Abstract
Sequestration of chemical defenses from host plants is a strategy widely used by herbivorous insects to avoid predation. Larvae of the arctiine moth Utetheisa ornatrix feeding on unripe seeds and leaves of many species of Crotalaria (Leguminosae) sequester N-oxides of pyrrolizidine alkaloids (PAs) from these host plants, and transfer them to adults through the pupal stage. PAs confer protection against predation on all life stages of U. ornatrix. As U. ornatrix also uses other Crotalaria species as host plants, we evaluated whether the PA chemical defense against predation is independent of host plant use. We fed larvae from hatching to pupation with either leaves or seeds of one of eight Crotalaria species (C. incana, C. juncea, C. micans, C. ochroleuca, C. pallida, C. paulina, C. spectabilis, and C. vitellina), and tested if adults were preyed upon or released by the orb-weaving spider Nephila clavipes. We found that the protection against the spider was more effective in adults whose larvae fed on seeds, which had a higher PA concentration than leaves. The exceptions were adults from larvae fed on C. paulina, C. spectabilis and C. vitellina leaves, which showed high PA concentrations. With respect to the PA profile, we describe for the first time insect-PAs in U. ornatrix. These PAs, biosynthesized from the necine base retronecine of plant origin, or monocrotaline- and senecionine-type PAs sequestered from host plants, were equally active in moth chemical defense, in a dose-dependent manner. These results are also partially explained by host plant phylogeny, since PAs of the host plants do have a phylogenetic signal (clades with high and low PA concentrations in leaves) which is reflected in the adult defense.
Introduction
Defenses evolved by animals to avoid predation are ubiquitous in nature, and different defensive strategies have evolved in response to different life styles. The myriad defensive strategies include avoiding detection, preventing attack, and deceiving predators [1]. Some herbivorous insects feeding on chemically protected host plants are able to overcome these plant defenses by sequestering plant secondary compounds, and using them for their own defense against predator attack [2–4]. Sequestration may have evolved independently in different taxa of herbivorous insects. It predominates in Coleoptera and Lepidoptera, but also occurs frequently in the Heteroptera, Hymenoptera, Orthoptera and Sternorrhyncha [4]. The sequestered defensive compounds comprise a vast array of natural products of different biosynthetic pathways, such as cardenolides, cyanogenic and iridoid glycosides, aristolochic acids, glucosinolates and pyrrolizidine alkaloids. These substances are effective against a variety of predators, ranging from invertebrates including spiders and ants, to vertebrates including birds and mammals [2–5].
Generally, the effectiveness of chemical defenses against predation is dose- and/or structure-dependent [3]. High concentrations of sequestered compounds in the herbivorous prey are more effective against predators, and their effectiveness is usually related to the concentration of these compounds in the host plants. Different structures of the same class of compounds may also show different activities against predation. For instance, a palatability spectrum of the monarch and queen butterflies, Danaus plexippus and D. gilippus, was found to be associated with the amount of cardenolides sequestered from different host plants [6,7]. This pattern has been also observed in the adults of the nymphalid butterfly Euphydryas phaeton, which acquired defensive iridoid glycosides as larvae from two different host plants [8,9]; and in the adults of the heliconiine butterfly Heliconius erato, whose larvae feed on four species of Passiflora [10]. Additionally, Silva and Trigo [11] demonstrated that pyrrolizidine alkaloids have a positive dose-dependent activity in the protection of insects against the orb-weaving spider Nephila clavipes (Nephilidae). In the same study, they showed that different PA structures had different antipredation activities.
Among the defensive compounds sequestered by herbivorous insects, the role of pyrrolizidine alkaloids (PAs) has been well documented [12]. These compounds are produced by plants in several families (e.g. Asteraceae, Boraginaceae and Leguminosae), and are sequestered by specialist grasshoppers, hemipterans, beetles, moths and butterflies [5,13,14], where they show defensive and sexual communication functions [14]. PAs in specialized insects are always present in N-oxide form [15]. Many arctiine moths convert PAs sequestered from their larval host plants into “insect-PAs” in which the acid components of the alkaloids are replaced by small, branched aliphatic 2-hydroxy acids of insect origin [16]. These PAs of insect origin are precursors of the male sex pheromone in these moths [16].
The arctiine rattlebox moth Utetheisa ornatrix is one of the most-studied species with respect to its ecological dependence on PAs [5,14,17–19]. U. ornatrix occurs in the Neotropics and warm Nearctic regions [20], where it feeds on many species of Crotalaria legumes [21]. The genus Crotalaria has a Pantropical distribution, and some members have colonized the warmer parts of the Nearctic region [22]. The larvae of U. ornatrix sequester these alkaloids, and pass them to pupae and adults. During mating, males transfer PAs to females, which transfer them to the eggs. Therefore, all life stages of U. ornatrix are protected by PAs against predators [14]. Eisner [23] first demonstrated that the unpalatability of U. ornatrix adults is due to PAs in their tissues, and that the alkaloid originates from the larval host plant, Crotalaria pallida (formerly C. mucronata). Eisner found that adults of U. ornatrix were protected against the orb-weaving spider Nephila clavipes, as well as from other spiders, and birds. Additional evidence of the defensive role of PAs in U. ornatrix came from bioassays, with PAs topically applied on palatable organisms or by offering diets with or without PAs to PA-specialist insects, and testing these organisms against various predators [5].
Although it is suggested that the unpalatability of U. ornatrix may be closely related to the amount of PAs in their host-plant tissues, few studies have demonstrated this relationship. Eisner et al. [14,23–25] tested the adults, with and without PAs, against spiders and birds, but no dose-activity bioassay was carried out. Ferro et al. [26] found that the differences in adult palatability increased when their larvae fed on unripe seeds and leaves of C. pallida. The leaves had a lower PA content than unripe seeds, and consequently the larvae fed on leaves were more consumed by N. clavipes than those fed on unripe seeds. Likewise, Hristov and Conner [27] also showed that U. ornatrix fed on leaves of C. spectabilis were more predated by the bat Eptesicus fuscus compared to moths fed on seeds; moths raised in a diet free of PAs were palatable to the bats.
An important issue that remains unclear is the role of PA structure in the chemical defense of U. ornatrix. Do different structures confer different levels of defense? Eisner et al. [23–25] raised U. ornatrix on its usaramine PA-containing host plant (C. pallida) and observed that the moth was rejected by the spiders N. clavipes and Lycosa ceratiola, while moths raised on a diet free of PAs were preyed upon. Similar results were found for L. ceratiola, which released U. ornatrix raised on a diet supplied with PA monocrotaline, the main PA of C. spectabilis [24,25]. In two other studies on chemical defense of U. ornatrix adults, larvae were also raised on C. pallida or C. spectabilis, and therefore the PAs involved in the defense were respectively, usaramine and monocrotaline [26,27]. However, larvae of U. ornatrix can feed on many Crotalaria species [21,28], with different PA concentrations and profiles [29]. The finding that C. pallida and C. spectabilis conferred similar levels of defense in spite of containing different PAs led us to hypothesize that the structure is unimportant in determining palatability. Our predictions were: (1) adults from larvae that fed on plants or plant parts with high PA contents would be better defended than those that fed on plants or plant parts with low PA contents, and (2) PA structure would play no role in the moth chemical defenses.
Another point that has not been examined in studies of chemical defenses of herbivores sequestered from their host plants is the role of host plant phylogeny. It would be expected that phylogenetically related host plants produce similar compounds available for sequestration by insects. Therefore we cannot consider each host plant species as independent for comparative analysis (e.g. [30]). If phylogenetic inertia is strong, the potential adaptations that related species may evolve will be similarly constrained, with the effect that species cannot be regarded as independent of each other [31]. Consequently, it was necessary to take into account the host plant phylogeny, in order to compare the sequestered chemical defenses in an herbivorous insect. To our knowledge, this approach has never been taken in published studies. For example, even for the monarch butterfly, a well-studied model of sequestered chemical compounds, the connection between insect defenses and host plant phylogenetic relationships has never been explored, although the phylogenetic trends related to chemical defenses are well known in Asclepias [32,33]. Therefore, we explored the question of whether Crotalaria phylogeny directs any trend for the chemical defenses of U. ornatrix. We hypothesized that moths that fed on phylogenetically related host plants would show similar defensive patterns.
