Abstract
Accumulations of dormant eggs in container habitats allow Aedes aegypti populations to survive harsh environmental conditions and may frustrate control interventions directed at larval and adult life stages. While sodium hypochlorite solutions (NaOCl) have long been recognized as ovicides for use against dengue vectors, the susceptibility of eggs to spray applications has not been robustly evaluated on substrate materials representative of the most frequently utilized artificial container habitats. Experiments were performed under controlled and natural conditions by applying dilutions of household bleach (52.5 ppt NaOCl) as a spray to eggs on plastic, rubber, and concrete surfaces, with and without a smectite clay thickener. Laboratory assays identified the minimum NaOCl concentrations required to eliminate eggs on plastic (10 ppt), rubber (20 ppt) and concrete (20 ppt) surfaces. Addition of smectite clay reduced the minimum effective concentration to 10 ppt NaOCl for all 3 substrates. A minimum exposure period of 24 h was required to completely eliminate egg viability on concrete surfaces, even at the highest NaOCl concentration (52.5 ppt). Field experiments verified that spray application of a 1:3 dilution of household bleach mixed with smectite clay can reduce egg hatching by ≥ 99% in shaded and sun-exposed plastic containers. Similarly, 4:1 dilution of household bleach (with or without smectite clay) eliminated ≥ 98% of eggs from concrete surfaces in outdoor, water-filled drums. In this study, we propose a practical, effective and safe strategy for using household bleach to eliminate Ae. aegypti eggs in a range of artificial container habitats.
Keywords: Aedes aegypti, control, ovicide, vector, dengue, sodium hypochlorite
Introduction
Eggs of Aedes aegypti (L.) are deposited in a variety of natural and artificial container habitats on or above the water surface (Wallis 1954). After completing early embryogenesis, unsubmerged eggs become resistant to desiccation and can remain viable for many months under favorable condition (Trpis 1972, Fox 1974, Sota and Mogi 1992, Rezende et al. 2008, Fisher et al. 2011). Accumulations of dormant eggs in the environment (aka “egg bank”) allow Ae. aegypti populations to avoid local extinction when suitable aquatic habitats are scarce, or seasonal temperatures are unfavorable for adult survival and juvenile development (Surtees 1958, Fox 1975, Vezzani et al. 2004). A large standing crop of dormant eggs may also allow vector populations to rapidly recover from short-term perturbations from control measures targeting the larval or adult stages.
Incorporating strategies for eliminating dormant eggs could provide potential benefits to integrated vector management programs for preventing dengue virus (DENV) transmission. Applications of ovicides to container habitats that are difficult to eliminate through source reduction or treat with larvicides (e.g., wash basins; Fernandez et al. 1998) may complement concurrent application of conventional tools against the larval or adult life stages, resulting in a more substantial or sustained impact on the vector population (Machaca et al. 2002). In regions where intraannual variation in vector abundance and DENV transmission are predictable and positively associated with seasonal changes in rainfall or temperature (Vezzani et al. 2004, Dibo et al. 2008, Barrera et al. 2011), intensive, preemptive vector control efforts targeting container habitats that harbor dormant eggs when the vector population is most vulnerable (i.e., dry or cool season) can reduce the rate of increase in vector abundance preceding epidemic transmission (Chadee 2009), potentially inhibiting dissemination of DENV in the host population. Preemptive ovicidal strategies also could significantly improve the success potential of large-scale release programs for Wolbachia infected or genetically modified vectors (Esteva and Yang 2005, Jacups et al. 2013). While several agents have been identified as potential ovicides against dengue vector mosquitoes (e.g., Hatchett 1946, Judson et al. 1962, Sinniah 1983, Luz et al. 2007, Leyva et al. 2009), there has been little effort to develop them into practical tools that can be integrated into vector control programs to complement existing strategies.
The outer layers of Ae. aegypti eggs (exochorion and endochorion) provide resistance to desiccation and insecticide penetration, protect the embryo from pathogenic bacteria and fungi, and anchor the egg to the substrate (Christophers 1960, Mohapatra et al. 1999, Russell et al. 2001). These structures are digestible by sodium hypochlorite (NaOCl), the active ingredient in household bleach (Trpis 1970). Household bleach is a widely available and inexpensive consumer product that is commonly used at the household level for domestic cleaning purposes and in community-based programs for disinfection of drinking water. Water treated with household bleach is considered safe for human consumption when residual chlorine concentrations do not exceed 4 ppm (USEPA 1998).
