Abstract
Fresh fruits and vegetables are an important part of a healthy diet. Melons have been associated with enteric infections. We reviewed outbreaks reported to the Centers for Disease Control and Prevention’s Foodborne Disease Outbreak Surveillance System during 1973–2011 in which the implicated food was a single melon type. We also reviewed published literature and records obtained from investigating agencies. During 1973–2011, 34 outbreaks caused by a single melon type were reported, resulting in 3602 illnesses, 322 hospitalizations, 46 deaths, and 3 fetal losses. Cantaloupes accounted for 19 outbreaks (56%), followed by watermelons (13, 38%) and honeydew (2, 6%). Melon-associated outbreaks increased from 0.5 outbreaks per year during 1973–1991 to 1.3 during 1992–2011. Salmonella was the most common etiology reported (19, 56%), followed by norovirus (5, 15%). Among 13 outbreaks with information available, melons imported from Mexico and Central America were implicated in 9 outbreaks (69%) and domestically grown melons were implicated in 4 outbreaks (31%). The point of contamination was known for 20 outbreaks; contamination occurred most commonly during growth, harvesting, processing, or packaging (13, 65%). Preventive measures focused on reducing bacterial contamination of melons both domestically and internationally could decrease the number and severity of melon-associated outbreaks.
Introduction
Consumption of fresh produce in the United States has increased over the last 40 years (United States General Accounting Office, 2002). Global trade has allowed American consumers to expect year-round availability of fresh fruits and vegetables (Pollack, 2001). In addition, initiatives to combat chronic diseases have brought awareness of the nutritional value of fresh produce as part of a healthy diet (United States General Accounting Office, 2002). At the same time, an increasing number of foodborne disease outbreaks have been associated with fresh produce (Lynch et al., 2009). Between 1973 and 1987, fresh produce caused only 2% of reported foodborne disease outbreaks (Sivapalasingam et al., 2004), but by 2009–2010 fresh produce was implicated in 23% (CDC, 2013b).
Melons are frequently implicated in produce-associated outbreaks (Sivapalasingam et al., 2004). For example, recurrent outbreaks of Salmonella enterica serotype Poona infections associated with Mexican cantaloupes occurred annually from 2000 to 2002, resulting in importation restrictions for implicated producers (CDC, 2002). More recently, the possible severity of outbreaks caused by contaminated melons was underscored by the 2011 outbreak of Listeria monocytogenes infections caused by contaminated cantaloupes; it was the deadliest foodborne outbreak in the United States since the 1920s (CDC, 2011b). To better understand the frequency and characteristics of melon-associated outbreaks, we examined data reported to the Centers for Disease Control and Prevention’s (CDC) Food-borne Disease Outbreak Surveillance System (FDOSS).
Materials and Methods
State, local, territorial, and tribal health departments voluntarily submit reports of foodborne disease outbreaks to FDOSS. We defined melon-associated outbreaks as the occurrence of similar illnesses in ≥ 2 persons resulting from consumption of a melon, including cantaloupe (or musk-melon), watermelon, or honeydew. We defined multistate outbreaks as those where illnesses linked to ingestion of melons occurred in more than one state. For this analysis, we defined precut melons as fresh whole melons that were sliced or cut, with or without washing, before use by the consumer or retail establishment (Fleming et al., 2005).
We analyzed outbreak frequency, size, month and year, geographic location, case demographics (i.e., sex and age group), number of hospitalizations and deaths, etiologies (confirmed and suspected), location of food preparation, and type of melon reported. We analyzed the points of melon contamination (production or point of service) as well as their origin (imported or domestic). We defined contamination at the point of service as contamination caused by either an implicated foodworker or cross-contamination with other foods. We assumed that if a food-worker was implicated as the source, contamination occurred during food preparation at the point of service. We defined contamination that occurred during production as contamination that occurred during growth, harvesting, processing, or packaging before the point of service. We categorized reported locations of food preparation into six groups: restaurant, private home, grocery store, institution (e.g., camp, school, and day care), other, and multiple locations (i.e., more than one location reported). We corrected missing or incompletely reported data by reviewing published literature and by contacting the agencies that conducted the investigation.