To address our hypothesis and predictions, we fed larvae on leaves or seeds of eight different Crotalaria species found in the Neotropics, which were native, non-native or of uncertain origin, with different PA concentrations and profiles [29]. The adults that emerged from larvae fed the different diets were offered to the spider N. clavipes in a predation bioassay. We analyzed the PA concentrations and profiles for U. ornatrix adults and Crotalaria species, and correlated the PA concentrations of adults with the PA contents of the plant parts they fed on as larvae. We also correlated the PA concentration and profile between the moths and the spider response in the predator bioassay. This correlation will or will not support our first prediction. Since we found different classes of PAs in U. ornatrix, we also bioassayed the three most common classes against the spider to test our second prediction. Finally, we mapped the PA profiles and concentrations in Crotalaria species, the PA concentrations in the moth, and the defensive response of N. clavipes against an independent phylogenetic hypothesis for the eight Crotalaria species, in order to test our last hypothesis.
Materials and Methods
Study System
The rattlebox moth Utetheisa ornatrix (Erebidae: Arctiinae) is primarily Neotropical and extends to warmer areas of the Nearctic region [20]. U. ornatrix, together with five species that occur only in the Galapagos Islands, are the extant Utetheisa species in the Neotropics [34,35]. The adults are generally found flying near patches of Crotalaria in pastures, the edge of woods and roadsides, where the larvae can be found feeding on both leaves or seeds inside the unripe pods of Crotalaria [21,26,28].
Crotalaria (Leguminosae: Papilionoideae: Crotalarieae) is a Pantropical genus of weeds, comprising approximately 702 species [22,36]. In the Neotropics, particularly in Brazil, 31 native and 11 non-native species have been recorded [37]. Crotalaria species are rich in PAs [34], which are found in higher concentrations in seeds than in leaves [26]. In addition to PAs, Crotalaria species have other defensive traits against herbivores including antifeeding deterrents such as lectins [38], non-protein amino acids [39] and protease inhibitors [40]. They also have extrafloral nectaries (EFNs) that attract predatory ants and wasps [26,41–43]. We used eight species of Crotalaria, including three natives, three non-natives, and two with uncertain origins (Table 1), that have been planted in an open area near the Animal Biology Department, Institute of Biology at the State University of Campinas, Campinas, São Paulo, Brazil (22°49'15.38"S, 047°04'8.87"W). In natural environments, we have observed U. ornatrix using C. incana, C. micans, C. spectabilis, C. pallida and C. vitelline as host plants. For the other three species, we have no information about their natural use by U. ornatrix.
Table 1. Species of Crotalaria used to feed larvae of Utetheisa ornatrix for Nephila clavipes bioassay and PA analysis.
| Species | Section | Native range |
|---|---|---|
| C. incana L. | Chrysocalycinae | Pantropical (uncertain origin) 2 |
| C. juncea L. | Calycinae | India, Asia 2 |
| C. micans Link | Chrysocalycinae | Neotropics 1 , 2 |
| C. ochroleuca G. Don | Hedriocarpae | Tropical Africa 2 |
| C. pallida Aiton | Hedriocarpae | Pantropical (uncertain origin) 2 |
| C. paulina Schrank | Calycinae | Neotropics 1 , 2 |
| C. spectabilis Roth | Crotalaria | Asia 2 |
| C. vitellina Ker Gawler | Chrysocalycinae | Neotropics 1 |
The neotropical orb-weaving spider Nephila clavipes (Nephilidae) is a predator that builds its web in forest clearings and corridors, which are flight paths for insects [44]. This spider preys on grasshoppers, bees, wasps, moths, and butterflies, but is able to discriminate PA-containing insects, releasing them unharmed [5] (Fig 1). In the edges of woods, this spider co-occurs with U. ornatrix.
Fig 1. Female of Nephila clavipes handling an adult male of Utetheisa ornatrix.
Note that the spider touches the moth with her pedipalps, probably evaluating the content of defensive pyrrolizidine alkaloids.
The permit for research with wild animals was provided by IBAMA-ICMBio (Ministério do Meio Ambiente, Brazil).
Rearing Utetheisa ornatrix Larvae
We obtained larvae of U. ornatrix from adults collected on the Fazenda Santa Mariana, Campinas, São Paulo, Brazil (22°47’02.91”S, 47°00’36.03”W), where the host plant C. pallida is abundant. We brought the adults to the laboratory, sexed them following Travassos [45], and allowed them to mate (20 males and 10 females per cage) in a paper cage (ca. 3.2 L) following Cogni [28]. We supplied food in a vial containing 5.0% aqueous honey. After 3–4 days, the adults started to oviposit on the paper-cage surfaces. The eggs were mixed to randomize the parental origin. After eclosion, we reared the first instars on leaves or unripe seeds of one of the eight species of Crotalaria. We also reared a group of first instars on a PA-free diet, following Cogni et al. [46]. For each treatment (diets of leaves or unripe seeds of each species of Crotalaria), we reared ca. 20 individuals in a plastic container (6 cm high, 20 cm diameter) until pupation. For some treatments, depending on the availability of seeds and leaves, we had 2 or 3 containers, giving 40–60 individuals per treatment. The larvae were fed ad libitum. We inspected the containers daily, removing feces and dead individuals, and replacing the leaves or seeds with new ones. We moved the pupae to another container with the same dimensions until adult emergence, since the larvae can cannibalize pupae [47]. We carried out the same procedure for pupae from the PA-free diet. We moved newly emerged adults to paper cages, separating them by treatment and sex, until the spider-predation bioassays. We kept both the paper cages with adults and the plastic containers with larvae and host plants, or pupae, at room temperature.
Predation Bioassay with Living Moths
We carried out the bioassays with Nephila clavipes in a small patch of woods in Campinas (22°48'21.26"S, 47°4'43.12"W) from March to May in 2012 and 2013, when the spiders were abundant. We conduced all bioassays between 09:00 and 16:00 hs. We used only adult female spiders that responded immediately when any prey was tossed into their web. We did not use spiders that were in the course of feeding on a prey insect, but we did not control for satiation before each bioassay. We used around 80 spiders for the bioassays. Since the number of spiders was a limiting factor, sometimes we repeated the bioassays with the same spider, but each individual was used only once per week. We bioassayed 481 adult moths, testing around 20 individuals for each sex, host plant and plant part. We made a small cut in the moth's hind wings before placing it on the web, to prevent the moth from flying away if released by the spider. We placed the moth on the web, and recorded if it was eaten or released. We considered the spider response to be predation when it bit, wrapped and killed the moth. When the spider cut the web around the moth after touching it and freed it unharmed, we recorded the response as a release. We did not record the rejection behavior described by Vasconcellos-Neto and Lewinsohn [48] (the prey was initially sucked and then freed), since the prey was removed from the web before this stage. If the spider released the moth, we offered a palatable freeze-killed mealworm Tenebrio molitor (Coleoptera: Tenebrionidae) as a control. We recorded the trial as a rejection only if the spider fed on the mealworm. If the spider killed the experimental moth, we did not use the control. When predation was recorded, we immediately removed the moth from the web and placed it in a 1.5-mL Eppendorf tube filled with MeOH for further PA analysis. All moths were recovered intact; even the parts bitten by the spider had no visible signs of damage. We also preserved the released moths in MeOH as above. We analyzed 310 individuals; 171 were not used for PA analysis, since they were lost after the spider bioassay.
Pyrrolizidine Alkaloids: Extraction, Quantification, Characterization and Isolation
To quantify the PAs, we extracted the freeze-dried samples of unripe seeds, leaves of host plants or adult moths three times with EtOH (10x volume:weight). We centrifuged the EtOH extract at 10,416 rcf for 10 min, and recovered the EtOH layer. We completed the EtOH layer to 20 mL and took an aliquot (0.1 or 1.0 mL) to carry out the colorimetric analysis according to Trigo et al. [49,50]. We used monocrotaline for the reference curve. The total PA concentration was given in μg of PAs/mg of dry weight.
For PA characterization by gas chromatography-mass spectrometry (GC-MS), we extracted the plant or insect samples using the acid-base procedures described by Trigo et al. [49,50]. The GC-MS analysis was carried out in electron impact mode according to Flores et al. [29]. The retention indices and mass fragmentation patterns were compared with published descriptions (see Table 2, and references [29,51,52]).
Table 2. Mass fragmentation pattern of pyrrolizidine alkaloids found in Crotalaria species and in adults of Utetheisa ornatrix fed on these host plants.
