Abstract
Purpose
To study the brief and reversible mood response to acute tryptophan depletion (ATD) as a trait marker in subjects considered at risk for major depressive disorder (MDD).
Procedures
ATD was administered to 64 subjects (54 European-Americans and 10 from other races) with a personal and family history of MDD. They were in remission and had been medication-free for at least 3 months. Subjects were randomly assignment to an active or sham condition in a double-blind crossover design. They were genotyped for serotonin-related candidate genes, and mood response was quantified with the Hamilton Depression Rating Scale (HDRS). Data were analyzed using Poisson regression with repeated measures and latent trajectory models.
Results
Compared to the sham controls, active ATD caused modest depressive changes showing significant main effects of test condition (χ2 = 5.14, d.f. = 1, p = 0.023) and time (χ2 = 12.22, d.f. = 3, p = 0.007), but no significant interaction of time and test condition. Latent trajectory analysis revealed two groups, identified as depletion responders and non-responders. Those with the HTR2A rs6313 CC genotype had significantly higher HDRS scores during ATD (χ2 = 11.72, d.f. = 1, p = 0.0006).
Conclusions
ATD may help identifying the biological subtypes of MDD. These data are consistent with imaging reports implicating 5-HT2A receptor function in ATD phenotypes.
Key Words: Neurotransmitter depletion, Depression endophenotype, Serotonin candidate genes, SLC6A4, 5-HTTLPR, STin2, HTR1A, HTR2A, TPH2
Introduction
The acute tryptophan depletion (ATD) test has been widely used to test hypotheses about the function of the brain serotonin (5-HT) system. The ATD test is based on the fact that tryptophan (Trp) is an essential amino acid, i.e. a necessary component of most proteins, that mammals cannot synthesize [1]. It is the foundational precursor for 5-HT synthesis, a pathway whose rate-limiting enzyme is usually not saturated, setting the stage for a rapid reduction in 5-HT synthesis as Trp availability diminishes [2]. When mammals ingest a bolus of amino acids, hepatic protein synthesis ensues; however, if the bolus is missing any essential amino acid, plasma stores of the missing amino acid are tapped, causing acute depletion. Ingestion of a 50-100 g solution of 15 amino acids without Trp induces a 4-5-hour-long, 60-80% depletion of plasma Trp in humans [3,4,5,6]. When such a solution is used in the ATD test, it is most often administered using a double-blind, sham-controlled, randomized crossover design, with each subject ingesting a Trp-free amino acid solution (true test ‘active’) and an identical but Trp-supplemented solution (‘sham’) on distinct test days separated by an interval of several days.
Human studies have largely confirmed that ATD acutely causes small to modest reductions in 5-HT neurotransmission. For example, positron emission tomography (PET) with a putative marker for 5-HT synthesis rate showed an 80% decrease after ATD [7], and three separate PET studies using radioligands for 5-HT2 receptors showed that ATD causes a reduction in 5-HT2 receptor binding in specific brain regions [8,9,10]. Measurement of the 5-HT metabolite 5-hydroxyindoleacetic acid (5-HIAA) in the cerebrospinal fluid of healthy subjects tested with ATD showed a 25% decrease when measured with either continuous cerebrospinal fluid sampling [11] or a single lumbar puncture 9 h after the Trp-free drink [12]. Although it has been suggested that non-5-HT-related processes may contribute to the human behavioral responses to ATD [13], it is generally accepted that the effects of ATD on 5-HT more fully explain the behavioral and biochemical effects [14].
The effects of ATD on behavior and cognition in healthy subjects have generally been consistent with the known functions of 5-HT. For example, ATD can induce depressive mood in some healthy subjects [15], modify pain sensitivity [16], impair learning [17], reduce punishment-induced inhibition [18], decrease long-term memory [19,20,21] and hinder executive function [22,23]. However, the effects on mood and cognition have been small and have not always been replicated [24,25,26].
In clinical populations, the effects of ATD are more pronounced, the most dramatic effect being the ability of ATD to cause a brief return of depressive symptoms in patients with major depressive disorder (MDD) who had improved with and were continuing to take 5-HT selective serotonin reuptake inhibitor (SSRI) antidepressants during the ATD test. Similar responses are seen in MDD patients with a seasonal pattern who have responded to light therapy [27,28]. In contrast, the lack of ATD mood response in healthy subjects dosed for 4 weeks with fluvoxamine as well as never-depressed SSRI-treated patients with obsessive-compulsive disorder [29,30], panic disorder [31] or generalized anxiety disorder [32] suggests that the mood response to ATD is not simply due to an adverse interaction with the antidepressant or a non-specific effect. ATD also showed no significant behavioral effects in symptomatic, medication-free patients with MDD [5,33] or obsessive-compulsive disorder [34].
Given the extensive body of data supporting involvement of the 5-HT system in the pathophysiology of MDD and the depressive responses to ATD in some healthy subjects and MDD patients, several groups have sought to determine whether a particularly intense mood response to ATD could be the phenotype of a set of individuals predisposed to future MDD. In this context, ATD induced mild to moderate depressive symptoms [35,36,37] or cognitive changes [37,38] to a much greater extent in subjects with a multigenerational family history of affective disorder but no personal history of MDD in comparison to subjects with no personal or family history of affective disorder. Most [6,39], but not all [40] studies have shown that compared to sham control, ATD causes mild depressive symptoms in untreated euthymic subjects with a past history of MDD compared with personal and family history-negative controls, although depressive symptoms are seen more often in healthy women compared to healthy men, irrespective of whether there is a history of MDD [41,42,43].
Given that MDD is a phenotypically heterogeneous, and likely biologically heterogeneous disorder, some of the variability in mood response to ATD may be due to genomic differences between subjects. For example, MDD patients homozygous for the long ‘l’ vs. short ‘s’ allele of the 5-HT transporter protein gene (SLC6A4) 5-HTTLPR polymorphism [44] were found to be more likely to have a depressive response to ATD [45,46,47], but the results have not always been replicated [48], and one study found that the ATD response in healthy subjects was greater in those who were homozygous for the ‘s’ allele of SLC6A4[49]. These data suggest that the presence of genetic factors that modify 5-HT neurotransmission may contribute to a greater likelihood of having a mood response to ATD, a finding supported by a retrospective study showing that subjects with an increased number of proposed risk genotypes for MDD have a greater risk of having the disorder [50]. Other studies in healthy subjects [8] and SSRI-treated MDD patients [9] have shown that mood response to ATD was associated with a failure to show a decrease in PET measures of 5-HT2 receptor binding during the procedure.
The present study was designed to test the hypothesis that the depressive response to ATD is associated with certain proposed risk genotypes in subsets of people with MDD, possibly identifying a subset of MDD patients with more homogeneous underlying causal mechanisms. Since ATD has minimal acute effects in symptomatic patients with MDD and the effects of ATD on MDD patients taking antidepressants are highly associated with the pharmacology of the antidepressant [51], the present study focused on medication-free non-depressed subjects who reported both a past personal and family history of MDD.