We used food availability, consumption, and supply data on fresh melons from 1973 to 2010 from the United States Department of Agriculture’s Economic Research Service (USDA, 2012) and from CDC’s FoodNet Population Survey (CDC, 2007) to estimate melon consumption to determine whether changes in consumption were correlated with changes in outbreak frequency using the Pearson correlation test. We analyzed data using SAS Version 9.3 (SAS Institute, Cary, NC) and Microsoft Access 2010 and Microsoft Excel 2010 (Microsoft, Redmond, WA).
Results
During 1973–2011, 34 outbreaks caused by the consumption of cantaloupes, watermelons, or honeydews were reported, resulting in 3602 illnesses, 322 hospitalizations, 46 deaths, and 3 fetal losses (Table 1). Outbreak reports specifying more than 1 type of melon (12 outbreaks), in which the melon type was unspecified (8), or in which melons and other fruits were reported (e.g., fruit salad, 43), were excluded from analysis. Cantaloupes were the most commonly reported melon type (19 outbreaks, 56%), followed by watermelons (13, 38%) and honeydews (2, 6%). Outbreaks occurred in 45 states; California reported the most (16), followed by Colorado (11), Oregon (11), and Washington (10).
Table 1.
Melon-Associated Outbreaks, Illnesses, Hospitalizations, Deaths, and Fetal Losses—United States, 1973–2011
|
Cantaloupea
|
Watermelon
|
Honeydew
|
All melons n |
||||
|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | ||
| Outbreaks | 19 | (56) | 13 | (38) | 2 | (6) | 34 |
| Illnesses | 1012 | (28) | 2466 | (68) | 124 | (3) | 3602 |
| Hospitalizations | 215 | (67) | 91 | (28) | 16 | (5) | 322 |
| Deaths | 37 | (80) | 7 | (15) | 2 | (4) | 46 |
| Fetal losses | 1 | (33) | 2 | (67) | 0 | (0) | 3 |
The high percentage of hospitalizations and deaths associated with cantaloupe is strongly influenced by a single 2011 Listeria outbreak (McCollum et al., 2013) with 147 illnesses, 143 hospitalizations, 33 deaths, and 1 fetal loss.
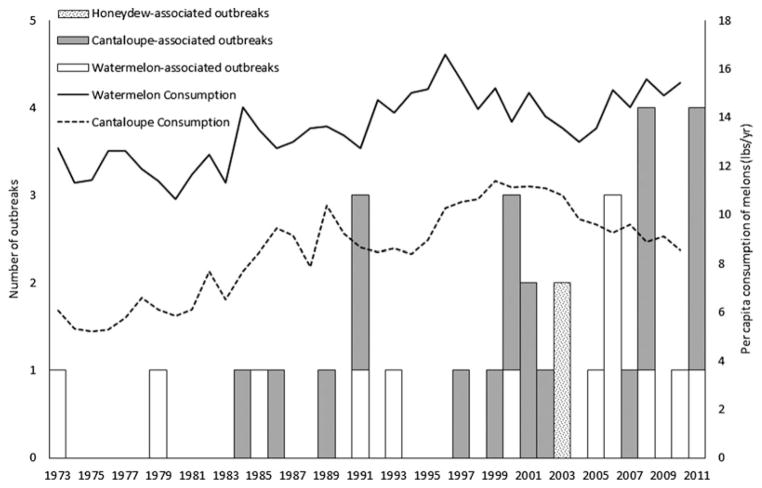
The frequency of melon-associated outbreaks increased from 0.5 outbreaks per year during 1973–1991 to 1.3 per year during 1992–2011 (Fig. 1). During the study period (1973–2011), the per capita consumption of watermelons (14 lb/y) was greater than that of cantaloupes (9 lb/y) or honeydews (2 lb/y) (Fig. 1). More recently, the per capita consumption of cantaloupes decreased from 11.1 lb in 2000 to 8.5 lb in 2010, while the per capita consumption of watermelons increased, from 13.8 lb in 2000 to 15.4 lb in 2010. Changes in consumption were weakly correlated with changes in the number of outbreaks caused by any type of melon (r = 0.35 for watermelon, r = − 0.15 cantaloupe).
FIG. 1.
Number of outbreaks* by melon type and per capita consumption† in pounds per year (lbs/yr) of cantaloupes and watermelons, United States, 1973–2011. *Source: Centers for Disease Control and Prevention, Foodborne Disease Outbreak Surveillance System. †Source: U.S. Department of Agriculture, Economic Research Service.