The analyses were carried out by gas chromatography-mass spectrometry (GC-MS) in electron impact mode and liquid chromatography-mass spectrometry (LC-MS) in electrospray ionization mode.
| Pyrrolizidine alkaloids | RI a | Rt b | Diagnostic ions for GC-MS c , m/z (%) | Diagnostic ions for LC-MS d , m/z (%) | Reference for GC-MS e |
|---|---|---|---|---|---|
| Retronecine | 1487 | 6.085 | [M+] 155 (23), 111 (60), 94 (17), 80 (100) | [2M+H]+ 343 (9), [M+H]+ 172 (100) | [29]1 |
| 9-(2’-Hydroxy)-ethanoylretronecine-like | 1795 | nd | [M+] 227 (2), 183 (6), 138 (62), 120 (6), 93 (100), 80 (40) | nd | [29]1 |
| 9-(2-Hydroxy-3-methylpentanoyl)-trachelanthamidine | 1831 | nd | [M]+ 255 (2), 142 (6), 125 (10), 124 (100), 96 (4), 83 (20), 82 (12) | nd | [52]1 |
| Creatonotine B-like | 1840 | nd | [M]+ 255 (3), 211 (7), 138 (99), 124 (13), 120 (11), 94 (40), 93 (100), 80 (25) | nd | [51]2 |
| 7-Senecioyl retronecine-like | 1861 | nd | [M]+ 237 (5), 137 (29), 136 (18), 94 (25), 93 (10), 80 (100) | nd | [29]1 |
| 9-Senecioyl retronecine-like | 1890 | nd | [M]+ 237 (3), 193 (9), 154 (15), 138 (27), 137 (28), 136 (20), 94 (24), 93 (100), 80 (20) | nd | [29]1 |
| Iso-creatonotine B | 2024 | 22.075 | [M]+ 269 (3), 251 (10), 138 (40), 124 (17), 120 (26), 111 (61), 106 (51), 94 (26), 80 (100) | [M+Na]+ 308 (11), [M+H]+ 286 (100) | [51]1 |
| 7-Octanoyl retronecine-like | 2031 | nd | [M]+ 281 (50), 220 (19), 154 (8), 136 (22), 124 (22), 111 (69), 106 (47), 94 (24), 80 (100) | nd | [29]1 |
| Creatonotine B | 2048 | 22.969 | [M]+ 269 (<1), 251 (1), 225 (5), 138 (100), 93 (88), 80 (18) | [M+Na+] 308 (6), [M+H]+ 286 (100) | [51]1 |
| 9-Octanoyl retronecine-like | 2052 | nd | [M]+ 281 (5), 236 (6), 138 (62), 120 (26), 106 (13), 94 (47), 93 (100), 80 (44) | nd | [29]2 |
| 1,2-Dihydrocreatonotine B | 2064 | nd | [M]+ 271 (4), 210 (12), 171 (20), 140 (25), 139 (11), 114 (10), 96 (34), 95 (70), 82 (100) | nd | [52]1 |
| 9-(5’-Hydroxy)-heptanoylretronecine-like | 2082 | 24.162 | [M]+ 283 (1), 224 (4), 155 (24), 138 (65), 93 (100), 80 (19) | [M+Na]+ 322 (14), [M+H]+ 300 (100) | [29]1 |
| Crispatine-like | 2175 | 22.598 | [M]+ 309 (2), 222 (7), 136 (88), 120 (83), 119 (100), 93 (62), 80 (29) | [M+Na]+ 348 (11), [M+H]+ 326 (100) | [29]3 |
| Unknown monocrotaline-type | 2243 | 24.936 | [M]+ 323 (3), 236 (4), 208 (10), 136 (97), 120 (87), 119 (100), 93 (54), 80 (27) | [M+Na]+ 362 (9), [M+H]+ 340 (100) | [29]3 |
| Incanine-like | 2315 | 27.280 | [M]+ 337 (6), 264 (11), 250 (5), 222 (8), 136 (100), 120 (76), 119 (79), 93 (49), 80 (27) | [M+Na+] 376 (15), [M+H+] 354 (100) | [29]1 |
| Monocrotaline | 2336 | 18.610 | [M+] 325 (1), 254 (3), 236 (46), 136 (52), 120 (100), 93 (38), 80 (19) | [M+Na]+ 364 (6), [M+H]+ 342 (100) | [29]1 |
| Senecionine / Integerrimine | 2339/2410 | 26.789/ 25.111 | [M]+ 335 (6), 291 (12), 248 (12), 220 (21), 136 (97), 120 (100), 93 (83), 80 (34) | [M+Na)+] 374 (13), [M+H]+ 352 (100) | [29]1 |
| Trichodesmine-like | 2341/2348 | 25.326 | [M]+ 353 (6), 264 (100), 136 (31), 120 (35), 93 (44), 80 (14) | [M+Na]+ 392 (17), [M+H]+ 370 (100) | [29]1 |
| Unknown monocrotaline-type | 2346 | 26.050 | [M]+ 337 (2), 222 (5), 136 (58), 120 (89), 119 (100), 93 (40), 80 (18) | [M+Na]+ 376 (20), [M+H]+ 354 (100) | [29]3 |
| Unknown monocrotaline-type | 2346 | nd | [M]+ 353 (2), 264 (45), 136 (75), 120 (100), 93 (70), 80 (25) | nd | [29]2 |
| Senecionine-like | 2376 | nd | [M]+ 337 (6), 222 (18), 136 (75), 120 (100), 93 (67), 80 (32), 55 (47) | nd | [29]2 |
| Methylmonocrotaline-like | 2384 | nd | [M]+ 339 (1), 250 (58), 136 (53), 120 (100), 93 (39), 80 (20) | nd | [29]1 |
| Senecionine-like | 2386 | nd | [M]+ 321 (13), 247 (7), 136 (98), 120 (100), 93 (76), 80 (37) | nd | [29]1 |
| Trichodesmine-like | 2418 | 23.415 | [M]+ 353 (2), 264 (60), 136 (47), 120 (100), 93 (35), 80 (18) | [M+Na]+ 392 (13), 370 [M+H]+ 370 (100) | [29]1 |
| Incanine-like | 2430 | nd | [M]+ 337 (5), 264 (27), 222 (20), 136 (62), 120 (100), 93 (65), 80 (27) | nd | [29]1 |
| Trichodesmine-like | 2437 | nd | [M]+ 353 (2), 264 (83), 222 (8), 136 (58), 120 (100), 93 (34), 80 (18) | nd | [29]1 |
| Senecionine-like | 2540 | nd | [M]+ 337 (1), 155 (12), 138 (71), 136 (28), 93 (100), 80 (19), 55 (27) | nd | [29]2 |
| Senecionine-like | 2551 | nd | [M]+ 351 (31), 220 (10), 136 (43), 120 (100), 119 (90), 93 (75), 80 (33) | nd | [29]2 |
| Platyphorine C-like | 2556 | 25.260 | [M]+ 383 (2), 281 (13), 267 (11), 252 (100), 138 (24), 136 (27), 120 (51), 93 (51), 80 (18) | [M+Na]+ 422 (19), [M+H]+ 400 (100) | [52]1 |
| Unknown seco-PA | 2615 | nd | [M]+ 365 (20), 321 (15), 276 (22), 238 (100), 168 (70), 122 (38), 110 (32), 94 (31), 83 (46) | nd | [29]1 |
| Retrorsine/Usaramine | 2621/2647 | 22.989/ 22.731 | [M]+ 351 (7), 246 (5), 136 (100), 120 (99), 119 (84), 93 (80), 80 (35) | [M+Na]+ 390 (7), [M+H]+ 368 (100) | [29]1 |
| Senecionine-like | 2675 | nd | [M]+ 351 (3), 224 (8), 143 (100), 136 (65), 120 (86), 119 (67), 93 (51), 80 (20) | nd | [29]2 |
| Senecionine-like | 2684 | nd | [M]+ 351 (2), 143 (24), 136 (51), 120 (100), 119 (91), 93 (79), 80 (23) | nd | [29]2 |
| Unknown seco-PA | 2728 | nd | [M]+ 381 (25), 338 (55), 320 (63), 250 (58), 238 (77), 168 (100), 150 (32), 122 (52), 110 (44) | nd | [29]1 |
| Unknown seco-PA | 2815 | nd | [M]+ 379 (43), 334 (26), 238 (22), 168 (74), 151 (29), 139 (57), 122 (100), 110 (62), 94 (57) | nd | [29]1 |
| Unknown seco-PA | 2866 | nd | [M]+ 421 (31), 376 (22), 168 (77), 150 (79), 139 (67), 122 (100), 110 (64), 94 (45), 43 (96) | nd | [29]1 |
| Unknown seco-PA | 2907 | nd | [M]+ 437 (34), 250 (79), 226 (41), 197 (78), 183 (78), 168 (44), 122 (100), 110 (52), 94 (52) | nd | [29]1 |
a. Retention index in GC-MS analyses.
b. Retention time (min) in LC-MS analyses. Some compounds detected by GC-MS were not detected (nd) by LC-MS.
c. in free base form.
d. in N-oxide form.
e. Characterization of PAs by mass spectra using: 1Mass spectra matching with literature, 2.Interpretation of the mass spectrum from literature, 3Erroneously described in 29 as unknown senecionine-type.