Subjects and Methods
Subjects
After review and approval of the protocol for this study by the Investigational Review Boards of the University of Arizona, Case Western Reserve University and the University of Texas Health Sciences Center at San Antonio, 64 psychotropic medication-free subjects aged 18-80 years (mean age 53 years, 66% women, 84% European-American [EA]) were recruited by advertisement and provided written informed consent to participate. Interested persons underwent initial screening with a brief, focused review of the inclusion and exclusion criteria as well as medical and psychiatric history. Prospective subjects then met with an investigator for informed consent and/or further assessment and received complete physical and neurological exams as well as pertinent laboratory studies (complete blood count, liver and thyroid function tests, urinalysis, urine drug screen and urine pregnancy test for females). Additional laboratory studies were obtained when indicated.
Subjects were selected for inclusion if, after a structured interview with the Diagnostic Interview for Genetic Studies (DIGS) and the Family Interview for Genetic Studies (FIGS) [52], they met Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) [53] criteria for at least one prior but not for a current major depressive episode, had a family history of MDD in at least one first-degree relative, and had been free of psychotropic medication for at least 3 months but less than 2 years at the time of enrollment. Subjects had to have a total Hamilton Depression Rating Scale (HDRS) score <10 on the 25-item version [54].
Subjects were excluded for (1) lifetime history of any DSM-IV axis I disorder except MDD, (2) borderline or antisocial personality disorder, (3) history or manifest signs of serious medical or neurological illness on examination or as a result of laboratory studies that might induce depressive symptoms or increase the risk of adverse events during testing, (4) current or prior psychosis, (5) need for any psychotropic medication, including the use of medication for hypnotic purposes (including antihistamines) or use of benzodiazepines for the treatment of anxiety, (6) ongoing suicidal ideation or assessed by one of the investigators to pose a risk of suicide based on clinical presentation and past history, or (7) not using an acceptable method of contraception if they were female and capable of childbearing. Neither sex nor ethnicity/race were exclusion criteria.
About 70% of the subjects were recruited and tested at the Arizona Health Sciences Center, 10% at Case Western Reserve University (Cleveland, Ohio, USA) and 20% at the University of Texas Health Sciences Center San Antonio.
Candidate Gene Selection
The following candidate genes were selected prospectively based on their mechanistic involvement in the brain synthesis (Trp hydroxylase-2), reuptake (5-HT transporter) and principal receptor activity of 5-HT (5-HT1A and 5-HT2A) as well as their suggestive evidence of risk for mood disorders based on evidence available at the time of study.
ATD Procedures
ATD tests were performed in a double blind, sham-controlled fashion and took place between 2003 and 2009. All subjects received two ATD procedures 1 week apart. Each ATD session involved three consecutive days: an amino acid drink day and two follow-up days (see table 1 for schedule of testing). One of the tests involved consumption of a 102.5-g Trp-free amino acid drink (active ATD), and the sham condition involved an identical mixture of amino acids but containing 2.3 g l-Trp (see table 2 for amino acid drink composition). Subjects were randomly assigned to receive either active ATD or sham condition on the first week and then crossed over to receive the alternative condition the week later. The order of conditions was unknown to the subjects, the supervising physicians and the study raters. Because of sex differences in tolerability of this amino acid drink, women received a 75% strength drink. Given that Trp depletion had been reported to exacerbate premenstrual dysphoric disorder symptomatology [55], the dates of depletion were assigned to coincide with the follicular phase of menstruating women to minimize confounding behavioral effects. The phase of the menstrual cycle was calculated with support from a daily temperature log.
Table 1.
Schedule for ATD and sham condition test sessions
| Day 1 | 8:00 a.m. | arrive at the research clinic (fasting after midnight) clinician and self-ratings, plasma Trp (10 cm3), vital signs |
| 8:30 a.m. | administration of amino acid drink | |
| 1:30 p.m. | clinician and self-ratings, plasma Trp (10 cm3), vital signs | |
| 3:30 p.m. | clinician and self-ratings, plasma Trp (10 cm3), vital signs | |
| 4:00 p.m. | return to unrestricted food intake | |
| Day 2 | 8:00 a.m. | clinician and self-ratings, plasma Trp (10 cm3) |
| Day 3 | 8:00 a.m. | clinician and self-ratings |
Table 2.
Amino acid content (100% strength)
| l-Alanine | 5.5 g |
| Glycine | 3.2 g |
| l-Histidine | 3.2 g |
| l-Isoleucine | 8.0 g |
| l-Leucine | 13.5 g |
| l-Lysine hydrochloride | 11.0 g |
| l-Phenylalanine | 5.7 g |
| l-Proline | 12.2 g |
| l-Serine | 6.9 g |
| l-Threonine | 6.9 g |
| l-Tyrosine | 6.9 g |
| l-Valine | 8.9 g |
| l-Methionine | 3.0 g |
| l-Arginine | 4.9 g |
| l-Cysteine | 2.7 g |
| With or without | |
| l-Trp | 2.3 g |
75% strength used in women.
For each test session, subjects arrived at the test site after an overnight fast on day 1 of the study. Depression ratings and blood sampling for Trp levels and vital signs were performed, followed by ingestion of the amino acid drink. Subjects remained in the testing room for the next 7 h but were free to walk about the room, use the toilet and drink water. All subjects remained fasted throughout the day until the test was completed (approximately 4:30 p.m.), when they returned to regular unrestricted food intake. At that time, they were provided a Trp-rich meal for immediate ingestion and an additional Trp-rich meal to take home for dinner. Research subjects were continuously monitored during the day of the amino acid drink by an experienced nurse clinician and one of the investigators was available at all times.
On the day of testing, the amino acid drink was prepared by mixing the blinded amino acid powder with enough water for a final volume of 350 ml and flavored to taste with Hershey's chocolate syrup. The three least palatable amino acids, cysteine, methionine and arginine, were encapsulated and given separately from the drink.
Compensation of USD 100 was offered for participation in each of the blinded procedures (ATD and sham condition.)
Depression Measurements
Depression ratings were obtained at specified intervals during each testing period (see table 1 for schedule of testing). Clinician-rated instruments included the HDRS [54,56,57]. The HDRS was used in this study because it provides the best comparison to earlier investigations utilizing neurotransmitter depletion paradigms in clinical populations. Experienced clinicians with extensive experience using the scale performed all ratings. The raters were blinded to the sequence of testing conditions.
Biochemical Measurements
Plasma for free Trp was obtained immediately prior to ingestion of the test drink and 5 h later. Free plasma Trp is assayed by obtaining an ultrafiltrate through a commercially available membrane system and subjecting the ultrafiltrate to high-performance liquid chromatography with fluorometric detection [58].