More illnesses in these outbreaks involved women than men (cantaloupes: 61% women; watermelons: 67%; honeydews: 58%). In outbreaks attributed to cantaloupes, 68% of illnesses were among persons aged > 50 years old, compared with 54% in outbreaks caused by honeydews and 19% in outbreaks caused by watermelons. Outbreaks caused by watermelons more commonly affected young children (1–4 years old: 42%) than outbreaks caused by cantaloupes (3%) and honeydews (5%).
Outbreaks were most often caused by Salmonella (19 outbreaks, 56%), followed by norovirus (5 outbreaks, 15%) (Table 2). The etiology was confirmed in all but one outbreak in which norovirus was suspected. Of the 12 Salmonella enterica serotypes reported, the two most common were Poona and Javiana. Their frequency varied by melon type; Poona was always associated with cantaloupes (four of four Poona outbreaks) and Javiana most commonly with watermelons (three of four Javiana outbreaks).
Table 2.
Characteristics of Melon-Associated Outbreaks by Melon Type—United States, 1973–2011
|
Cantaloupe
|
Watermelon
|
Honeydew
|
All melons
|
|||||
|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | |
| Etiology (no. with information) | 19 outbreaks | 13 outbreaks | 2 outbreaks | 34 outbreaks | ||||
| Salmonellaa | 11 | (58) | 7 | (54) | 1 | (50) | 19 | (56) |
| Norovirusb | 3 | (16) | 2 | (15) | 0 | (0) | 5 | (15) |
| Unknown | 2 | (11) | 1 | (8) | 0 | (0) | 3 | (9) |
| Campylobacter jejuni | 1 | (5) | 1 | (8) | 0 | (0) | 2 | (6) |
| Shiga toxin–producing Escherichia coli | 1 | (5) | 1 | (8) | 0 | (0) | 2 | (6) |
| Aldicarb (pesticide) | 0 | (0) | 1 | (8) | 0 | (0) | 1 | (3) |
| Listeria monocytogenes | 1 | (5) | 0 | (0) | 0 | (0) | 1 | (3) |
| Shigella sonnei | 0 | (0) | 0 | (0) | 1 | (50) | 1 | (3) |
| Locations of food preparation (no. with information) | 17 outbreaks | 10 outbreaks | 2 outbreaks | 29 outbreaks | ||||
| Multiple locationsc | 9 | (53) | 2 | (20) | 1 | (50) | 12 | (41) |
| Restaurant | 3 | (18) | 2 | (20) | 1 | (50) | 6 | (21) |
| Grocery store | 2 | (12) | 2 | (20) | 0 | (0) | 4 | (14) |
| Private home | 2 | (12) | 1 | (10) | 0 | (0) | 3 | (10) |
| Institution | 0 | (0) | 2 | (20) | 0 | (0) | 2 | (7) |
| Catered event or picnic | 1 | (6) | 1 | (10) | 0 | (0) | 2 | (7) |
| Point of contamination (no. with information) | 19 outbreaks | 13 outbreaks | 2 outbreaks | 34 outbreaks | ||||
| Unknown | 6 | (32) | 7 | (54) | 1 | (50) | 14 | (41) |
| Production | 10 | (52) | 2 | (15) | 1 | (50) | 13 | (38) |
| Point of service | 3 | (16) | 4 | (31) | 0 | (0) | 7 | (21) |
Serotypes were Poona (four outbreaks), Javiana (four), Newport (two), and Chester, Oranienburg, Saphra, Litchfield, Saintpaul, Typhimurium, Panama, Uganda, and Anatum (one each).
All laboratory-confirmed except one suspected norovirus outbreak.
Food served at more than one location.
The location of preparation was reported for 29 outbreaks (85%) (Table 2). Among the 17 (59%) with a single preparation location, restaurant or deli (6 outbreaks, 35%), grocery store (4, 24%), and private home (3, 18%) were most commonly reported.
