Summary
Progression to AIDS is driven by CD4 T-cell depletion, mostly involving pyroptosis elicited by abortive HIV infection of CD4 T cells in lymphoid tissues. Inefficient reverse transcription in these cells leads to cytoplasmic accumulation of viral DNAs that are detected by the DNA sensor IFI16, resulting in inflammasome assembly, caspase-1 activation, and pyroptosis. Unexpectedly, we found that peripheral blood-derived CD4 T cells naturally resist pyroptosis. This resistance is partly due to their deeper resting state, resulting in fewer HIV-1 reverse transcripts and lower IFI16 expression. However, when co-cultured with lymphoid-derived cells, blood-derived CD4 T cells become sensitized to pyroptosis, likely recapitulating interactions occurring within lymphoid tissues. Sensitization correlates with higher levels of activated NF-κB, IFI16 expression, and reverse transcription. Blood-derived lymphocytes re-purified from co-cultures lose sensitivity to pyroptosis. These differences highlight how the lymphoid tissue microenvironment encountered by trafficking CD4 T lymphocytes dynamically shapes their biological response to HIV.
Introduction
Abortive HIV infection is a key driver of “bystander” CD4 T-cell depletion in lymphoid tissues. Recent studies indicate that HIV fuses normally to these quiescent cells; however, because of their resting state, the elongation step of reverse transcription is inefficient, and consequently, short HIV DNA transcripts accumulate in the cytosol (Doitsh et al., 2010). The DNA sensor IFI16 detects these viral DNAs, triggers an innate interferon-β response, and inflammasome assembly that leads to caspase-1 activation (Doitsh et al., 2010; Doitsh et al., 2014; Gariano et al., 2012; Kerur et al., 2011; Monroe et al., 2014; Schoder and Tschopp, 2010; Steele et al., 2014; Unterholzner et al., 2010). Activated caspase-1 induces pyroptosis, a highly inflammatory form of programmed cell death associated with pro-interleukin-1β processing, plasma membrane pore formation, and extrusion of cytoplasmic contents (Doitsh et al., 2014; Fink and Cookson, 2005; Lamkanfi and Dixit, 2009; Miao et al., 2011). While resting CD4 T cells derived from tonsil, spleen, and gut-associated lymphatic tissue (GALT) infected with X4- or R5-tropic HIV undergo pyroptosis (Steele et al., 2014), it is not known whether blood-derived CD4 T cells are similarly susceptible to this pathway of programmed cell death. Since naive CD4 T cells often reside in lymphoid tissues for 12–18 h before returning to peripheral blood (Cyster, 2005), we considered the possibility that differences in the microenvironments found in these two tissues might affect the sensitivity of CD4 T cells to abortive HIV infection-mediated pyroptosis.
Results
Blood-Derived CD4 T Cells Are Naturally Resistant to HIV-Mediated Depletion
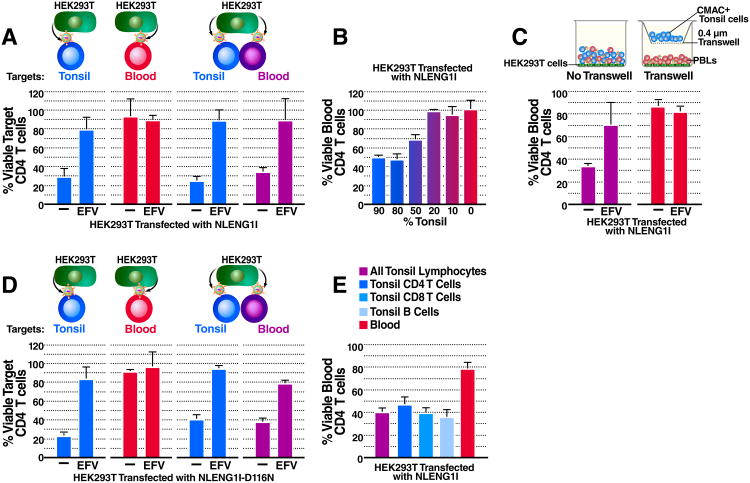
The sensitivity of blood- and lymphoid tissue-derived CD4 T cells to HIV-mediated depletion was assessed in the human lymphoid aggregated culture (HLAC) system (Figure 1A) (Doitsh et al., 2010; Jekle et al., 2003). Effector tonsil cells were infected with the lab adapted CXCR4-tropic virus NL4-3. As expected, carboxyfluoroscein diacetate succinimydyl ester (CFSE)-labeled (target) tonsil CD4 T cells were massively depleted when co-cultured with productively infected (effector cells) tonsil cells (Figure 1B). In agreement with prior results, CD4 T-cell depletion persisted in the presence of azidothymidine (AZT), a nucleoside reverse transcriptase inhibitor that allows the accumulation of short reverse transcripts but blocks the generation of full-length late transcripts though chain termination. These findings with AZT indicate that the observed cell death was not a consequence of productive infection. However, cell death was blocked by efavirenz (EFV), a non-nucleoside reverse transcriptase inhibitor that allosterically inhibits reverse transcriptase thereby preventing accumulation of the short viral DNA transcripts (Figure 1B)(Doitsh et al., 2010; Quan et al., 1999). This pattern of drug sensitivity where EFV but not AZT blocks cell death is characteristic of pyroptosis triggered by abortive HIV infection and is consistent with prior studies (Doitsh et al., 2010).