For PA characterization by liquid chromatography-mass spectrometry the samples were extracted in EtOH as above. The EtOH layer was evaporated in a rotaevaporator at 40°C, recovered with 1.5 mL 2% aqueous acetic acid and cleaned 3 times with the same volume of hexane. The acid solution was added to a clean 2-mL vial, capped, and stored at -20°C until LC-MS analysis. We used an Agilent 1260 Infinity Quaternary LC, equipped with an Eclipse Plus C-18 column, 4.6 x 250 mm, 5 μm, and a guard column with the same phase, coupled with an Agilent 6130 single quadrupole in electrospray ionization mode. The column was kept at 40°C, and the injection volume was 5 to 50 μl. The PA was separated using a linear gradient containing aqueous 20 mM ammonium acetate and MeOH at flow rate 0.5 mL/min. The gradient started at 95% ammonium acetate:5% MeOH (3 min), MeOH was raised to 100% in 25 min, and kept at 100% for 3 min. The mass spectrometer was run in positive mode, scanning the product ions from 100–500 amu (see Table 2).
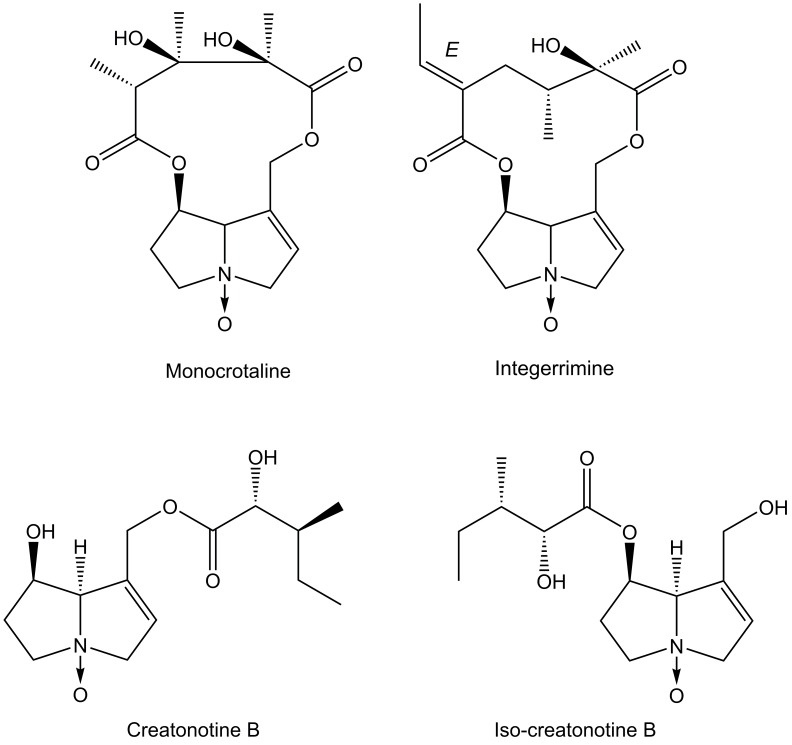
For the predation bioassays with pure PAs in N-oxide form, we isolated monocrotaline from C. spectabilis, integerrimine from Senecio brasiliensis (Asteraceae: Senecioneae) and the putative mixture insect PAs creatonotine B: iso-creatonotine B (Fig 2) from adults of U. ornatrix that fed as larvae on leaves and unripe seeds of C. vitellina. We extracted PAs using the acid-base procedure as described above, and isolated them using an adsorption column chromatograph (40 cm length, 2.5 cm diameter), using Silica Gel 70–325 mesh as the stationary phase, and a gradient from CHCl3 to CHCl3:MeOH:Et3N 85:14:1 as the mobile phase. We followed the PAs in 10-mL fractions by silica-gel thin-layer chromatography with CHCl3:MeOH:NH4OH 85:14:1 as eluent and Dragendorff's reagent for detection. We N-oxidized the PAs and purified them using the procedure described by Craig and Puroshothaman [53].
Fig 2. Some pyrrolizidine alkaloids found in this study.
The alkaloids are drawn in their N-oxide form. For more structures, see, e.g., Flores et al. [29].
Predation Bioassay with Pure Pyrrolizidine Alkaloids
We found that adults of U. ornatrix generally contained PA N-oxides of monocrotaline and integerrimine types, and the specific mixture of insect PAs creatonotine B: iso-creatonotine B (see Results). We carried out predation bioassays with N. clavipes to determine if PA N-oxides with different structures had similar activity against the spider. The bioassays were carried out in the same area where we carried out the predation bioassay with living moths.
We treated palatable freeze-killed larvae of the mealworm T. molitor (hereafter baits) with 3.0 μg/mg dry weight of each alkaloid, which were obtained as described above. This concentration was determined by calculating the logistic regression of the N. clavipes response in relation to PA concentration in adults of U. ornatrix fed as larvae on one of the eight different host plants (see Statistical analyses), where at 3.0 μg/mg the probability of release by the spider was 87%. To evaluate whether the PA concentration also played any role in the chemical protection against the spider, we also bioassayed these PA N-oxides in a one-tenth concentration. Additionally, we conducted bioassays with 5.0 μg/mg N-oxide for the insect PA. All bioassays were conducted using 10 baits for each PA concentration. The PAs were diluted in MeOH and applied topically with a 10-μL syringe on the surface of the bait, which was killed by freezing. We used a MeOH-treated bait as a control to determine whether to accept the trial, as described above for the N. clavipes predation bioassay with living moths.
Host Plant Phylogenetic Hypothesis and Character Mapping
We mapped the characters of PAs involved in the chemical defenses of U. ornatrix in an independent phylogenetic hypothesis for the eight Crotalaria species. This procedure may reveal the evolutionary trends of mapped characters [54]. We did this only for those that were fed on leaves, as all individuals fed on seeds were well protected against the spider, regardless of the host plant. As PA characters we used PA concentration (high > 3.0 μg/mg and low < 1.0 μg/mg) and PA type (monocrotaline, senecionine, senkerkine or insect PA) in all eight Crotalaria species and U. ornatrix, and the response of the predator N. clavipes in relation to U. ornatrix (percentage of release).
We constructed a phylogenetic hypothesis based on ITS sequences retrieved from GenBank for seven Crotalaria species and for the out-group Bolusia amboensis [36]. Total genomic DNA of C. vitellina (unavailable in GenBank) was extracted from fresh leaves, collected in the garden of UNICAMP, using the two-fold hexadecyltrimethylammonium bromide (CTAB) method [55]. The nuclear ribosomal ITS region (ca. 700 bp) was amplified as described by le Roux et al. [36], using the primers ITS 17SE and ITS 26SE [56,57]. PCR products were purified with a GFX PCR DNA and Gel Band Purification Kit (GE Healthcare) and sequenced at the Center of Molecular Biology and Genetic Engineering, UNICAMP, using an automated capillary sequencer (ABI PRISM 3700 DNA Analyzer, Applied Biosystems). Complementary sequences were assembled and edited with the Muscle algorithm [58] in MEGA 6 [59]. The C. vitellina ITS sequence was submitted to GenBank (KR013000). This sequence was aligned visually with the sequences gathered in GenBank, using MEGA 6, and the most appropriate nucleotide substitution model for the set of sequences was determined with the same software. Phylogenetic analyses were carried out using Bayesian Inference (maximum posterior probability, MPP) in Beast v1.8.1 [60] for a total of 10 million generations, with tree sampling every 1 million generations. A Yule speciation process was assumed, as recommended for species-level phylogenies [61]. The HKY+I substitution model was used for the substitution rate, and the default prior distribution was used for all other parameters. Tracer v.1.6 [62] was used to assess convergence using a minimum ESS value of 200. After the analyses were completed, 10% of the trees were removed as “burn-in”. The tree was visualized in FigTree v.1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/).