The activity of hypochlorite compounds against eggs of dengue vector mosquitoes has been demonstrated in several laboratory and field studies (Hatchett 1946, Fernandez et al. 1998, Ritchie 2001, Domenico et al. 2006, Jacups et al. 2013); however, there have been few attempts to quantify the susceptibility of eggs on surfaces representative of common container habitats using application methods practical for routine use in a large-scale control program. Many artificial container habitats utilized by Ae. aegypti are constructed from nonpolar (e.g., plastic, vulcanized rubber) or porous (e.g., concrete) materials. Uniform application and adherence of aqueous solutions to vertical surfaces in these containers poses a significant challenge to the effective use of household bleach as an ovicide. Previous attempts to overcome this limitation have used household bleach mixed with powdered detergent to increase viscosity and adherence to vertical surfaces (Sherman et al. 1998). Though effective, this formulation requires a laborious manual application followed by scrubbing and rinsing of treated surfaces. To improve upon this approach, we have evaluated inert, mineral clays in the smectite group as an alternative thickening agent for household bleach. Natural and synthetic smectite clays are used as thixotropic additives in many home and personal care products, including bleach-based cleaners (Braun and Rosen 1999), and represent a low or negligible toxicity to humans through oral consumption (Carretero 2002).
In this study we tested the ovicidal activity of different dilutions of household bleach, with and without smectite clay thickeners, when applied as a spray against the eggs of Ae. aegypti on 3 types of surfaces. The overall purpose was to identify effective application rates for a range of common container habitats that result in an aqueous chlorine concentration in treated container habitats that is of minimal risk to human and animal health and to the environment. Our specific objectives were to 1) identify concentrations of sodium hypochlorite, with and without smectite clay, that prevent the hatching of Ae. aegypti eggs on substrates representative of common container habitats (plastic, rubber, and concrete), 2) determine the minimum effective exposure period to household bleach (without smectite clay) that prevents hatching of eggs on surfaces where bleach efficacy was lowest in objective 1 (i.e., concrete), and 3) evaluate the effect of spray applications of household bleach on Ae. aegypt egg survival and aqeuous chlorine concentration in plastic pails and concrete-lined drums in a natural setting.
Materials and Methods
Survival of Ae. aegypti eggs to spray applications of household bleach, with and without smectite clay
Six laboratory bioassays were performed to test the efficacy of household bleach, with and without smectite clay, on 3 substrate types representative of important container habitats for Ae. aegypti production. Plastic and rubber surfaces (≈ 10 × 11 cm) were constructed from halved high-density, polyethylene (HDPE) drink cups and pieces cut from the sidewall of an automobile tire, respectively. A commercial mix (Quickrete®, Atlanta, GA) was used to mold concrete surfaces of similar dimensions. To eliminate any preexisting mosquito eggs and remove contaminants that could affect egg survival, new and reused surfaces were cleaned with detergent, soaked overnight in a 1:9 dilution of household bleach, then rinsed with tap water several times before each use.
Test surfaces were prepared by affixing them to the inner wall of a screen-covered plastic cup (473 ml) containing 210 ml of tap water, such that only 1 side of the surface was available as an oviposition substrate. Six gravid Ae. aegypti females, from the F2 to F4 generation of a colony established from eggs collected from Patillas, Puerto Rico, were placed in each cup for 24 h. Three days after the adult females were removed from cups, surfaces with eggs were transferred to ≈ 75% RH and 25°C for an additional 2 days prior to use.
For each bioassay, serial dilutions containing 1.25, 2.5, 5, 10, 20, and 40 ppt of NaOCl were prepared immediately before use by adding deionized water and/or a 6–12.6% (w/w) slurry of smectite clay (final concentration 3%; VanGel O, Vanderbilt Chemicals, Norwalk, CT) to Clorox® regular bleach (Clorox Company, Oakland, CA; hereafter referred to as “household bleach”). Four replicate surfaces representing each treatment were vertically suspended, and a compression sprayer (RL Flo-Master®, Root-Lowell Manufacturing Co., Lowell, MI) was used to apply test formulations to one side of the surface (i.e., “egg side”) to the point of saturation (≈ 2 ml per surface). Deionized water or a 3% suspension of smectite clay was applied to control surfaces. One day after application, eggs on each surface were counted and examined for evidence of chorion damage (i.e., complete depigmentation). Surfaces were maintained at 25°C and 75% RH for 2 days after application, placed at high humidity (≈ 95% RH) for 24 h, then immersed in a 0.1% yeast extract solution for 22 h to stimulate hatching of viable eggs. Hatching success was calculated by dividing the number of live larvae by the total number of eggs, excluding those eggs that were either broken or completely collapsed when examined the day following application. Data from each experiment were fit to a probit regression (SPSS Statistics Ver. 21, IBM Corporation, Armonk, NY) to calculate LC50 values for both formulations, on each surface type.
Minimum exposure period to spray applications of household bleach for eliminating viability of eggs on concrete
Two experiments were performed to identify the minumum exposure time required to kill eggs on the type of substrate where the efficacy of the bleach treatments was observed to be lowest (i.e., concrete) in the previous experiments (objective 1). Egg surfaces were prepared and treated with household bleach and control formulations as described above. In the first experiment, hatching success of eggs on concrete surfaces treated with a 1:1 dilution of household bleach (26.25 ppt NaOCl) was assessed at 0.5, 2, 6, 24, and 72 h posttreatment. In the second experiment, undiluted household bleach (52.5 ppt NaOCl) was applied to concrete surfaces, and viability was assessed 3, 6, and 24 h posttreatment. In each experiment, four control and four bleach-treated surfaces were randomly selected at each time point. To prevent mortality of first instars from exposure to residual bleach in the hatching media, surfaces were rinsed three times in distilled water, in a container with a fine mesh bottom to retain eggs that detached from the concrete. Immediately after rinsing, the concrete surface and any detached eggs were immersed in a 0.1% yeast extract solution to stimulate hatching. Hatching success was assessed 22 h after imersion, as described previously.