Figure 1. Blood-Derived CD4 T Cells Are Naturally Resistant to HIV-Mediated Depletion.
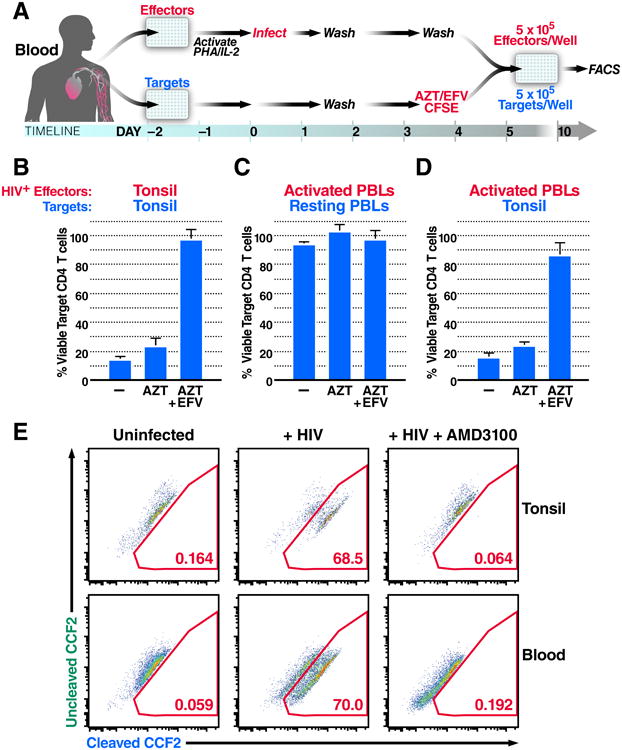
(A) The HLAC system. Uninfected cells were labeled with CFSE (target cells) and treated with medium, azidothymidine (AZT), or AZT and efavirenz (EFV), and then co-cultured with NL4-3 productively infected (effector) cells for 5 days. Cells were harvested and analyzed by flow cytometry.
(B) Percent viable target tonsil CD4 T cells co-cultured with infected tonsil cells.
(C) Percent viable target blood CD4 T cells co-cultured with infected PBLs.
(D) Percent viable target tonsil CD4 T cells co-cultured with infected PBLs.
(E)Virion based fusion assays were performed with BLAM-Vpr-NL4-3-infected tonsil lymphocytes or PBLs. Cells were then loaded with the CCF2-AM dye. Gated populations represent the percentage of fused CD4 T cells scoring positive for BLAM-dependent CCF2-AM cleavage. Data presented in B-D reflect cumulative results from three experiments; data in E are representative of a single experiment performed three times with similar results. Error bars, SEM. See also Figure S1.
To determine if resting blood-derived CD4 T cells are susceptible to this mechanism of HIV-induced cell death, effector peripheral blood lymphocytes (PBLs) were stimulated with phytohemagglutin (PHA) and interleukin-2 (IL-2) for 48h to render them susceptible to productive HIV infection. Effector PBLs were co-cultured with resting target PBLs 5 days post infection (Figure 1A). Strikingly, resting target blood CD4 T cells were not depleted (Figure 1C), even though these same effector cells readily induced target tonsil CD4 T cell depletion (Figure 1D). These results imply that the resistance of target PBLs to depletion is not due to inefficient viral production or transfer from effector PBLs.
Since HIV-infected subjects exhibit higher levels of overall immune activation compared to healthy subjects even when their viral load is controlled by effective combination anti-retroviral therapy (cART) (Lederman et al., 2013), we explored whether higher levels of caspase-1 activation might be present in HIV-infected patient blood samples. Caspase-1 activation was monitored in CD4 T cells from the blood of 11 healthy and 15 HIV-infected (8 untreated and 7 cART treated) donors by staining with cell-permeable fluorogenic caspase-1−specific substrates (CaspLux-1) followed by flow cytometric analyses. In all cases, less than 1% of patient blood-derived CD4 T cells scored positive for caspase-1 activation (Figure S1A). Lysates from HIV infected donors, and healthy monocytes were also compared by immunoblotting with anti-caspase-1 antibodies to identify the inactive pro-enzyme (p50) and the active (p20 and p10) subunits that form during capase-1 activation (Figure S1B). None of the HIV-infected donors showed evidence of activated caspase-1, whereas the p10 and p20 subunits were readily detected in monocytes where caspase-1 is constitutively active. Thus, the vast majority of blood-derived CD4 T cells from HIV-infected subjects, on and off cART do not exhibit caspase-1 activation.
Next, we assessed the level of HIV fusion to blood- and lymphoid tissue−derived CD4 T cells. Using a virion-based fusion assay (Cavrois et al., 2002), we found that NL4-3 fused similarly to CD4 T cells from both tissues and fusion was effectively inhibited by addition of the CXCR4 co-receptor antagonist, AMD3100 (Figure 1E). Thus, differences in viral entry into target blood CD4 T cells (versus tonsil cells) do not explain their resistance to pyroptosis.
