Abstract
Background
Encephalitozoon cuniculi, a microsporidial species most commonly recognized as a cause of renal, respiratory, and central nervous system infections in immunosuppressed patients, was identified as the cause of a temporally associated cluster of febrile illness among 3 solid organ transplant recipients from a common donor.
Objective
To confirm the source of the illness, assess donor and recipient risk factors, and provide therapy recommendations for ill recipients.
Design
Public health investigation.
Setting
Two transplant hospitals and community interview with the deceased donor’s family.
Patients
Three transplant recipients and the organ donor.
Measurements
Specimens were tested for microsporidia by using culture, immunofluorescent antibody, polymerase chain reaction, immunohistochemistry, and electron microscopy. Donor medical records were reviewed and a questionnaire was developed to assess for microsporidial infection.
Results
Kidneys and lungs were procured from the deceased donor and transplanted to 3 recipients who became ill with fever 7 to 10 weeks after the transplant. Results of urine culture, serologic, and polymerase chain reaction testing were positive for Encephalitozoon cuniculi of genotype III in each recipient; the organism was also identified in biopsy or autopsy specimens in all recipients. The donor had positive serologic test results for Encephalitozoon cuniculi. Surviving recipients received albendazole. Donor assessment did not identify factors for suspected Encephalitozoon cuniculi infection.
Limitation
Inability to detect organism by culture or polymerase chain reaction in donor due to lack of autopsy specimens.
Conclusion
Transmission of microsporidiosis through organ transplantation is described. Microsporidiosis is now recognized as an emerging transplant-associated disease and should be considered in febrile transplant recipients when tests for routinely encountered agents are unrevealing. Donor-derived disease is critical to assess when multiple recipients from a common donor are ill.
Primary Funding Source
None.
Microsporidia are spore-forming, obligately intracellular organisms related to fungi. Enterocytozoon bieneusi and 3 Encephalitozoon species (Encephalitozoon cuniculi, Encephalitozoon hellem, and Encephalitozoon intestinalis) are the most common microsporidia identified as pathogens of humans (1, 2). Studies indicate that asymptomatic microsporidial disease occurs in humans, and seroprevalence data suggest that humans may be frequently exposed to these organisms (3–5). Microsporidia cause a spectrum of disease, from self-limiting diarrhea to disseminated, life-threatening infections. Microsporidiosis is recognized predominantly among HIV-infected patients but has more recently been noted in non–HIV-infected individuals as an emerging pathogen (6, 7). Several reports describe microsporidial infections involving organ transplant recipients, but to our knowledge, these infections have not been proved to be transplant-transmitted (8–11).
In February 2012, the Centers for Disease Control and Prevention (CDC) was notified of a cluster of 3 transplant recipients with febrile illness onset 7 to 10 weeks after receiving organs from a common donor. Initial evaluation included serologic, molecular, and culture-based assays against a broad range of bacterial, fungal, and viral pathogens. Concomitant illnesses, such as urinary tract infection and organ rejection, were appropriately treated, yet recipients remained ill. Subsequent diagnostic evaluation of recipients suggested infections with a Brucella species that were presumed to be donor-derived. However, despite directed therapy for brucellosis and continued empirical treatment for other infectious etiologies, the patients did not improve, and assistance was requested from public health authorities in determining a common cause for the illness cluster. Kidney biopsy samples sent to CDC from 1 recipient confirmed the diagnosis as microsporidiosis with Encephalitozoon cuniculi, and a public health investigation was initiated to confirm the diagnosis in the other recipients, assess donor and recipient risk factors, and provide therapy recommendations for ill recipients.
Methods
Histopathology, Immunohistochemistry, and Electron Microscopy
Sections from formalin-fixed, paraffin-embedded tissue specimens were cut at 4 μm and stained using hematoxylin and eosin, Lillie-Twort and Grocott methenamine silver, and Warthin–Starry techniques. We examined tissues for evidence of infection with a Brucella or Encephalitozoon species by using an immunoalkaline phosphatase staining method. The primary antibodies included a monoclonal anti–Brucella abortus antibody diluted at 1/1000; an anti–Brucella melitensis antibody diluted at 1/200 (12); and a rabbit hyperimmune anti–Encephalitozoon cuniculi antiserum, diluted at 1/1000, that reacts with Encephalitozoon cuniculi, Encephalitozoon hellem, and Encephalitozoon intestinalis (13). For examination by electron microscopy, formalin-fixed tissues were transferred to buffered 2.5% glutaraldehyde and 1% osmium tetroxide, embedded in a mixture of Epon substitute and Araldite, and stained with uranyl acetate and lead citrate.
Culture and Serum Testing
Encephalitozoon cuniculi was cultured in human lung fibroblast cells. Inocula consisted of urine sediment washed by centrifugation in Hanks balanced salt solution or renal tissue triturated in Modified Eagle Medium; cultures were incubated at 37.8 °C. Serum samples were tested for antibodies to Encephalitozoon cuniculi by an indirect immunofluorescent antibody (IFA) assay using spores of the organism; total antibody titers greater than 1:16 were considered positive in the setting of immunodeficiency (14). We tested serum samples by using a Brucella microagglutination test with minor modifications (15). A titer of 160 or greater was considered positive, 20 to 80 was considered borderline, and less than 20 was considered negative.
