Abstract
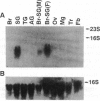
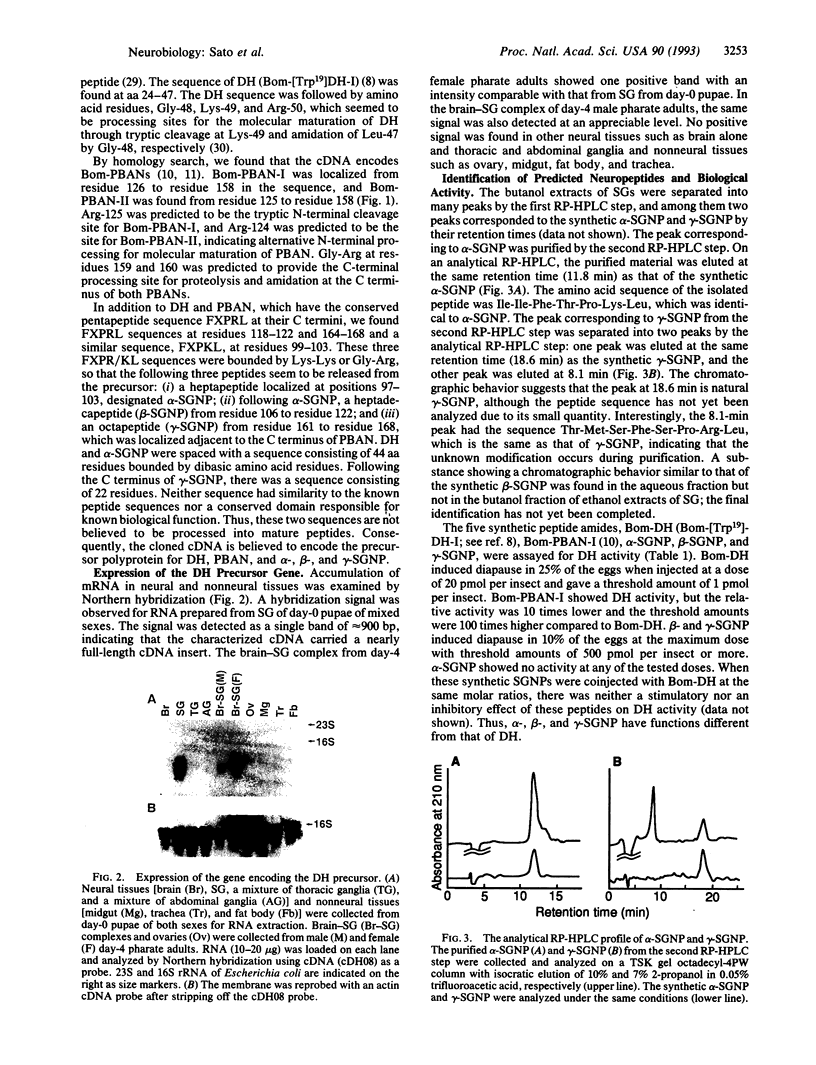
Peptidergic neurons, which serve as source of various endocrine neuropeptides, were identified in the suboesophageal ganglion (SG) and brain of insects. In the silkworm Bombyx mori, SG is known to secrete two neuropeptides, diapause hormone (DH) responsible for induction of embryonic diapause and pheromone biosynthesis-activating neuropeptide, which share a pentapeptide amide, Phe-Xaa-Pro-Arg-Leu-NH2 (Xaa = Gly or Ser), at the C terminus. We have isolated cDNA clones for DH from the cDNA library of SG by using oligonucleotide probes. The molecular characterization of the cDNA reveals that the mRNA encodes an open reading frame consisting of 192 aa residues in which the 24-aa DH peptide is localized at the N-terminal region just after the signal peptide. A homology search proposed that the cDNA encodes pheromone biosynthesis-activating neuropeptide and three other neuropeptides [alpha-, beta-, and gamma-SG neuropeptide (SGNP)] in the region following DH, all of which are flanked by possible tryptic cleavage sites and share the Phe-Xaa-Pro-Arg-Leu-Gly sequence at the C terminus. Northern hybridization analysis clearly showed that the gene expression was limited to SG. We chemically synthesized alpha-, beta-, and gamma-SGNP and used them to identify components in extracts of SG and to examine biological functions, alpha- and gamma-SGNP were identified in extracts of SG, and the synthetic beta- and gamma-SGNP expressed weak DH activity. These results indicate that DH, along with four other neuropeptides, is generated from a common precursor polyprotein that is encoded by a single mRNA transcribed in neurosecretory cells of SG.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradbury A. F., Finnie M. D., Smyth D. G. Mechanism of C-terminal amide formation by pituitary enzymes. Nature. 1982 Aug 12;298(5875):686–688. doi: 10.1038/298686a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Davis M. T., Vakharia V. N., Henry J., Kempe T. G., Raina A. K. Molecular cloning of the pheromone biosynthesis-activating neuropeptide in Helicoverpa zea. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):142–146. doi: 10.1073/pnas.89.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass J., Civelli O., Herbert E. Polyprotein gene expression: generation of diversity of neuroendocrine peptides. Annu Rev Biochem. 1984;53:665–715. doi: 10.1146/annurev.bi.53.070184.003313. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Holman G. M., Cook B. J., Nachman R. J. Primary structure and synthesis of a blocked myotropic neuropeptide isolated from the cockroach, Leucophaea maderae. Comp Biochem Physiol C. 1986;85(1):219–224. doi: 10.1016/0742-8413(86)90077-0. [DOI] [PubMed] [Google Scholar]
- Holman G. M., Nachman R. J., Wright M. S. Insect neuropeptides. Annu Rev Entomol. 1990;35:201–217. doi: 10.1146/annurev.en.35.010190.001221. [DOI] [PubMed] [Google Scholar]