Statistical Analysis
We analyzed the frequency of individuals predated or released, using a generalized linear model (GLM) with binomial distribution and logit function link, using the package “bbmle” in R 3.1.0 for Windows [63,64]. Different models were generated to assess the effects of the explanatory variables (host plant, plant part, and sex of moth) as well as interactions between and among these variables in the response variable frequency of individuals predated or released. The model host plant, host plant part, and interaction between these factors provided the lowest AIC (Akaike’s Information Criterion) value (= 0.0). Therefore, we used an 8 x 2 design, where the factors were host plants (eight levels) and the part of the host plants where the larvae fed (two levels).
We compared the response variable concentration of PAs (μg/mg) in moths between parts of plant (two levels) and host-plant treatments (eight levels) by a two-way ANOVA, using the Tukey post-hoc test [65]. We discharged the explanatory variable sex, using AIC as above. We ln-transformed the PA concentration (in μg/g to avoid values < 1 in the ln transformation) to meet ANOVA assumptions [65]. A similar analysis was used to compare the PA concentration in the host plants. We calculated the nonlinear relationship between the PA concentration in U. ornatrix and the PA concentration in the leaves or seeds of the host plants, using the CurveExpert Professional 2.2.0.
We assessed the relationship between the N. clavipes response (predation or release) for individual adults of U. ornatrix feeding on each of eight different host plants and their PA concentrations, by a simple logistic regression [65]. We also tested this relationship after pooling the data for adults feeding on all host plants.
We asked if there is a relationship between the PA concentration in adults whose larvae fed on leaves or seeds and N. clavipes release, and if this relationship depended on the species of Crotalaria. We calculated this nonlinear relationship, as described above, using the mean PA concentration in adults whose larvae fed on leaves or seeds of each host plant and the percentage of adults released by the spider for each host plant.
As adults reared on different Crotalaria species showed a different PA profile with three PA classes (monocrotaline, senecionine and insect PA), we compared the response-variable percentage of adults released by N. clavipes in relation to the explanatory variables different PA structures (two levels) and concentration (two levels), topically applied to a palatable bait. We used a GLM binomial analysis as described above.
Results
Predation Bioassay with Living Moths
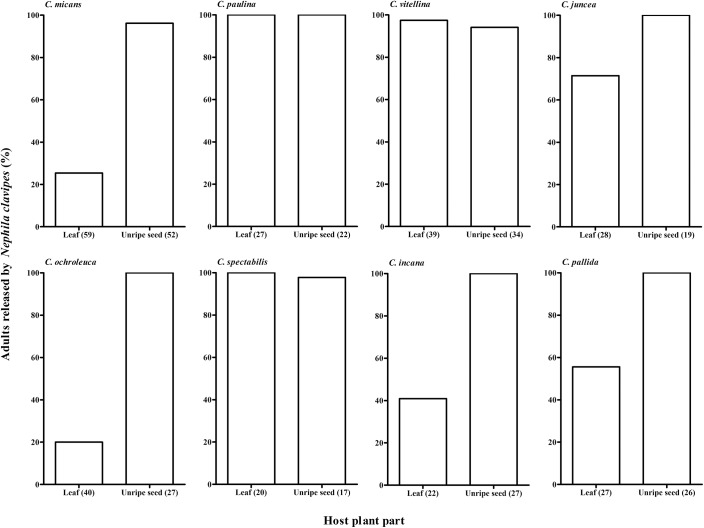
The frequency of predation or release was affected by the host plant (GLM binomial, χ2 = 104.714, df = 7, P <0.001), the part of the plant consumed by the larvae (χ2 = 147.355, df = 1, P <0.001) and by the interaction between these factors (χ2 = 19.274, df = 7, P = 0.007). Across all eight hosts, the spiders released 57.2% of the moths whose larvae fed on leaves, whereas 97.8% of the moths whose larvae fed on unripe seeds were released. The release percentage was lower for leaf feeders compared to seed feeders, for adults when larvae fed on C. incana, C. micans, C. juncea, C. ochroleuca and C. pallida (Fig 3). Adults whose larvae fed on either leaves or seeds of C. paulina, C. vitellina and C. spectabilis were equally well protected against N. clavipes attacks; almost all individuals were released (Fig 3).
Fig 3. Percentage of adults of Utetheisa ornatrix released by Nephila clavipes with respect to eight host-plant species and plant parts (leaf or unripe seed) fed to the larvae.
The natives Crotalaria micans, C. paulina, and C. vitellina, non-natives C. juncea, C. ochroleuca, and C. spectabilis, and species of uncertain origin C. incana and C. pallida. Numbers in parentheses in "plant part" represent the number of adults bioassayed for each diet.
Pyrrolizidine Alkaloids: Sequester from Larval Host Plants and Transformation
Pyrrolizidine alkaloid concentration in moth and host plants
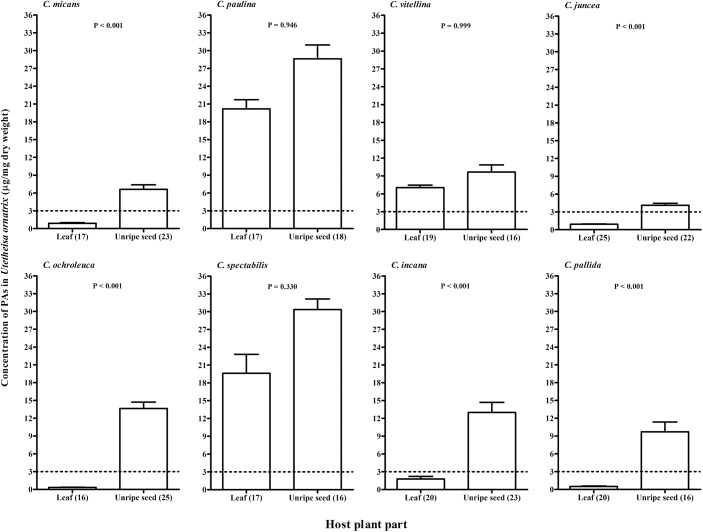
The concentration of PAs differed among adults whose larvae fed on different host plants (two-way Anova, F7,294 = 118.50, P < 0.001). Adults feeding as larvae on C. paulina and C. spectabilis had higher concentrations of PAs compared with adults reared on other species (Tukey test P < 0.001, Fig 4). The concentrations of PAs in adults also differed between plant parts fed to the larvae (F1,294 = 585.73, P < 0.001). The concentration of PAs was significantly higher in larvae feeding on seeds than in those feeding on leaves, but for larvae fed on C. paulina, C. spectabilis and C. vitellina we found no significant differences (Fig 4). We found an interaction between host plants and plant parts (F7,294 = 39.51, P < 0.001). Adults whose larvae fed on leaves of C. paulina, C. spectabilis and C. vitellina showed significantly higher PA concentrations than those reared on leaves of other Crotalaria species (Tukey test, P<0.001, Fig 4); however, larvae fed on C. vitellina showed a significantly lower concentration than both C. paulina and C. spectabilis feeders (P < 0.001, Fig 4).
Fig 4. Concentration of pyrrolizidine alkaloids (mean ± se) in adults of Utetheisa ornatrix with respect to eight host-plant species and plant parts (leaves or unripe seeds) fed to the larvae.
Numbers in parentheses in "plant part" represent the number of adults analyzed for each diet. The dotted line is the concentration at which baits treated with monocrotaline and integerrimine in the N-oxide form (3.0 μg/mg) were 100% released by Nephila clavipes. The probability that the PA concentration in adults fed as larvae on leaves or seeds, for each host plant, is significantly different is given above the bars (post hoc Tukey test). For other statistics, see Results.