Genetic Analysis
DNA was extracted by standard techniques from whole blood. Blood from subjects at all sites was frozen and shipped to the Arizona Health Sciences Center for genotyping. Assuming selectivity of ATD for serotonin neurotransmission, the following candidate genes were selected a priori based on their role in serotonin synthesis, transport, and recognition: SLC6A4 gene including the promoter region polymorphism (5-HTTLPR) [44], its triallelic LA-G polymorphism (rs25531) [59], and the intron-2 variable number tandem repeat of the 5-HT transporter (STin2) [60,61], the serotonin receptor-1A (rs6295) (HTR1A)[62], the serotonin receptor-2A (rs6313) (HTR2A)[63] and the Trp hydroxylase-2 gene (rs1386494) (TPH-2)[64].
Adverse Events
No significant adverse events were observed in any of the subjects during testing. One patient died the week after sham testing for reasons unrelated to this study.
Statistical Analysis
The effect of ATD on Trp levels was analyzed using repeated measures ANOVA with measures at baseline and 5 h. The effect of ATD on mood was assessed using a generalized linear regression model with generalized estimating equations (SAS GENMOD) specifying an overdispersed Poisson distribution (non-normal distribution of the error). Fixed design effects were test condition (depletion vs. sham), time (5, 7, 24 and 48 h after ingestion) and the condition by time interaction. Baseline measures were used as covariates in each test condition. Supplemental analyses explored the effects of participants' sex on HDRS by adding main and interaction effects to the model. Two subjects who completed only the active depletion session were included in these analyses. Genetic data were analyzed by adding genotype as an additional classification factor to these models. Preliminary models were run including order of testing (depletion vs. sham first) as an additional fixed design effect. Neither main nor interaction effects of note were observed, so it was dropped from the final models. A simple confirmatory analysis used a paired-samples t test to test the difference between the mean of each post-baseline measure in depletion vs. sham. We also conducted a latent trajectory analysis on the data from the active depletion condition using SAS PROC TRAJ [65]. Rather than characterizing average trends as a function of depletion condition, latent trajectory analysis attempts to identify subgroups that have homogeneous trends over time. We fit multiple models specifying increasing numbers of latent groups, and selected a ‘best’ model based on Schwarz's Bayesian information criterion [66]. The Akaike information criterion and the Bayesian information criterion are well-known statistics used to quantify goodness of fit in regression models that use likelihood-based estimation, and are very widely used to evaluate and choose among models of differing complexity. Given the inclusion of a mixed racial/ethnic subject sample, the genetic analyses were also done excluding non-EA subjects (10/64) to control for possible population stratification.
Results
Trp Levels
The Trp-free test caused an 80.81% decrease from baseline in free plasma levels of Trp (from 8.17 ± 4.01 to 1.57 ± 1.8 μm/l), and the Trp-supplemented sham test caused free Trp to increase from baseline by 81.77% (from 8.41 ± 4.7 to 15.29 ± 7.67 μm/l). ANOVA with repeated measures showed a highly significant test by time interaction (F = 110.46, d.f. = 1,120, p < 0.001).
Depressive Response
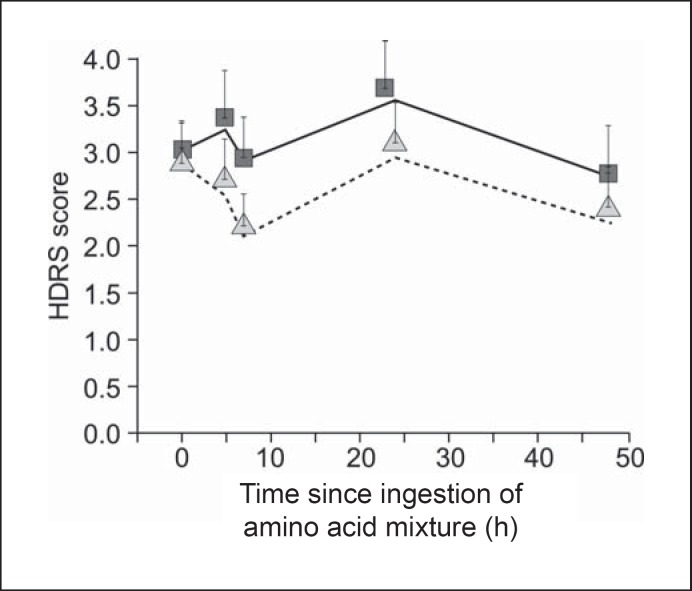
Generalized estimating equation analysis of HDRS showed a significant main effect of test condition (χ2 = 5.14, d.f. = 1, p = 0.023). The result is depicted in figure 1, which displays both the actual means and the model estimates (the slight differences between line and triangle alignment reflect effects of missing data). Mean HDRS was almost identical at baseline in both conditions and higher across time in the ATD than in the sham condition. The main effect of time was also significant (χ2 = 12.22, d.f. = 3, p = 0.007), but the interaction of condition and time was not (χ2 = 0.89, d.f. = 3, p = 0.829). Supplemental analyses exploring differences between men (n = 22) and women (n = 42) revealed no significant effects (sex main effect: χ2 = 0.95, d.f. = 1, p = 0.33; sex by task: χ2 = 0.07, d.f. = 1, p = 0.79; sex by time: χ2 = 0.71, d.f. = 3, p = 0.87; sex by task by time: χ2 = 2.99, d.f. = 3, p = 0.39).
Fig. 1.
Depressive response during ATD and sham condition (n = 64). The figure displays both the actual mean HDRS scores during ATD (squares) and sham condition (triangles) and the model estimates utilizing the generalized linear regression model with generalized estimating equations during ATD (solid line) and sham condition (dotted line).