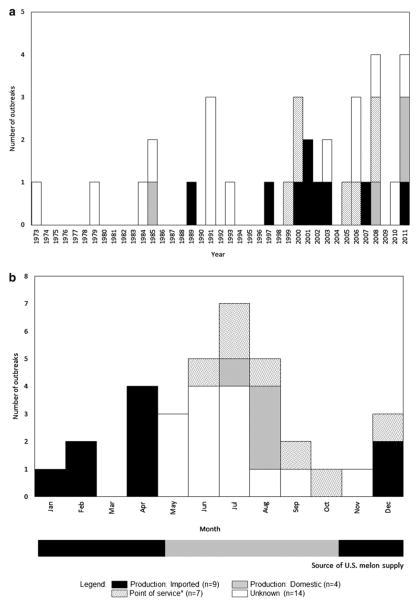
Among 20 outbreaks (59%) with available contamination information, melons were contaminated during production in 13 (65%) and at the point of service in 7 (35%). Among 22 outbreaks with information available, precutting of melons was reported as contributing to contamination for 17 (77%). Among 13 outbreaks attributed to contamination during production, 9 outbreaks (69%) involved imported melons and 4 outbreaks (31%) involved domestically grown melons. Among the outbreaks caused by imported melons, 8 (88%) were caused by cantaloupes and 1 (13%) by honeydews. The annual occurrence of outbreaks caused by melons from domestic and imported sources varied, but followed production patterns (Fig. 2a). Melon-associated outbreaks occurred most often during June–August (50%) (Fig. 2b). Outbreaks associated with melons contaminated during production and imported (n = 9) were only reported during winter months; outbreaks associated with melons contaminated during production and domestically grown (n = 4) were only reported during summer months.
FIG. 2.
(a) Number of melon-associated outbreaks by contamination source and year—United States, 1973–2011. (b) Number of melon-associated outbreaks by contamination source and the usual source of the melon supply, and by month†—United States, 1973–2011. *Point of service includes five outbreaks, which were caused by implicated foodworkers and one outbreak caused by E. coli O157:H7-contaminated watermelons that were cross-contaminated by raw meat. †Source: U.S. Department of Agriculture, Economic Research Service.
There were 13 (38%) multistate outbreaks (Table 3). Of these, 11 (85%) were caused by cantaloupes, 1 (8%) by watermelon, and 1 (8%) by honeydew. The median number of states involved in multistate outbreaks was 10 (range: 5–30). Multistate outbreaks (median number of cases: 50 [range: 10–949]) were generally larger than single-state outbreaks (18 [range: 4–736]). Many multistate outbreaks (8, 62%) were caused by melons imported from Mexico and Central America (Table 3). Two multistate outbreaks were caused by domestically grown melons from Colorado and California. For most of the multistate outbreaks (10, 77%), the initial contamination occurred during production; for 3 (23%) the source of contamination could not be determined.
Table 3.
Multistate Melon-Associated Outbreaks—United States, 1973–2011
| Year | Melon type | Domestic cases | Month | Etiology | Source of melon | Initial point of contaminationa | Public health action | Reference |
|---|---|---|---|---|---|---|---|---|
| 1985 | Watermelon | 949b | July | Aldicarb (pesticide) | California (probable) | Production | Embargo | (CDC, 1986) |
| 1989 | Cantaloupe | 295 | December |
Salmonella Chester |
Mexico, Guatemala | Production | (Ries et al., 1990) | |
| 1991 | Cantaloupe | 143b | May |
Salmonella Poona |
Texas, Mexico (probable) | Unknown | (CDC, 1991) | |
| 2000 | Cantaloupe | 46 | April |
Salmonella Poonac |
Mexico | Production | (CDC, 2002) | |
| 2001 | Cantaloupe | 50 | April |
Salmonella Poonac |
Mexico | Production | Embargo, recall | (CDC, 2002) |
| 2001 | Cantaloupe | 35 | April |
Salmonella Anatumc |
Mexico (probable) | Production | ||
| 2002 | Cantaloupe | 26b | April |
Salmonella Poonac |
Mexico | Production | Recall, import alert | (CDC, 2002) |
| 2003 | Honeydew | 68 | January |
Salmonella Newport |
Central America | Production | ||
| 2007 | Cantaloupe | 53b | December |
Salmonella Litchfield |
Honduras | Production | Recall, import alert | (CDC, 2008a) |
| 2008 | Cantaloupe | 10 | November |
Salmonella Javiana |
Unknown | Unknown | ||
| 2011 | Cantaloupe | 20 | February |
Salmonella Panama |
Guatemala | Production | Recall | (CDC, 2011a) |
| 2011 | Cantaloupe | 25 | June |
Salmonella Uganda |
Unknown | Unknown | ||
| 2011 | Cantaloupe | 146 | August | Listeria monocytogenes | Colorado | Production | Recall | (CDC, 2011b) |
Production includes growth, harvesting, processing, and packaging before the point of service.