Blood-Derived CD4 T Cells Accumulate Fewer Reverse Transcripts and Express Less IFI16 than Lymphoid Tissue-Derived CD4 T Cells
To further dissect the basis for the resistance of blood-derived CD4 T cells to HIV-mediated depletion, we used a flexible experimental system where HEK293T cells are transfected with the viral plasmid pNLENG1I and then overlaid with blood- or tonsil-derived cells (Figure 2A) (Doitsh et al., 2014; Levy et al., 2004). In this system, tonsil-derived CD4 T cells were massively depleted. Efavirenz prevented depletion of these cells−confirming that the depletion requires steps of the viral life cycle beyond gp120 engagement and reverse transcription initiation. Conversely, overlaid blood-derived CD4 T cells remained resistant to depletion (Figure 2B). As shown by qPCR analysis, 5 h after overlay on NLENG1I-transfected HEK293T cells, tonsil lymphocytes generated 3–10-fold more HIV reverse transcripts than PBLs (Figures 2C and 2D), suggesting that the accumulation of fewer HIV reverse transcripts may explain the resistance of blood CD4 T cells to HIV-mediated depletion.
Figure 2. Blood-Derived CD4 T Cells Accumulate Fewer Reverse Transcripts and Express Less IFI16 DNA Sensor than Tonsil CD4 T Cells.
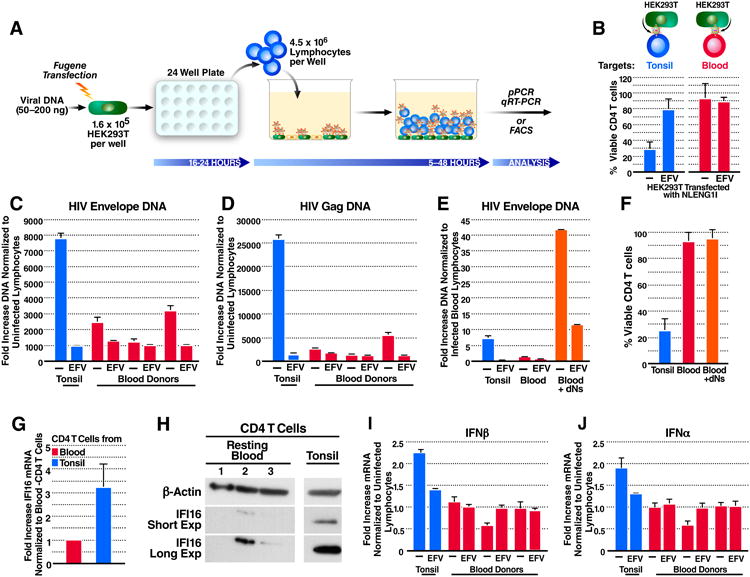
(A) The HEK293T overlay culture system. Lymphocytes were overlaid on NLENG1I-transfected HEK293T cells for 5-48 h. Samples were then immunostained and analysed by flow cytometry, or lysed for DNA or mRNA purification and analyzed by qPCR or RT-qPCR.
(B) Percentages of viable tonsil- or blood-derived CD4 T cells 48 h after overlay.
(C and D) Fold-increase in HIV DNA versus the corresponding uninfected lymphocytes 5 h after overlay. (C) Envelope or (D) Gag HIV DNA.
(E) Virus-producing HEK293T cells were overlaid with dNs-treated PBLs for 5 h. Shown is the fold-increase in HIV Envelope DNA versus the infected untreated PBLs.
(F) Percentages of viable CD4 T cells 48 h post overlay on virus-producing HEK293T.
(G) Fold-increase IFI16 mRNA in purified CD4 T cells from three tonsil versus three blood donors.
(H) Immuno-blot of IFI16 expression in resting blood- or tonsil-purified CD4 T cells. IFI16 expression varied among different cellular compartments (25-99% less IFI16 in blood than tonsil CD4 T cells). Tonsil- and blood-purified CD4 T samples were analyzed on the same gel exposed for the same length of time, but are shown separately for graphical clarity.
(I and J) Fold increase type I interferon mRNA versus corresponding uninfected lymphocytes 24 h post overlay on virus-producing HEK293T cells (I) interferon-β or (J) interferon-α mRNA. Data shown in B and F-G represent cumulative results from at least three experiments, while representative results from a single experiment repeated three times with similar results are shown in C-E and H-J. Error bars, SEM.
To determine if boosting HIV reverse transcription was sufficient to induce blood CD4 T-cell depletion, we treated PBLs with deoxynucleosides (dNs, 2.5–5 mM), for 0–1 h before overlaying them on virus-producing HEK293T cells (Plesa et al., 2007; Zack et al., 1990). Infected dNs-treated PBLs produced up to 40-fold more HIV Envelope DNA than their untreated counterparts (Figure 2E). However, the dNs-dependent increase in reverse transcription was not sufficient to sensitize blood-derived CD4 T cells to depletion (Figure 2F). These data suggest that restriction of reverse transcription is not the only factor underlying the intrinsic resistance of blood-derived CD4 T cells to HIV-mediated depletion.
Next, we compared expression levels of the DNA sensor IFI16, in blood- and lymphoid tissue–derived CD4 T cells. IFI16 transcription was approximately three fold higher in tonsil compared to blood-derived CD4 T cells (Figure 2G); the reduction in IFI16 protein expression was even greater (Figure 2H). With insufficient IFI16 expression, HIV reverse transcripts would not be sensed, and caspase-1 would not be activated to induce pyroptotic cell death.
