Abstract
Background
Naegleria fowleri is a climate-sensitive, thermophilic ameba found in warm, freshwater lakes and rivers. Primary amebic meningoencephalitis (PAM), which is almost universally fatal, occurs when N. fowleri–containing water enters the nose, typically during swimming, and N. fowleri migrates to the brain via the olfactory nerve. In August 2013, a 4-year-old child died of meningoencephalitis of unknown etiology in a Louisiana hospital.
Methods
Clinical and environmental testing and a case investigation were initiated to determine the cause of death and to identify potential exposures.
Results
Based on testing of CSF and brain specimens, the child was diagnosed with PAM. His only reported water exposure was tap water; in particular, tap water that was used to supply water to a lawn water slide on which the child had played extensively prior to becoming ill. Water samples were collected from both the home and the water distribution system that supplied the home and tested; N. fowleri were identified in water samples from both the home and the water distribution system.
Conclusions
This case is the first reported PAM death associated with culturable N. fowleri in tap water from a U.S. treated drinking water system. This case occurred in the context of an expanding geographic range for PAM beyond southern tier states with recent case reports from Minnesota, Kansas, and Indiana. This case also highlights the role of adequate disinfection throughout drinking water distribution systems and the importance of maintaining vigilance when operating drinking water systems using source waters with elevated temperatures.
Keywords: Naegleria fowleri, primary amebic meningoencephalitis, free-living ameba
Introduction
Naegleria fowleri is a climate sensitive, thermophilic free-living ameba found naturally in freshwater environments [1]. N. fowleri causes the disease, primary amebic meningoencephalitis (PAM), when Naegleria-containing water enters the nose, usually during swimming, and then migrates along the olfactory nerve through the cribriform plate to the brain. In the United States, 0–8 cases are recognized each year [2]. Infection occurs primarily in males and children with a median age of 12 years in U.S. case patients [2]. Symptoms start an average of five days after exposure and are indistinguishable from those of bacterial meningitis [2]. Early on, they include fever, headache, nausea, and vomiting, progressing to altered mental status, seizures, and coma. Death typically occurs within 5 days of symptom onset [2]. Most PAM cases are associated with exposure to warm, untreated freshwater while participating in recreational water activities such as swimming and diving. Recently, other types of water exposures have been reported to be associated with PAM including using contaminated tap water in a neti pot for sinus irrigation and performing ritual nasal rinsing with contaminated tap water [3–5]. This report summarizes the first documented PAM death associated with exposure to water from a U.S. treated public drinking water system colonized with culturable Naegleria fowleri.
Case Report
On 27 July 2013, a previously healthy 4 year-old boy from Mississippi, who was visiting relatives in St. Bernard Parish, Louisiana developed one episode of diarrhea. The next day, he had multiple episodes of vomiting, poor oral intake and a severe headache. He was febrile (temperature, 104°F) at home and was given acetaminophen with little relief. That afternoon, he was noted by his mother to have two staring spells lasting several seconds each. During these episodes, he appeared to be unresponsive with his eyes fixed and open. There were no abnormal movements of his eyes, mouth or body and he quickly returned to baseline between episodes. After the second episode, he was brought to a New Orleans hospital where a third staring spell occurred. The child was alert and reported an intermittent headache but denied neck pain or light sensitivity. His mother noted he was tired and not as playful as usual.
In the emergency department, he was febrile (temperature, 103°F) and pale, with a heart rate of 122 beats per minute, blood pressure of 122/85 mmHg, and respirations of 22/minute. He was alert and appropriate with a normal physical and neurologic exam. He was admitted to the Pediatric Intensive Care Unit early in the morning on 28 July where he complained of intermittent headaches with a waxing and waning mental status and positive Kernig’s and Brudzinski’s signs. A non-contrast computed tomography (CT) of the head showed slight symmetric prominence of the ventricular system without evidence of acute intracranial hemorrhage or space-occupying lesion. His peripheral WBC count was elevated with a neutrophilic predominance. He was started on intravenous vancomycin and ceftriaxone at bacterial meningitis doses four hours before undergoing a lumbar puncture, which revealed colorless, hazy cerebrospinal fluid (CSF) with an opening pressure greater than 35 cm H20. CSF analysis showed elevated protein (172 mg/dL) and elevated white blood cell count (1139 cells/μL) with a neutrophilic predominance. Gram stain did not show any organisms. After the lumbar puncture, piperacillin/tazobactam and acyclovir were added.
