Abstract
BACKGROUND
Cardiac troponin concentrations are used to identify patients who would benefit from urgent revascularization for acute coronary syndromes. We hypothesized that they might be used in patients with stable ischemic heart disease to identify those at high risk for cardiovascular events who might also benefit from prompt coronary revascularization.
METHODS
We measured the cardiac troponin T concentration at baseline with a high-sensitivity assay in 2285 patients who had both type 2 diabetes and stable ischemic heart disease and were enrolled in the Bypass Angioplasty Revascularization Investigation in Type 2 Diabetes trial. We tested for an association between the troponin T concentration and a composite end point of death from cardiovascular causes, myocardial infarction, or stroke; we then evaluated whether random assignment to prompt revascularization reduced the rate of the composite end point in patients with an abnormal troponin T concentration (≥14 ng per liter) as compared with those with a normal troponin T concentration (<14 ng per liter).
RESULTS
Of the 2285 patients, 2277 (99.6%) had detectable (≥3 ng per liter) troponin T concentrations and 897 (39.3%) had abnormal troponin T concentrations at baseline. The 5-year rate of the composite end point was 27.1% among the patients who had had abnormal troponin T concentrations at baseline, as compared with 12.9% among those who had had normal baseline troponin T concentrations. In models that were adjusted for cardiovascular risk factors, severity of diabetes, electrocardiographic abnormalities, and coronary anatomy, the hazard ratio for the composite end point among patients with abnormal troponin T concentrations was 1.85 (95% confidence interval [CI], 1.48 to 2.32; P<0.001). Among patients with abnormal troponin T concentrations, random assignment to prompt revascularization, as compared with medical therapy alone, did not result in a significant reduction in the rate of the composite end point (hazard ratio, 0.96; 95% CI, 0.74 to 1.25).
CONCLUSIONS
The cardiac troponin T concentration was an independent predictor of death from cardiovascular causes, myocardial infarction, or stroke in patients who had both type 2 diabetes and stable ischemic heart disease. An abnormal troponin T value of 14 ng per liter or higher did not identify a subgroup of patients who benefited from random assignment to prompt coronary revascularization. (Funded by the National Institutes of Health and Roche Diagnostics; BARI 2D ClinicalTrials.gov number, NCT00006305.)
Cardiac troponin concentration is the preferred marker of myocardial necrosis.1 Elevated concentrations of cardiac troponin have a strong association with an adverse prognosis in patients with acute coronary syndromes and are used to identify patients who are likely to benefit from an early invasive management strategy.2–4 High-sensitivity assays that allow the measurement of very low cardiac troponin levels in patients with stable heart disease are now available for clinical and research use. These low, previously undetectable troponin concentrations have shown strong associations with myocardial infarction, stroke, and death in a variety of primary and secondary prevention populations, including in patients with stable ischemic heart disease.5–10 We hypothesized that a high-sensitivity cardiac troponin assay might be used to identify a subgroup of patients with both stable ischemic heart disease and diabetes who are at high risk for cardiac events and, furthermore, might predict who would benefit from prompt coronary revascularization. We also hypothesized that coronary revascularization would lower subsequent measurements of circulating troponin concentrations.
To test these hypotheses, we used a high-sensitivity assay to measure cardiac troponin T concentrations at baseline and at the 1-year follow-up in 2368 patients with both type 2 diabetes and stable ischemic heart disease who underwent randomization to either prompt coronary revascularization plus intensive medical therapy or intensive medical therapy alone in the Bypass Angioplasty Revascularization Investigation in Type 2 Diabetes (BARI 2D) trial.
METHODS
STUDY POPULATION AND DESIGN
The inclusion and exclusion criteria for the BARI 2D trial are described in detail elsewhere.11 In brief, starting on January 1, 2001, a total of 2368 patients who had both type 2 diabetes and stable ischemic heart disease with mild or no symptoms of angina were selected as candidates for either percutaneous coronary intervention or coronary-artery bypass grafting and were then randomly assigned to either prompt coronary revascularization plus intensive medical therapy (revascularization group) or intensive medical therapy alone (medical-therapy group). The current study is an ancillary study of the BARI 2D trial. All enrolled BARI 2D participants provided written informed consent.
STUDY OVERSIGHT
This study was approved by the Partners Human Research Committee at Brigham and Women’s Hospital, Boston. The study was designed by the first, second, and last authors. All the authors collected the data. The initial independent data analysis was conducted by the second and third author. Subsequent interpretation and analyses were conducted by all authors. All authors take responsibility for the accuracy and completeness of the analyses. The first author wrote the first draft of the manuscript; no one who is not an author contributed to the writing of the manuscript. All the authors made the decision to submit the manuscript for publication. The high-sensitivity electrochemiluminescence assays used to measure cardiac troponin T concentration were donated by Roche Diagnostics, which had no other role in the study.
STUDY END POINTS
The primary end point of this ancillary study of the BARI 2D trial was a composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke. A core laboratory adjudicated myocardial infarction, and an independent mortality and morbidity committee classified the cause of death. The criteria that were used to adjudicate myocardial infarction and stroke are described elsewhere.11 Secondary end points of this study were the rates of death from any cause, the individual components of the primary composite outcome, heart failure, and the composite of death from any cause, myocardial infarction, stroke, or heart failure. Details of the ascertainment of heart failure are provided in the Methods section in the Supplementary Appendix, available with the full text of this article at NEJM.org.