Molecular Techniques
We extracted DNA from clinical samples by using a modified version of the FastDNA method (MP Biomedicals, Solon, Ohio), with further purification with the QIAquick polymerase chain reaction (PCR) purification kit (QIAGEN, Valencia, California) (16). Polymerase chain reactions were performed in 50-μL total volumes by using species-specific diagnostic primers based on the small subunit ribosomal RNA gene of Enterocytozoon bieneusi, Encephalitozoon cuniculi, Encephalitozoon intestinalis, and Encephalitozoon hellem (17–20). We performed amplification with the diagnostic primers by using AmpliTaq PCR gold mix (PerkinElmer, Foster City, California); 5 μmol of each primer; and annealing temperatures of 55 °C for Encephalitozoon hellem PCR and 65 °C for Enterocytozoon bieneusi, Encephalitozoon cuniculi, and Encephalitozoon intestinalis. Genotyping of Encephalitozoon cuniculi was determined by analysis of the GTTT repeats in the ITS region (21). Brucella DNA was detected by real-time PCR using TaqMan probes targeting the IS711 gene (22). We considered a sample to be positive if the fluorescent growth curves crossed the threshold line within 45 cycles.
Epidemiologic Investigation
We reviewed medical records of the donor and organ recipients to construct a timeline of events and descriptions of clinical illness. A donor questionnaire was developed to assess potential risk factors or evidence of infection with microsporidia. The questionnaire focused on illnesses or exposures that would be consistent with microsporidial infection and included medical history, review of symptoms, recent gastrointestinal illness in the donor or family members, and exposures to pets or other animals.
Role of the Funding Source
This study received no funding.
Results
Organ Donor
The organ donor was a woman in her thirties who had moved to the United States from Mexico within the year before her death. In the autumn of 2011, she became unresponsive and was diagnosed with a subarachnoid hemorrhage. She progressed to brain death on the day of hospitalization. No illness was reported in the weeks preceding her death, and her medical history was unremarkable. The patient donated both kidneys and lungs, which were transplanted into 3 recipients; no corneas or other tissues were procured. Stored donor serum was tested with serologic and molecular assays, and the results showed elevated Encephalitozoon cuniculi titers (Table) and were negative for infection with a Brucella species. No autopsy was performed; however, a liver biopsy specimen obtained during organ procurement showed no immunohistochemical evidence of infection with a Brucella species or Encephalitozoon cuniculi.
Table.
Laboratory Testing of Clinical Samples for Encephalitozoon cuniculi
| Patient | Serum Titer* (Total Antibody) | Urine Culture Result | Urine PCR Result | Genotype |
|---|---|---|---|---|
| Lung recipient | 1:128 to 1:256 | Positive | Positive | III |
| Right kidney recipient | 1:32 to 1:128 | Positive | Positive | III |
| Left kidney recipient | 1:64 | Positive | Positive | III |
| Organ donor† | 1:4096 | NA | NA | NA |
NA = not available for testing; PCR = polymerase chain reaction.
>1:16 considered positive.
Stored splenocytes and lymphocytes were tested with PCR, but Encephalitozoon cuniculi was not detected
Left Kidney Recipient
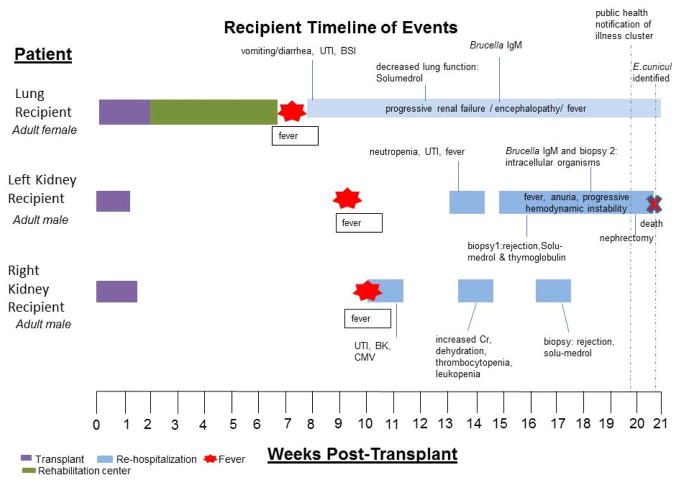
The recipient was a man with a history of polycystic kidney disease and hypertension who did well immediately after the transplant. He received induction therapy with basiliximab and solumedrol and maintenance therapy with tacrolimus, mycophenolic acid, and prednisone. He developed fever, myalgia, and fatigue approximately 9 weeks after the transplant (Figure 1). He was treated for urinary tract infection, neutropenia, and organ rejection over several hospitalizations; results of all initial tests for infectious diseases were negative. His first kidney biopsy specimen showed rejection, which was treated with corticosteroids and thymoglobulin, and his other immunosuppressive agents were stopped. Because of a presumptive diagnosis of transplant-acquired brucellosis in the lung recipient, serum from the left kidney recipient was tested at a commercial laboratory and showed Brucella IgM antibodies, for which he received doxycycline and ceftriaxone. A second kidney biopsy specimen was obtained, and abundant intracellular organisms in renal tubular cells were interpreted as brucellae at a commercial laboratory by immunohistochemistry. The patient’s graft became tender and enlarged, and he underwent a nephrectomy 20 weeks after the transplant (Figure 2, A). Despite broad-spectrum antimicrobial coverage, his illness progressed to anuria, persistent fever, and hemodynamic instability and he died approximately 21 weeks after the transplant. Examination of the kidney biopsy and allograft nephrectomy specimen at CDC revealed histopathology consistent with microsporidiosis (Figure 2, B). Results of immunohistochemistry for Brucella in allograft nephrectomy specimens were negative. Immunohistochemistry and electron microscopy showed infection with an Encephalitozoon species in multiple tissues (Figure 2, C and D). Autopsy specimens showed disseminated infection with Encephalitozoon cuniculi (Figure 2, E and F). Encephalitozoon cuniculi was detected in a urine specimen by culture, IFA, and PCR and was identified as genotype III (Table and Figure 3). Serum was confirmed to be negative for Brucella by antibody and PCR testing but positive for Encephalitozoon cuniculi by antibody testing at CDC (Table).