Latent Trajectory Modeling of Mood
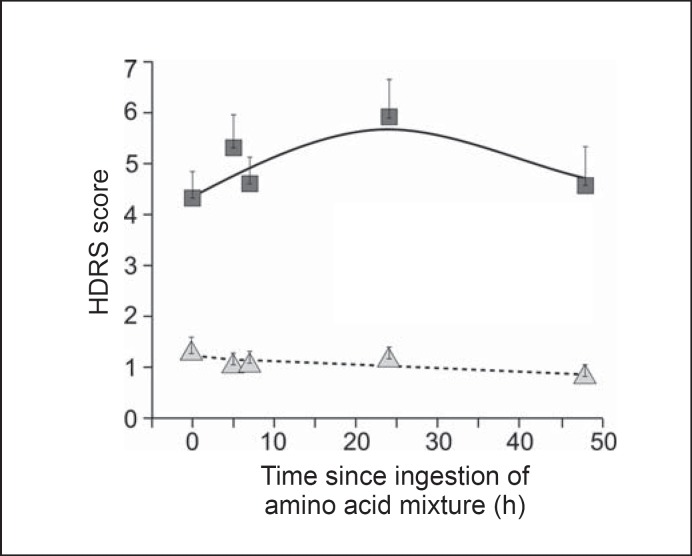
The final latent trajectory model had two latent classes. The results from PROC TRAJ are presented in table 3. Group 1 was defined by an intercept-only trajectory, with no significant trend component (p = 0.22), meaning no change over time. Group 2 began with a higher baseline score (intercept) than group 1 and was defined by a significant (p = 0.02) inverted quadratic function, i.e. scores that went up and then down, with a peak at roughly 24 h post baseline. Group 1 comprised 46% of the cases and group 2 comprised 54%. We interpret the inverted U response in group 2 as ‘depletion responders’ and the flat trajectory of group 1 as ‘non-responders’. Mean PEAK D (the highest value of HDRS from 5-48 h post depletion in group 2 was 8.4 ± 4.0 (median = 7, range = 2-21), while in group 1 it was 1.9 ± 1.1 (median = 2, range = 0-4). Figure 2 is a graphic of this analysis with actual sample means and model-based trajectories.
Table 3.
Latent trajectory analysis of HDRS over time
| Group | Parameter | Estimate | SE | T for Hθ | p value |
|---|---|---|---|---|---|
| 1 | intercept | 0.293 | 0.153 | 1.914 | 0.056 |
| linear | –0.007 | 0.006 | –1.229 | 0.220 | |
| 2 | intercept | 1.579 | 0.065 | 24.391 | <0.000 |
| linear | 0.020 | 0.008 | 2.444 | 0.015 | |
| quadratic | <–0.000 | <0.000 | –2.345 | 0.020 | |
| alpha | –2.170 | 0.280 | –7.741 | <0.000 | |
| Group membership | |||||
| 1 | % | 45.692 | 6.630 | 6.892 | <0.000 |
| 2 | % | 54.308 | 6.630 | 8.192 | <0.000 |
Group 1 represents individuals considered non-responders; group 2 represents individuals considered responders.
Likelihood criteria: Bayesian information criterion = −726.47 (n = 314); Bayesian information criterion = −720.91 (n = 64); Akaike information criterion = −713.35; likelihood criterion = −706.35.
SE = Standard error; T for Hθ = test for null hypothesis (parameter = 0).
Fig. 2.
Depressive response during ATD in latent trajectory groups (n = 64). The figure displays both the actual mean HDRS scores for ATD responders (squares) and non-responders (triangles) at each time point and the model estimates for responders (solid line) and non-responders (dotted line) utilizing generalized linear regression model with generalized estimating equations.
Genotype and Depletion Response
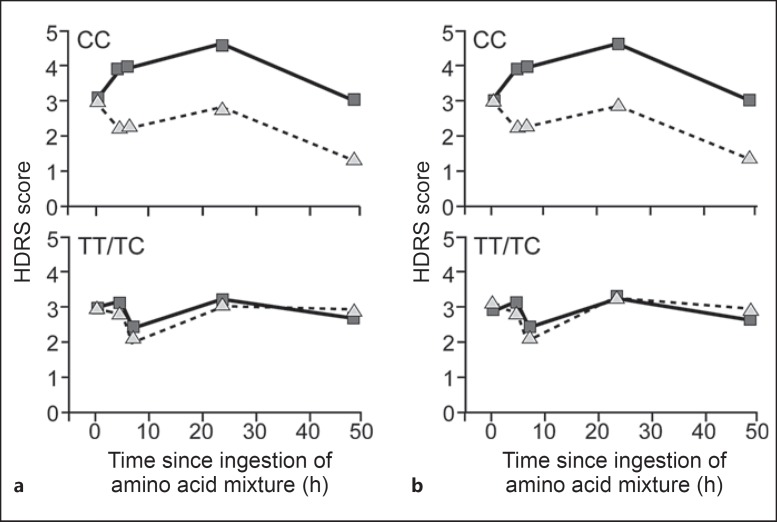
Primary analyses of genotypes were done using data only from EA subjects (54/64) to decrease the stratification risk. Six SNPs were analyzed, so the Bonferroni-adjusted p value was 0.05/6 = 0.008. Only the rs6313 SNP in HTR2A yielded a statistically significant association with depression response (χ2 = 11.85, d.f. = 2, p = 0.003). The depletion response (mean difference between depletion and sham conditions) was highly significant only for those with the CC genotype (32.1%, z = 4.2, p < 0.0001), and non-significant for those with the TC (52.8%, z = 1.06, p = 0.29) or TT genotypes (15.1%, z = 0.14, p = 0.89). When the TC and TT groups were pooled and compared with the CC group, the genotype by task interaction yielded χ2 = 11.72, d.f. = 1, p = 0.0006. Furthermore, the HTR2A genotype groups were associated with the latent trajectory classes as expected. Individuals with the CC genotype were more likely to be classified as ATD responders, with an odds ratio of 6.5, a positive predictive value of 82%, a negative predictive value of 58% and a kappa of 0.34 (χ2 = 7.71, d.f. = 1, p = 0.006). These results were weaker when the analysis was done using data from the entire racially heterogeneous sample, but HTR2A (CC vs. TC/TT) still had a nominally significant genotype by test condition interaction (χ2 = 5.65, d.f. = 1, p = 0.017) for the HDRS, and individuals with the CC genotype were more likely to be considered ATD responders, with an odds ratio of 3.9, a positive predictive value of 76%, a negative predictive value of 55% and a kappa of 0.265 (χ2 = 5.43, d.f. = 1, p = 0.02). This result is depicted in figure 3, which suggests that the depletion response in depressive mood occurs primarily in those with the CC genotype. For the rest of the genes, the genotype frequencies are 5-HTTLPR ll 32.8%, ls 50%, ss 17.2%; LA-G triallelic sa/sa 15.5% + sa/lg 3.4% = 18.9%, sa/la 46.5% + la/lg 6.9%, and la/la 27.6%; STin2 ss 17.2%, sl 46.9%, ll 35.9%; HTR1A cc 14%, cg 26%, gg 22%; TPH2 intron-5 tt 1.6%, tc 22.6%, cc 75.8%. None of these SNPs achieved statistical significance for an association with depressive response during ATD.
Fig. 3.
Depressive response during ATD (squares) and sham condition (triangles) by HTR2A (rs6313) genotype. The figure displays differences in HDRS scores between risk genotype (CC) and non-risk genotype (TT/TC) for HTR2A rs6313 for both the racially heterogeneous sample (n = 64, a) and the European descent sample (n = 54, b). CC = Homozygous C allele; TT = homozygous T allele; TC = heterozygous.