IFI16 also induces the type I interferon response upon DNA sensing (Unterholzner et al., 2010). Blood-derived CD4 T cells produced less type I interferon mRNA than their tonsil counterparts, 24 h after overlay on virus-producing HEK293T cells (Figures 2I and 2J). These findings suggest that lower reverse transcription coupled with lower IFI16 expression results in less HIV DNA sensing. Of note, Berg et al., reported that human primary T cells fail to induce strong type I interferon responses and concluded that the defect involves impaired DNA sensing machinery (Berg et al., 2014). While these findings raise the possibility that diminished reverse transcription and decreased IFI16 expression contribute to the overall resistance of blood-derived CD4 T cells to pyroptosis, they do not exclude the possible involvement of additional, yet unidentified intracellular blocks.
Blood-Derived CD4 T Cells Co-Cultured with Lymphoid Tissue-Derived Cells Are Susceptible to HIV-Mediated Depletion
The microenvironments of lymphoid tissues and peripheral blood are quite distinct (Cyster, 2005; Eckstein et al., 2001; Kreisberg et al., 2006; Moutsopoulos et al., 2006). To test if the lymphoid tissue microenvironment is necessary and sufficient to induce abortive HIV infection-mediated depletion of CD4 T cells, we mimicked this environment by co-culturing PBLs with tonsil-derived cells and PBLs using the HLAC and HEK293T overlay culture systems. Remarkably, blood-derived CD4 T cells were rendered sensitive to depletion when co-cultured with tonsil cells (Figures 3A and S2A). This effect was not the result of allogenic cellular activation alone, as co-culture of PBLs from mismatched donors was not sufficient to sensitize target blood-derived CD4 T cells for depletion (Figure S2B). Thus, blood CD4 T cells are highly resistant to pyroptosis unless co-cultured with tonsil cells−raising the possibility that the lymphoid microenvironment sensitizes CD4 T cells abortive HIV-mediated depletion. Interestingly, the percent depletion of blood CD4 T cells increased with the fraction of tonsil lymphocytes (Figure 3B). Splenocytes also sensitized blood CD4 T cells to HIV-mediated depletion, suggesting that this phenotype is a general property of lymphoid tissues (Figure S2C).
Figure 3. Blood-Derived CD4 T Cells Co-Cultured with Lymphoid Tissue-Derived Cells Are Susceptible to HIV-Mediated Depletion.
(A) Percent viable target CD4 T cells 48 h post overlay on pNLENG1I-transfected HEK293T cells. The data presented for isolated cultures of tonsil or blood cells are the same as presented in Figure 2B, which corresponded to a portion of the larger experiment shown here. (See also Figure S2).
(B) Percent viable blood CD4 T cells 48 h post co-culture with different percentages of CMAC+ tonsil cells and overlay on virus producing HEK293T cells.
(C) Percent viable blood CD4 T cells 48 h post overlay on virus-producing HEK293T in direct contact (left panel) with or separated by a transwell insert (right panel) from CMAC+ tonsil cells.
(D) Percent viable target-CD4 T cells 48 h post overlay on D116N-NLENG1I-transfected HEK293T.
(E) Percent viable blood CD4 T cells 48 h post co-culture with enriched CMAC+ tonsil-derived-B cells, -CD8 T cells, or -CD4 T cells and overlay on virus-producing HEK293T cells. Data shown in A-E represent cumulative results from at least three experiments. Error bars, SEM. See also Figure S2.
To determine if sensitization of blood CD4 T cells requires close cell-to-cell interactions, PBLs were allowed to interact directly with virus-producing HEK293T cells and 7-amino-4-chloromethylcoumarin (CMAC) labeled tonsil cells, or were separated from tonsil cells by a transwell insert. When separated from tonsil cells, blood CD4 T cells were resistant to depletion (Figures 3C and S2D), suggesting that close interactions between lymphoid- and blood-derived cells are required for sensitization. These results are consistent with our finding that supernatants from tonsil cultures do not render blood CD4 T cells susceptible to pyroptosis (data not shown). However, these findings do not exclude the possibility that cell-to-cell signaling induces expression of soluble factor(s) that in turn mediate sensitization.
We recently found that cell-to-cell spread of HIV-1 is required for pyroptosis of lymphoid-derived CD4 T cells (Galloway et al., 2015). If blood-derived CD4 T cell depletion requires direct delivery of virions from productively infected lymphoid tissue-derived CD4 T cells, the transwell system could have interrupted this infection pathway. To investigate this possibility, HEK293T cells were transfected with an integrase-defective viral plasmid, NLENG1I-D116N (Gelderblom et al., 2008). Thus, transfected HEK293T cells produce virions that fuse to, but do not integrate into target lymphocytes. When overlaid on NLENG1I-D116N-transfected HEK293T cells, tonsil CD4 T cells were effectively depleted. This findings confirm that viral integration and productive infection are not required for lymphoid tissue-derived CD4 T-cell depletion (Doitsh et al., 2010). When the same conditions were replicated with PBLs, blood CD4 T cells were not depleted unless they were co-cultured with tonsil cells (Figure 3D). Thus, although the virus need not emanate from lymphoid tissue-derived CD4 T cells, close interactions between tonsil and blood cells are required for sensitization to HIV-mediated depletion (Figures 3C, 3D, and S2C).