Field assessment of a household bleach–smectite clay formulation in plastic pails
Ten gravid Ae. aegypti females were allowed to oviposit for 24 h in each of 20 screen-covered pails (HDPE, 18.9 liters capacity) containing 12 liters of tap water and 0.6 g of ground rabbit chow. Six days after the female mosquitoes were removed, pails were emptied, gently rinsed with tap water, a hole was drilled into each pail (the lower margin 2 cm below the previous 12 liter water level) and fresh tap water was added to the level of the drainage hole (≈ 11 liter). Ten pails each were randomly assigned to receive ≈ 40 ml of a 1:3 dilution of household bleach and deionized water (i.e., ≈ 10 ml of household bleach per pail) + 3% smectite clay (VanGel O). The remaining pails (controls) were treated with a 3% suspension of smectite clay in deionized water. A compression sprayer was used to apply control and treatment suspensions to the inner surface of pails above the water line, as described previously. Immediately after application, control and treatment pails were randomly divided among 2 outdoor sites; an open location that maximized exposure to direct sunlight (“sun-exposed”), and a location underneath a canopy constructed from a double layer of 80% shade cloth (“shaded”). Water samples were collected from pails daily from 0 to 4 days after treatment. Concentrations of free and combined chlorine in samples were measured by colorimetry (DPD method; LaMotte Company, Chesterton, MA) and summed to calculate total chlorine concentration. Four days after treatment, drainage holes in pails were sealed and 0.8 g of ground rabbit chow and ≈ 4 lister of tap water (to a total volume of 16 liters) were added to stimulate larval eclosion. Pails contents were sieved 3 days after submerging eggs and the number of live larvae was counted.
Field assessment of household bleach formulations in concrete-lined drums
Three field experiments were performed to evaluate the efficacy of household bleach spray applications in drums. In each experiment, 6 concrete-lined, metal drums, sheltered from direct exposure to sunlight or rainfall, were filled with 164 liters of tap water (≈ 3/4 capacity) and sealed with a screen cover. Twenty (experiment 1 and 2) or 25 (experiment 3) gravid Ae. aegypti females were added to each drum and allowed to oviposit for 24 h. After 4 to 5 days, the water level in each drum was marked and drums were emptied and gently rinsed to remove any larvae present (from hatched eggs) before refilling drums with fresh tap water to their previous volume.
In each experiment, household bleach was diluted ≈ 4:1 with deionized water (experiment 1) or deionized water and smectite clay (experiment 2 and 3), to a final concentration of 42 ppt NaOCl. A synthetic smectite clay (Laponite RD, Byk-Chemie GmbH, Wessel, Germany) replaced VanGel O as the thickening agent, and the final clay concentration was reduced from 3 to 1% (w/w) to achieve an equivalent viscosity. A handheld, pump-action sprayer was used to apply test formulations in a ≈ 12 cm–wide band above the water line in 3 randomly selected drums; 36 ml was applied to each drum in experiments 1 and 2, and 72 ml was applied in experiment 3 (i.e., representing 28.8 and 57.6 ml of household bleach per drum, respectively). An equal volume of deionized water (with or without smectite clay) was applied to the control drums in each experiment. Twenty-four hours after application, 40 liters of tap water and 20 g of ground rabbit chow was added to drums to submerge eggs and stimulate eclosion. Five days after treatment, the contents of each drum were emptied through a fine-mesh sieve, and all live larvae present were counted. To determine total chlorine concentration (as described previously), a 1.5 m long, 0.5 cm diam glass tube was used to collect samples of the water column in each drum 0, 1 (immediately after submerging eggs), and 2 days after treatment.
Results
Survival of Ae. aegypti eggs to spray applications of household bleach, with and without smectite clay
Spray applications of household bleach without smectite clay were least efficacious against eggs on concrete, and most efficacious against eggs on plastic (Table 1). When formulated with smectite clay, household bleach had a similar efficacy on all 3 substrates. Reduction in hatching success was positively associated with chorion loss (Fig. 1). With the exception of nonthickened bleach treatments applied to concrete surfaces treated, a majority of eggs on surfaces treated with ≥ 20 ppt NaOCl were completely depigmented after a 24 h exposure.
Table 1.