Figure 1.
Timeline of Events for Recipients
Initial posttransplant recovery was unremarkable for all recipients until fever onset 7 to 10 weeks after transplant. BK = BK polyomavirus viremia; BSI = bloodstream infection; CMV = cytomegalovirus infection; UTI = urinary tract infection.
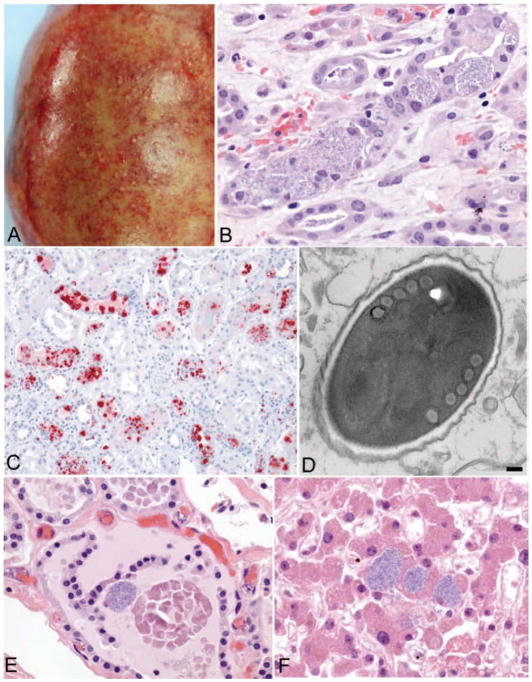
Figure 2.
Gross and microscopic pathology of tissues from the left kidney recipient.
A: Explanted kidney showing multiple microabscesses and hemorrhages of the renal capsule. B: Intracellular masses of Encephalitozoon cuniculi within renal tubular epithelium in the explanted kidney (original magnification × 100). C: Immunohistochemical staining of Encephalitozoon cuniculi (red) in the explanted kidney (original magnification × 25). D: Electron micrograph of a spore of Encephalitozoon cuniculi, surrounded by a dense exospore and electron-lucent endospore and containing 5 cross-sections through the coils of the polar filament, as is typical for this microsporidian (bar, 100 nm). E and F: Evidence of disseminated microsporidiosis involving thyroid gland (E) and liver (F) (original magnification × 100). Encephalitozoon cuniculi organisms were detected in several other tissues, including the central nervous system, heart, trachea, lung, pancreas, adrenal gland, gallbladder, prostate gland, stomach, colon, and vascular smooth muscle.
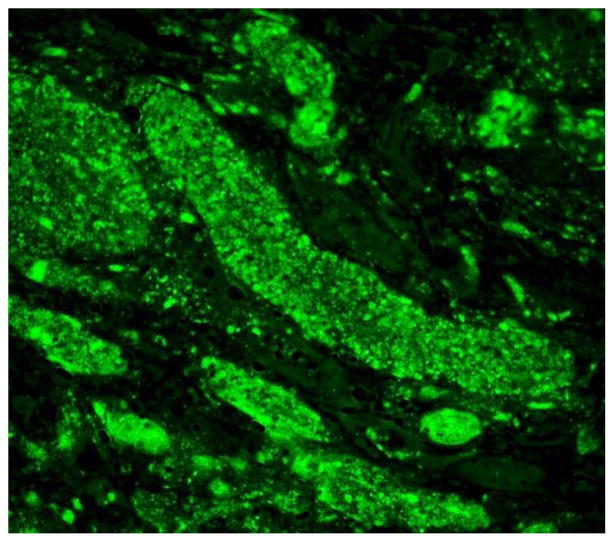
Figure 3.
Encephalitozoon cuniculi immunofluorescent antibody in kidney section.
The spores were birefringent and variably Gram-positive. Stained smears of all initial urine specimens from all recipients revealed oval spores characteristic of microsporidia. The spores fluoresced brightly when reacted with a 1:400 dilution of the rabbit antiserum made against Encephalitozoon cuniculi.