Next, we investigated whether the sensitization of blood cells reflects a specific property of CD4 T cells in lymphoid tissue. Tonsil CD4 T, CD8 T, and B cells were enriched with MACS Miltenyi beads (Figure S2E). Interestingly, all three enriched populations equally sensitized blood-derived CD4 T cells to depletion (Figure 3E). Thus, the ability to sensitize is not limited to lymphoid tissue−derived CD4 T cells. Since B cells and CD8 T cells are not susceptible to HIV infection or depletion, these results further exclude the possibility that sensitization requires productive infection or death of lymphoid-derived CD4 T cells. Additionally, these findings further confirm that sensitization of blood CD4 T cells does not require the virus to emanate from lymphoid tissue-derived CD4 T cells.
Co-culture with Lymphoid Tissue-derived Cells Sensitizes Blood CD4 T Cells to Pyroptosis Induced by Multiple Inflammasomes
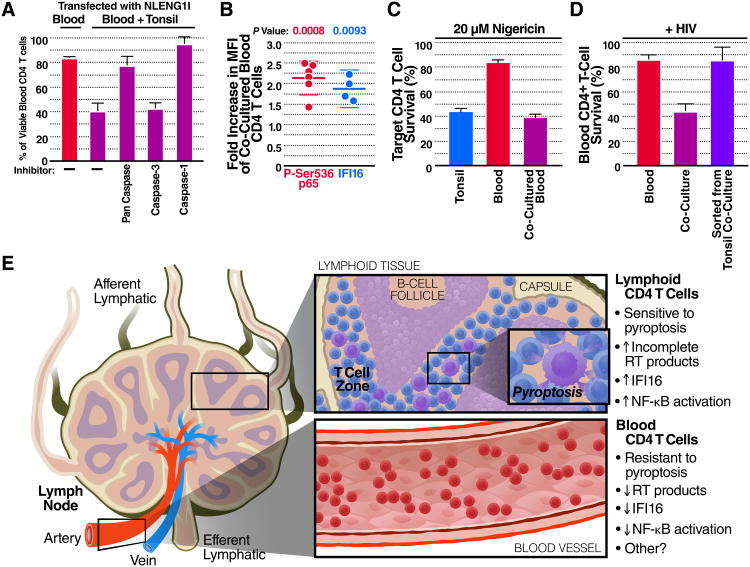
Although abortive HIV infection of lymphoid tissue-derived CD4 T cells leads to caspase-1− dependent pyroptosis, it was important to determine if sensitized blood CD4 T cells die by the same mechanism or another pathway of programmed cell death, such as apoptosis or necroptosis (Fink and Cookson, 2005; Miao et al. 2011). To test this, NLENG1I-transfected HEK293T cells were overlaid with co-cultured tonsil lymphocytes and PBLs in the presence of a pan-caspase, caspase-1, or caspase-3 inhibitor. The death of co-cultured blood-derived CD4 T cells was efficiently prevented by the caspase-1 and pan-caspase inhibitors, but not by the caspase-3 inhibitor (Figure 4A) implying that caspase-1 activation is essential for the death of these cells.
Figure 4. Co-culture with Lymphoid Tissue-Derived Cells Sensitizes Blood CD4 T Cells to Pyroptosis Induced by Multiple Inflammasomes.
(A) Co-cultured PBLs were treated with media, or inhibitors of caspase-1, caspase-3, or a pan-caspase inhibitor and then overlaid on NLENG1I-transfected HEK293T for 48 h. Of note, the caspase-1 inhibitor also weakly inhibits caspase-4 and -5. Shown is the percentage of viable blood-derived CD4 T cells in the presence of these different inhibitors.
(B) PBLs were co-cultured with CMAC+ tonsil cells for 48 h and stained intracellularly for P-Ser536 RelA or IFI16. Shown is the fold increase median fluorescence intensity (MFI) for each protein in co-cultured PBLs versus PBLs cultured alone.
(C) Percent viable CD4 T cells 6 h post nigericin treatment (20 μM).
(D) PBLs cultured alone, co-cultured with tonsil cells, or sorted from tonsil co-cultures were overlaid on pNLENG1I-transfected HEK293T cells for 48. Shown is the percentage of viable blood CD4 T cells. Cumulative results from (A, C-D) three, or (B) four experiments are presented. Error bars, SEM. See also Figure S3.
(E) Blood-trafficking CD4 T cells are resistant to HIV mediated pyroptosis due at least in part to less reverse transcription and IFI16 expression. When these cells return to the lymphoid tissues, cell-cell interactions sensitize them to this mechanism of HIV induced depletion.
Next, we analyzed CD4 T cells from tonsil, PBLs, or co-cultured PBLs, for activated caspase-1 and propidium iodide (PI) uptake after 48 h of overlay on virus-producing HEK293T cells (Figure S3A). After HIV infection, only a very small percentage of blood CD4 T cells stained positive for caspase-1 activation and PI uptake. Conversely, co-culture with tonsil cells induced a 4-5-fold increase in the percentage of blood CD4 T cells that stained positive for caspase-1 activation and a 5-6-fold increase for PI up-take. In addition, co-culture with tonsil cells induced a 3-fold increase in the percentage of blood CD4 T cells that took up ethidium bromide (EtBr) presumably though membrane pores formed during pyroptosis while none of the samples took up ethidium homodimer-2 (EtD2) (Figure S3B). Uptake of EtBr coupled with exclusion of EthD2 is characteristic of pyroptosis (Fink and Cookson, 2006). These results further suggest co-cultured blood- and tonsil-derived CD4 T cells undergo pyroptosis following abortive HIV infection.