LABORATORY ANALYSIS
The plasma samples that were obtained at baseline and at the 1-year follow-up were stored at −80°C in the BARI 2D Fibrinolysis Core Laboratory at the University of Vermont. Cardiac troponin T concentration was measured with the use of high-sensitivity electrochemiluminescence assays. According to the manufacturer, the limit of detection of the troponin T assay is 3 ng per liter, and the 99th percentile (upper reference limit) in a healthy population of volunteers is 14 ng per liter. The 10% coefficient of variation is reported to be less than this value. The methods for measuring N-terminal pro–brain natriuretic peptide (NT-proBNP) levels are described in the Methods section in the Supplementary Appendix.
STATISTICAL ANALYSIS
High-sensitivity cardiac troponin T concentrations were categorized as normal (<14 ng per liter) or abnormal (≥14 ng per liter) according to the manufacturer’s established upper reference limit. We compared the baseline characteristics of the group with normal troponin T concentrations with those of the group with abnormal troponin T concentrations using the Wilcoxon rank-sum test for continuous variables and the chi-square test for categorical variables. Kaplan–Meier analysis and log-rank statistics were used for between-group comparisons of the unadjusted rates of each of the study end points. We constructed Cox proportional-hazards models to estimate the adjusted association between troponin T concentration and the risk of the primary composite end point of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke, as well as the risks of the secondary end points of death from any cause, heart failure, and the composite of death from any cause, myocardial infarction, stroke, or heart failure. A two-sided P value of less than 0.05 was considered to indicate statistical significance. The approach that was taken to adjust for possible confounding variables is described in the Methods section in the Supplementary Appendix. We tested for evidence of heterogeneity of the effect of random assignment to prompt revascularization among patients with normal baseline troponin T concentrations and those with abnormal baseline troponin T concentrations in a model that included a multiplicative interaction term along with the main-effect terms for revascularization and bio-marker category. Sensitivity analyses and the methods for assessing change in troponin T concentrations, along with a landmark analysis involving the 1984 participants with baseline and 1-year follow-up troponin T values, are described in the Methods section in the Supplementary Appendix.
RESULTS
PATIENTS
Of the 2368 patients enrolled in the BARI 2D trial, 2314 (97.7%) had baseline plasma samples available, and samples from 2285 of those patients (98.7%) were successfully analyzed for troponin T concentration. Of these 2285 patients, 1984 (86.8%) also had 1-year follow-up samples that were available and successfully analyzed (Fig. S1 in the Supplementary Appendix). The median follow-up time for the 2285 patients with baseline plasma samples that could be analyzed for troponin T concentration was 5 years (interquartile range, 4.1 to 6.0). Of these patients, 2277 (99.6%) had troponin T concentrations above the limit of detection (≥3 ng per liter) and 897 (39.3%) had concentrations in the abnormal range (≥14 ng per liter). Median troponin T concentrations were similar among patients in the revascularization group (11.7 ng per liter; inter-quartile range, 8.0 to 19.9) and those in the medical-therapy group (11.6 ng per liter; inter-quartile range, 7.8 to 19.3) (P = 0.41). The baseline characteristics of the study population are shown in Table 1.
Table 1.
Baseline Characteristics of the Study Population, According to High-Sensitivity Cardiac Troponin T Concentration.*
| Characteristic | Troponin T <14 ng/liter (N = 1388) | Troponin T ≥14 ng/liter (N = 897) | P Value |
|---|---|---|---|
| Median age (IQR) — yr | 61 (55–68) | 64 (58–71) | <0.001 |
| Male sex — no./total no. (%) | 903/1388 (65.1) | 698/897 (77.8) | <0.001 |
| Race or ethnic group — no./total no. (%)† | 0.006 | ||