Discussion
Active but not sham Trp depletion caused depressive mood reactions in this sample of euthymic, psychotropic medication-free subjects with both a personal and family history of MDD. As a group, our subjects showed a smaller depressive response during active depletion than we had anticipated, and our latent trajectory analysis showed that a modest mood response occurred in a subgroup of about one-half of the subjects. One variable that may help explain this is the selection of subjects who were in sustained remission from depression from 3 to 24 months. Recently remitted individuals may be more likely to experience a depressive response during active depletion. We tested women with a 75% strength drink, which may have led to less dramatic brain serotonin depletion than the 100-g drink previously utilized. Additionally, in women of reproductive age we conducted our testing only during the first phase of their menstrual cycle. It has been suspected that women undergoing ATD during the late luteal phase are more likely to experience negative mood changes [55].
Genetic analyses showed that in a mixed racial sample, subjects with the CC genotype of the rs6313 SNP in the HTR2A gene accounted for a proportion of the mood responses; the result was only nominally significant. When controlling for the effect of race by testing EA subjects only (84% of sample), the association of CC genotype and mood response to ATD was stronger and met corrected significant levels. The present study - while small - is still the largest prospective study to date in which an association between genotype and the depressive response to ATD was analyzed in subjects in remission from MDD.
The HTR2A*C allele is associated with lower quantity of mRNA and protein made [67] and higher 5-HT2A receptor density in the cerebral cortex [68], and it has been considered a risk allele for various psychiatric conditions including schizophrenia, alcohol dependence and mood disorders by some investigators. Recent PET study findings suggest that the status of 5-HT2 receptor availability correlates with the depressive response during ATD in SSRI-treated MDD patients and healthy volunteers [8,9]. The fact that subjects who showed greater decreases in 5-HT2 binding during ATD were less likely to experience depressive responses is consistent with observations in treated animal models and patients who are in remission from MDD after treatment [69]. Antagonists of the 5-HT2A receptor are effective as single or combined clinical treatments for MDD, and various other antidepressants with different receptor or transporter affinity profiles, including electroconvulsive therapy, reportedly lead to downregulation of postsynaptic 5-HT2 receptors, a phenomenon believed to be relevant to their mechanism of action [70]. Yatham et al. [9] hypothesized that the decrease in 5-HT2A binding observed during ATD is a compensatory mechanism that prevents induction of depression. This phenomenon may be similar to the increase in brain-derived neurotrophic factor plasma levels observed during ATD in healthy volunteers, which is believed to be a compensatory mood-stabilizing temporary adaptation [71].
Although theoretically HTR2A ‘CC’, which results in less mRNA and protein, may explain mechanistically a greater decrease in 5-HT2A receptor availability during ATD, the dynamic adaptability of 5-HT2A appears to explain more broadly changes in expression, and response to both agonists and antagonists of 5-HT2A leads to downregulation of 5-HT2A receptor [69]. Should future efforts utilizing ATD or other methods be able to identify specific depression subgroups, the potential for advanced understanding of the mechanism of illness may facilitate the identification of specific risk factors and the development of tailored therapeutic interventions.
This study did not confirm prior reports [45,46] of increased depressive response to ATD in subjects homozygous to the ‘l’ allele of SLC6A4. According to findings from EA female-female twin pairs over the course of illness, the onset of depressive episodes may become increasingly autonomous, suggesting a sensitization or ‘kindling’ to the depressive state [72]. If this is the case, subjects without vulnerability genes may be equally likely to express depressive episodes after experiencing recurrent lifetime episodes. Note that we included subjects who had experienced several depressive episodes.
Population stratification is a well-known source of artifact in genetic association studies. In the present study, when we considered only self-identified EAs, the result was statistically significant when taking into account the testing of six SNPs. When we considered the entire heterogeneous sample, the results were only nominally significant. It is impossible to draw specific conclusions because of the limited sample size, but the results are consistent with this variant having an effect on Trp depletion response only in EA subjects. Larger groups of non-EA subjects would need to be evaluated to draw a more definitive conclusion.
In summary, this study replicated consistent reports of robust and safe depletion of plasma Trp and the induction of depressive symptoms in a subgroup of patients recovered from depression. Among the group experiencing depressive changes, the HTR2A CC genotype predicted greater symptom induction.
Disclosure Statement
All authors declare the absence of any competing financial interests in relation to the work described.
Acknowledgements
The authors acknowledge support from NIMH grant 1R01MH66235 ‘Tryptophan Depletion: A Phenotypic Marker for Depression’ to Francisco A. Moreno, MD.
References
- 1.Rose WC, Lambert GF, Coon MJ. The amino acid requirements of man. VII. General procedures; the tryptophan requirement. J Biol Chem. 1954;211:815–827. [PubMed] [Google Scholar]
- 2.Fernstrom JD, Wurtman RJ. Brain serotonin content: physiological dependence on plasma tryptophan levels. Science. 1971;173:149–152. doi: 10.1126/science.173.3992.149. [DOI] [PubMed] [Google Scholar]