In the morning of 29 July, the patient was found to be irritable with continued waxing and waning mental status. He was started on levetiracetam for the repeat staring spells suggestive of seizures. His vital signs demonstrated episodes of hypertension and bradycardia. A second non-contrast head CT showed no acute change from the prior study. He acutely decompensated that afternoon, becoming obtunded with multiple generalized tonic-clonic seizures. The next morning, he required intubation and placement of an external ventricular drain for increased intracranial pressure. Brain magnetic resonance imaging with contrast revealed scattered areas of edema which were most prominent in the right frontal lobe and the gray-white matter junction. There was no evidence of herniation. He continued to have focal seizures and posturing with intracranial pressures measuring 20–50 cm H20.
On 30 July he had a fixed, dilated pupil and repeat head CT imaging revealed diffuse sulcal effacement suggestive of cerebral edema and interval compression of the lateral and third ventricles without herniation. He was taken to the operating room for an emergency decompressive frontal craniectomy, and the dura was found to be tight with areas of petechial hemorrhage. Diffuse cerebral edema and herniation of the brain out of the dural opening was noted. To improve intracranial pressure, hyperosmolar therapy, CSF drainage and barbiturate coma were instituted. Over the next day, he developed progressively worsening hypotension, decreased urinary output and pulmonary edema despite vasopressors and fluid support. Continuous electroencephalography showed absence of any clear electrocerebral activity and after complete evaluation, he was declared brain dead. On the afternoon of 1 August, five days after presentation to the emergency department, the family made the decision to withdraw life support.
CSF bacterial and fungal cultures showed no growth as did blood bacterial cultures. All infectious (including CSF and blood arboviral encephalitis panels, serum Mycoplasma antibodies, CSF Cryptococcal antigen, and CSF Epstein-Barr virus, herpes simplex virus 1 and 2, and enterovirus PCR)and autoimmune studies (including CSF oligoclonal bands, N-methyl-D-aspartic acid [NMDA]-receptor antibody and serum antinuclear antibody and anti-neutrophil cytoplasmic antibody profiles, scleroderma antibody, and thyroid peroxidase antibody) were negative. Autopsy brain specimens were sent to the Centers for Disease Control and Prevention (CDC) for further investigation. On 14 August, the CDC made a diagnosis of PAM due to N. fowleri after identification of the amebic trophozoites in the brain tissue by histopathologic evaluation and immunohistochemical testing [6]. This diagnosis was further supported by positive results in a real-time polymerase chain reaction assay [7] that distinguishes N. fowleri from other pathogenic free-living amebae; both the brain tissue and CSF tested positive for N. fowleri and negative for other free-living amebae. Further studies on the patient’s clinical specimens identified N. fowleri genotype III [8].
On 15 August 2013, the Louisiana Department of Health and Hospitals (DHH) began an epidemiologic and environmental investigation of the case. The investigation focused on water contact the child had during the two weeks prior to becoming ill. During this time, he was visiting relatives in St. Bernard Parish. According to his mother, the child had no contact with surface water (lake, pond, river, ditch, or puddle) during the entire period. In addition to contact with tap water while inside the home, on 18 July 2013, he played all day in the yard adjacent to the house on a commercially purchased lawn water slide (irrigated plastic sheet) which was supplied with water from two garden hoses connected to the home’s outdoor faucet. The child played on the slide, going down both head first and feet first into a pool of water and mud that collected at the bottom. Given that the child’s reported water exposure during the incubation period was only tap water and that a case of PAM associated with sinus irrigation and neti pot use had previously been identified in St. Bernard Parish in 2011 [3], further investigation focused on testing of both the home and municipal water supply.