Probit analysis of Aedes aegypti egg viability on 3 surfaces after exposure to spray applications of household bleach and household bleach formulated with smectite clay.
| Surface | Formulation | N | Coefficient (SE) | LC50 (NaOCl ppt; 95% CI) | Chi-square | df | P |
|---|---|---|---|---|---|---|---|
| Plastic | Bleach only | 24 | 2.6 (0.2) | 1.8 (1.4, 2.1) | 104.7 | 22 | < 0.001 |
| Bleach + smectite clay | 24 | 3.9 (0.3) | 2.4 (2.0, 3.0) | 132.4 | 22 | < 0.001 | |
| Rubber | Bleach only | 24 | 2.6 (0.1) | 4.1 (3.5, 4.7) | 94.8 | 22 | < 0.001 |
| Bleach + smectite clay | 20 | 2.6 (0.1) | 2.4 (1.7, 3.1) | 230.4 | 18 | < 0.001 | |
| Concrete | Bleach only | 24 | 3.4 (0.1) | 8.1 (6.6, 10.5) | 685.4 | 22 | < 0.001 |
| Bleach + smectite clay | 24 | 6.1 (0.2) | 3.2 (2.9, 3.4) | 91.8 | 22 | < 0.001 |
Fig. 1.
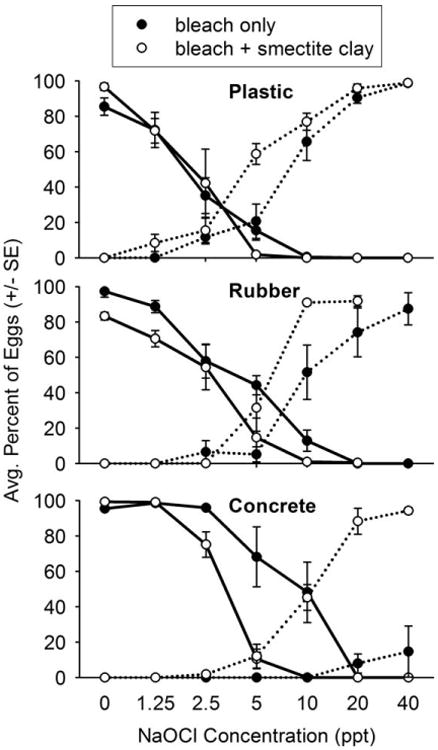
Influence of a thickening agent on the hatching success (solid lines) and chorion depigmentation (broken lines) of Ae. aegypti eggs exposed to spray applications of diluted household bleach on plastic, rubber, and concrete surfaces (efficacy of the highest NaOCl concentration formulated with smectite clay was not evaluated on a rubber surface).
Minimum exposure period to spray applications of household bleach for eliminating viability of eggs on concrete
Between ≈ 10% and 40% eggs on concrete surfaces exposed to household bleach formulations for ≤ 6 h remained viable (Fig. 2). Hatching success was reduced to less than 1% of eggs exposed to either a 1:1 bleach dilution (26.25 ppt NaOCl) for 72 h, or undiluted bleach (52.5 ppt NaOCl) for 24 h.
Fig. 2.
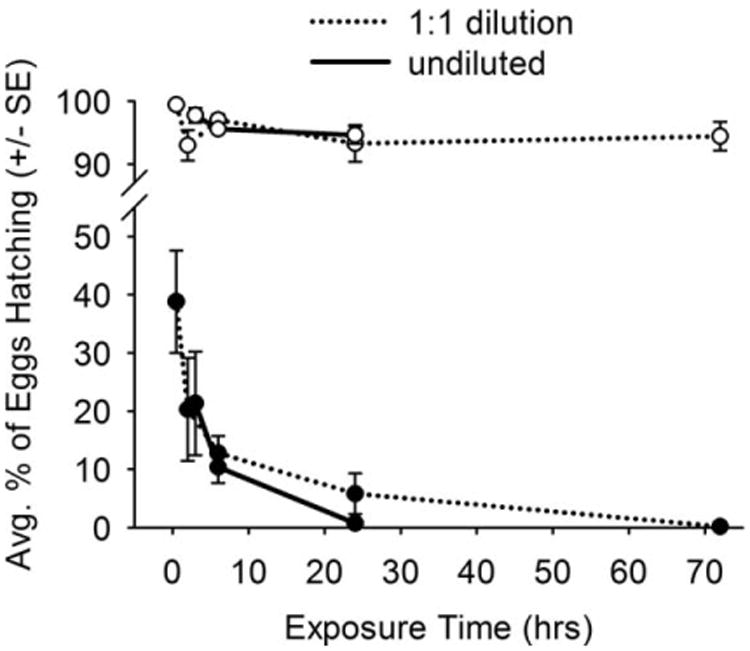
Influence of exposure period on the efficacy of spray applications of a 1:1 dilution (26.25 ppt NaOCl) and undiluted (52.5 ppt NaOCl) household bleach against eggs of Ae. aegypti on concrete surfaces (closed symbols = eggs on treated surfaces, open symbols = eggs on control surfaces).
