by research grants NIH R01AI37562 and the LeRoy Schecter Research Foundation to H.C. and the Arthritis National Research Foundation to H.-J.K. A provisional patent (U.S. patent application 62/170,379) has been filed pertaining to biological applications relating to the conversion of regulatory T cells into effector T cells for immunotherapy.
Footnotes
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
Literature cited
- 1.Rubtsov YP, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010 Sep 24;329:1667. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyao T, et al. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012 Feb 24;36:262. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Kim HJ, Verbinnen B, Tang X, Lu L, Cantor H. Inhibition of follicular T helper cells by CD8 + Treg is essential for self tolerance. Nature. 2010;467:328. doi: 10.1038/nature09370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thornton AM, et al. Expression of Helios, an Ikaros transcription factor family member,differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. Journal of Immunology. 2010;184:3433. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai Q, Dierich A, Oulad-Abdelghani M, Chan S, Kastner P. Helios deficiency has minimal impact on T cell development and function. Journal of Immunology. 2009;183:2303. doi: 10.4049/jimmunol.0901407. [DOI] [PubMed] [Google Scholar]
- 6.Kim HJ, Cantor H. Regulation of self tolerance by Qa-1-restricted CD8+ regulatory T cells. Semin Immunol. 2011;23:446. doi: 10.1016/j.smim.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurachi M, et al. The transcription factor BATF operates as an essential differentiation checkpoint in early effector CD8+ T cells. Nat Immunol. 2014 Apr;15:373. doi: 10.1038/ni.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernst J, et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature. 2011 May 5;473:43. doi: 10.1038/nature09906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng Y, et al. Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell. 2014 Aug 14;158:749. doi: 10.1016/j.cell.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Liang Y, LeBlanc M, Benner C, Zheng Y. Function of a Foxp3 cis-element in protecting regulatory T cell identity. Cell. 2014 Aug 14;158:734. doi: 10.1016/j.cell.2014.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez RJ, et al. Arthritogenic self-reactive CD4+ T cells acquire an FR4hiCD73hi anergic state in the presence of Foxp3+ regulatory T cells. J Immunol. 2012 Jan 1;188:170. doi: 10.4049/jimmunol.1101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. J Exp Med. 2012 Feb 13;209:243. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tripathi P, et al. STAT5 is critical to maintain effector CD8+ T cell responses. J Immunol. 2010 Aug 15;185:2116. doi: 10.4049/jimmunol.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castro I, Yu A, Dee MJ, Malek TR. The basis of distinctive IL-2- and IL-15-dependent signaling: weak CD122-dependent signaling favors CD8+ T central-memory cell survival but not T effector-memory cell development. J Immunol. 2011 Nov 15;187:5170. doi: 10.4049/jimmunol.1003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol. 2009 Jul;10:689. doi: 10.1038/ni.1760. [DOI] [PubMed] [Google Scholar]
- 17.Ohkura N, et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012 Nov 16;37:785. doi: 10.1016/j.immuni.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Hill JA, et al. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27:786. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Rudra D, et al. Transcription factor Foxp3 and its protein partners form a complex regulatory network. Nat Immunol. 2012 Oct;13:1010. doi: 10.1038/ni.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierson W, et al. Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3(+) regulatory T cells. Nat Immunol. 2013 Sep;14:959. doi: 10.1038/ni.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golding A, Hasni S, Illei G, Shevach EM. The percentage of FoxP3+Helios+ Treg cells correlates positively with disease activity in systemic lupus erythematosus. Arthritis Rheum. 2013 Nov;65:2898. doi: 10.1002/art.38119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bin Dhuban K, et al. Coexpression of TIGIT and FCRL3 Identifies Helios+ Human Memory Regulatory T Cells. J Immunol. 2015 Mar 11; doi: 10.4049/jimmunol.1401803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malchow S, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013 Mar 8;339:1219. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.