Environmental Investigation Methods and Results
DHH staff collected 28 samples for shipment to CDC for N. fowleri testing. Twelve of these samples were from in and around the home of the case-patient’s relative (Table 1) and 16 were from various locations around the parish’s water distribution system (Table 2). In addition, DHH staff performed field water quality testing at each sample collection site. Presence of total chlorine residual was tested according to Hach Method 8167 using DPD-3 powder packets and a Pocket Colorimeter II, prior to N. fowleri sample collection. Records were obtained from the water utility and the Environmental Protection Agency (EPA) to describe the water source, treatment methods, and other characteristics of the municipal drinking water system.
Table 1.
Environmental sample test results for household samples, St. Bernard Parish, Louisiana, 2013
| Sample ID | Sample Type | Volume Collected | Total Chlorine (mg/L) | Temp. (°C) | Direct PCR results for N. fowleri | Culture results followed by PCR for N. fowleri | Genotyping results for N. fowleri |
|---|---|---|---|---|---|---|---|
| Yard soil #1 | Soil | ~1 kg | NA | NT | Positive | Negative | genotype I |
| Yard soil #2 | Soil | ~1 kg | NA | NT | Negative | Negative | NT |
| Garden Hose #1 | Entire hose | NA | NT | NT | Positive | Positive | genotype III |
| Garden Hose #2 | Entire hose | NA | NT | NT | Positive | Positive | genotype III |
| Lawn water slide | Entire slide | NA | NT | NT | Negative | Negative | NT |
| Service line hose bib | Ultrafilter | 158 L | NT | NT | Positive | Positive | genotype III |
| Service line hose bib | Grab | 0.7 L | < LOD | 29 | Negative | Negative | NT |
| Kitchen sink hot water | Grab | 0.7 L | < LOD | 46 | Negative | Negative | NT |
| Bathtub faucet | Grab | 0.7 L | < LOD | NT | Negative | Negative | NT |
| Bathtub faucet, sink, showerhead | Swab | NA | NA | NT | Negative | Negative | NT |
| Toilet tank | Grab | 0.7 L | < LOD | NT | Negative | Positive | genotype III |
| Hot water heater | Grab | 0.7 L | NT | 46 | Negative | Positive | genotype III |
Abbreviations: NA, not applicable; NT, not tested; <LOD, below limit of detection of 0.02 mg/L; PCR, polymerase chain reaction.
Table 2.
Environmental sample test results for water distribution system samples, St. Bernard Parish, Louisiana, 2013
| Map Location Number | Sample Description | Sample Type | Volume Collected | Total Chlorine (mg/L) | Temp. (°C) | Direct PCR results for N. fowleri | Culture results followed by PCR for N. fowleri | Genotyping results for N. fowleri |
|---|---|---|---|---|---|---|---|---|
| 1 | Water tower, Town A | Ultrafilter | 119 L | NT | NT | Negative | Negative | NT |
| 1 | Water tower, Town A | Grab | 0.7 L | 1.7 | 30 | Negative | Negative | NT |
| 2 | Flushing station, Town A | Grab | 0.7 L | 1.2 | NT | Negative | Negative | NT |
| 3 | Parish Water Plant Reservoir | Ultrafilter | 119 L | NT | NT | Negative | Negative | NT |
| 3 | Parish Water Plant Reservoir | Grab | 0.7 L | 3.8 | 29 | Negative | Negative | NT |
| 4 | Service Line, Town A1 | Ultrafilter | 510 L | 0.24 | 28 | Negative | Negative | NT |
| 4 | Hot water heater, Town A | Grab | 0.5 L | NT | 43 | Negative | Negative | NT |
| 5 | Service Line, Town A1 | Ultrafilter | 340 L | 0.53 | 30 | Negative | Negative | NT |
| 6 | Service Line, Town A1 | Ultrafilter | 418 L | < LOD | 31 | Negative | Negative | NT |
| 7 | Service Line, Town B1 | Ultrafilter | 350 L | < LOD | 32 | Positive | Positive | genotype I |
| 8 | Service Line, Town B1 | Ultrafilter | 302 L | < LOD | 34 | Positive | Positive | genotype I |
| 9 | Main Line2 | Ultrafilter | 146 L | < LOD | 29 | Negative | Negative | NT |
| 10 | Main Line2 | Ultrafilter | 116 L | 0.3 | NT | Negative | Negative | NT |
| 11 | Main Line2 | Ultrafilter | 136 L | 0.2 | NT | Negative | Negative | NT |
| 12 | Fire Hydrant #1 | Ultrafilter | 236 L | < LOD | 30 | Positive | Positive | genotype III |
| 13 | Fire Hydrant #2 | Ultrafilter | 201 L | < LOD | NT | Negative | Positive | genotype I |