Next, we examined wether co-culture with lymphoid cells alters the activation state of blood-derived CD4 T cells. As shown by immuno-staining for CD69, CD25, and HLA-DR, cellular activation was greater in tonsil- versus blood-derived CD4 T cells. However, expression of these same activation markers was not increased in the majority of co-cultured blood CD4 T cells (Figure S3C).
To test for more subtle changes in activation, PBLs were stained intracellularly with antibodies specific for the RelA (p65) subunit of NF-κB phosphorylated at Ser-536. This site-specific phosphorylation only occurs after stimulus-coupled degradation of IκBα (Chen and Greene, 2004; Huang et al., 2010). In blood CD4 T cells co-cultured with tonsil lymphocytes, Ser-536 phosphorylation of RelA was significantly increased by 2-fold compared to blood CD4 T cells cultured alone (Figure 4B).
To test if this level of RelA-Ser-536 phosphorylation was functionally significant, tonsil lymphocytes, PBL, or co-cultured PBLs were treated with nigericin (10 or 20 μM for 3 h) (Figure S3D). Nigericin is a potent inducer of pyroptosis involving assembly of the NF-κB–inducible gene, NLRP3, and other inflammasome components (Qiao et al., 2012). Under these conditions, higher percentages of tonsil- and co-cultured blood-derived CD4 T cells displayed caspase-1 activation and PI uptake, and became susceptible to depletion versus PBL cultured alone (Figure S3D and 4C). These results imply that CD4 T cells circulating in the blood are resistant to pyroptosis elicited either by abortive HIV infection or nigericin exposure. The low level of NF-κB activation occurring in co-cultured blood CD4 T cells is likely important for sensitization as this factor can regulate expression of key inflammasome components. Finally, reverse transcription (Figure S3E) and expression of the IFI16 (Figure 4B) were increased 3- and 2-fold respectively, in co-cultured blood CD4 T cells. This increase in reverse transcription and up-regulation of IFI16 is likely required for induction of pyroptosis.
The frequent trafficking of naive CD4 T cells between blood and lymphoid tissues suggests that the gain and loss of sensitivity to pyroptosis may be quite dynamic. To test for potential lability of sensitization, blood-derived CD4 T cells were sorted from co-cultures and analyzed for IFI16 transcription by RT-qPCR. These sorted blood CD4 T cells transcribed less IFI16 than tonsil-derived CD4 T cells (Figure S3F). Remarkably, sorted PBLs that were overlaid on pNLENG1I-transfected HEK293T cells for 48 h reacquired resistance to pyroptosis (Figure 4D). These results imply that the sensitization of CD4 T cells to HIV-mediated pyroptosis requires continuous signaling and is rapidly lost when this signaling is interrupted, as would occur when CD4 T cells in lymphoid tissues return to the bloodstream (Figure 4E).
Discussion
Our studies reveal that blood-derived CD4 T cells are naturally resistant to abortive HIV infection mediated pyroptosis, a finding that stands in sharp contrast to the sensitivity of CD4 T cells residing in lymphoid tissues. This intrinsic resistance of blood CD4 T cells may be explained by decreased reverse transcription and IFI16 sensor expression needed to induce pyroptosis, but other unidentified factors may also contribute. These findings underscore a fundamental biological difference between CD4 T cells circulating in the blood versus cells within lymphoid tissues. Had we initiated our studies with blood-derived CD4 T cells, we would have not detected the pyroptotic cell death pathway of abortively HIV infected cells, which promotes a massive CD4 T-cell depletion during HIV infection in lymphoid tissues (Doitsh et al., 2010; Doitsh et al., 2014).
Our findings illustrate how the state of cellular activation, which is influenced by the tissue microenvironment, critically determines how CD4 T cells die during HIV infection (Kreisberg et al., 2006; Moutsopoulos et al., 2006). For example, many CD4 T cells in the lamina propria of GALT exhibit a high activation state that allows productive HIV infection culminating in death by apoptosis (Brenchley et al., 2004; Li et al., 2005; Mattapallil et al., 2005). Conversely, although “primed”, more than 95% of CD4 T cells derived from spleen, lymph node, and tonsil are too quiescent to support productive infection (Kreisberg et al., 2006; Moutsopoulos et al., 2006). However, these cells are activated sufficiently to support an abortive form of infection culminating in caspase-1–dependent pyroptosis. Most CD4 T cells in the blood, however, are in a deeper resting state (Moutsopoulos et al., 2006; Zack et al., 1990) than those residing in lymphoid tissues and are therefore naturally resistant to both productive infection and pyroptosis. Although blood CD4 T cells from HIV-infected subjects have been reported to exhibit a higher state of activation, less than 1% of these cells displayed caspase-1activation. The natural resistance of blood CD4 T cells to pyroptosis may benefit the host by confining the intense inflammation associated with abortive HIV infection to lymphoid tissues. These results further underscore AIDS as a disease of lymphoid tissues, not blood.