Bilateral Lung Recipient
The recipient was a woman who had a bilateral lung transplant. She received induction therapy with basiliximab and maintenance therapy with tacrolimus, azathioprine, and prednisone. Seven weeks after the transplant, she developed fever, nausea, vomiting, and diarrhea and became encephalopathic with confusion (Figure 1). She was treated for a urinary tract infection and a catheter-associated bloodstream infection during her illness, but her fever continued. She received solumedrol for a decrease in lung function 12 weeks after the transplant. Secondary to continued neurologic decline and liver dysfunction, her therapy was changed to cyclosporine and mycophenolate mofetil at decreasing doses. Because of potential consumption of unpasteurized cheese from Mexico, Brucella testing was performed on serum and cerebrospinal fluid at a commercial laboratory and revealed IgM antibodies reactive with Brucella. Despite broad-spectrum coverage, including doxycycline and streptomycin for presumed brucellosis, she continued to have low-grade fever and encephalopathy and developed kidney failure. Results of pathologic evaluation of tissue specimens from the left kidney recipient prompted testing for microsporidia. Encephalitozoon cuniculi was subsequently detected in a urine specimen by culture, IFA, and PCR and was identified as genotype III (Table and Figure 3). It was also detected in a stored colon biopsy specimen collected approximately 13 weeks after the transplant. Serum samples were found to be positive for Brucella by microagglutination test and PCR at CDC. The patient also had positive serologic test results for Encephalitozoon cuniculi (Table). She began receiving albendazole, 400 mg twice daily, and her immunosuppression was further decreased; her encephalopathy and kidney function slowly improved. During month 4 of therapy, her medication was changed to nitazoxanide because of bone marrow suppression believed to be induced by albendazole. Her urine samples remained positive by PCR for Encephalitozoon cuniculi until month 5 of therapy. Brucella therapy was stopped at month 6 because of continued neutropenia. She developed a central nervous system posttransplant lymphoproliferative disorder and was treated with radiation and rituximab. She continued to receive nitazoxanide in a rehabilitation facility until her death 20 months after the transplant, secondary to the posttransplant lymphoproliferative disorder.
Right Kidney Recipient
The recipient was a man with diabetes and hypertension. He received induction therapy with basiliximab and solumedrol and maintenance therapy with tacrolimus, mycophenolic acid, and prednisone. Approximately 10 weeks after the transplant, he presented with fever, fatigue, myalgia, tremors, and joint pain. He was treated for cytomegalovirus, which was complicated by ganciclovir-associated thrombocytopenia and leukopenia, urinary tract infection, and biopsy-diagnosed organ rejection over several hospitalizations, during which he was treated with solumedrol; he was also noted to have BK polyomavirus viremia, for which his mycophenolic acid was stopped (Figure 1). Brucella testing was pursued on the basis of positive results in the lung recipient, but no serum IgG or IgM antibodies were detected. The right kidney recipient’s fatigue and weakness persisted. Urine specimens were evaluated after microsporidiosis was confirmed in the left kidney recipient, and infection with Encephalitozoon cuniculi of genotype III was confirmed by culture, IFA, and PCR (Table and Figure 3). A biopsy specimen of the transplanted kidney revealed immunohistochemical evidence of infection with Encephalitozoon cuniculi; serum was negative for Brucella by Brucella microagglutination test and PCR at CDC but positive for Encephalitozoon cuniculi by serologic testing (Table). The patient began receiving albendazole (400 mg orally twice daily) and gradually improved. He discontinued therapy after an initial 2-week course of albendazole but resumed 5 days later after presenting with fever, nausea, vomiting, and diarrhea; a urine specimen was positive for Encephalitozoon cuniculi by PCR. Results of his urine PCR were negative after 2 months of therapy. Albendazole was stopped 1 year after the transplant after a total of 6 months of therapy, and the patient remains healthy.
Epidemiologic Evaluation
Assessment with a questionnaire given to the donor’s husband did not reveal donor illnesses, gastrointestinal illness in other household members, or other concerning exposures consistent with microsporidial infection (for example, pets, wild animals, or farm animals).
Discussion
To our knowledge, we report the first recognized cluster of transplant-transmitted microsporidiosis linked to a common organ donor. The presence of the same Encephalitozoon cuniculi genotype among all 3 recipients coupled with the extremely elevated Encephalitozoon cuniculi titer in the organ donor confirms that this infection was transplant-transmitted. Increasing reports of microsporidiosis as an infectious complication after transplantation, now coupled with recognition of donor-derived disease, should heighten clinician awareness of this infection among transplant recipients. The search for a cause of this illness cluster, culminating in the diagnosis of microsporidiosis among these organ recipients, was complicated by initial presumption of brucellosis due to positive Brucella test results in 2 recipients at commercial laboratories. The lung recipient was confirmed to be positive by serologic and PCR testing at CDC laboratories; this may represent a reactivation of disease or detection of a distant infection because positive results of Brucella PCR and antibody testing have been reported up to 2 years after infection (23, 24). The left kidney recipient’s Brucella IgM result was not confirmed and is considered to be falsely positive; Brucella serologic testing requires confirmation and has been described as prone to yielding false-positive results (25).
Past reports of microsporidial infections primarily describe disease in HIV-infected individuals. Microsporidiosis has been reported in transplant recipients of solid organs, corneas, or bone marrow (8–11, 26, 27). However, the organ donor was not investigated as a potential source of infection in these reports. The symptoms of microsporidial infection vary on the basis of species, site of infection, and the immune status of the infected host (28). Enterocytozoon bieneusi is most commonly associated with persistent diarrhea, whereas Encephalitozoon species, including Encephalitozoon cuniculi, have been associated with diverse clinical syndromes, such as encephalitis, interstitial nephritis, hepatitis, tracheobronchitis, keratoconjunctivitis, myositis, and sinusitis (29). Asymptomatic disease has been reported in both immunocompetent and immunodeficient hosts (5, 30, 31). Studies have described latent disease among otherwise healthy individuals with intermittent spore shedding from stool and urine specimens; detection of microsporidial DNA in urine suggests that the organism is able to disseminate despite the protective effects of an intact immune system (5).