- 3.Delgado PL, Charney DS, Price LH, Aghajanian GK, Landis H, Heninger GR. Serotonin function and the mechanism of antidepressant action. Reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch Gen Psychiatry. 1990;47:411–418. doi: 10.1001/archpsyc.1990.01810170011002. [DOI] [PubMed] [Google Scholar]
- 4.Delgado PL, Price LH, Miller HM, Salomon RM, Licinio J, Krystal JH, Heninger GR, Charney DS. Rapid serotonin depletion as a provocative challenge test for patients with major depression: relevance to antidepressant action and the neurobiology of depression. Psychopharmacol Bull. 1991;27:321–330. [PubMed] [Google Scholar]
- 5.Delgado PL, Price LH, Aghajanian GK, Miller HM, Salomon RM, Heninger GR, Charney DS. Serotonin and the neurobiology of depression: effects of tryptophan depletion in drug-free depressed patients. Arch Gen Psychiatry. 1994;51:865–874. doi: 10.1001/archpsyc.1994.03950110025005. [DOI] [PubMed] [Google Scholar]
- 6.Moreno FA, Gelenberg AJ, Heninger GR, Potter RL, McKnight K, Allen J, Phillips AP, Delgado P. Tryptophan depletion and depressive vulnerability. Biol Psychiatry. 1999;46:498–505. doi: 10.1016/s0006-3223(99)00095-5. [DOI] [PubMed] [Google Scholar]
- 7.Nishizawa S, Benkelfat C, Young SN, Leyton M, Mzengeza S, de Montigny C, Blier P, Diksec M. Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci USA. 1997;94:5308–5313. doi: 10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yatham LN, Liddle PF, Shiah IS, Lam RW, Adam MJ, Zis AP, Ruth TJ. Effects of rapid tryptophan depletion on brain 5-HT(2) receptors: a PET study. Br J Psychiatry. 2001;178:448–453. doi: 10.1192/bjp.178.5.448. [DOI] [PubMed] [Google Scholar]
- 9.Yatham LN, Liddle PF, Sossi V, Erez J, Vafai N, Lam RW, Blinder S. Positron emission tomography study of the effects of tryptophan depletion on brain serotonin(2) receptors in subjects recently remitted from major depression. Arch Gen Psychiatry. 2012;69:601–609. doi: 10.1001/archgenpsychiatry.2011.1493. [DOI] [PubMed] [Google Scholar]
- 10.Talbot PS, Slifstein M, Hwang DR, Huang Y, Scher E, Abi-Dargham A, Laruelle M. Extended characterisation of the serotonin 2A (5-HT2A) receptor-selective PET radiotracer 11C-MDL100907 in humans: quantitative analysis, test-retest reproducibility, and vulnerability to endogenous 5-HT tone. Neuroimage. 2012;59:271–285. doi: 10.1016/j.neuroimage.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carpenter LL, Anderson GM, Pelton GH, Gudin JA, Kirwin PD, Price LH, Heninger GR, McDougle CJ. Tryptophan depletion during continuous CSF sampling in healthy human subjects. Neuropsychopharmacology. 1998;19:26–35. doi: 10.1016/S0893-133X(97)00198-X. [DOI] [PubMed] [Google Scholar]
- 12.Moreno FA, Parkinson D, Palmer C, Castro WL, Misiaszek J, El Khoury A, Mathe AA, Wright R, Delgado PL. CSF neurochemicals during tryptophan depletion in remitted depressive subjects and healthy controls. Eur Neuropsychopharmacol. 2010;20:18–24. doi: 10.1016/j.euroneuro.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Donkelaar EL, Blokland A, Ferrington L, Kelly PAT, Steinbusch HWM, Prickaerts J. Mechanism of acute tryptophan depletion: is it only serotonin? Mol Psychiatry. 2011;16:695–713. doi: 10.1038/mp.2011.9. [DOI] [PubMed] [Google Scholar]
- 14.Crockett MJ, Clark L, Roiser JP, Robinson OJ, Cools R, Chase HW, den Ouden H, Apergis-Schoute A, Campbell-Meikeljohn D, Seymour B, Sahakian BJ, Rogers RD, Robbins TW. Converging evidence for central 5-HT effects in acute tryptophan depletion. Mol Psychiatry. 2012;17:121–123. doi: 10.1038/mp.2011.106. [DOI] [PubMed] [Google Scholar]
- 15.Young SN, Smith SE, Pihl RO, Ervin FR. Tryptophan depletion causes a rapid lowering of mood in normal males. Psychopharmacology (Berl) 1985;87:173–177. doi: 10.1007/BF00431803. [DOI] [PubMed] [Google Scholar]
- 16.Abbott FV, Etienne P, Franklin KB, Morgan MJ, Sewitch MJ, Young SN. Acute tryptophan depletion blocks morphine analgesia in the cold-pressor test in humans. Psychopharmacology (Berl) 1992;108:60–66. doi: 10.1007/BF02245286. [DOI] [PubMed] [Google Scholar]
- 17.Park SB, Coull JT, McShane RH, Young AH, Sahakian BJ, Robbins TW, Cowen PJ. Tryptophan depletion in normal volunteers produces selective impairments in learning and memory. Neuropharmacology. 1994;33:575–588. doi: 10.1016/0028-3908(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 18.Crockett MJ, Clark L, Robbins TW. Reconciling the role of serotonin in behavioral inhibition and aversion: acute tryptophan depletion abolishes punishment-induced inhibition in humans. J Neurosci. 2009;29:11993–11999. doi: 10.1523/JNEUROSCI.2513-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riedel WJ, Klaassen T, Deutz NEP, van Someren A, van Praag HM. Tryptophan depletion in normal volunteers produces selective impairment in memory consolidation. Psychopharmacology (Berl) 1999;141:362–369. doi: 10.1007/s002130050845. [DOI] [PubMed] [Google Scholar]
- 20.Schmitt JAJ, Jorissen BL, Sobczak S, van Boxtel MPJ, Hogervorst E, Deutz NEP, Riedel WJ. Tryptophan depletion impairs memory consolidation but improves focused attention in healthy young volunteers. J Psychopharmacol. 2000;14:21–29. doi: 10.1177/026988110001400102. [DOI] [PubMed] [Google Scholar]
- 21.McAllister-Williams RH, Massey AE, Rugg MD. Effects of tryptophan depletion on brain potential correlates of episodic memory retrieval. Psychopharmacology (Berl) 2002;160:434–442. doi: 10.1007/s00213-001-0996-8. [DOI] [PubMed] [Google Scholar]
- 22.Rubinsztein JS, Rogers RD, Riedel WJ, Mehta MA, Robbins TW, Sahakian BJ. Acute dietary tryptophan depletion impairs maintenance of ‘affective set’ and delayed visual recognition in healthy volunteers. Psychopharmacology (Berl) 2001;154:319–326. doi: 10.1007/s002130000655. [DOI] [PubMed] [Google Scholar]
- 23.Murphy FC, Smith KA, Cowen PJ, Robbins TW, Sahakian BJ. The effects of tryptophan depletion on cognitive and affective processing in healthy volunteers. Psychopharmacology (Berl) 2002;163:42–53. doi: 10.1007/s00213-002-1128-9. [DOI] [PubMed] [Google Scholar]
- 24.Harmer CJ, Rogers RD, Tunbridge E, Cowen PJ, Goodwin GM. Tryptophan depletion decreases the recognition of fear in female volunteers. Psychopharmacology (Berl) 2003;167:411–417. doi: 10.1007/s00213-003-1401-6. [DOI] [PubMed] [Google Scholar]