Strikingly, co-culture with lymphoid tissue-derived cells sensitizes blood CD4 T cells to pyroptosis. This sensitization is rapidly lost when the co-cultures are disassembled. Since CD4 T cells traffic in and out of lymphoid tissues, they likely rapidly gain and lose sensitivity to pyroptosis (Figure 4D). Our findings suggest the key signals sensitizing cells to pyroptosis are dependent on cell-to-cell interactions and that such interactions may extend to other inducers of pyroptosis like nigericin that involves assembly of a different inflammasome, NLRP3 (Figure S3D). With a better understanding of these yet unidentified signaling events, it might be possible to block signal transduction thereby conferring upon lymphoid-CD4 T cells the same resistance to pyroptosis that is naturally present in circulating blood CD4 T cells. This could potentially result in a host-directed approach to control progression of HIV disease to AIDS that could be combined with cART or potentially used in cases of multi-drug resistance where cART options are limited.
Experimental Procedures
Statistical Analysis
To exclude other causes of cell death not involving HIV infection, we normalized CD4 T-cell absolute counts to that of CD8 T cells. These cells do not undergo pyroptosis in the presence of HIV. Since ratios do not follow a normal distribution, we used a log-transformation on the CD4/CD8 ratios to uninfected controls. The assumption of normality of the log-transformed values was examined with the Shapiro-Wilk test. One-sample t test and t-confidence intervals were used for normally distributed data. A one-sample Wilcoxon rank-sum test to calculate the P value of non-normally distributed data. In all experiments involving infected tonsil, PBLs, or co-cultured tonsil and PBLs CD4 T cell depletion was significantly higher versus the corresponding uninfected control (p< 0.05), while depletion of blood CD4 T cells cultured alone was not significant (p> 0.05).
Preparation of Viral Stocks
To generate viral stocks, HEK293T cells (ATCC) were transfected with pNL4-3 (NIH AIDS Reagent Program) (80 μg/T175 flask) using the calcium-phosphate method. Please see our Supplemental Experimental Procedures for detailed experimental protocols.
Fusion Assay
Tonsil cells or PBL were infected with NL4-3 BLAM-Vpr virus (50 ng/μl) for 1 h at 37°C. Cells were washed with CO2-independent medium, stained with CCF2-AM for 1 h at room temperature, and incubated overnight in probenecid/CO2-independent medium at room temperature as described (Cavrois et al., 2002). The next day, cells were stained for surface CD4 expression and fixed with 2-4% paraformaldehyde for at least 1 h prior to measurement of cell fusion indicated by BLAM cleavage of CCF2-AM.
qPCR
Lymphocytes were treated with EFV or left untreated and overlaid on DNase I-treated pNLENG1I-transfected HEK293T. The cells were harvested 5 h later, and DNA was purified with the Qiagen DNeasy Blood and Tissue Kit. A β-actin probe was used as an internal reference. Corresponding uninfected controls served as the calibrator to calculate ΔΔCT values. The probes for HIV envelope and Gag have been described (Doitsh et al., 2010). Please see Supplemental Experimental Procedures for detailed experimental protocols.
Supplementary Material
Acknowledgments
We thank David N. Levy for the multiple-round NLENG1I and single-round NLENG1I-D116N HIV reporter clones. We are grateful for technical assistance from the Gladstone Flow Core including Dr. Marielle Cavrois, Marianne Gesner, and Jaime Tawney. Efavirenz, azidothymidine, and the NL4-3 plasmid were from the AIDS Reagent Program, Division of AIDS, NIAID, NIH. We thank Drs. Brian Webster, Silke Wissing, Brianna Williams, Nicole Galloway, Nadia Roan, and Kate Monroe for technical advice. We also thank Dr. Peter Hunt and the staff of the UCSF SCOPE cohort and its study participants for supporting this study. We thank Gary C. Howard, Anna Lisa Lucido, and Stephen Ordway for editorial assistance; John C.W. Carroll, Giovanni Maki, and Teresa Roberts for graphic arts; Teresa Liu, Robin Givens, and Sue Cammack for administrative assistance; and Grisell Diaz-Ramirez for assistance with the statistical analysis. This work was also made possible with help from the University of California at Berkeley, University of California at the San Francisco-Gladstone Institute of Virology and Immunology Center for AIDS Research (CFAR), an NIH-funded program (P30 AI027763) and grant NIH S10 RR028962-01. I.M.A. is a University of California Berkeley graduate student supported by the UC Berkeley Institutional Fund Block Grant and NIH T32 Al007620-04. S.S. is funded by UCSF-GIVI CFAR and PhRMA Foundation. Various phases of this work were also supported by NIH R21AI102782, 1DP1036502, and U19AI0961133 to W.C.G.