Abbreviations: NT, not tested; <LOD, below limit of detection; PCR, polymerase chain reaction
collected from service line hose bib
collected from tap directly off the water system main line normally used for routine coliform testing
Sampling and dead-end ultrafiltration (DEUF) were performed as previously described [3, 9]. Samples were stored at room temperature and shipped priority overnight in a non-chilled container for testing at CDC. Water grab samples were concentrated by centrifugation and immunomagnetic separation (IMS) for ameba recovery according to procedures described previously [10]. Ultrafilters were backflushed and the inside of the garden hoses, entire lawn water slide, 300 g of soil samples, and the swab were all washed with WB saline containing 0.01% Tween 80 to dislodge any amebae present [11]. The backflush and wash solutions were then processed and assayed using the same procedures as referenced above [10]. After IMS, samples were assayed by real-time PCR to detect N. fowleri, and by culture at 42°C on non-nutrient agar coated with E. coli cells for viable N. fowleri isolation. The sample pellets were also assayed by real-time PCR and cultured directly without IMS processing. Two TaqMan® real-time PCR assays (each of which amplify a different genomic target) were used to confirm the presence of N. fowleri [7, 10]. Organisms obtained by culture were genotyped by sequencing the 5.8S ribosomal RNA gene and internal transcribed spacers 1 and 2 (ITS1 and ITS2) [8].
Tap water to the case patient’s home was supplied by a treated municipal water system, whose source water came from the Mississippi River. Treatment processes at the water facility included filtration, disinfection with chlorine, and addition of ammonia to produce monochloramine for maintaining residual disinfectant in the distribution system. Disinfectant levels were not boosted after leaving the treatment plant. Following Hurricane Katrina in 2005, the population of St. Bernard Parish declined by 51% (from 67,900 to 33,000). It has since increased to 65% (44,000) of the pre-Katrina population as of April 2013, but the municipal water system for this community continues to serve fewer customers than it did prior to August 2005. The system has had water quality exceedances that have led to two violations for inadequate Total Organic Carbon (TOC) reduction, one violation for exceeding the Maximum Contaminant Level (MCL) for Total Coliform bacteria, and six minor monitoring violations for failure to calibrate turbidity meters between 2011 and 2013.
Total chlorine levels throughout the house were below the limit of detection of the test (<0.02 mg/L). The measured temperature of water was 29 °C in the service line to the house (at outside hose bib) and 46 °C in the house hot water heater. Of the 12 household samples, six (50%) were positive for N. fowleri by direct real-time PCR (direct analysis of water concentrate), culture (with cultures tested by real-time PCR), or both. The two real-time PCR assays used in this investigation were in agreement for each household sample tested. The positive samples included a soil sample taken from the yard (genotype I), the two garden hoses that supplied water to the lawn water slide and water samples taken from an outdoor hose bib connected to the main water service line to the home, the toilet tank, and the hot water heater (all genotype III) (Table 1). While both a 0.7-L grab sample and 158-L DEUF sample were collected from the service line hose bib, only the large-volume ultrafiltration sample was positive for N. fowleri.
Four (25%) of 16 water distribution system samples (all collected by DEUF) were positive for culturable N. fowleri (genotypes I and III). The two real-time PCR assays used in this investigation were in agreement for each distribution system sample tested. There was no detectable total chlorine residual and the water temperature was greater than 30°C at three of the four sampling locations where N. fowleri was found to be present (Table 2 and Figure).
Figure 1.