Seroprevalence studies have shown a frequency of 1.3% to 8% for Encephalitozoon species among blood donors, pregnant women, slaughterhouse workers, and dog breeders (32–34); seroprevalence of Enterocytozoon bieneusi was 10% among healthy blood donors but as high as 33% in animal keepers reporting exposure to excrement (4). These studies suggest that humans are frequently exposed to microsporidia and indicate that exposure to animal excrement could be a risk factor (4). The wide array of symptoms, the possibility of asymptomatic carriage, and a relatively high seroprevalence in several populations suggest that an unrecognized infection occurred in the organ donor; this is further supported by her elevated anti–Encephalitozoon cuniculi titers. Few studies have evaluated risk factors for microsporidial infections, but infectious sources are believed to include other infected humans, animals, water, and insect vectors (35). Foodborne outbreaks have also recently been reported (36). Exposures to microsporidia are probably common among humans given that the organism is ubiquitous (35, 37), but re-interview of the donor’s family did not reveal an obvious source of her infection or an illness potentially compatible with microsporidiosis.
Microsporidiosis should be considered in the differential diagnosis when an infectious cause is suspected, evaluation for common pathogens is unrevealing, and response to standard therapies is poor. Furthermore, transplant-transmitted disease should be considered in recipients when the illness is common to other patients receiving organs from the same donor. The diagnosis of microsporidiosis can be difficult because it requires a high index of suspicion, ascertainment of appropriate samples, and specialized testing. The diagnosis depends on identifying spores in clinical samples that are obtained on the basis of patient symptoms but may include stool, urine, sputum, bronchoalveolar lavage fluid, and cerebrospinal fluid (28). Because the kidney is a site of disseminated infection and urine is easily obtainable, evaluation of urine for spores may be appropriate, especially in the transplant recipient (1, 11). Intermittent shedding has been described; therefore, multiple specimens of urine and stool collected over several months may be necessary to adequately evaluate patients for active infection (28). The diagnosis of microsporidiosis from urine or stool requires identification of spores in samples by using specialized methods that include Ritchie concentration, Weber trichrome stain, and fluorochrome stain, which are generally accessible at most medical facilities (28, 38, 39). In tissue, microsporidia stain variably with hematoxylin and eosin; however, Gram stain and Warthin–Starry techniques considerably enhance the visualization of these organisms. A species-specific identification typically requires electron microscopy, molecular assays, or indirect immunofluorescence or immunohistochemical stains that may only be available at reference laboratories. Clinicians can request this testing from CDC through their state and local health departments. Species-level identification is generally needed to appropriately tailor therapy (28, 38–40). Because no autopsy was performed on the donor, it is unknown whether microsporidia could be detected in any donor tissue before transplantation; however, complete autopsies that include microscopic examination should be pursued routinely for organ donors, particularly in otherwise healthy individuals who die unexpectedly. Unexpected findings on autopsy specimens should be communicated immediately to the organ procurement organization (OPO) and public health officials. In some cases, an autopsy can identify an unsuspected or subclinical infection in the donor before its manifestation in recipients, thus providing the opportunity to initiate prophylaxis or targeted therapy to recipients.
Therapy for microsporidiosis is species-dependent, and finding a tolerable option can be challenging in a transplant recipient. Encephalitozoon species, including Encephalitozoon cuniculi, are treatable with albendazole, but this agent is not effective against Enterocytozoon bieneusi (28, 40, 41). For severely ill patients, which transplant recipients often are, oral medications may be unsuitable because of poor gastrointestinal function. In such cases, physicians may consider Fumagillin, which is efficacious against Encephalitozoon species and Enterocytozoon bieneusi (28, 40, 41). Fumagillin is not approved for use in humans in the United States but is available commercially for veterinary purposes; however, a compassionate use application would need to be filed through the U.S. Food and Drug Administration. Alternatively, centers can request an emergency investigational new drug application from the U.S. Food and Drug Administration to obtain the drug from the manufacturer. The adverse effects associated with both drugs, including gastrointestinal upset and variable degrees of bone marrow suppression, further complicate management of the condition.
The appropriate duration of therapy required to eradicate infection with Encephalitozoon cuniculi in transplant recipients is unknown. There have been reports of relapse as long as 1 year after cessation of therapy and positive urine PCR results after more than 5 months of therapy. In this cluster, we chose to monitor the recipients by using PCR testing on urine specimens. The surviving kidney recipient had no detectable organisms in urine specimens within 2 months of starting therapy, whereas the lung recipient remained positive until completing 5 months of therapy. Variable degrees of immunosuppression among transplant recipients may also be considered as a reason for differences in response; immune reconstitution in HIV-positive individuals has proved to be an effective treatment method but may not be feasible in transplant recipients (39).