| White non-Hispanic | 926/1388 (66.7) | 571/897 (63.7) | |
| Black non-Hispanic | 218/1388 (15.7) | 170/897 (19.0) | |
| Hispanic | 164/1388 (11.8) | 127/897 (14.2) | |
| Asian non-Hispanic | 71/1388 (5.1) | 24/897 (2.7) | |
| Other non-Hispanic | 9/1388 (0.6) | 5/897 (0.6) | |
| Median body-mass index (IQR)‡ | 31.0 (27.7–34.7) | 30.9 (27.3–35.0) | 0.60 |
| Median blood pressure (IQR) — mm Hg | |||
| Systolic | 129 (118–141) | 130 (119–145) | 0.07 |
| Diastolic | 74 (68–81) | 73 (67–81) | 0.18 |
| Hypertension requiring treatment — no./total no. (%) | 1107/1371 (80.7) | 757/888 (85.2) | 0.006 |
| Hypercholesterolemia requiring treatment — no./total no. (%) | 1126/1367 (82.4) | 721/889 (81.1) | 0.45 |
| History of cigarette smoking — no./total no. (%) | 919/1388 (66.2) | 608/897 (67.8) | 0.44 |
| History of myocardial infarction — no./total no. (%) | 389/1369 (28.4) | 334/881 (37.9) | <0.001 |
| History of congestive heart failure requiring treatment — no./total no. (%) | 55/1377 (4.0) | 93/893 (10.4) | <0.001 |
| History of stroke or TIA — no./total no. (%) | 127/1388 (9.1) | 94/897 (10.5) | 0.29 |
| Median duration of diabetes mellitus (IQR) — yr | 7.2 (3.3–13.8) | 10.4 (4.9–17.9) | <0.001 |
| Chronic renal dysfunction — no./total no. (%) | 13/1385 (0.9) | 57/887 (6.4) | <0.001 |
| Median glycated hemoglobin (IQR) — % | 7.3 (6.4–8.4) | 7.4 (6.5–8.7) | 0.03 |
| Median lipids (IQR) — mg/dl | |||
| Total cholesterol | 165 (142–190) | 163 (139–194) | 0.68 |
| HDL cholesterol | 37 (32–43) | 36 (31–42) | 0.003 |
| LDL cholesterol | 93 (73–114) | 90 (72–115) | 0.30 |
| Triglycerides | 146.0 (100.0–215.0) | 151.5 (109.0–223.0) | 0.02 |
| Median estimated GFR (IQR) — ml/min/1.73 m2 | 80.3 (66.7–94.9) | 69.6 (55.1–83.3) | <0.001 |
| Median NT-proBNP (IQR) — ng/liter | 93.0 (46.4–185.8) | 280.2 (104.2–719.8) | <0.001 |
| Current medications — no./total no. (%) | |||
| Insulin | 322/1388 (23.2) | 314/897 (35.0) | <0.001 |
| Aspirin | 1234/1381 (89.4) | 771/895 (86.1) | 0.02 |
| Statin | 1036/1385 (74.8) | 666/895 (74.4) | 0.84 |
| Median myocardial jeopardy index score (IQR)§ | 41.0 (24.0–60.0) | 46.0 (28.0–65.0) | 0.001 |
| Number of lesions with ≥50% stenosis — no./total no. (%) | <0.001 | ||
| 0–1 | 470/1388 (33.9) | 216/897 (24.1) | |
| 2–3 | 581/1388 (41.9) | 366/897 (40.8) | |
| >3 | 337/1388 (24.3) | 315/897 (35.1) | |
| Number of vessels with lesions with ≥50% stenosis — no./total no. (%) | <0.001 | ||
| 1 | 574/1386 (41.4) | 284/896 (31.7) | |
| 2 | 491/1386 (35.4) | 326/896 (36.4) | |
| 3 | 321/1386 (23.2) | 286/896 (31.9) | |
| Proximal LAD stenosis of ≥50% — no./total no. (%) | 174/1388 (12.5) | 130/897 (14.5) | 0.18 |
| LVEF <50% — no./total no. (%) | 163/1388 (11.7) | 219/897 (24.4) | <0.001 |
| Any ECG abnormality — no./total no. (%) | 656/1349 (48.6) | 575/869 (66.2) | <0.001 |
| CABG revascularization stratum — no./total no. (%)¶ | 429/1388 (30.9) | 322/897 (35.9) | 0.01 |
| Assignment to prompt revascularization — no./total no. (%) | 675/1388 (48.6) | 453/897 (50.5) | 0.38 |
To convert the values for cholesterol to millimoles per liter, multiply by 0.02586. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129. CABG denotes coronary-artery bypass grafting, ECG electrocardiogram, GFR glomerular filtration rate, HDL high-density lipoprotein, IQR interquartile range, LAD left anterior descending coronary artery, LDL low-density lipoprotein, LVEF left ventricular ejection fraction, NT-proBNP N-terminal pro–brain natriuretic peptide, and TIA transient ischemic attack.
Race or ethnic group was self-reported.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
The myocardial jeopardy index is the proportion (0 to 100%) of the myocardium supplied by significantly diseased coronary arteries. Thus, a higher score represents more severe coronary artery disease and is associated with worse prognosis.
In the Bypass Angioplasty Revascularization Investigation in Type 2 Diabetes trial, before randomization, patients were stratified according to the most appropriate method of coronary revascularization (percutaneous coronary intervention vs. CABG).