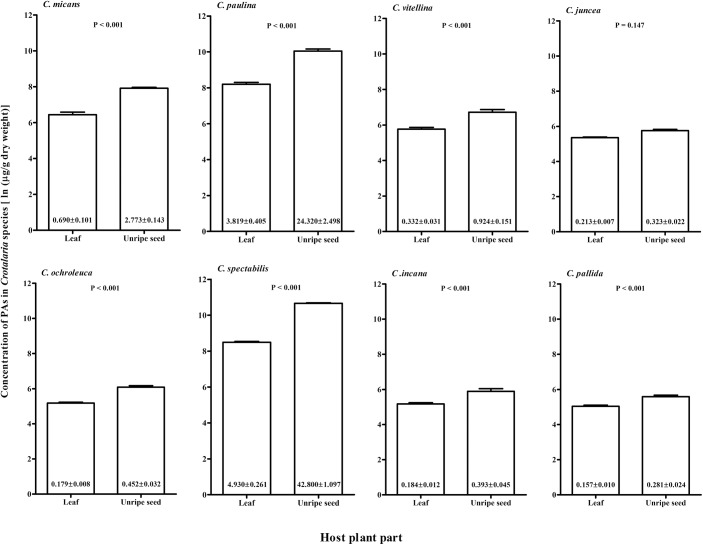
The PA concentrations in host plants differed significantly among the Crotalaria species (two-way Anova, F7,149 = 678.2, P < 0.001), and were highest in C. paulina and C. spectabilis (Fig 5). The concentration of PAs was significantly higher in seeds than in leaves (F1,149 = 596.2, P < 0.001, Fig 5). A significant interaction between host species and host plant part occurred (F7,149 = 23.8, P < 0.001), due to the lack of a significant difference between plant parts for C. juncea (Tukey test, P = 0.147).
Fig 5. Concentration of pyrrolizidine alkaloids (mean ± se) in leaves and unripe seeds of Crotalaria species, which were used to rear Utetheisa ornatrix larvae.
The concentration is given in ln transformed data (μg/g dry weight). The numbers inside the bars represent the mean ± SE of untransformed data in μg/mg dry weight. The probability that the PA concentration in leaves and seeds, for each host plant, is significantly different is given above the bars (post hoc Tukey test).
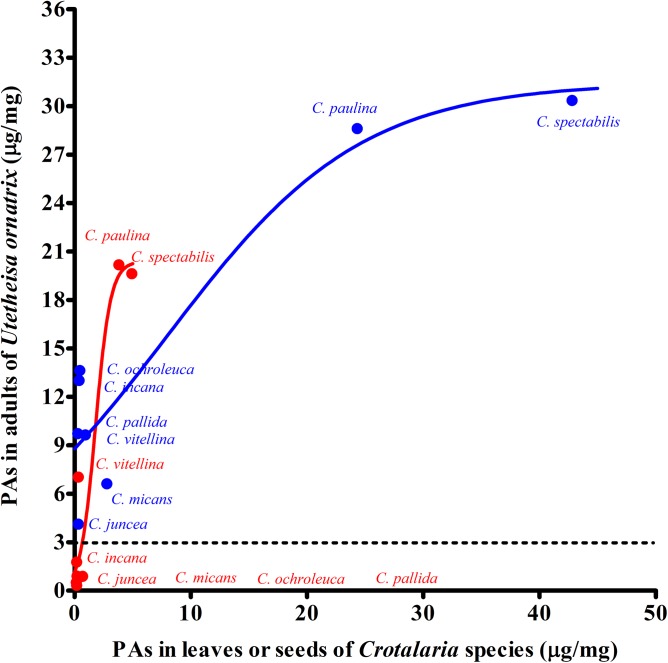
There was a positive relationship between the PA concentration in Crotalaria species and U. ornatrix, regardless of the part of the plant that the larvae fed on [nonlinear regression for larvae fed on leaves y = 20.39/(1+16.48e-1.55x), r2 = 0.93, and for larvae fed on unripe seeds y = 31.47/(1+2.58e-0.11x), r2 = 0.88, Fig 6]. However, we noted a maximum PA sequestration in U. ornatrix (around 30 μg/mg), independently of an increase in the concentration of PAs in their host-plant seeds.
Fig 6. Non-linear relationship between the mean of pyrrolizidine alkaloid concentration (μg/mg dry weight) in Crotalaria species and in adults of Utetheisa ornatrix.
Red symbols are leaves and blue are seeds. The dotted line is the concentration at which baits treated with monocrotaline and integerrimine in the N-oxide form (3.0 μg/mg) were 100% released by Nephila clavipes.
Pyrrolizidine alkaloid sequestration and transformation
The transformation of sequestered PAs into insect PAs is host-plant dependent. Adults whose larvae fed on host plants with monocrotaline-type (C. juncea, C. paulina or C. spectabilis) or integerrimine-type PAs (C. incana or C. ochroleuca) sequestered a large amount of unchanged alkaloids; small amounts of biosynthesized insect PAs were also found (Table 3). Adults whose larvae fed on C. vitellina, a host plant whose alkaloids are mainly seco-PAs (alkaloids with an otonecine base, such as senkirkine) and the necine base retronecine, biosynthesize large amounts of insect PA iso-creatonotine and creatonotine B; the seco-PAs, which are not N-oxidized, were not sequestered, but eliminated in the feces (data not shown) (Table 3). Adults whose larvae fed on C. micans or C. pallida showed high amounts of insect PAs together with unchanged PAs originating from the host plant; C. micans contained retronecine and integerrimine as the main PAs, and C. pallida contained usaramine (Table 3). All PAs in moths and seeds were present in the N-oxide form, except for the seco-PAs in C. vitellina and 34.7% of the monocrotaline in C. spectabilis, which was present in free base form.
Table 3. Relative abundance (%) of pyrrolizidine alkaloids in adults of Utetheisa ornatrix (male: M, female: F) and the species of Crotalaria used as larval host plants (HP).
C. micans: mic; C. paulina: pau; C. vitellina: vit; C. juncea: jun; C. ochroleuca: och; C. spectabilis: spe; C. incana: inc; C. pallida: pal. Five individuals were used for GC-MS analysis. Only seeds were analysed; the alkaloid profile of leaves was similar. The data are shown as mean ± standard error.
| Pyrrolizidine alkaloids | RI | mic M | mic F | mic HP | pau M | pau F | pau HP | vit M | vit F | vit HP | jun M | jun F | jun HP | och M | och F | och HP | spe M | spe F | spe HP | inc M | inc F | inc HP | pal M | pal F | pal HP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retronecine | 1487 | 19±2 | 22±3 | 21±6 | - | 2±1 | - | 26±2 | 32±4 | 3±1 | - | 2±1 | - | 6± 1 | 8±1 | 2±1 | - | - | - | 3±1 | 6±1 | 13±1 | 16±1 | - | |
| 9-(2’-Hydroxy)-ethanoyl retronecine-like | 1795 | - | - | - | - | 2±1 | 2±1 | - | - | - | 9±2 | 4±1 | 3±1 | - | - | - | - | - | - | - | - | - | - | - | - |
| 9-(2’-Hydroxy-3’-methylpentanoyl)-trachelanthamidine | 1831 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 2±1 | - | - | 2±1 | - | - |
| Creatonotine B-like | 1840 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1±1 | - | |
| 7-Senecioyl retronecine-like | 1861 | - | - | 3±1 | - | - | - | - | - | - | - | - | - | - | - | 2±1 | - | - | - | - | - | 2±1 | - | - | - |
| 9-Senecioyl retronecine-like | 1890 | - | - | 10±2 | - | - | - | - | - | - | - | - | - | - | - | 2±1 | - | - | - | - | - | 3±1 | - | - | - |
| Iso-creatonotine B | 2024 | 9±1 | 13±3 | - | - | - | - | 9±1 | 10±1 | - | - | - | - | 2±1 | 2±1 | - | - | 1±1 | - | - | 1±1 | - | 5±1 | 5±1 | - |
| 7-Octanoyl retronecine-like | 2031 | - | - | - | 2±1 | 1±1 | - | - | - | - | 6±1 | 11±4 | 13±1 | - | - | - | - | - | - | - | - | - | - | - | - |
| Creatonotine B | 2048 | 42±4 | 38±5 | - | 5±1 | 3±1 | - | 61±2 | 56±3 | - | 3±1 | 3±1 | - | 8±2 | 4±2 | - | - | 4±1 | - | 6±2 | 5±1 | - | 20±1 | 22±1 | - |
| 9-Octanoyl retronecine-like | 2052 | - | - | - | - | - | - | - | - | - | 8±1 | 10±1 | 7±1 | - | - | - | - | - | - | - | - | - | - | - | - |
| 1,2 Dihydrocreatonotine B | 2064 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1±1 | - | - | - | - | - |
| 9-(5’-Hydroxy)-heptanoyl retronecine-like | 2082 | - | - | - | - | - | - | - | - | - | 21±3 | 24±3 | 14±1 | - | - | - | - | - | - | - | - | - | - | - | - |