Field assessment of a household bleach–smectite clay formulation in plastic pails
The average number (± SE) of larvae recovered from shaded pails treated with a 1:3 dilution of household bleach (13.125 ppt NaOCl) formulated with smectite clay was 0.2 ± 0.2, representing a > 99.9% reduction compared to shaded control pails (312.8 ± 57.1 per pail). No larvae were present in treated pails exposed to the sun (control = 199.8 ± 22.0 larvae per pail). Average total chlorine concentrations (ppm ± SE) in sun-exposed and shaded pails immediately after bleach treatment were 21.5 ± 3.3 and 23.6 ± 3.2, respectively. While the concentration of total chlorine dropped to < 1 ppm in all sun-exposed pails 1 day after application, the average concentration in shaded pails remained high, even 4 days after application (24.3 ± 2.7 ppm).
Field assessment of household bleach formulations in concrete-lined drums
Spray applications of 36 ml of a 4:1 dilution of household bleach (42 ppt NaOCl), with or without smectite clay, resulted in a ≥ 98% reduction in larval abundance in drums, compared with controls (experiments 1 and 2; Table 2). Average total chlorine concentration declined to less than 1 ppm in treated drums within 1 day (bleach alone) or 2 days (bleach + smectite clay) following application (Fig. 3). No larvae were recovered from drums treated with 72 ml of the household bleach–smectite clay formation (experiment 3; Table 2), and average total chlorine concentration declined to < 2 ppm, 2 days after treatment (Fig. 3).
Table 2.
Efficacy of household bleach spray formulations against Aedes aegypti eggs when applied to surfaces above the water line in concrete-lined drums.
| Application | Household bleach (ml/drum) | Avg. larvae per drum ± SE | % reduction in larval abundance | |
|---|---|---|---|---|
|
| ||||
| Control (n = 3) | Treatment (n = 3) | |||
| Exp. 1: 36 ml of 42 ppt NaOCl | 28.8 | 1,154.0 ± 27.8 | 21.0 ± 9.1 | 98 |
| Exp. 2: 36 ml of 42 ppt NaOCl + smectite clay | 28.8 | 432.3 ± 24.3 | 4.3 ± 1.9 | 99 |
| Exp. 3: 72 ml of 42 ppt NaOCl + smectite clay | 57.6 | 1,273.0 ± 99.6 | 0 | 100 |
Fig. 3.
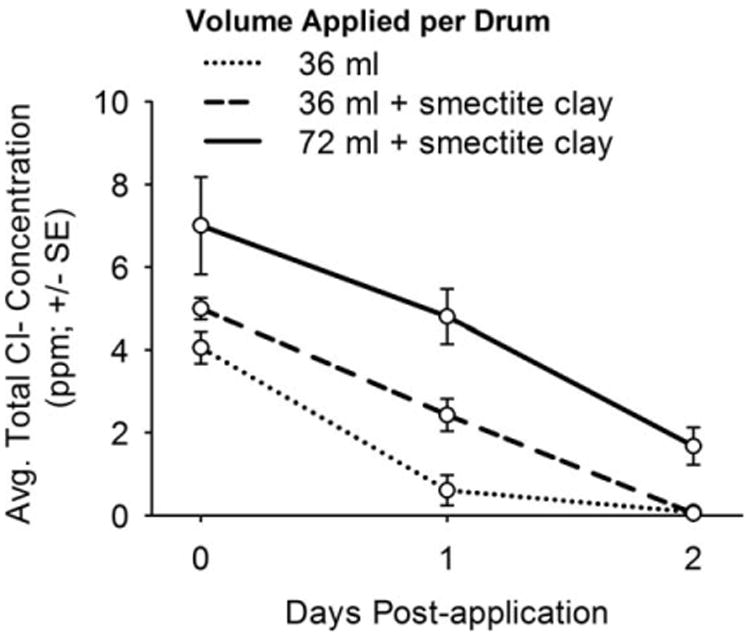
Total chlorine concentration in water-filled drums after spray application of a 4:1 dilution of household bleach (42 ppt NaOCl) to inner wall above water surface. Water samples from 1 day posttreatment were collected immediately after adding 40 liters of tap water and 20 g of ground rabbit chow to immerse eggs and stimulate larval eclosion.
Discussion
The current study represents the first systematic comparison of Ae. aegypti egg susceptibility to spray applications of household bleach on substrates representative of common container habitats. Our laboratory results demonstrated that a spray application of ≈ 180 ml/m2 of solutions containing 20 ppt NaOCl (≈ 38% household bleach v/v), or 10 ppt NaOCl (≈ 19% household bleach v/v) and smectite clay, prevent hatching of Ae. aegypti eggs on plastic, rubber, and concrete surfaces. However, efficacy against eggs on concrete surfaces was reduced when the exposure period was less than 24 h, even when undiluted household bleach (52.5 ppt NaOCl) was used. In previous reports, complete loss of Aedes spp. egg viability was observed after a brief (< 1 min) immersion in bleach solutions containing 10 to 37.5 ppt NaOCl (Ritchie 2001, Domenico et al. 2006). A much weaker concentrations of NaOCl (0.105 ppt) was reported to be effective against Ae. aegypti eggs immersed for a 48 h period (Sherman et al. 1998). Our results suggest that direct immersion of eggs is not a realistic surrogate for evaluating the ovicidal efficacy of bleach solutions applied as a spray.