- Innis M. A., Myambo K. B., Gelfand D. H., Brow M. A. DNA sequencing with Thermus aquaticus DNA polymerase and direct sequencing of polymerase chain reaction-amplified DNA. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9436–9440. doi: 10.1073/pnas.85.24.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura A., Nagasawa H., Kataoka H., Ando T., Suzuki A. Amino acid sequence of pheromone biosynthesis activating neuropeptide-II (PBAN-II) of the silkmoth, Bombyx mori. Agric Biol Chem. 1990 Sep;54(9):2495–2497. [PubMed] [Google Scholar]
- Kitamura A., Nagasawa H., Kataoka H., Inoue T., Matsumoto S., Ando T., Suzuki A. Amino acid sequence of pheromone-biosynthesis-activating neuropeptide (PBAN) of the silkworm, Bombyx mori. Biochem Biophys Res Commun. 1989 Aug 30;163(1):520–526. doi: 10.1016/0006-291x(89)92168-2. [DOI] [PubMed] [Google Scholar]
- Lynch D. R., Snyder S. H. Neuropeptides: multiple molecular forms, metabolic pathways, and receptors. Annu Rev Biochem. 1986;55:773–799. doi: 10.1146/annurev.bi.55.070186.004013. [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Fónagy A., Kurihara M., Uchiumi K., Nagamine T., Chijimatsu M., Mitsui T. Isolation and primary structure of a novel pheromonotropic neuropeptide structurally related to leucopyrokinin from the armyworm larvae, Pseudaletia separata. Biochem Biophys Res Commun. 1992 Jan 31;182(2):534–539. doi: 10.1016/0006-291x(92)91765-i. [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Isogai A., Suzuki A. N-terminal amino acid sequence of an insect neurohormone, melanization and reddish coloration hormone (MRCH): heterogeneity and sequence homology with human insulin-like growth factor II. FEBS Lett. 1985 Sep 9;189(1):115–118. doi: 10.1016/0014-5793(85)80853-x. [DOI] [PubMed] [Google Scholar]
- Mounier N., Prudhomme J. C. Isolation of actin genes in Bombyx mori: the coding sequence of a cytoplasmic actin gene expressed in the silk gland is interrupted by a single intron in an unusual position. Biochimie. 1986 Sep;68(9):1053–1061. doi: 10.1016/s0300-9084(86)80179-1. [DOI] [PubMed] [Google Scholar]
- Nachman R. J., Roberts V. A., Dyson H. J., Holman G. M., Tainer J. A. Active conformation of an insect neuropeptide family. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4518–4522. doi: 10.1073/pnas.88.10.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B. K., Ray A. Synthesis of a highly efficient cDNA library. Nucleic Acids Res. 1991 Aug 25;19(16):4559–4559. doi: 10.1093/nar/19.16.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoofs L., Tips A., Holman G. M., Nachman R. J., De Loof A. Distribution of locustamyotropin-like immunoreactivity in the nervous system of Locusta migratoria. Regul Pept. 1992 Feb 18;37(3):237–254. doi: 10.1016/0167-0115(92)90618-5. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]