- 25.Riedel WJ. Cognitive changes after acute tryptophan depletion: what can they tell us? Psychol Med. 2004;34:3–8. doi: 10.1017/s0033291703008924. [DOI] [PubMed] [Google Scholar]
- 26.Clark L, Roiser JP, Cools R, Rubinsztein DC, Sahakian BJ, Robbins TW. Stop signal response inhibition is not modulated by tryptophan depletion or the serotonin transporter polymorphism in healthy volunteers: implications for the 5-HT theory of impulsivity. Psychopharmacology (Berl) 2005;182:570–578. doi: 10.1007/s00213-005-0104-6. [DOI] [PubMed] [Google Scholar]
- 27.Lam RW, Zis AP, Grewal A, Delgado PL, Charney DS, Krystal JH. Effects of rapid tryptophan depletion in patients with seasonal affective disorder in remission after light therapy. Arch Gen Psychiatry. 1996;53:41–44. doi: 10.1001/archpsyc.1996.01830010043007. [DOI] [PubMed] [Google Scholar]
- 28.Neumeister A, Praschak-Rieder N, Hebelman B, Rao ML, Gluck J, Kasper S. Effects of tryptophan depletion on drug-free patients with seasonal affective disorder during a stable response to bright light therapy. Arch Gen Psychiatry. 1997;54:133–138. doi: 10.1001/archpsyc.1997.01830140043008. [DOI] [PubMed] [Google Scholar]
- 29.Kulz AK, Meinzer S, Kopasz M, Voderholzer U. Effects of tryptophan depletion on cognitive functioning, obsessive-compulsive symptoms and mood in obsessive-compulsive disorder: preliminary results. Neuropsychobiology. 2007;56:127–131. doi: 10.1159/000115778. [DOI] [PubMed] [Google Scholar]
- 30.Barr LC, Goodman WK, McDougle CJ, Delgado PL, Heninger GR, Charney DS, Price LH. Tryptophan depletion in patients with obsessive-compulsive disorder who respond to serotonin reuptake inhibitors. Arch Gen Psychiatry. 1994;51:309–317. doi: 10.1001/archpsyc.1994.03950040053007. [DOI] [PubMed] [Google Scholar]
- 31.Toru I, Shlik J, Maron E, Vasar V, Nutt DJ. Tryptophan depletion does not modify response to CCK-4 challenge in patients with panic disorder after treatment with citalopram. Psychopharmacology (Berl) 2006;186:107–112. doi: 10.1007/s00213-006-0351-1. [DOI] [PubMed] [Google Scholar]
- 32.Hood SD, Hince DA, Davies SJ, Argyropoulos S, Robinson H, Potokar J, Nutt DJ. Effects of acute tryptophan depletion in serotonin reuptake inhibitor-remitted patients with generalized anxiety disorder. Psychopharmacology (Berl) 2010;208:223–232. doi: 10.1007/s00213-009-1722-1. [DOI] [PubMed] [Google Scholar]
- 33.Berman RM, Sanacora G, Anand A, Roach LM, Fasula MK, Finkelstein CO, Wachen RM, Oren DA, Heninger GR, Charney DS. Monoamine depletion in unmedicated depressed subjects. Biol Psychiatry. 2002;51:469–473. doi: 10.1016/s0006-3223(01)01285-9. [DOI] [PubMed] [Google Scholar]
- 34.Barr LC, Goodman WK, Price LH, McDougle CJ, Charney DS. The serotonin hypothesis of obsessive-compulsive disorder: implications of pharmacologic challenge studies. J Clin Psychiatry. 1992;53(suppl):17–28. [PubMed] [Google Scholar]
- 35.Benkelfat C, Ellenbogen MA, Dean P, Palmour RM, Young S. Mood-lowering effect of tryptophan depletion: enhanced susceptibility in young men at genetic risk for major affective disorders. Arch Gen Psychiatry. 1994;51:687–697. doi: 10.1001/archpsyc.1994.03950090019003. [DOI] [PubMed] [Google Scholar]
- 36.Quintin P, Benkelfat C, Launay JM, Arnulf I, Pointereau-Bellenger A, Barbault S, Alvarez JC, Varoquaux O, Perez-Diaz F, Jouvent R, Leboyer M. Clinical and neurochemical effect of acute tryptophan depletion in unaffected relatives of patients with bipolar affective disorder. Biol Psychiatry. 2001;50:184–190. doi: 10.1016/s0006-3223(01)01140-4. [DOI] [PubMed] [Google Scholar]
- 37.Sobczak S, Riedel WJ, Booij I, Aan Het Rot M, Deutz NE, Honig A. Cognition following acute tryptophan depletion: difference between first-degree relatives of bipolar disorder patients and matched healthy control volunteers. Psychol Med. 2002;32:503–515. doi: 10.1017/s0033291702005342. [DOI] [PubMed] [Google Scholar]
- 38.Feder A, Skipper J, Blair JR, Buchhilz K, Mathew SJ, Schwarz M, Doucette JT, Alonso A, Collins KA, Neumeister A, Charney D. Tryptophan depletion and emotional processing in healthy volunteers at high risk for depression. Biol Psychiatry. 2011;69:804–807. doi: 10.1016/j.biopsych.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith KA, Fairburn CG, Cowen PJ. Relapse of depression after rapid tryptophan depletion. Lancet. 1997;349:915–919. doi: 10.1016/s0140-6736(96)07044-4. [DOI] [PubMed] [Google Scholar]
- 40.Leyton M, Young SN, Blier P, Ellenbogen MA, Palmour RM, Ghadirian AM, Benkelfat C. The effect of tryptophan depletion on mood in medication-free, former patients with major affective disorder. Neuropsychopharmacology. 1997;16:292–297. doi: 10.1016/S0893-133X(96)00262-X. [DOI] [PubMed] [Google Scholar]
- 41.Van der Does AJ. The effects of tryptophan depletion on mood and psychiatric symptoms. J Affect Disord. 2001;64:107–119. doi: 10.1016/s0165-0327(00)00209-3. [DOI] [PubMed] [Google Scholar]
- 42.Ellenbogen MA, Young SN, Dean P, Palmour RM, Benkelfat C. Mood response to tryptophan depletion in healthy volunteers: sex differences and temporal stability. Neuropsychopharmacology. 1996;15:465–474. doi: 10.1016/S0893-133X(96)00056-5. [DOI] [PubMed] [Google Scholar]
- 43.Moreno FA, McGahuey C, Freeman M, Delgado PL. Gender effects in mood during monoamine depletion. J Clin Psychiatry. 2006;67:1618–1623. doi: 10.4088/jcp.v67n1019. [DOI] [PubMed] [Google Scholar]
- 44.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 45.Neumeister A, Hu XZ, Luckenbaugh DA, Schwarz M, Nugent AC, Bonne O, Herscovitch P, Goldman D, Drevets WC, Charney DS. Differential effects of 5-HTTLPR genotypes on the behavioral and neural responses to tryptophan depletion in patients with major depression and controls. Arch Gen Psychiatry. 2006;63:978–986. doi: 10.1001/archpsyc.63.9.978. [DOI] [PubMed] [Google Scholar]
- 46.Moreno FA, Rowe DC, Kaiser B, Chase D, Michaels T, Gelernter J, Delgado PL. Association between a serotonin transporter promoter region polymorphism and mood response during tryptophan depletion. Mol Psychiatry. 2002;7:213–216. doi: 10.1038/sj.mp.4000962. [DOI] [PubMed] [Google Scholar]