The efficacy of household bleach treatments without smectite clay significantly differed among substrate types, possibly reflecting relative differences in surface area and texture. Female mosquitoes preferentially deposited eggs in grooves on rubber surfaces, and cracks or cavities on the concrete surfaces, which may have provided some protection from direct exposure to spray droplets. The high alkaline buffering of concrete also may have reduced the oxidation strength of the treatment products by raising the equilibrium ratio of hypochlorite to hypochlorous acid (Frazer et al. 2013). Addition of smectite clay negated the effect of substrate type on efficacy, most likely by allowing a greater proportion of the NaOCl to remain on the treated surface. Though the efficacy of household bleach formulated with the natural (Van Gel O) and synthetic (Laponite RD) smectite clays used in this study were not directly compared, we do not expect that the type of inert thickener formulated with bleach will have a substantial influence on the activity of the sodium hypochlorite or adhesion to surfaces. Further testing will be required to validate whether other thickening agents can be used without compromising efficacy.
Field trials confirmed that a spray application of a 1:3 dilution of household bleach (13.125 ppt NaOCl) formulated with smectite clay can reduce Ae. aegypti egg viability in plastic containers by 99–100% under natural conditions. A 4:1 dilution of household bleach (42 ppt NaOCl) was similarly efficacious when applied to eggs on concrete surfaces in water-filled drums. Measurements of initial, posttreatment chlorine levels in treated pails and drums suggest these applications could additionally result in lethal or sublethal conditions for first-stage larvae (Barrera et al. 2004) and might remain toxic to this life stage for up to 4 days posttreatment in containers not exposed to sunlight. Application of this mixture to containers holding small volumes of water, such as discarded tires and pot-plant bases, will most likely also kill the larvae and pupae of Ae. aegypti or destroy their larval food resources (microorganisms; Barrera et al. 2004), but field trials are required to evaluate the joint impact on eggs, larvae, and pupae.
Sodium hypochlorite is registered with the US Environmental Protection Agency (USEPA) as an antimicrobial for treating potable water and is one of the most commonly used chemical disinfectants for household water treatment in developing countries (WHO/UNICEF 2011). Although a maximum concentration of 4 ppm is permitted, it is recommended that chlorine concentrations in drinking water not exceed 2 ppm to avoid any unpleasant taste or odor (USEPA 1998, WHO 2011). In an effort to minimize runoff from treated surfaces, ovicide applications to water storage drums in the current study employed a high NaOCl concentration applied at a lower volume. Chlorine concentrations in drums treated with 32 ml of a 4:1 dilution of household bleach briefly reached ≈ 4 ppm chlorine after application and dropped to below 2 ppm immediately after flooding. These results demonstrate an effective application of household bleach treatments for removing eggs in water storage containers can also potentially meet disinfection requirements for improving water quality without resulting in residual chlorine concentrations that are unpalatable or a health risk to humans.
Published guidelines for eliminating Aedes spp. eggs in containers for quarantine purposes recommend spraying a 10 ppt hypochlorite solution to surfaces in containers, with a minimum exposure period of 5 sec (Lamache and Whelan 2002). Our findings indicate that this concentration and exposure period may not be sufficient to provide an acceptable level of efficacy on all surface materials. Addition of a thickening agent (e.g., smectite clay), however, can allow an equivalent NaOCl concentration (≈ 10 ppt) to be effective in a broad range of surfaces, and thus should be appropriate for treating common container classes likely to collect nonpotable water that cannot be eliminated or otherwise prevented from supporting vector mosquito development. Addition of smectite clay also increases the visibility of household bleach on surfaces during application, reducing the risk of inadvertent retreatment of containers and potentially enabling the operator to achieve a more uniform application.
While the household bleach and bleach-smectite treatments evaluated in this study were effective in laboratory and small-scale field trials, several operational limitations must be considered if they are to be used in a vector control program. Direct exposure to NaOCl can cause corrosion of metallic surfaces, and damage to paint, wood, and natural fabrics, so household bleach applications may be unsuited for some types of containers and in certain settings. As exposure of treated surfaces to rainfall or refilling soon after application could compromise efficacy, uncovered containers should be turned over after treatment when possible or a higher NaOCl concentration may be considered to reduce the minimum exposure time required for a satisfactory level of efficacy. Most commercial bleach products remain stable for long periods under favorable storage conditions; however, a rapid decline in NaOCl concentration can occur after dilution, especially when directly exposed to sunlight or high temperatures, so formulations should be prepared shortly before use if a dilution of household bleach is being applied. While products containing sodium hypochlorite have widespread approval for use as antimicrobial pesticides for disinfection of indoor and outdoor surfaces, regulatory agencies in some countries may require additional registration before household bleach can be applied to container habitats for the specific purpose of eliminating mosquito eggs.