The frequency of transplant-transmitted microsporidiosis is unknown. Investigation of microsporidia transmission from the donor in previously reported transplant patients with microsporidiosis may not have been done, especially if other recipients from a common donor were not known to be ill. Recognition of donor-derived illness clusters can only occur when notification is given to the OPO and public health authorities. Organ Procurement and Transplantation Network (OPTN) policy requires reporting of potential donor-derived disease by both OPOs and transplant centers. Transplant centers should notify the OPO of any illness in a recipient believed to be transplant-transmitted so that an assessment of the health status of other recipients can take place; the OPO and transplant center should also notify OPTN as required. Should it be discovered that other organ recipients are ill or if the pathogen is a nationally notifiable disease, public health authorities should be notified to aid in the investigation. Any illness of unknown cause or poorly responsive disease found to be present in multiple organ recipients should be immediately reported to the OPO, OPTN, and public health authorities upon discovery by treating physicians. This process is particularly crucial in circumstances such as the cluster described in this report, when an unusual, unsuspected, or previously unknown transplant-associated pathogen is involved. Rapid diagnosis and appropriate therapy may improve outcomes in recipients with transplant-transmitted pathogens and may also reduce the risk for further transmission.
Sidebar.
Context
Microsporidia, such as Encephalitozoon cuniculi, are ubiquitous organisms related to fungi and may be acquired by animal contact. They can cause protean systemic disease that is especially severe in immunocompromised hosts.
Contribution
Investigation of a temporal cluster of severe febrile illness in 3 solid organ transplant recipients revealed infection with Encephalitozoon cuniculi of genotype III. Two patients died. The donor had serologic evidence of infection with the same organism.
Caution
Donor tissue was not available for additional testing. No risk factors for acquisition were found.
Implication
Microsporidiosis should be considered in febrile transplant patients when commonly encountered infections are not found or when response to standard therapies is poor, and, donor-derived disease should be considered when multiple recipients who received organs from the same donor are ill.
—The Editors
Acknowledgments
The authors thank all members of the clinical care teams involved in treatment of the patients, the organ procurement organization staff, and the organ donor’s family.
Appendix: Microsporidia Transplant Transmission Investigation Team
The members of the Microsporidia Transplant Transmission Investigation Team are Neil Pascoe RN, BSN, CIC and Tom Sidwa DVM, MPH, Texas Department of State Health Services, Austin, Texas; Mam Ibraheem MD, MPH, CPH, New Mexico Department of Health, Santa Fe, New Mexico, Epidemic Intelligence Service, Centers for Disease Control and Prevention, Atlanta, GA; Joe Vidales RN, BSN, Las Palmas Medical Center, El Paso, Texas; and Barun Kumar De PhD, Alex R. Hoffmaster PhD, William A. Bower MD, Marta A. Guerra DVM, MPH, PhD, and Meredith G. Morrow MSPH, Centers for Disease Control and Prevention, Atlanta, Georgia.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential Conflicts of Interest: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M13-2226.
Reproducible Research Statement: Study protocol, statistical code, and data set: Not available.
Author Contributions: Conception and design: S.N. Hocevar, C.D. Paddock, M.J. Kuehnert.
Analysis and interpretation of the data: S.N. Hocevar, C.D. Paddock, R. Rosenblatt, S. Antony, R.A. Stoddard, D.M. Blau, M. de Almeida, M.J. Kuehnert.
Drafting of the article: S.N. Hocevar, C.D. Paddock, R. Rosenblatt, S. Antony, M.J. Kuehnert.
Critical revision of the article for important intellectual content: S.N. Hocevar, C.D. Paddock, C.W. Spak, S. Antony, R.A. Stoddard, D.M. Blau, M. de Almeida, M.J. Kuehnert.
Final approval of the article: S.N. Hocevar, C.D. Paddock, C.W. Spak, R. Rosenblatt, G.C. Friedman, S. Antony, R.A. Stoddard, M.J. Kuehnert.
Provision of study materials or patients: C.W. Spak, R. Rosenblatt, T. Peterson.
Administrative, technical, or logistic support: S.N. Hocevar, C.D. Paddock, T. Benedict.
Collection and assembly of data: S.N. Hocevar, C.D. Paddock, C.W. Spak, R. Rosenblatt, I. Castillo, S. Luna, G.C. Friedman, R.A. Stoddard, T. Peterson, D.M. Blau, M. de Almeida, C.S. Goldsmith, M.J. Kuehnert.