| Desoxymonocrotaline | 2175 | - | - | - | 17±1 | 21±1 | 42±2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Unknown monocrotaline-type | 2243 | - | - | - | 38±2 | 52±1 | 29±2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Incanine-like | 2315 | - | - | - | - | - | - | - | - | - | 6±1 | 2±1 | 12±1 | - | - | - | - | - | - | - | - | - | - | - | - |
| Monocrotaline | 2336 | - | - | - | 18±2 | 9±1 | 15±1 | - | - | - | - | - | - | - | - | - | 100 | 95±1 | 100 | - | - | - | - | - | - |
| Senecionine | 2339 | 3±1 | 4±2 | - | - | - | - | - | - | - | - | - | - | - | - | 9±1 | - | - | - | - | - | - | 1±1 | 2±1 | 1±1 |
| Trichodesmine-like | 2341 | - | - | - | - | - | - | - | - | - | 11±1 | 14±2 | 16±1 | - | - | - | - | - | - | - | - | - | - | - | - |
| Methylmonocrotaline | 2346 | - | - | - | 2±1 | 1±1 | 5±1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Trichodesmine-like | 2348 | - | - | - | - | - | - | - | - | - | 20±1 | 16±1 | 17±1 | - | - | - | - | - | - | - | - | - | - | - | - |
| Senecionine-like | 2376 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 8±2 | - | - | - |
| 14-Methylmonocrotaline-like | 2384 | - | - | - | 18±2 | 9±2 | 7±1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Senecionine-like | 2386 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1±1 | 1±1 | - |
| Integerrimine | 2410 | 22±6 | 17±4 | 66±4 | - | - | - | - | - | - | - | - | - | 65±4 | 70±2 | 50±1 | - | - | - | 53±3 | 87±1 | 76±4 | 1±1 | - | 7±1 |
| Trichodesmine-like | 2418 | - | - | - | - | - | - | - | - | - | 5±1 | 5±1 | 6±1 | - | - | - | - | - | - | - | - | - | - | - | - |
| Incanine-like | 2430 | - | - | - | - | - | - | - | - | - | 2±1 | 1±1 | 5±1 | - | - | - | - | - | - | - | - | - | - | - | - |
| Trichodesmine-like | 2437 | - | - | - | - | - | - | - | - | - | 9±1 | 8±2 | 7±2 | - | - | - | - | - | - | - | - | - | - | - | - |
| Senecionine-like | 2540 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 6±1 | - | - | - |
| Senecionine-like | 2551 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 2±1 | 2±1 | - |
| Platyphorine C-like | 2556 | 5±1 | 6±1 | - | - | - | - | 4±1 | 2±1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Unknown seco-PA | 2615 | - | - | - | - | - | - | - | - | 8±1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Retrorsine | 2621 | - | - | - | - | - | - | - | - | - | - | - | - | 15±1 | 13±2 | 30±1 | - | - | - | - | - | - | - | - | - |
| Usaramine | 2647 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 29±2 | 1±1 | 5±1 | 27±3 | 15±2 | 81±1 |
| Senecionine-like | 2675 | - | - | - | - | - | - | - | - | - | - | - | - | 4±1 | 3±1 | 5±1 | - | - | - | 3±1 | - | - | 26±2 | 34±2 | 11±1 |
| Senecionine-like | 2684 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 3±1 | - | - | 2±1 | 2±1 | - |
| Unknown seco-PA | 2728 | - | - | - | - | - | - | - | - | 6±1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Unknown seco-PA | 2815 | - | - | - | - | - | - | - | - | 61±2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Unknown seco-PA | 2866 | - | - | - | - | - | - | - | - | 20±2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Unknown seco-PA | 2907 | - | - | - | - | - | - | - | - | 2±1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
Pyrrolizidine Alkaloids and Predator Response
Across all adults whose larvae fed on different host plants and on different plant parts, the concentration of individual PAs in the moths had a significant positive relationship with release by N. clavipes (Table 4). For adults whose larvae fed on each species of Crotalaria, this pattern persisted, but for C. vitellina we found no significant relationship. For C. paulina and C. spectabilis, no statistical analyses were possible, since adults whose larvae fed on these two host plants had a high PA concentration regardless of plant part, and 98 to 100% of them were released.
Table 4. Logistic regression results for the Nephila clavipes response (predation or release) in relation to the concentration of pyrrolizidine alkaloids in adults of Utetheisa ornatrix fed as larvae on leaves or unripe seeds of eight host plants.
| Host plant | Equation | χ2 | P a |
|---|---|---|---|
| C. micans | Logit Pi = -2.655 + (1.641 Xi) | 29.279 | < 0.001 |
| C. paulina | Not calculated: all individuals were released | - | - |
| C. vitellina | Logit Pi = 3.696 - (0.020 Xi) | 0.006 | 0.940 |
| C. ochroleuca | Logit Pi = -2.547 + (1.772 Xi) | 40.464 | < 0.001 |
| C. juncea | Logit Pi = 0.526 + (0.887 Xi) | 5.384 | 0.020 |
| C. spectabilis | Not calculated: only 1 of 37 individuals was preyed | - | - |
| C. incana | Logit Pi = -1.586 + (1.040 Xi) | 21.594 | < 0.001 |
| C. pallida | Logit Pi = -0.646 + (1.779 Xi) | 13.585 | < 0.001 |
| All species pooled together | Logit Pi = -0.751 + (0.890 Xi) | 129.535 | < 0.001 |
aThe likelihood ratio test
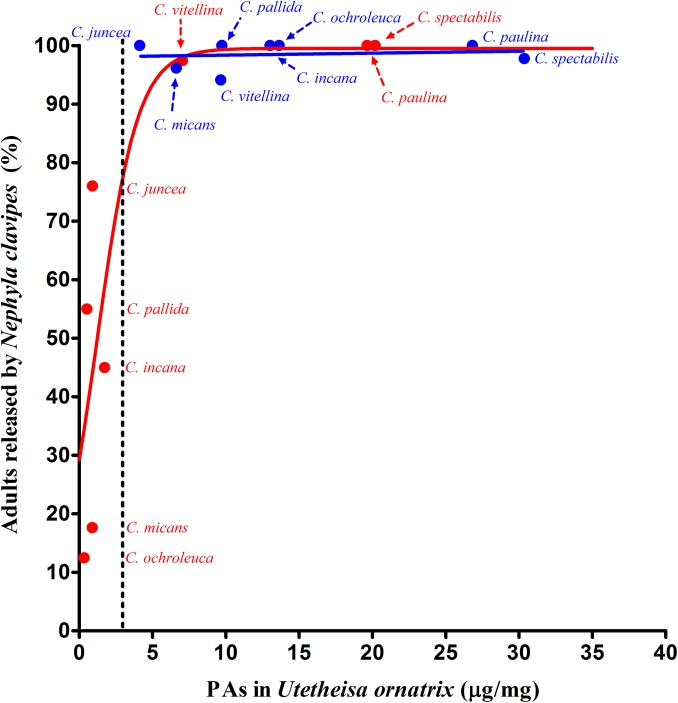
We found a significant positive relationship between the PA concentration of adults fed as larvae on leaves of different host plants and the percentage of adults released by N. clavipes [y = 99.5/(1+2.40e-0.72x), r2 = 0.83, Fig 7]. When larvae fed on host plants with a low PA concentration (C. incana, C. micans, C. juncea, C. ochroleuca and C. pallida), the percentage of release was low; conversely, when they fed on plants with a high PA concentration (C. paulina, C. spectabilis and C. vitellina), the percentage of release was high, nearly 100%.
Fig 7. Non-linear relationship between the mean of pyrrolizidine alkaloid concentration (μg/mg dry weight) in adults of Utetheisa ornatrix fed on leaves of different Crotalaria species and the percentage of these adults released by Nephila clavipes.
Red symbols stand for larvae fed on leaves and blue symbols stand for larvae fed on seeds. The dotted line is the concentration at which baits treated with monocrotaline and integerrimine in the N-oxide form (3.0 μg/mg) were 100% released by Nephila clavipes.