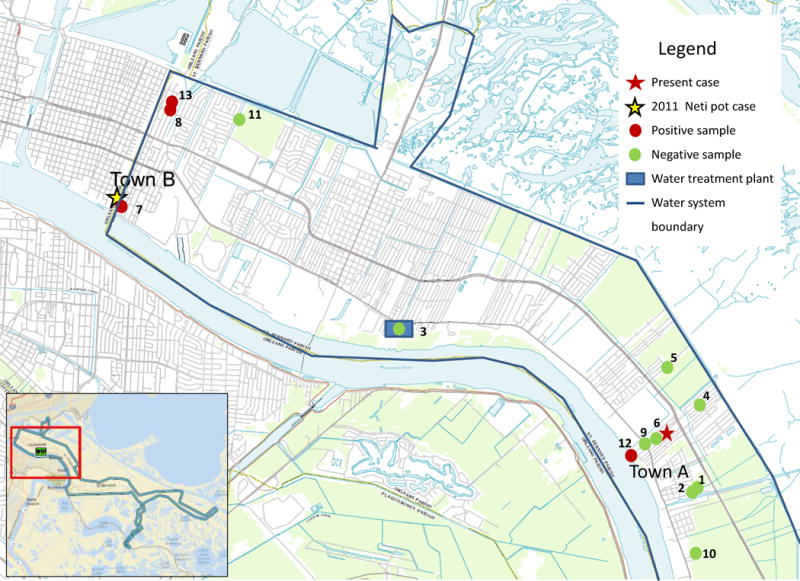
Four sampling locations where Naegleria fowleri was present
Discussion
While PAM remains a rare disease and is most often associated with swimming in warm untreated freshwater, this case and its association with tap water use highlights the evolving epidemiology of PAM and N. fowleri in the United States. This investigation marks the first detection of culturable N. fowleri in a treated drinking water distribution system in the United States that was linked to a fatal infection. However, previous PAM cases were linked to tap water exposure. A PAM case that occurred in 2012 was associated with the use of untreated household water for use in ritual nasal rinsing [4]. In 2011, two cases of PAM in Louisiana were associated with tap water use in neti pots but N. fowleri was not detected in the public drinking water system at that time [3]. Two Arizona PAM cases in 2002 were associated with exposure to tap water; however, at the time of the case-patients’ exposure, the tap water source was an untreated geothermal municipal well [12]. PAM cases associated with tap water exposure have previously been documented in Pakistan and Australia [5, 13]. In response to several deaths of children in the 1960s and 1970s who had played in backyard wading pools or had submerged their heads while bathing, parts of Australia implemented a protocol to monitor for thermophilic amebae and maintain adequate chlorine levels throughout the water distribution system [13, 14]. The primary response strategy for N. fowleri was increased water quality monitoring, ameba testing, and adding chlorine booster stations to maintain a disinfectant residual of ≥0.5 mg/L [15]. Australian officials also developed the following messages for the public: persons living in households supplied by water systems where N. fowleri has been detected should consider taking additional precautions to limit the amount of water that goes up the nose, especially those activities that involve children. These precautions might include not allowing water to go up the nose when bathing, showering, washing the face, or swimming in small hard plastic or blow-up pools that have been filled with tap water; not jumping into or putting the head under bathing water (bathtubs, small hard plastic/blow-up pools); and not allowing children to play unsupervised with hoses, sprinklers or other devices that can force water up their nose.
It is important to note that there were no detectable total chlorine residuals and water temperatures were greater than 30°C at the sampling locations in the water distribution system where N. fowleri was found to be present. These disinfectant “dead spots” corresponded to areas of the system that supplied both the current case-patient’s residence and the 2011 neti pot case-patient’s residence. The combination of no detectable disinfectant residual and warm water temperature likely created conditions for N. fowleri colonization of the distribution system and premise plumbing at the case patient’s residence. N. fowleri cysts and trophozoites, while somewhat tolerant to free chlorine [16, 17], can be inactivated with free chlorine concentrations ≥0.5 mg/L. While it is not known when or how N. fowleri entered and colonized the water distribution system in St. Bernard Parish, no drinking water distribution system is a completely closed system, and all distribution systems experience pipe breaks and pressure fluctuations. On these occasions, N. fowleri may gain entry from the environment and, if water temperature is warm and disinfectant levels are not maintained, could colonize the system, putting users at risk. More specifically, the area in which this water system is located was heavily damaged by Hurricane Katrina and may have been particularly vulnerable to system intrusions while the region was flooded for several weeks. Additionally, the area’s population remains below pre-Katrina levels, introducing the possibility that water may sit stagnant for longer periods in some areas of the distribution system, reducing disinfectant levels and creating an environment conducive for N. fowleri activity.