Additional cases in Canada.
Outbreaks were all traced to same distributor.
Discussion
The average annual number of outbreaks caused by melons in the United States increased during 1973–2011. Changes in melon consumption are unlikely to explain the observed increase in melon outbreaks; cantaloupe was the most common melon type reported in outbreaks, yet in recent years cantaloupe consumption has decreased. Several other explanations for the increase in melon-associated outbreaks are possible. Enhancements in outbreak detection, investigation, and reporting may have led to an increase in the number of reported outbreaks in recent years. In 1996, the national molecular subtyping network (PulseNet) was established to identify cases of enteric illnesses with similar bacterial strains and improve outbreak detection (Swaminathan et al., 2001). National participation in PulseNet for Salmonella isolates was reached in 2001, and the number of Salmonella isolates subtyped increased almost ninefold between 2001 and 2012 (Peter Gerner-Smidt, CDC, personal communication). Increased PulseNet participation likely contributed to an increased number of multistate outbreaks detected, including those attributed to melons (CDC, 2013a). In addition, reporting of foodborne disease outbreaks transitioned from paper-based to electronic reporting in 1998, and the number of outbreaks reported doubled (CDC, 2006). The increase in melon-associated outbreaks may in part be a result of these enhancements in detection and reporting. These changes have also been noted for other food commodities (CDC, 2013b).
Cantaloupes accounted for more than half of outbreaks attributed to melons; this is likely due in part to differences in the physical attributes of cantaloupe compared with other melon types. Cantaloupes have rough, netted surfaces that make them more difficult to clean. Surface irregularities such as roughness, crevices, and pits increase bacterial adherence and reduce the ability of washing treatments to remove bacterial cells (Frank et al., 1990; Austin et al., 1995). Once present on the surface of a cantaloupe, specifically on and within the ridges and corky tissues of the netting, pathogens cannot be completely eliminated by washing (Parnell et al., 2005). In comparison, watermelons and honeydews have smoother surfaces that are more amenable to pathogen removal by washing; also, efforts to reduce microbial contamination on the rind have been shown to be more effective with honeydews than cantaloupes (Ukuku, 2004). In addition, biofilms readily form on cantaloupe rinds and are resistant to antimicrobial agents (Annous et al., 2005). Precut melons were implicated in many of these outbreaks. During the 1990s, changes in retail marketing made melons more widely available to consumers as precut, convenience products (Boriss et al., 2006). In 2003, melons, including fruit salads and melon mixes, accounted for 65% of precut products (Mayen et al., 2003). Precutting melons leads to additional opportunities for contamination and pathogen amplification. Slicing into a melon can transfer pathogens from the surface to the edible flesh (Patil et al., 2013), or may lead to cross-contamination of other melons. Bacterial pathogens grow rapidly on the edible flesh of cut melons held at room temperatures (Golden et al., 1993; Ukuku et al., 2012).
The initial source of contamination in most outbreaks, particularly those that were multistate, was during production. Because melons are grown on the ground, their surfaces can become contaminated with dirt, chemicals, animal excreta, and bacteria. The most common Salmonella serotypes implicated in outbreaks, Poona and Javiana, have been associated with reptilian reservoirs (Jackson et al., 2013). In a recent outbreak investigation, cultures of samples of cantaloupe collected from the fields yielded Salmonella (FDA, 2012a). Poor sanitary practices in packing sheds and inadequate monitoring of chlorinated wash water have also contributed to contamination (Castillo et al., 2004). Improper cooling and cold storage practices and equipment that is difficult to clean may lead to contamination of melons (FDA, 2011). Although the cantaloupe industry took actions in 2005 to address contamination during production (Fleming et al., 2005), it is unknown how widely they were implemented. The Food and Drug Administration (FDA) continues to urge cantaloupe production facilities to review their practices in the context of current food safety guidance documents developed by FDA (FDA, 2009), industry trade organizations, and academic institutions to address common risk factors for contamination in their operations (FDA, 2013). Given the difficulty in removing pathogens during food preparation and the fact that melons are usually consumed raw, efforts should focus on the prevention of microbial contamination at all steps from production to distribution.