- 47.Nugent AC, Neumeister A, Goldman D, Herscovitch P, Charney DS, Drevets WC. Serotonin transporter genotype and depressive phenotype determination by discriminant analysis of glucose metabolism under acute tryptophan depletion. Neuroimage. 2008;43:764–774. doi: 10.1016/j.neuroimage.2008.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roiser JP, Levy J, Fromm SJ, Goldman D, Hodgkinson CA, Hasler G, Sahakian BJ, Drevets WC. Serotonin transporter genotype differentially modulates neural responses to emotional words following tryptophan depletion in patients recovered from depression and healthy volunteers. J Psychopharmacol. 2012;26:1434–1442. doi: 10.1177/0269881112442789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neumeister A, Konstantinidis A, Stastny J, Schwarz MJ, Vitouch O, Willeit M, Praschak-Rieder N, Zach J, de Zwaan M, Bondy B, Ackenheil M, Kasper S. Association between serotonin transporter gene promoter polymorphism (5HTTLPR) and behavioral responses to tryptophan depletion in healthy women with and without family history of depression. Arch Gen Psychiatry. 2002;59:613–620. doi: 10.1001/archpsyc.59.7.613. [DOI] [PubMed] [Google Scholar]
- 50.Garriock HA, Delgado P, Kling MA, Carpenter LL, Burke M, Burke WJ, Schwartz T, Marangell LB, Husain M, Erickson RP, Moreno FA. Number of risk genotypes is a risk factor for major depressive disorder: a case control study. Behav Brain Funct. 2006;2:24. doi: 10.1186/1744-9081-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delgado PL. Neurobiology of serotonin norepinephrine reuptake inhibitors. Prim Psychiatry. 2009;16:8–15. [Google Scholar]
- 52.Nurnberger JI Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic Interview for Genetic Studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- 53.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. ed 4. Washington: American Psychiatric Association; 2005. [Google Scholar]
- 54.Mazure CM, Nelson JC, Price LH. Reliability and validity of the symptoms of major depressive illness. Arch Gen Psychiatry. 1986;43:451–456. doi: 10.1001/archpsyc.1986.01800050053006. [DOI] [PubMed] [Google Scholar]
- 55.Menkes DB, Coates DC, Fawcett JP. Acute tryptophan depletion aggravates premenstrual syndrome. J Affect Disord. 1994;32:37–44. doi: 10.1016/0165-0327(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 56.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams JB. Standardizing the Hamilton Depression Rating Scale: past, present, and future. Eur Arch Psychiatry Clin Neurosci. 2001;251(suppl 2):II6–II12. doi: 10.1007/BF03035120. [DOI] [PubMed] [Google Scholar]
- 58.Anderson GM, Young JG, Cohen DJ, Schlicht KR, Patel N. Liquid-chromatographic determination of serotonin and tryptophan in whole blood and plasma. Clin Chem. 1981;27:775–776. [PubMed] [Google Scholar]
- 59.Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- 60.Battersby S, Ogilvie AD, Smith CA, Blackwood DH, Muir WJ, Quinn, Fink G, Goodwin GM, Harmar AJ. Structure of a variable number tandem repeat of the serotonin transporter gene and association with affective disorder. Psychiatr Genet. 1996;6:177–181. doi: 10.1097/00041444-199624000-00001. [DOI] [PubMed] [Google Scholar]
- 61.Lesch KP, Balling U, Gross J, Strauss K, Wolozin BL, Murphy DL, Riederer P. Organization of the human serotonin transporter gene. J Neural Transm Gen Sect. 1994;95:157–162. doi: 10.1007/BF01276434. [DOI] [PubMed] [Google Scholar]
- 62.Le François B, Czesak M, Steubl D, Albert PR. Transcriptional regulation at a HTR1A polymorphism associated with mental illness. Neuropharmacology. 2008;55:977–985. doi: 10.1016/j.neuropharm.2008.06.046. [DOI] [PubMed] [Google Scholar]
- 63.Zhang HY, Ishigaki T, Tani K, Chen K, Sheh JC, Miyasato K, Ohara K, Ohara K. Serotonin2A receptor gene polymorphism in mood disorders. Biol Psychiatry. 1997;41:768–773. doi: 10.1016/S0006-3223(96)00160-6. [DOI] [PubMed] [Google Scholar]
- 64.Haghighi F, Bach-Mizrachi H, Huang YY, Arango V, Shi S, Dwork AJ, Rosoklija G, Sheng HT, Morozova I, Ju J, Russo JJ, Mann JJ. Genetic architecture of the human tryptophan hydroxylase 2 gene: existence of neural isoforms and relevance for major depression. Mol Psychiatry. 2008;13:813–820. doi: 10.1038/sj.mp.4002127. [DOI] [PubMed] [Google Scholar]
- 65.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29:374–393. [Google Scholar]
- 66.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–464. [Google Scholar]
- 67.Polesskaya OO, Sokolov BP. Differential expression of the ‘C’ and ‘T’ alleles of the 5-HT2A receptor gene in the temporal cortex of normal individuals and schizophrenics. J Neurosci Res. 2002;67:812–822. doi: 10.1002/jnr.10173. [DOI] [PubMed] [Google Scholar]
- 68.Turecki G, Briere R, Dewar K, Antonetti T, Lesage AD, Seguin M, Chawky N, Vanier C, Aida M, Joober R, Benkelfat C, Rouleau GA. Prediction of level of serotonin 2A receptor binding by serotonin 2A genetic variation in postmortem brain samples from subjects who did or did not commit suicide. Am J Psychiatry. 1999;156:1456–1458. doi: 10.1176/ajp.156.9.1456. [DOI] [PubMed] [Google Scholar]
- 69.Gray JA, Roth BL. Paradoxical trafficking and regulation of 5-HT(2A) receptors by agonists and antagonists. Brain Res Bull. 2001;56:441–451. doi: 10.1016/s0361-9230(01)00623-2. [DOI] [PubMed] [Google Scholar]
- 70.Marek GJ, Martín-Ruiz R, Abo A, Artigas F. The selective 5-HT2A receptor antagonist M100907 enhances antidepressant-like behavioral effects of the SSRI fluoxetine. Neuropsychopharmacology. 2005;30:2205–2215. doi: 10.1038/sj.npp.1300762. [DOI] [PubMed] [Google Scholar]
- 71.Neumeister A, Yuan P, Young TA, Bonne O, Luckenbaugh DA, Charney DS, Manji H. Effects of tryptophan depletion on serum levels of brain-derived neurotrophic factor in unmedicated patients with remitted depression and healthy subjects. Am J Psychiatry. 2005;162:805–807. doi: 10.1176/appi.ajp.162.4.805. [DOI] [PubMed] [Google Scholar]
- 72.Kendler KS, Thornton LM, Gardner CO. Stressful life events and previous episodes in the etiology of major depression in women: an evaluation of the ‘kindling’ hypothesis. Am J Psychiatry. 2000;157:1243–1251. doi: 10.1176/appi.ajp.157.8.1243. [DOI] [PubMed] [Google Scholar]