Genotyping was performed on both clinical and environmental specimens during this investigation. The N. fowleri identified in the clinical specimens was found to be genotype III. Isolates from the household premise plumbing were also genotype III, but genotype I N. fowleri was isolated from a backyard soil sample and both genotypes I and III were isolated from the distribution system. However, current N. fowleri genotyping tools are insufficient for molecular epidemiology purposes because they lack the discriminatory power to definitively link the clinical and environmental isolates in this case, considering this is a free-living organism that is naturally occurring in the environment. Therefore, the identification of drinking water as the likely exposure leading to PAM in this case relied on the epidemiologic investigation with support from the environmental investigation.
The issue of N. fowleri in treated public drinking water systems is emerging in the United States. The response to the situation in St. Bernard Parish relied heavily on the Australian experience where government officials responded to water system-associated PAM cases by establishing an ameba monitoring program and implementing boosting of disinfectant levels in vulnerable drinking water systems. The key goal for officials was to treat all parts of the water distribution system so that “dead spots”, like those found in the St Bernard Parish water distribution system samples, did not occur. This response strategy was implemented in Australia in 1981; no further PAM cases have been associated with use of the implicated South Australia water system since that time. Shortly after the discovery of N. fowleri in its water distribution system, St. Bernard Parish increased the levels of disinfectant throughout the system.
The public can take actions to reduce the risk of N. fowleri infection. Persons practicing nasal or sinus rinsing, whether for medical or religious purposes, should not use tap water or untreated freshwater. Water that is put up the nose should be sterile, distilled, filtered (using a filter with an absolute pore size of ≤1 micron), or previously boiled and left to cool. Devices such as neti pots that are used in the practice of nasal rinsing should be rinsed after each use using the same sterile, distilled, filtered, or boiled water.
In addition to newly emerging routes of transmission, the geographic range of N. fowleri infection has increased. Once limited primarily to 15 southern-tier states, PAM cases have recently been reported from Minnesota (2010 [18] and 2012), Kansas (2011), and Indiana (2012). As a climate-sensitive, thermophilic ameba, predictions of a warming climate have implications for the ecology of Naegleria fowleri and for infections, which warrants further research, monitoring, and awareness of this pathogen. Clinicians in all regions of the United States should be aware of this infection and recognize that not all patients will have the traditional exposure to warm recreational freshwater. Additional research needed to prepare water utilities for this pathogen include testing for occurrence in source waters; understanding how this organism colonizes the biofilm of distribution systems and premise plumbing; developing optimal disinfection strategies; understanding the potential advantages of different residual disinfectants (e.g., chlorine, monochloramine); and developing optimal management and response strategies for N. fowleri control.
Acknowledgments
We would like to acknowledge the following people for their contributions to this investigation: Caryn Benjamin, Amanda Laughlin, Johan Forsman, John Williams, Yuanda Zhu, Frank Naquin, David Boggs, Yoland Brumfield, Sean Nolan, Louisiana Department of Health and Hospitals.
The findings and conclusions in this report are those of the authors and do not necessarily represent those of the Centers for Disease Control and Prevention, State of Louisiana, or U.S. Department of Health and Human Resources.
Funding: The authors completed this work in the course of their regular duties for their affiliated institutions and received no additional funding.
Footnotes
Potential conflicts of interest
All authors: no reported conflicts.