After melons have been transported to the point of service, they can be cross-contaminated by a foodworker (CDC, 2008b) using improperly cleaned work surfaces and cutting utensils or by another food; bacterial contamination can also be amplified by improper storage (CDC, 1979). For example, an outbreak in a restaurant in 2000 was attributed to watermelons cross-contaminated with Escherichia coli O157:H7 by raw meat products on food preparation surfaces (CDC, Foodborne Disease Outbreak Surveillance System (unpublished data) 2013). Efforts to intervene at the point of service should continue. Current recommendations for preparing fresh produce both at retail and at home include thorough washing under running water before cutting and storage at temperatures of 40°F or below. In addition, for firm produce, such as melons, scrubbing with a clean produce brush is recommended (Fleming et al., 2005). While scrubbing a melon with a produce brush has been shown to significantly reduce contamination on the rind (Parnell et al., 2005), it is not known whether this practice decreases the transfer of the organism to the edible part of the melon. In addition, when melons are scrubbed in water, contamination may spread from a localized region on the rind to surrounding areas (Parnell et al., 2005). Understanding the most likely source of melons during a particular period can be useful for rapid outbreak investigation and identification of implicated foods. The source of melons implicated in these outbreaks was reflected in known distribution patterns for domestic and imported melons. For example, although most outbreaks were reported during the summer months, when the consumption of melons is also the highest (CDC, 2007), outbreaks caused by imported melons only occurred during December–April, coinciding with the months during which most of the United States melon supply is sourced from foreign locales, particularly Latin American countries, including Mexico and Central America (Boriss et al., 2006). In addition to being more common in the winter months, outbreaks caused by imported melons were often geographically widespread. Through import alerts and laboratory-based monitoring systems for imported cantaloupes, FDA and the Mexican government have reduced the number of outbreaks in the United States caused by Salmonella and attributed to Mexican cantaloupes (FDA, 2012b); since 2005, none have been reported. However, outbreaks continue to be associated with imported cantaloupes from Central America.
The findings in this report are subject to limitations. The number of outbreaks caused by melons is likely underestimated. Because melons, like other produce items, have a limited shelf life, the implicated melon might be consumed or discarded before an outbreak investigation begins. In addition, because cantaloupe, watermelon, and honeydew are often consumed together (e.g., fruit salad) or alongside other foods, it can be difficult to implicate a specific type of melon. Even when a single melon type is suspected, poor labeling and comingling of melons during repacking and distribution can make traceback difficult. Finally, the source of melon contamination was unknown for one third of outbreaks; many of these outbreaks occurred during the summer months with either inconclusive or no information on traceback reported.
Conclusions
Preventive measures focused on reducing contamination of melons by bacterial pathogens during production on domestic and international farms and in packinghouses and processing facilities could decrease the number and severity of melon-associated outbreaks. Retail establishments should review policies related to sick leave for ill foodworkers, practices to improve proper storage of melons, especially precut melons, and methods to reduce cross-contamination during food preparation and storage. In addition, populations at high risk for severe complications of infections caused by enteric pathogens, including the young, old, and immunocompromised, or those preparing their meals, should be targeted for food safety education.
Acknowledgments
We would like to acknowledge the contributions made by Caitlin Monahan, Katherine Vierk, and Katherine Kreil from FDA’s Core Post Response Team; Bill Keene, formerly from the Oregon Department of Health; and Pat Kennelly from the California Department of Public Health.
Footnotes
Disclosure Statement
The findings and conclusions are those of the authors and do not necessarily represent those of the Centers for Disease Control and Prevention. No competing financial interests exist.