Footnotes
Author Contributions: I.M.A. designed and performed the experiments to generate all the figures and wrote the manuscript with guidance and supervision from W.C.G. G.D. generated the initial observations that led to this project, and provided reagents, guidance and advice on the project. Z.Y. analyzed cell lysates by Western blots for IFI16 and p-actin. S.S. analyzed caspase-1 activation in samples from HIV-infected patients. D.R. provided key materials. W.C.G. supervised the study design, analysis of data, and manuscript preparation. The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berg RK, Rahbek SH, Kofod-Olsen E, Holm CK, Melchjorsen J, Jensen DG, Hansen AL, Jorgensen LB, Ostergaard L, Tolstrup M, et al. T cells detect intracellular DNA but fail to induce type I IFN responses: implications for restriction of HIV replication. PLoS One. 2014;9:e84513. doi: 10.1371/journal.pone.0084513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavrois M, De Noronha C, Greene W. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat Biotechnol. 2002;20:1151–1154. doi: 10.1038/nbt745. [DOI] [PubMed] [Google Scholar]
- Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–159. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- Doitsh G, Cavrois M, Lassen KG, Zepeda O, Yang Z, Santiago ML, Hebbeler AM, Greene WC. Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell. 2010;143:789–801. doi: 10.1016/j.cell.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doitsh G, Galloway N, Geng X, Yang Z, Monroe K, Zepeda O, Hunt PW, Hatano H, Sowinski S, Munoz-Arias I, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein DA, Penn ML, Korin YD, Scripture-Adams DD, Zack JA, Kreisberg JF, Roederer M, Sherman MP, Chin PS, Goldsmith MA. HIV-1 actively replicates in naive CD4(+) T cells residing within human lymphoid tissues. Immunity. 2001;15:671–682. doi: 10.1016/s1074-7613(01)00217-5. [DOI] [PubMed] [Google Scholar]
- Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- Galloway NL, Doitsh G, Monroe KM, Yang Z, Munoz-Arias I, Levy DN, Greene WC. Cell-to-Cell Transmission of HIV-1 Is Required to Trigger Pyroptotic Death of Lymphoid-Tissue-Derived CD4 T Cells. Cell reports. 2015 doi: 10.1016/j.celrep.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariano GR, Dell'Oste V, Bronzini M, Gatti D, Luganini A, De Andrea M, Gribaudo G, Gariglio M, Landolfo S. The intracellular DNA sensor IFI16 gene acts as restriction factor for human cytomegalovirus replication. PLoS Pathog. 2012;8:e1002498. doi: 10.1371/journal.ppat.1002498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom HC, Vatakis DN, Burke SA, Lawrie SD, Bristol GC, Levy DN. Viral complementation allows HIV-1 replication without integration. Retrovirology. 2008;5:60. doi: 10.1186/1742-4690-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Yang XD, Lamb A, Chen LF. Posttranslational modifications of NF-kappaB: another layer of regulation for NF-kappaB signaling pathway. Cell Signal. 2010;22:1282–1290. doi: 10.1016/j.cellsig.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jekle A, Keppler OT, De Clercq E, Schols D, Weinstein M, Goldsmith MA. In vivo evolution of human immunodeficiency virus type 1 toward increased pathogenicity though CXCR4-mediated killing of uninfected CD4 T cells. J Virol. 2003;77:5846–5854. doi: 10.1128/JVI.77.10.5846-5854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerur N, Veettil MV, Sharma-Walia N, Bottero V, Sadagopan S, Otageri P, Chandran B. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe. 2011;9:363–375. doi: 10.1016/j.chom.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisberg JF, Yonemoto W, Greene WC. Endogenous factors enhance HIV infection of tissue naive CD4 T cells by stimulating high molecular mass APOBEC3G complex formation. J Exp Med. 2006;203:865–870. doi: 10.1084/jem.20051856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. Manipulation of host cell death pathways during microbial infections. Cell Host Microbe. 2009;8:44–54. doi: 10.1016/j.chom.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol. 2013;119:51–83. doi: 10.1016/B978-0-12-407707-2.00002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DN, Aldrovandi GM, Kutsch O, Shaw GM. Dynamics of HIV-1 recombination in its natural target cells. Proc Natl Acad Sci USA. 2004;101:4204–4209. doi: 10.1073/pnas.0306764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe KM, Yang Z, JR J, Geng X, Doitsh G, Krogan NJ, Greene WC. IFI16 DNA Sensor is Required for Death of Lymphoid CD4 T cells Abortively Infected with HIV. Science. 2014a;343:428–432. doi: 10.1126/science.1243640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsopoulos NM, Vazquez N, Greenwell-Wild T, Ecevit I, Horn J, Orenstein J, Wahl SM. Regulation of the tonsil cytokine milieu favors HIV susceptibility. J Leukoc Biol. 2006;80:1145–1155. doi: 10.1189/jlb.0306142. [DOI] [PubMed] [Google Scholar]
- Plesa G, Dai J, Baytop C, Riley JL, June CH, O'Doherty U. Addition of deoxynucleosides enhances human immunodeficiency virus type 1 integration and 2LTR formation in resting CD4+ T cells. J Virol. 2007;81:13938–13942. doi: 10.1128/JVI.01745-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y, Wang P, Qi J, Zhang L, Gao C. TLR-induced NF-kappaB activation regulates NLRP3 expression in murine macrophages. FEBS Lett. 2012;586:1022–1026. doi: 10.1016/j.febslet.2012.02.045. [DOI] [PubMed] [Google Scholar]
- Quan Y, Rong L, Liang C, Wainberg MA. Reverse transcriptase inhibitors can selectively block the synthesis of differently sized viral DNA transcripts in cells acutely infected with human immunodeficiency virus type 1. J Virol. 1999;73:6700–6707. doi: 10.1128/jvi.73.8.6700-6707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Steele AK, Lee EJ, Manuzak JA, Dillon SM, Beckham JD, McCarter MD, Santiago ML, Wilson CC. Microbial exposure alters HIV-1-induced mucosal CD4+ T cell death pathways Ex vivo. Retrovirology. 2014;11:14. doi: 10.1186/1742-4690-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, Sirois CM, Jin T, Latz E, Xiao TS, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack JA, Arrigo SJ, Weitsman SR, Go AS, Haislip A, Chen IS. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.