BASELINE TROPONIN T CONCENTRATION AND CARDIOVASCULAR EVENTS
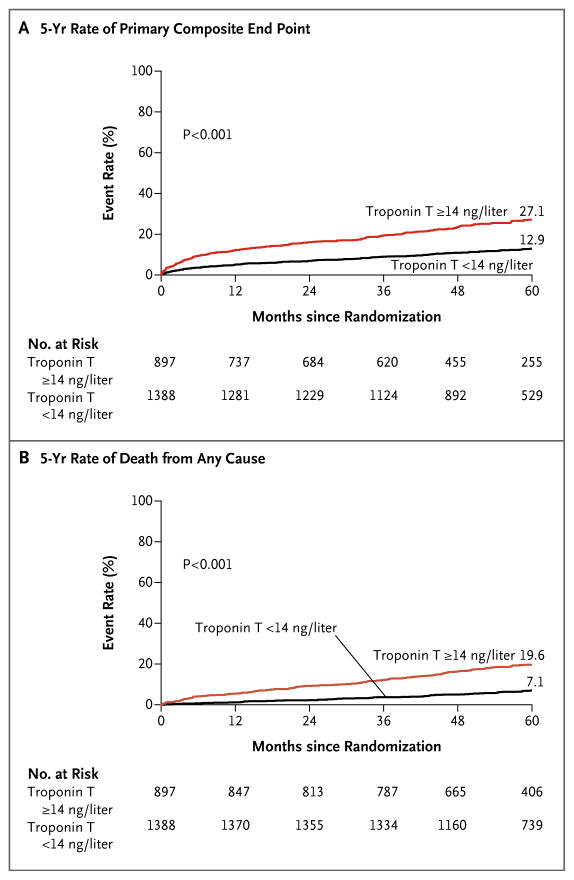
In unadjusted Kaplan–Meier analyses, we observed a significantly higher 5-year rate of the composite end point of death from cardiovascular causes, myocardial infarction, or stroke (Fig. 1A) among patients who had had abnormal baseline troponin T concentrations than among those who had had normal baseline concentrations. The 5-year incidence of the primary composite end point was 27.1% among patients with abnormal baseline troponin T concentrations, versus 12.9% among those with normal baseline troponin T concentrations (P<0.001). There were similar large between-group differences in the rates of the individual components of the primary end point (i.e., death from cardiovascular causes, myocardial infarction, and stroke; Fig. S2, S3, and S4, respectively, in the Supplementary Appendix), as well as in the rate of death from any cause (Fig. 1B), the rate of heart failure (Fig. S5 in the Supplementary Appendix), and the rate of the secondary composite end point of death from any cause, myocardial infarction, stroke, or heart failure (Fig. S6 in the Supplementary Appendix).
Figure 1. Unadjusted Kaplan–Meier Estimates of Adverse Outcomes over 5 Years.
The primary end point (Panel A) was a composite of death from cardiovascular causes, myocardial infarction, or stroke. Death from any cause (Panel B) was a secondary end point. Data are shown for patients with an abnormal high-sensitivity cardiac troponin T level (≥14 ng per liter) at baseline, as compared with those with a normal level (<14 ng per liter) at baseline, who were enrolled in the Bypass Angioplasty Revascularization Investigation in Type 2 Diabetes (BARI 2D) trial.
After adjustment for traditional cardiovascular risk factors, history of myocardial infarction, history of heart failure, factors related to the severity of type 2 diabetes, glomerular filtration rate, electrocardiographic abnormalities, myocardial jeopardy index (the proportion [0 to 100%] of the myocardium supplied by diseased coronary arteries),12 the number of coronary lesions, and abnormal ejection fraction, the adjusted hazard ratio for the composite end point among patients with abnormal baseline troponin T concentrations remained robust and significant (1.85; 95% confidence interval [CI], 1.48 to 2.32; P<0.001) (Table 2). The association of abnormal troponin T concentrations with death from cardiovascular causes and myocardial infarction, as well as with death from any cause, heart failure, and the composite of death from any cause, myocardial infarction, stroke, or heart failure remained significant after adjustment (Table S1); additional adjustment for log-transformed NT-proBNP concentrations led to attenuation of the point estimates. However, in the case of each of these end points, the risk estimates remained significant (Table S1, Model 4, in the Supplementary Appendix).
Table 2.
Hazard Ratio for Major Cardiovascular Events in Adjusted Models, According to Baseline Cardiac Troponin T Concentration.*
| Model | Troponin T <14 ng/liter (N = 1388) | Troponin T ≥14 ng/liter (N = 897) | P Value |
|---|---|---|---|
| hazard ratio (95% CI) | |||
| Model 1† | Reference | 2.35 (1.91–2.89) | <0.001 |
|
| |||
| Model 2‡ | Reference | 2.00 (1.61–2.49) | <0.001 |
|
| |||
| Model 3§ | Reference | 1.85 (1.48–2.32) | <0.001 |
|
| |||
| Model 4¶ | Reference | 1.56 (1.22–1.99) | <0.001 |
In the subgroup of patients who had baseline high-sensitivity cardiac troponin T concentrations of less than 14 ng per liter, 158 events of the primary composite end point (death from cardiovascular causes, myocardial infarction, or stroke) occurred, representing an event rate of 12.9%; in the subgroup with baseline troponin T concentrations of 14 ng per liter or more, 210 events of the primary composite end point occurred, representing an event rate of 27.1% (P<0.001 for the comparison between the two subgroups).
Model 1 was adjusted for age, race, sex, and assignment to revascularization.
Model 2 was adjusted for age, race, sex, assignment to revascularization, systolic blood pressure, history of smoking, history of stroke or transient ischemic attack, history of myocardial infarction, history of heart failure, total and high-density lipoprotein cholesterol, glycated hemoglobin, baseline insulin use, body-mass index, and estimated glomerular filtration rate.
Model 3 was adjusted for the factors in model 2 plus the myocardial jeopardy index, the number of coronary-artery lesions, left ventricular ejection fraction less than 50%, and major electrocardiographic abnormalities.
Model 4 was adjusted for the factors in model 3 plus natural log-transformed N-terminal pro–brain natriuretic peptide concentration.