References
- 1.Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol. 2007;50:1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 2.Yoder JS, Eddy BA, Visvesvara GS, Capewell L, Beach MJ. The epidemiology of primary amoebic meningoencephalitis in the USA, 1962–2008. Epidemiol Infect. 2010;138:968–975. doi: 10.1017/S0950268809991014. [DOI] [PubMed] [Google Scholar]
- 3.Yoder JS, Straif-Bourgeois S, Roy SL, et al. Primary amebic meningoencephalitis deaths associated with sinus irrigation using contaminated tap water. Clin Infect Dis. 2012;55:e79–85. doi: 10.1093/cid/cis626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Notes from the field: primary amebic meningoencephalitis associated with ritual nasal rinsing—St. Thomas, U.S. Virgin islands, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:903. [PMC free article] [PubMed] [Google Scholar]
- 5.Shakoor S, Beg MA, Mahmood SF, et al. Primary amebic meningoencephalitis caused by Naegleria fowleri, Karachi, Pakistan. Emerg Infect Dis. 2011;17:258–261. doi: 10.3201/eid1702.100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guarner J, Bartlett J, Shieh WJ, Paddock CD, Visvesvara GS, Zaki SR. Histopathologic spectrum and immunohistochemical diagnosis of amebic meningoencephalitis. Mod Pathol. 2007;20:1230–1237. doi: 10.1038/modpathol.3800973. [DOI] [PubMed] [Google Scholar]
- 7.Qvarnstrom Y, Visvesvara GS, Sriram R, da Silva AJ. Multiplex real-time PCR assay for simultaneous detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri. J Clin Microbiol. 2006;44:3589–3595. doi: 10.1128/JCM.00875-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou L, Sriram R, Visvesvara GS, Xiao L. Genetic variations in the internal transcribed spacer and mitochondrial small subunit rRNA gene of Naegleria spp. J Eukaryot Microbiol. 2003;50(Suppl):522–526. doi: 10.1111/j.1550-7408.2003.tb00617.x. [DOI] [PubMed] [Google Scholar]
- 9.Smith CM, Hill VR. Dead-end hollow-fiber ultrafiltration for recovery of diverse microbes from water. Appl Environ Microbiol. 2009;75:5284–5289. doi: 10.1128/AEM.00456-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mull BJ, Narayanan J, Hill VR. Improved method for the detection and quantification of Naegleria fowleri in water and sediment using immunomagnetic separation and real-time PCR. J Parasitol Res. 2013;2013:608367. doi: 10.1155/2013/608367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Visvesvara GS, Balamuth W. Comparative studies on related free-living and pathogenic amebae with special reference to Acanthamoeba. J Protozool. 1975;22:245–256. doi: 10.1111/j.1550-7408.1975.tb05860.x. [DOI] [PubMed] [Google Scholar]
- 12.Marciano-Cabral F, MacLean R, Mensah A, LaPat-Polasko L. Identification of Naegleria fowleri in domestic water sources by nested PCR. Appl Environ Microbiol. 2003;69:5864–5869. doi: 10.1128/AEM.69.10.5864-5869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorsch MM, Cameron AS, Robinson BS. The epidemiology and control of primary amoebic meningoencephalitis with particular reference to South Australia. Trans R Soc Trop Med Hyg. 1983;77:372–377. doi: 10.1016/0035-9203(83)90167-0. [DOI] [PubMed] [Google Scholar]
- 14.Government of Western Australia, Department of Health, Public Health and Clinical Services. Amoeba response protocol. Available at: http://www.public.health.wa.gov.au/cproot/3649/2/Amoeba%20Response%20Protocol%20v3.pdf. Accessed 23 September 2014.
- 15.Robinson BS, Christy PE. Disinfection of water for control of amoebae. Water. 1984;11:21–24. [Google Scholar]
- 16.Chang SL. Resistance of pathogenic Naegleria to some common physical and chemical agents. Appl Environ Microbiol. 1978;35:368–375. doi: 10.1128/aem.35.2.368-375.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarkar P, Gerba CP. Inactivation of Naegleria fowleri by chlorine and ultraviolet light. J Am Water Works Assoc. 2012;104:51–52. [Google Scholar]
- 18.Kemble SK, Lynfield R, DeVries AS, et al. Fatal Naegleria fowleri infection acquired in Minnesota: possible expanded range of a deadly thermophilic organism. Clin Infect Dis. 2012;54:805–809. doi: 10.1093/cid/cir961. [DOI] [PubMed] [Google Scholar]