References
- 1.Didier ES, Weiss LM. Microsporidiosis: current status. Curr Opin Infect Dis. 2006;19:485–92. doi: 10.1097/01.qco.0000244055.46382.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Didier ES, Didier PJ, Snowden KF, Shadduck JA. Microsporidiosis in mammals. Microbes Infect. 2000;2:709–20. doi: 10.1016/s1286-4579(00)00354-3. [DOI] [PubMed] [Google Scholar]
- 3.Sak B, Brady D, Pelikánová M, Kvetonová D, Rost M, Kostka M, et al. Unapparent microsporidial infection among immunocompetent humans in the Czech Republic. J Clin Microbiol. 2011;49:1064–70. doi: 10.1128/JCM.01147-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sak B, Kucerova Z, Kvac M, Kvetonova D, Rost M, Secor EW. Seropositivity for Enterocytozoon bieneusi, Czech Republic. Emerg Infect Dis. 2010;16:335–7. doi: 10.3201/eid1602.090964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sak B, Kvác M, Kucerová Z, Kvetonová D, Saková K. Latent microsporidial infection in immunocompetent individuals - a longitudinal study. PLoS Negl Trop Dis. 2011;5:e1162. doi: 10.1371/journal.pntd.0001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lobo ML, Xiao L, Antunes F, Matos O. Microsporidia as emerging pathogens and the implication for public health: a 10-year study on HIV-positive and -negative patients. Int J Parasitol. 2012;42:197–205. doi: 10.1016/j.ijpara.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Didier ES, Weiss LM. Microsporidiosis: not just in AIDS patients. Curr Opin Infect Dis. 2011;24:490–5. doi: 10.1097/QCO.0b013e32834aa152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson JR, Li L, Helton CL, Munn RJ, Wasson K, Perez RV, et al. Disseminated microsporidiosis in a pancreas/kidney transplant recipient. Arch Pathol Lab Med. 2004;128:e41–3. doi: 10.5858/2004-128-e41-DMIAKT. [DOI] [PubMed] [Google Scholar]
- 9.Lanternier F, Boutboul D, Menotti J, Chandesris MO, Sarfati C, Mamzer Bruneel MF, et al. Microsporidiosis in solid organ transplant recipients: two Enterocytozoon bieneusi cases and review. Transpl Infect Dis. 2009;11:83–8. doi: 10.1111/j.1399-3062.2008.00347.x. [DOI] [PubMed] [Google Scholar]
- 10.Kakrania R, Joseph J, Vaddavalli PK, Gangopadhyay N, Sharma S. Microsporidia keratoconjunctivitis in a corneal graft [Letter] Eye (Lond) 2006;20:1314–5. doi: 10.1038/sj.eye.6702178. [DOI] [PubMed] [Google Scholar]
- 11.Galván AL, Sánchez AM, Valentín MA, Henriques-Gil N, Izquierdo F, Fenoy S, et al. First cases of microsporidiosis in transplant recipients in Spain and review of the literature. J Clin Microbiol. 2011;49:1301–6. doi: 10.1128/JCM.01833-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrington M, Choe U, Ubillos S, Stanek D, Campbell M, Wansbrough L, et al. Fatal case of brucellosis misdiagnosed in early stages of Brucella suis infection in a 46-year-old patient with Marfan syndrome. J Clin Microbiol. 2012;50:2173–5. doi: 10.1128/JCM.00573-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.del Aguila C, Moura H, Fenoy S, Navajas R, Lopez-Velez R, Li L, et al. In vitro culture, ultrastructure, antigenic, and molecular characterization of Encephalitozoon cuniculi isolated from urine and sputum samples from a Spanish patient with AIDS. J Clin Microbiol. 2001;39:1105–8. doi: 10.1128/JCM.39.3.1105-1108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Croppo GP, Visvesvara GS, Leitch GJ, Wallace S, De Groote MA. Western blot and immunofluorescence analysis of a human isolate of Encephalitozoon cuniculi established in culture from the urine of a patient with AIDS. J Parasitol. 1997;83:66–9. [PubMed] [Google Scholar]
- 15.Brown SL, Klein GC, McKinney FT, Jones WL. Safranin O-stained antigen microagglutination test for detection of Brucella antibodies. J Clin Microbiol. 1981;13:398–400. doi: 10.1128/jcm.13.2.398-400.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.da Silva AJ, Bornay-Llinares FJ, Moura IN, Slemenda SB, Tuttle JL, Pieniazek NJ. Fast and reliable extraction of protozoan parasite DNA from fecal specimens. Mol Diagn. 1999;4:57–64. doi: 10.1016/s1084-8592(99)80050-2. [DOI] [PubMed] [Google Scholar]
- 17.da Silva AJ, Bornay-Llinares FJ, del Aguila de la Puente Cdel A, Moura H, Peralta JM, Sobottka I, et al. Diagnosis of Enterocytozoon bieneusi (microsporidia) infections by polymerase chain reaction in stool samples using primers based on the region coding for small-subunit ribosomal RNA. Arch Pathol Lab Med. 1997;121:874–9. [PubMed] [Google Scholar]
- 18.da Silva AJ, Schwartz DA, Visvesvara GS, de Moura H, Slemenda SB, Pieniazek NJ. Sensitive PCR diagnosis of infections by Enterocytozoon bieneusi (microsporidia) using primers based on the region coding for small-subunit rRNA. J Clin Microbiol. 1996;34:986–7. doi: 10.1128/jcm.34.4.986-987.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Groote MA, Visvesvara G, Wilson ML, Pieniazek NJ, Slemenda SB, daSilva AJ, et al. Polymerase chain reaction and culture confirmation of disseminated Encephalitozoon cuniculi in a patient with AIDS: successful therapy with albendazole. J Infect Dis. 1995;171:1375–8. doi: 10.1093/infdis/171.5.1375. [DOI] [PubMed] [Google Scholar]