In secondary analyses that used baseline quintiles of troponin T concentration, the unadjusted rate of the primary end point at 5 years differed significantly across the five quintile groups; event rates were similar in the three lowest strata of troponin T concentration (<7.3 ng per liter, ≥7.3 to <10.0 ng per liter, and ≥10.0 to <14.0 ng per liter) and were substantially higher in the fourth quintile (≥14.0 to <23.0 ng per liter) and the fifth quintile (≥23.0 ng per liter) (Fig. S7 in the Supplementary Appendix). Adjusted models confirmed the observation that cardiovascular risk was elevated only in patients with abnormal baseline troponin T concentrations (Table S2 in the Supplementary Appendix). In exploratory analyses, we observed that patients with abnormal baseline troponin T concentrations and NT-proBNP concentrations of 300 ng per liter or higher were at increased risk for adverse outcomes (Table S3 in the Supplementary Appendix).
REVASCULARIZATION AND CARDIOVASCULAR EVENTS
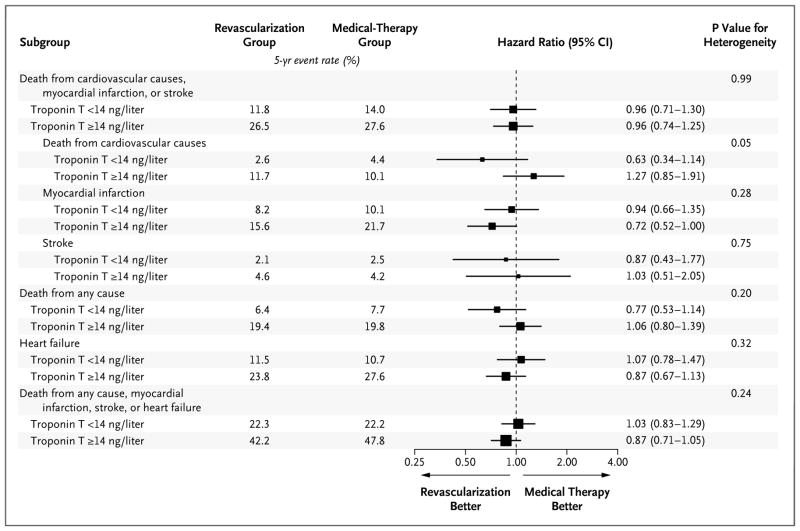
Among the patients included in this BARI 2D ancillary study, prompt revascularization was not associated with a significantly lower risk of the primary composite end point than that with medical therapy alone (hazard ratio, 0.98; 95% CI, 0.80 to 1.19; P = 0.83), a finding that is similar to the overall results of the BARI 2D trial11; this lack of significant benefit was seen both among patients with normal troponin T concentrations and among those with abnormal troponin T concentrations (Fig. 2). The effect of revascularization according to the two strata of troponin T concentration appeared to be consistent for each of the components of the primary composite end point, with no significant evidence of heterogeneity. Similar results were observed with respect to death from any cause, heart failure, and the composite end point of death from any cause, myocardial infarction, stroke, or heart failure (Fig. 2). In a series of sensitivity analyses, we again observed no benefit of revascularization across increasing quintiles of troponin T concentration (Table S4), when follow-up was truncated at 1 year (Table S5) or when patients with high concentrations of both troponin T and NT-proBNP were evaluated (Table S6 in the Supplementary Appendix). Finally, no evidence of heterogeneity was observed in analyses in which we used natural log-transformed troponin T concentration instead of categorical troponin T concentration (data not shown). We repeated these analyses separately in strata of patients who were eligible for revascularization with coronary-artery bypass grafting and those who were eligible for revascularization with percutaneous coronary intervention, and there was no differential benefit of revascularization among those with abnormal troponin T concentrations (Fig. S8 and S9 in the Supplementary Appendix).
Figure 2. Hazard Ratios for the Primary Composite End Point and Selected Secondary End Points.
Data are shown for the patients who were randomly assigned to either prompt revascularization plus intensive medical therapy (revascularization group) or intensive medical therapy alone (medical-therapy group), stratified according to a normal (<14 ng per liter) or an abnormal (≥14 ng per liter) high-sensitivity cardiac troponin T concentration at baseline. The size of the boxes is proportional to the size of the subgroups.
REVASCULARIZATION AND FOLLOW-UP TROPONIN T CONCENTRATIONS
The 1984 patients with both a baseline and 1-year follow-up troponin T concentration available tended to be healthier than those for whom only one value was available (Table S7 in the Supplementary Appendix). There was a numerically small but statistically significant increase in troponin T concentration between baseline and 1 year among all the patients in the BARI 2D trial who had measures available at both time points (median, 3.4%; interquartile range, −4.8 to 11.1; P<0.001). This increase was similar among patients in the revascularization group (median, 3.7%; interquartile range, −5.1 to 11.5) and those in the medical-therapy group (median, 3.1%; interquartile range, −4.7 to 10.6) (P = 0.40) (Table S8 in the Supplementary Appendix). Most patients (86.4%) had troponin T concentrations at the 1-year follow-up that changed (increased or decreased) by 25% or less from the baseline value. The proportion of patients who had an increase of more than 25% in troponin T concentration was 6.7%, and the proportion who had a decrease of more than 25% in troponin T concentration was 6.9%. These proportions were similar in the revascularization and medical-therapy groups (Table S8 in the Supplementary Appendix).