References
- Annous BA, Soloman EB, Cooke PH, Burke A. Biofilm formation by Salmonella spp. on cantaloupe melons. J Food Safety. 2005;25:276–287. [Google Scholar]
- Austin J, Bergeron G. Development of biofilms in dairy processing lines. J Dairy Res. 1995;62:509–519. doi: 10.1017/s0022029900031204. [DOI] [PubMed] [Google Scholar]
- Boriss H, Brunke H, Kreith M. Commodity Profile: Melons. Davis, CA: University of California Agricultural Issues Center; 2006. pp. 1–7. [Google Scholar]
- Castillo A, Mercado I, Lucia L. Salmonella contamination during production of cantaloupe: A binational study. J Food Prot. 2004;67:713–720. doi: 10.4315/0362-028x-67.4.713. [DOI] [PubMed] [Google Scholar]
- [CDC] Centers for Disease Control and Prevention. Salmonella Oranienburg gastroenteritis associated with pre-cut watermelons—Illinois. MMWR. 1979;28:522–523. [Google Scholar]
- CDC. Aldicarb food poisoning from contaminated melons—California. MMWR. 1986;35:254–258. [PubMed] [Google Scholar]
- CDC. Multistate outbreak of Salmonella Poona infections—United States and Canada, 1991. MMWR. 1991;40:549–552. [PubMed] [Google Scholar]
- CDC. Multistate outbreaks of Salmonella serotype Poona infections associated with eating cantaloupe from Mexico—United States and Canada, 2000–2002. MMWR. 2002;51:1044–1047. [PubMed] [Google Scholar]
- CDC. Surveillance for foodborne-disease outbreaks—United States, 1998–2002. MMWR. 2006;55:1–42. [PubMed] [Google Scholar]
- CDC. FoodNet Population Survey Data. Atlanta, GA: U.S. Department of Health and Human Services; 2007. [Google Scholar]
- CDC. Investigation of outbreak of infections caused by Salmonella Litchfield. [accessed May 2, 2014];National Center for Emerging and Zoonotic Infectious Diseases. 2008a Available at: http://www.cdc.gov/salmonella/litchfield/
- CDC. Salmonella Litchfield outbreak associated with a hotel restaurant—Atlantic City, New Jersey, 2007. MMWR. 2008b;57:775–779. [PubMed] [Google Scholar]
- CDC. Investigation update: multistate outbreak of Salmonella Panama infections linked to cantaloupe. [accessed May 2, 2014];National Center for Emerging and Zoonotic Infectious Diseases. 2011a Available at: http://www.cdc.gov/salmonella/panama0311/062311/index.html.
- CDC. Multistate outbreak of Listeriosis associated with Jensen Farms cantaloupe—United States, August–Septermber 2011. MMWR. 2011b;60:1357–1358. [PubMed] [Google Scholar]
- CDC. Surveillance for foodborne disease outbreaks—United States, 1998–2008. MMWR. 2013a;62:1–34. [PubMed] [Google Scholar]
- CDC. Surveillance for foodborne disease outbreaks—United States, 2009–2010. MMWR. 2013b;62:41–47. [PMC free article] [PubMed] [Google Scholar]
- Fleming P, Pool B, Fruit UF. Commodity Specific Food Safety Guidelines for the Melon Supply Chain. Produce Marketing Association; Newark, DE: United Fresh Fruit and Vegetable Association; Washington, DC: 2005. pp. 1–39. [Google Scholar]
- [FDA] Food and Drug Administration. [accessed May 2, 2014];Guidance for industry: Guide to minimize microbial food safety hazards of melons; draft guidance. 2009 Available at: http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ProducePlantProducts/ucm174171.htm.
- FDA. [accessed May 2, 2014];Memorandum to the file of the EA conducted at Jensen Farms at Granada, Colorado on September 22–23, 2011. 2011 Available at: http://www.fda.gov/Food/guidanceComplianceRegulatoryInformation/GuidanceDocuments/ProduceandPlanProducts/ucm278456.htm.
- FDA. Form 483, Inspectional Observations at Chamberlain Farms; Owensville, IN. August 14–31, 2012; 2012a. [accessed May 2, 2014]. Available at: http://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofGlobalRegulatoryOperationsandPolicy/ORA/ORAElectronicReadingRoom/UCM322103.pdf. [Google Scholar]
- FDA. Import Alert 22-01: Dentention Without Physical Examination of Cantaloupes from Mexico. Vol. 2012. Silver Spring, MD: U.S. Food and Drug Administration; 2012b. [accessed May 2, 2014]. Available at: http://www.accessdata.fda.gov/cms_ia/importalert_67.html. [Google Scholar]
- FDA. [accessed May 2, 2014];Letter to Cantaloupe Industry on Produce Safety. 2013 Available at: http://www.fda.gov/AboutFDA/CentersOffices/OfficeofFoods/CFSAN/CFSANFOIAElectronicReadingRoom/ucm341029.htm.