We believe that the method we have described for applying household bleach and bleach-smectite formulations to eliminate the egg stage of Ae. aegypti in container habitats is a promising tool for integration into a community-based vector control program. Household bleach is inexpensive, readily available, and most people are already familiar with and may be more likely to accept the application of bleach to containers outside their home, compared with many alternative control measures. With minimal training, these materials can be applied using a simple compression or pump sprayer and standard personal protective equipment (impermeable gloves and eye protection). However, additional studies will be required to evaluate whether the prescribed methods identified in the current study are effective and practical when used in a large-scale field trials to eliminate Ae. aegypti eggs from naturally colonized containers.
Acknowledgments
We would like to express our gratitude to Angel Berrios, Belkis Caban, Jesus Flores, Jose Gonzales, Orlando Gonzalez, Juan F. Medina, and Luis Riviera for providing assistance with the laboratory and field assays. We would also like to thank Vanderbilt Chemicals for providing a sample of VanGel O smectite clay.
References Cited
- Barrera R, Amador A, Clark GG. The use of household bleach to control Aedes aegypti. J Am Mosq Control Assoc. 2004;20:444–448. [PubMed] [Google Scholar]
- Barrera R, Amador A, Mackay AJ. Population dynamics of Aedes aegypti and dengue as influenced by weather and human behavior in San Juan, Puerto Rico. PLoS Negl Trop Dis. 2011;5:e1378. doi: 10.1371/journal.pntd.0001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun DB, Sosen MR. Rheology modifiers handbook: practical use and application. Norwich, NY: William Andrew Publishing; 1999. p. 512. [Google Scholar]
- Carretero MI. Clay minerals and their beneficial effects upon human health. A review. Appl Clay Sci. 2002;21:155–163. [Google Scholar]
- Chadee DD. Impact of pre-seasonal focal treatment on population densities of the mosquito Aedes aegypti in Trinidad, West Indies: a preliminary study. Acta Tropica. 2009;109:236–240. doi: 10.1016/j.actatropica.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Christophers SR. Aedes aegypti (L) The yellow fever mosquito, its life history, bionomics and structure. London, United Kingdom: Cambridge Univ. Press; 1960. [Google Scholar]
- Dibo MR, Chierotti AP, Ferrari MS, Mendonça AL, Neto FC. Study of the relationship between Aedes (Stegomyia) aegypti egg and adult densities, dengue fever and climate in Mirassol, state of São Paulo, Brazil. Mem Inst Oswaldo Cruz. 2008;103:554–560. doi: 10.1590/s0074-02762008000600008. [DOI] [PubMed] [Google Scholar]
- Domenico DD, Ruggeri L, Trentini M. The use of sodium hypochlorite as ovicide against Aedes albopictus. J Am Mosq Control Assoc. 2006;22:346–348. doi: 10.2987/8756-971X(2006)22[346:TUOSHA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Esteva L, Yang HM. Mathematical model to assess the control of Aedes aegypti mosquitoes by the sterile insect technique. Math Biosci. 2005;198:132–147. doi: 10.1016/j.mbs.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Fernandez EA, Leontsini E, Sherman C, Chan AST, Reyes CE, Lozano RC, Fuentes BA, Nichter M, Winch PJ. Trial of a community-based intervention to decrease infestation of Aedes aegypti mosquitoes in cement washbasins in El Progreso, Honduras. Acta Tropica. 1998;70:171–183. doi: 10.1016/s0001-706x(98)00033-3. [DOI] [PubMed] [Google Scholar]
- Fisher S, Alem IS, De Majo MS, Campos RE, Schweigmann N. Cold season mortality and hatching behavior of Aedes aegypti L. (Diptera: Culicidae) eggs in Buenos Aires City, Argentina. J Vector Ecol. 36(2011):94–99. doi: 10.1111/j.1948-7134.2011.00145.x. [DOI] [PubMed] [Google Scholar]
- Fox I. Viability of Puerto Rican Aedes aegypti eggs after long periods of storage. Mosq News. 1974;43:274–275. [Google Scholar]
- Fox I. Aedes aegypti reared from dry artificial habitats during drought in Puerto Rico in 1974. Mosq News. 1975;35:202–203. [Google Scholar]
- Frazer AC, Smyth JN, Bhupathiraju VK. Sporicidal efficacy of pH-adjusted bleach for control of bioburden on production facility surfaces. J Ind Microbiol Biotechnol. 2013;40:601–611. doi: 10.1007/s10295-013-1257-7. [DOI] [PubMed] [Google Scholar]
- Hatchett SP. Chlorine as a possible ovicide for Aedes aegypti eggs. Publ Hlth Rep. 1946;63:683–685. [PubMed] [Google Scholar]