CHANGES IN TROPONIN T CONCENTRATION AND CARDIOVASCULAR RISK
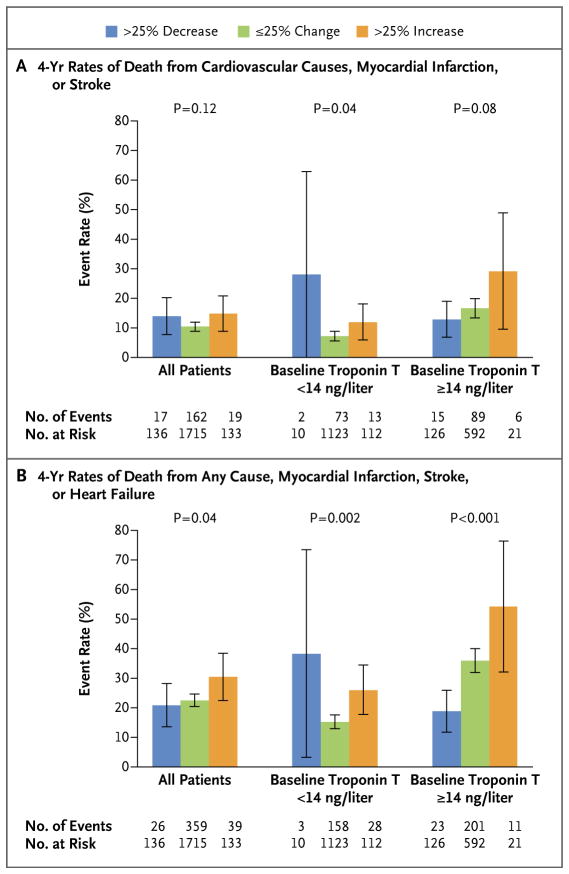
The baseline characteristics of the patients who had a decrease of more than 25% in troponin T concentration at 1 year, an increase of more than 25%, or a change (increase or decrease) of 25% or less are shown in Table S9 in the Supplementary Appendix. A baseline troponin T concentration of 14 ng per liter or higher, a longer duration of diabetes, and a higher baseline glycated hemoglobin level were independent predictors of an increase of more than 25% in troponin T concentration from baseline to 1 year (Table S10 in the Supplementary Appendix). In unadjusted landmark analyses, among the 1984 patients with both baseline and follow-up troponin T values, the 4-year rate of the primary composite end point of death from cardiovascular causes, myocardial infarction, or stroke (Fig. 3A) was nonsignificantly higher and the rate of the secondary composite end point of death from any cause, myocardial infarction, stroke, or heart failure (Fig. 3B) was significantly higher in those who had an increase of more than 25% in troponin T concentration from baseline to 1 year, as compared with those who had an increase or decrease of 25% or less and with those who had a decrease of more than 25%. In adjusted models, in which an increase or decrease in troponin T concentration of 25% or less was considered the reference category, both baseline troponin T concentration and an increase of more than 25% in troponin T concentration were independent predictors of the primary composite end point and the secondary composite end point (Table S11 in the Supplementary Appendix).
Figure 3. Unadjusted Kaplan–Meier Estimates.
Unadjusted Kaplan–Meier estimates are shown for the rate of the primary composite end point of death from cardiovascular causes, myocardial infarction, or stroke (Panel A) and for the rate of the secondary composite end point of death from any cause, myocardial infarction, stroke, or heart failure (Panel B), according to the change in high-sensitivity cardiac troponin T concentration from baseline to 1-year follow-up. The 4-year event rates are derived from a landmark analysis starting at the time of plasma sample collection at 1 year and continuing to the close of the study 4 years later. I bars indicate 95% confidence intervals. Data on the numbers of events and numbers at risk are for the 1984 patients in the BARI 2D trial who had both baseline and 1-year follow-up troponin T concentrations available.
DISCUSSION
In this study involving patients with both type 2 diabetes and stable ischemic heart disease, baseline cardiac troponin T concentrations above the upper limit of normal were associated with approximately a doubling of the risks of myocardial infarction, stroke, heart failure, death from cardiovascular causes, and death from any cause. Nearly 40% of the patients in our study had a high-sensitivity cardiac troponin T concentration at baseline that was above the upper reference limit used to define myocardial injury. The incidence of the primary composite end point of death from cardiovascular causes, myocardial infarction, or stroke at 5 years in this group was 27%, which was double the rate in the group with normal baseline troponin T concentrations. Similar results were seen with respect to other important outcomes, such as the secondary composite outcome of death from any cause, myocardial infarction, stroke, or heart failure. The addition of prompt coronary revascularization to intensive medical therapy did not improve the outcome in these patients. Despite aggressive medical therapy for type 2 diabetes and stable ischemic heart disease, the median troponin T concentration increased over 1 year of follow-up, and no significant reductions in troponin T concentrations were observed in patients who underwent coronary revascularization. Finally, the patients in the BARI 2D trial who had an increase of more than 25% in troponin T concentration from baseline to the 1-year follow-up had a worse outcome than those who had a stable or decreasing troponin T concentration.