- Frank J, Koffi R. Surface adherent growth of Listeria monocytogenes is associated with increased resistance to surfactant sanitizer and heat. J Food Prot. 1990;53:550–554. doi: 10.4315/0362-028X-53.7.550. [DOI] [PubMed] [Google Scholar]
- Golden D, Rhodehamel E, Kautter D. Growth of Salmonella spp. in cantaloupe, watermelon, and honeydew melons. J Food Prot. 1993;56:194–196. doi: 10.4315/0362-028X-56.3.194. [DOI] [PubMed] [Google Scholar]
- Jackson B, Griffin P, Cole D, Walsh K, Chai S. Outbreak-associated Salmonella enterica serotypes and food commodities, United States, 1998–2008. Emerg Infect Dis. 2013;19:1239–1244. doi: 10.3201/eid1908.121511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M, Tauxe R, Hedberg C. The growing burden of food-borne outbreaks due to contaminated fresh produce: Risks and opportunities. Epidemiol Infect. 2009;137:307–315. doi: 10.1017/S0950268808001969. [DOI] [PubMed] [Google Scholar]
- Mayen C, Marshall M. Opportunities in the Fresh-Cut Fruit Sector for Indiana Melon Growers. West Lafayette, IN: Purdue University, Department of Agricultural Economics; 2003. pp. 1–7. [Google Scholar]
- McCollum J, Cronquist A, Silk B, et al. Multistate outbreak of Listeriosis associated with cantaloupe. N Engl J Med. 2013;369:944–953. doi: 10.1056/NEJMoa1215837. [DOI] [PubMed] [Google Scholar]
- Parnell T, Harris L, Suslow T. Reducing Salmonella on cantaloupes and honeydew melons using wash practices applicable to postharvest handling, foodservice, and consumer preparation. Int J Food Microbiol. 2005;99:59–70. doi: 10.1016/j.ijfoodmicro.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Patil R, Thorns J, Ryser E. Extent of Listeria monocytogenes transfer during cutting of cantaloupe and honeydew melon. Program Abstract: International Association of Food Protection. J Food Prot. 2013;76(Suppl A):p3–p99. [abst] [Google Scholar]
- Pollack S. Changing Structure of Global Food Consumption and Trade. Washington, DC: United States Department of Agriculture Economic Research Service; 2001. Consumer demand for fruit and vegetables: The U.S. example; pp. 49–54. [Google Scholar]
- Ries A, Zaza S, Langkop C, Tauxe R, Blake P. Program Abstract: 30th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, DC: American Society of Microbiology; 1990. A multistate outbreak of Salmonella Chester linked to imported cantaloupe; p. 238. [Google Scholar]
- Sivapalasingam S, Friedman CR, Cohen L, Tauxe RV. Fresh produce: A growing cause of outbreaks of foodborne illness in the United States, 1973 through 1997. J Food Prot. 2004;67:2342–2353. doi: 10.4315/0362-028x-67.10.2342. [DOI] [PubMed] [Google Scholar]
- Swaminathan B, Barrett T, Hunter S, Tauxe R CDC PulseNet Task Force. PulseNet: The molecular subtyping network for foodborne bacterial disease surveillance, United States. Emerg Infect Dis. 2001;7:382–389. doi: 10.3201/eid0703.010303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukuku D. Effect of hydrogen peroxide treatment on microbial quality and appearance of whole and fresh-cut melons contaminated with Salmonella spp. J Food Microbiol. 2004;95:137–146. doi: 10.1016/j.ijfoodmicro.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Ukuku D, Olanya M, Geveke D, Sommers C. Effect of native microflora, waiting period, and storage temperature on Listeria monocytogenes serovars transferred from cantaloupe rind to fresh-cut pieces during preparation. J Food Prot. 2012;75:1912–1919. doi: 10.4315/0362-028X.JFP-12-191. [DOI] [PubMed] [Google Scholar]
- [USDA] United States Department of Agriculture. Food Availability Per Capita Data System. Economic Research Service; 2012. [accessed May 15, 2014]. Available at: http://ers.usda.gov/data-products/food-availability-(per-capita)-data-system.aspx. [Google Scholar]
- United States General Accounting Office. Fruits and vegetables: Enhanced federal efforts to increase consumption could yield health benefits for Americans. Washington, DC: General Accounting Office (GAO); 2002. pp. 1–6. Volume GAO-02-657. [Google Scholar]