- Jacups SP, Ball TS, Paton CJ, Johnson PH, Ritchie SA. Operational use of household bleach to “crash and release” Aedes aegypti prior to Wolbachia-infected mosquito release. J Med Entomol. 2013;50:344–351. doi: 10.1603/me12043. [DOI] [PubMed] [Google Scholar]
- Judson CL, Hokami Y, Bray AD. The effects of various chemicals on eggs of the yellow-fever mosquito, Aedes aegypti. J Econ Entomol. 1962;55:805–807. [Google Scholar]
- Lamache G, Whelan P. Recommended chlorination procedures for receptacles containing mosquito eggs for quarantine purposes. Bull Mosq Control Assoc Aust. 2002;14:14–18. [Google Scholar]
- Leyva M, Tacoronte JE, Marquetti MC, Scull R, Tiomno O, Mesa A, Montada D. Utilización de aceites esenciales de pinaceas endémicas como una alternativa en el control del Aedes aegypti. Rev Cubana Med Trop. 2009;61:239–243. [Google Scholar]
- Luz C, Tai MHH, Santos AH, Rocha LFN, Albernaz DAS, Silva HHG. Ovicidal activity of entomopathogenic hyphomycetes on Aedes aegypti (Diptera: Culicidae) under laboratory conditions. J Med Entomol. 2007;44:799–804. doi: 10.1603/0022-2585(2007)44[799:oaoeho]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Machaca J, Llontop F, Pasapera F, Castañeda C. Eliminación mecánica de huevos del Aedes aegypti para la erradicación del Dengue urbano. Localidad de Sechura - Piura, Abril - Diciembre 2001. Rev Peru Epidemiol. 2002;10:1–5. [Google Scholar]
- Mohapatra R, Ranjit MR, Dash AP. Evaluation of cyfluthrin and fenfluthrin for their insecticidal activity against three vector mosquitoes. J Commun Dis. 1999;31:91–99. [PubMed] [Google Scholar]
- Rezende GL, Martins AJ, Gentile C, Farnesi LC, Pelajo-Machado M, Peixoto AA, Valle D. Embryonic desiccation resistance in Aedes aegypti: presumptive role of the chitinized serosal cuticle. BMC Developmental Biol. 2008;8:182. doi: 10.1186/1471-213X-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie SA. Efficacy of Australian quarantine procedures against the mosquito Aedes aegypti. J Am Mosq Control Assoc. 2001;17:114–117. [PubMed] [Google Scholar]
- Russell BM, Kay BH, Shipton W. Survival of Aedes aegypti (Diptera: Culicidae) eggs in surface and subterranean breeding sites during the northern Queensland dry season. J Med Entomol. 2001;38:441–445. doi: 10.1603/0022-2585-38.3.441. [DOI] [PubMed] [Google Scholar]
- Sherman C, Fernandez EA, Chan AS, Lozano RC, Leontsini E, Winch PJ. La untadita: a procedure for maintaining washbasins and drums free of Aedes aegypti based on modifications of existing practices. Am J Trop Med Hyg. 1998;58:257–262. doi: 10.4269/ajtmh.1998.58.257. [DOI] [PubMed] [Google Scholar]
- Sinniah B. Insecticidal effect of aliphatic alcohols against aquatic stages of Aedes mosquitoes. Trans R Soc Trop Med Hyg. 1983;77:35–38. doi: 10.1016/0035-9203(83)90007-x. [DOI] [PubMed] [Google Scholar]
- Sota T, Mogi M. Interspecific variation in desiccation survival time of Aedes (Stegomyia) mosquito eggs is correlated with habitat and egg size. Oecologia. 1992;90:353–358. doi: 10.1007/BF00317691. [DOI] [PubMed] [Google Scholar]
- Surtees G. Laboratory studies on the survival of the eggs of Aedes (Stegomyia) aegypti under adverse conditions. W Afr Med J. 1958;7:52–53. [PubMed] [Google Scholar]
- Trpis M. A new bleaching and decalcifying method for general use in zoology. Can J Zool. 1970;48:892–893. [Google Scholar]
- Trpis M. Dry season survival of Aedes aegypti eggs in various breeding sites in the Dar es Salaam area, Tanzania. Bull World Health Organ. 1972;47:433–437. [PMC free article] [PubMed] [Google Scholar]
- USEPA [U.S. Environmental Protection Agency] National primary drinking water regulations: disinfectants and disinfection byproducts. Fed Reg. 1998;63:69390–69476. [Google Scholar]
- Vezzani D, Velázquez SM, Schweigmann N. Seasonal pattern of abundance of Aedes aegypti (Diptera: Culicidae) in Buenos Aires city, Argentina. Mem Inst Oswaldo Cruz. 2004;99:351–356. doi: 10.1590/s0074-02762004000400002. [DOI] [PubMed] [Google Scholar]
- Wallis RC. A study of oviposition activity of mosquitoes. Amer J Hyg. 1954;60:135–168. doi: 10.1093/oxfordjournals.aje.a119710. [DOI] [PubMed] [Google Scholar]
- WHO [World Health Organization] Guidelines for drinking-water quality. 4th. WHO; Geneva, Switzerland: 2011. p. 541. [Google Scholar]
- WHO/UNICEF [World Health Organization and United Nations Children's Fund] Drinking water: equity, safety and sustainability—joint monitoring program thematic report on drinking water. WHO/UNICEF; Geneva, Switzerland: 2011. p. 62. [Google Scholar]