The strength of the relationship between troponin T concentration and the subsequent risk of myocardial infarction, stroke, heart failure, death from cardiovascular causes, and death from any cause suggests that high-sensitivity cardiac troponin T concentration is a powerful prognostic marker in patients who have both type 2 diabetes and stable ischemic heart disease. The relationship that we observed in our study was independent of the possible confounding variables of traditional risk factors, measures of diabetes severity, the extent of coronary artery disease, electrocardiographic abnormalities, left ventricular ejection fraction, and renal function. All the patients enrolled in the BARI 2D trial received intensive medical therapy, with substantial and clinically significant reductions in blood pressure and in levels of low-density lipoprotein cholesterol, triglycerides, and glycated hemoglobin during the course of the trial.13 Despite this intensive medical therapy, patients with high-sensitivity troponin T concentrations of 14 ng per liter or higher at baseline were at substantial residual risk for death from cardiovascular causes, myocardial infarction, or stroke, with an absolute risk of more than 27% at 5 years.
Prompt revascularization did not appear to alter the risk of adverse outcomes, including death from any cause and heart failure, in patients with abnormal concentrations of troponin T. These results differ from those of another randomized trial of an early invasive strategy for patients with acute coronary syndromes, in which the benefit of that strategy was seen only among patients with elevated troponin I concentrations.2 The use of high-sensitivity troponin assays facilitates the diagnosis of myocardial infarction,14,15 but the effects of this improved accuracy on patient outcomes remain uncertain. In one recent study involving patients with acute coronary syndromes, troponin T concentration was a predictor of adverse outcomes only among those who received noninvasive treatment, and the use of this measure identified patients who were more likely to benefit from treatment with ticagrelor than from treatment with clopidogrel.16 In our study involving patients with both type 2 diabetes and stable ischemic heart disease, troponin T concentration was a predictor of adverse cardiovascular outcomes regardless of revascularization strategy, and prompt revascularization did not lead to reductions in the composite outcome in patients with abnormal troponin T concentrations at baseline. The circulating concentrations of cardiac troponin T observed in the BARI 2D trial, however, were much lower than those that are seen in patients with acute coronary syndromes.2,16 Indeed, conventional troponin T assays would have been unable to detect any circulating cardiac troponin in most of the patients in the BARI 2D trial. Our results suggest that the factors leading to troponin release in patients with stable ischemic heart disease, including chronic injury from small-vessel ischemia, hypertension, metabolic abnormalities, and renal dysfunction, may be less responsive to epicardial coronary revascularization than the ischemic injury that leads to troponin release in patients with acute coronary syndromes.7–9,16–18
The association of an abnormal troponin T concentration with adverse outcome bears special emphasis because of the 40% prevalence of abnormal troponin T concentration in the BARI 2D population. In this study, patients with known coronary anatomy, stable coronary heart disease, diabetes, and abnormal troponin T concentration did not benefit from percutaneous coronary intervention, and the potential benefit of coronary-artery bypass grafting was the same regardless of baseline troponin T concentration. These results, when viewed in the context of other trials involving patients with chronic stable angina, emphasize the importance of taking a detailed history in distinguishing stable from unstable coronary heart disease and support initial medical therapy as a reasonable first approach in patients who do not have unstable coronary artery disease.11,19,20 The high prevalence of abnormal troponin T concentration, at least according to the established threshold of 14 ng per liter, raises the possibility that the widespread use of this assay in populations similar to the population enrolled in the BARI 2D trial could lead to an increase in the use of revascularization procedures that, on the basis of our findings, appear to offer little benefit with respect to the reduction of key outcomes, including a composite of death from any cause, myocardial infarction, stroke, or heart failure. However, increasing the threshold at which troponin T concentration would be considered abnormal ignores the risk of adverse cardiovascular outcomes in patients with troponin T concentrations between 14 and 23 ng per liter, for whom there was also no evident benefit of revascularization.
Revascularization did not appear to reduce the proportion of patients who had an abnormal troponin T concentration at follow-up. An increase of more than 25% in troponin T concentration at follow-up was uncommon among the patients in our study (approximately 7%), but we found that the patients with this percentage increase, as compared with those with a stable troponin T concentration, were at an increased risk for death from cardiovascular causes, myocardial infarction, or stroke, even after the analysis was adjusted for a wide array of possible confounding variables. This observation is consistent with the findings from previous studies of the association between change in troponin concentration and outcome in a population of patients with hypercholesterolemia21 and in a population of elderly patients.9 However, troponin concentrations in our study appeared to be more stable over time than they were in those populations. These observations raise the possibility that serial measurements of troponin concentration may improve its prognostic value and that persistently elevated and increasing troponin concentrations may be the best predictors of adverse outcomes.