- 20.Visvesvara GS, da Silva AJ, Croppo GP, Pieniazek NJ, Leitch GJ, Ferguson D, et al. In vitro culture and serologic and molecular identification of Septata intestinalis isolated from urine of a patient with AIDS. J Clin Microbiol. 1995;33:930–6. doi: 10.1128/jcm.33.4.930-936.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Didier ES, Visvesvara GS, Baker MD, Rogers LB, Bertucci DC, De Groote MA, et al. A microsporidian isolated from an AIDS patient corresponds to Encephalitozoon cuniculi III, originally isolated from domestic dogs. J Clin Microbiol. 1996;34:2835–7. doi: 10.1128/jcm.34.11.2835-2837.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinic V, Brodard I, Thomann A, Holub M, Miserez R, Abril C. IS711-based real-time PCR assay as a tool for detection of Brucella spp. in wild boars and comparison with bacterial isolation and serology. BMC Vet Res. 2009;5:22. doi: 10.1186/1746-6148-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vrioni G, Pappas G, Priavali E, Gartzonika C, Levidiotou S. An eternal microbe: Brucella DNA load persists for years after clinical cure. Clin Infect Dis. 2008;46:e131–6. doi: 10.1086/588482. [DOI] [PubMed] [Google Scholar]
- 24.Buchanan TM, Faber LC. 2-mercaptoethanol Brucella agglutination test: usefulness for predicting recovery from brucellosis. J Clin Microbiol. 1980;11:691–3. doi: 10.1128/jcm.11.6.691-693.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC) Public health consequences of a false-positive laboratory test result for Brucella---Florida, Georgia, and Michigan, 2005. MMWR Morb Mortal Wkly Rep. 2008;57:603–5. [PubMed] [Google Scholar]
- 26.Ambrosioni J, van Delden C, Krause KH, Bouchuiguir-Wafa C, Nagy M, Passweg J, et al. Invasive microsporidiosis in allogeneic haematopoietic SCT recipients [Letter] Bone Marrow Transplant. 2010;45:1249–51. doi: 10.1038/bmt.2009.315. [DOI] [PubMed] [Google Scholar]
- 27.Meissner EG, Bennett JE, Qvarnstrom Y, da Silva A, Chu EY, Tsokos M, et al. Disseminated microsporidiosis in an immunosuppressed patient. Emerg Infect Dis. 2012;18:1155–8. doi: 10.3201/eid1807.120047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anane S, Attouchi H. Microsporidiosis: epidemiology, clinical data and therapy. Gastroenterol Clin Biol. 2010;34:450–64. doi: 10.1016/j.gcb.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Didier ES. Microsporidiosis: an emerging and opportunistic infection in humans and animals. Acta Trop. 2005;94:61–76. doi: 10.1016/j.actatropica.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Scaglia M, Gatti S, Sacchi L, Corona S, Chichino G, Bernuzzi AM, et al. Asymptomatic respiratory tract microsporidiosis due to Encephalitozoon hellem in three patients with AIDS. Clin Infect Dis. 1998;26:174–6. doi: 10.1086/516264. [DOI] [PubMed] [Google Scholar]
- 31.Franzen C, Schwartz DA, Visvesvara GS, Müller A, Schwenk A, Salzberger B, et al. Immunologically confirmed disseminated, asymptomatic Encephalitozoon cuniculi infection of the gastrointestinal tract in a patient with AIDS. Clin Infect Dis. 1995;21:1480–4. doi: 10.1093/clinids/21.6.1480. [DOI] [PubMed] [Google Scholar]
- 32.van Gool T, Vetter JC, Weinmayr B, Van Dam A, Derouin F, Dankert J. High seroprevalence of Encephalitozoon species in immunocompetent subjects. J Infect Dis. 1997;175:1020–4. doi: 10.1086/513963. [DOI] [PubMed] [Google Scholar]
- 33.del Aguila C, Rueda C, De la Camara C, Fenoy S. Seroprevalence of anti-Encephalitozoon antibodies in Spanish immunocompetent subjects. J Eukaryot Microbiol. 2001;(Suppl):75S–78S. doi: 10.1111/j.1550-7408.2001.tb00459.x. [DOI] [PubMed] [Google Scholar]
- 34.Kucerová-Pospísilová Z, Ditrich O. The serological surveillance of several groups of patients using antigens of Encephalitozoon hellem and E. cuniculi antibodies to microsporidia in patients. Folia Parasitol (Praha) 1998;45:108–12. [PubMed] [Google Scholar]
- 35.Didier ES, Stovall ME, Green LC, Brindley PJ, Sestak K, Didier PJ. Epidemiology of microsporidiosis: sources and modes of transmission. Vet Parasitol. 2004;126:145–66. doi: 10.1016/j.vetpar.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Decraene V, Lebbad M, Botero-Kleiven S, Gustavsson AM, Löfdahl M. First reported foodborne outbreak associated with microsporidia, Sweden, October 2009. Epidemiol Infect. 2012;140:519–27. doi: 10.1017/S095026881100077X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Didier ES. Microsporidiosis. Clin Infect Dis. 1998;27:1–7. doi: 10.1086/514607. [DOI] [PubMed] [Google Scholar]
- 38.Garcia LS. Laboratory identification of the microsporidia. J Clin Microbiol. 2002;40:1892–901. doi: 10.1128/JCM.40.6.1892-1901.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mofenson LM, Brady MT, Danner SP, Dominguez KL, Hazra R, Handelsman E, et al. Centers for Disease Control and Prevention. Guidelines for the prevention and treatment of opportunistic infections among HIV-exposed and HIV-infected children: recommendations from CDC, the National Institutes of Health, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. MMWR Recomm Rep. 2009;58(RR-11):1–166. [PMC free article] [PubMed] [Google Scholar]
- 40.Didier ES, Maddry JA, Brindley PJ, Stovall ME, Didier PJ. Therapeutic strategies for human microsporidia infections. Expert Rev Anti Infect Ther. 2005;3:419–34. doi: 10.1586/14787210.3.3.419. [DOI] [PubMed] [Google Scholar]
- 41.Gross U. Treatment of microsporidiosis including albendazole. Parasitol Res. 2003;90(Supp 1):S14–8. doi: 10.1007/s00436-002-0753-x. [DOI] [PubMed] [Google Scholar]