The strengths of our study include the random assignment to prompt revascularization plus intensive medical therapy or to intensive medical therapy alone and the relatively large number of patients in the subgroup with abnormal troponin T concentrations. The limitations include the lack of power to detect a treatment effect of revascularization in subgroups such as those defined according to baseline troponin concentration and the fact that 13% of the patients who had troponin T concentrations available at baseline did not have follow-up values available. Although 14 ng per liter is the current established upper reference limit for troponin T in both men and women, there has been interest in developing more age- and sex-specific reference limits.1,22,23
In conclusion, the results of this study showed a strong, consistent association between baseline concentrations of circulating cardiac troponin T and the risk of death from any cause, myocardial infarction, stroke, and heart failure in patients with both type 2 diabetes and stable ischemic heart disease. In this population, 40% of patients had troponin T concentrations above the upper reference limit of 14 ng per liter, and no differential benefit of prompt coronary revascularization was observed.
Supplementary Material
Acknowledgments
Supported by grants (U01HL061744, U01HL061746, U01HL061748, U01HL063804, and R21HL121495) from the National Heart, Lung, and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health and by an investigator-initiated research grant from Roche Diagnostics (to Dr. Everett).
Dr. Everett reports receiving grant support from Roche and Novartis and fees for serving on a clinical events committee from Genzyme. Dr. Brooks reports receiving grant support from Gilead Sciences. Dr. Chaitman reports receiving fees for serving on clinical events committees from Merck, Eli Lilly, Novo Nordisk, and Roche and fees for serving on data safety monitoring boards from Pfizer and Sanofi-Aventis. Dr. Bhatt reports serving on advisory boards for Cardax, Medscape Cardiology, and Regado Biosciences, receiving fees for serving on a steering committee from WebMD, receiving research funding through his institution from Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Forest Laboratories, Ischemix, Medtronic, Pfizer, Roche, Sanofi-Aventis, and the Medicines Company; participating in unfunded research collaborations with FlowCo, PLx Pharma, and Takeda; and serving as an investigator in studies funded by Biotronik and St. Jude Medical through his institution.
Footnotes
No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–98. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Morrow DA, Cannon CP, Rifai N, et al. Ability of minor elevations of troponins I and T to predict benefit from an early invasive strategy in patients with unstable angina and non-ST elevation myocardial infarction: results from a randomized trial. JAMA. 2001;286:2405–12. doi: 10.1001/jama.286.19.2405. [DOI] [PubMed] [Google Scholar]
- 3.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139–e228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt DL, Roe MT, Peterson ED, et al. Utilization of early invasive management strategies for high-risk patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative. JAMA. 2004;292:2096–104. doi: 10.1001/jama.292.17.2096. [DOI] [PubMed] [Google Scholar]
- 5.Omland T, de Lemos JA, Sabatine MS, et al. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361:2538–47. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Omland T, Pfeffer MA, Solomon SD, et al. Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J Am Coll Cardiol. 2013;61:1240–9. doi: 10.1016/j.jacc.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 7.Everett BM, Cook NR, Magnone MC, et al. Sensitive cardiac troponin T assay and the risk of incident cardiovascular disease in women with and without diabetes mellitus: the Women’s Health Study. Circulation. 2011;123:2811–8. doi: 10.1161/CIRCULATIONAHA.110.009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–12. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–76. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–15. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bypass Angioplasty Revascularization Investigation 2 Diabetes Study Group. Baseline characteristics of patients with diabetes and coronary artery disease enrolled in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Am Heart J. 2008;156:528–36. doi: 10.1016/j.ahj.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaitman BR, Hardison RM, Adler D, et al. The Bypass Angioplasty Revascularization Investigation 2 Diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease: impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation. 2009;120:2529–40. doi: 10.1161/CIRCULATIONAHA.109.913111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–67. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 15.Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868–77. doi: 10.1056/NEJMoa0903515. [DOI] [PubMed] [Google Scholar]
- 16.Wallentin L, Lindholm D, Siegbahn A, et al. Biomarkers in relation to the effects of ticagrelor in comparison with clopidogrel in non-ST-elevation acute coronary syndrome patients managed with or without in-hospital revascularization: a sub-study from the Prospective Randomized Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2014;129:293–303. doi: 10.1161/CIRCULATIONAHA.113.004420. [DOI] [PubMed] [Google Scholar]
- 17.White HD. Pathobiology of troponin elevations: do elevations occur with myocardial ischemia as well as necrosis? J Am Coll Cardiol. 2011;57:2406–8. doi: 10.1016/j.jacc.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 18.Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–16. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 20.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126(25):e354–e471. doi: 10.1161/CIR.0b013e318277d6a0. [DOI] [PubMed] [Google Scholar]
- 21.White HD, Tonkin A, Simes J, et al. Association of contemporary sensitive troponin I levels at baseline and change at 1 year with long-term coronary events following myocardial infarction or unstable angina: results from the LIPID Study (Long-Term Intervention With Pravastatin in Ischaemic Disease) J Am Coll Cardiol. 2014;63:345–54. doi: 10.1016/j.jacc.2013.08.1643. [DOI] [PubMed] [Google Scholar]
- 22.Gore MO, Seliger SL, Defilippi CR, et al. Age- and sex-dependent upper reference limits for the high-sensitivity cardiac troponin T assay. J Am Coll Cardiol. 2014;63:1441–8. doi: 10.1016/j.jacc.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Apple FS, Jaffe AS. Men are different than women: it’s true for cardiac troponin too. Clin Biochem. 2014;47:867–8. doi: 10.1016/j.clinbiochem.2014.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.