Predation Bioassay with Pure Pyrrolizidine Alkaloids
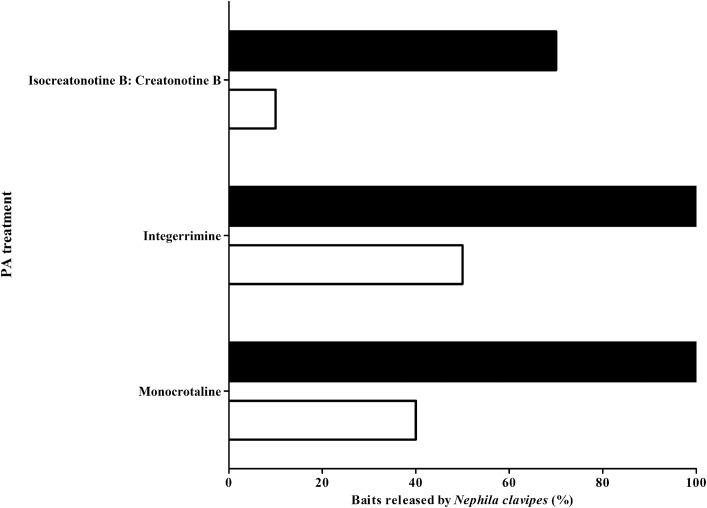
Baits treated with different N-oxide PA structures affected the percentages of baits (mealworm painted with PAs) released by N. clavipes (GLM binomial, χ2 = 9.692, P = 0.008). different PA concentrations also influenced the percentage of baits released by the spiders (χ2 = 27.442, df = 1, P < 0.001); while no interaction between factors was observed (χ2 = 0.008, df = 2, P = 0.996). N. clavipes released 100% of the baits treated with 3.0 μg/mg N-oxides of monocrotaline or integerrimine, and 70% of the baits treated with the insect PA mixture creatonotine B: iso-creatotine B. For 0.3 μg/mg, we found 60% release for monocrotaline, 50% for integerrimine, and 10% for the insect PAs (Fig 8). At the concentration of 5.0 μg/mg, N. clavipes released 100% of baits treated with creatonotine B: iso-creatotine B.
Fig 8. Percentage of baits treated with different PA N-oxides, which were released by the spider Nephila clavipes.
In black, the concentration of PAs was 3.0 μg/mg; in white 0.3 μg/mg.
Host Plant Phylogenetic Hypothesis and Character Mapping
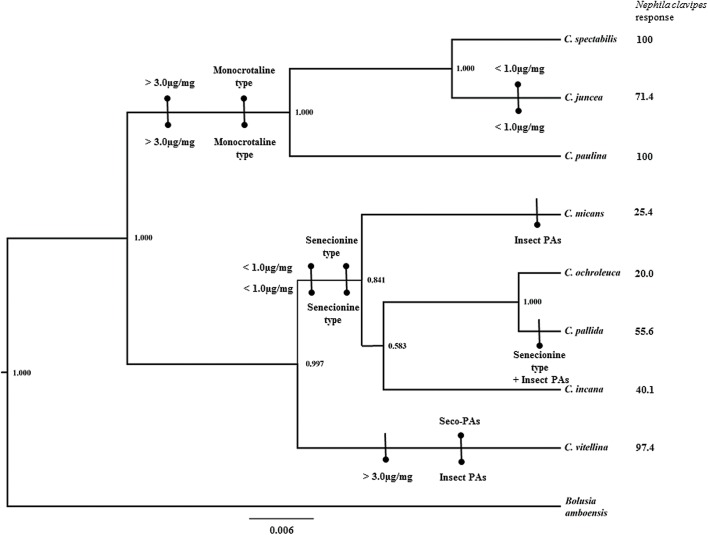
The eight species of Crotalaria assessed in this study were separated into three main clades, all highly supported by posterior probability values (Fig 9). Clade 1 included the species C. paulina, C. spectabilis and C. juncea, which showed monocrotaline-type PAs. High PA concentrations in leaves were found in C. paulina and C. spectabilis, but not in C. juncea. The moths feeding on leaves of plants of Clade 1 showed monocrotaline type PAs and a high PA concentration; most of these moths were released unharmed by the spiders. Clade 2 included the species C. micans, C. ochroleuca, C. pallida and C. incana, which contained senecionine-type PAs and small amounts of PA in the leaves. The moths reared on plants of this clade showed small PA amounts compared to those from plants of Clade 1, but a variation according to the class of PAs: adults reared on C. micans and C. pallida showed large amounts of insect PAs in the body, while the class senecionine was the main PA type found in adults reared on C. ochroleuca and C. incana; some of the adults were released by the spiders. Clade 3 included a single species, C. vitellina, which had seco-PAs and small amounts of PAs in the leaves. However, the PA content in moths reared on leaves of C. vitellina was high, with large amounts of insect PAs, and the spiders released almost all adults reared on this host species.
Fig 9. Bayesian inference topology for eight species of Crotalaria based on ITS sequences onto which are mapped the amount and type of PAs in leaves of the Crotalaria host plants (above) and in adults of Utetheisa ornatrix whose larvae fed on these plants (below).
Response of the spider Nephila clavipes in relation to adults of Utethesia ornatrix is given beside each Crotalaria species. Numbers in each node indicate Bayesian posterior probabilities. The scale below the tree indicates the mean number of substitutions per site.
Discussion
Our results indicated that PAs sequestered by U. ornatrix provide active chemical defense against predation, in a dose-dependent manner. The concentration of PAs in host plants where the larvae fed is a crucial factor for enhancing adult defense. Adults whose larvae fed on unripe seeds were almost 100% protected against N. clavipes, and showed high PA contents in their tissues. However, for those adults whose larvae fed on leaves, the protection against spider predation decreased nearly 40%; these adults, not coincidentally, showed low PA contents in their tissues. These results demonstrated that the high PA concentration in host plant seeds and low concentration in leaves might drive the spider response to U. ornatrix. Importantly, those adults whose larvae fed on leaves of C. paulina, C. spectabilis and C. vitellina had PA contents above 7.0 μg/mg in their tissues, and were well protected against the spider. The high content of PAs in C. paulina and C. spectabilis leaves may explain this protective effect. However, this was not the case for C. vitellina; this species had seco-PAs as the main compounds, together with retronecine. U. ornatrix did not sequester the seco-PAs, but they showed a high content of insect PAs biosynthesized from retronecine, which maximized their chemical defense. Although it is well known that PAs are responsible for the chemical defense of U. ornatrix and other arctiine moths [5,14,17], a dose-dependent approach has been less thoroughly documented. For instance, Hristov and Conner [27] demonstrated that when larvae fed on C. spectabilis seeds, the adults were less palatable to bats compared to adults whose larvae fed on leaves. Ferro et al. [26] found similar results when bioassaying U. ornatrix reared on C. pallida against the spider N. clavipes. Additionally, Dussourd et al. [66] described the same pattern for U. ornatrix eggs: those with a high amount of monocrotaline were rejected by the predaceous coccinellid beetle Coleomegilla maculata, but those with a low content were preyed upon. Pure PAs topically applied in a dose-dependent manner on palatable baits and offered to N. clavipes showed similar results [11]; there was a positive relationship between PA concentration and the N. clavipes release response using pure PAs.
The dose-dependence response raised other questions: what is the amount needed to elicit the predator's release response, and is there a threshold of sequestration by the moth? The spiders usually released adults that contained over 3.0 μg/mg PAs. However, adults whose larvae fed on C. paulina and C. spectabilis seeds showed ten times more PAs in their bodies. Feeding on high PA concentrations may not impose costs to U. ornatrix [47], and therefore larvae may prefer diets with a high PA content over diets with a low PA content [67]. However, larvae feeding on host plants with a very high PA concentration, such as in leaves and seeds of C. paulina and C. spectabilis, showed a sequestration threshold. Above 30 μg/mg of PA concentration in their diet, the larvae were unable to sequester more PAs. Malcolm [68] found a similar pattern for the monarch butterfly Danaus plexippus fed as larvae on milkweed Asclepias host plants with different cardenolide levels. Monarchs sequester cardenolides from milkweed species with low cardenolide contents, but when the plant increases the cardenolide content more than three to fourfold, monarchs reach an upper asymptote for sequestration. The likelihood of an uptake threshold in U. ornatrix, to reduce the costs of sequestration and metabolism (e.g. N-oxidation), deserves further evaluation. Additionally, we know little about the threshold response of other potential predators of U. ornatrix. Our results showed that above 3.0 μg/mg, adults were released by N. clavipes. However, the bat Eptesicus fuscus preyed on 70% of the adults of U. ornatrix from larvae fed on C. spectabilis leaves, and on 55% of the adults whose larvae fed on seeds [27]. Assuming that all C. spectabilis have a similar PA content, E. fuscus seems to tolerate a high concentration of PAs in relation to N. clavipes, which released all moths whose larvae fed on C. spectabilis. Therefore, sequestering a high amount of PAs may maximize the chemical defense against a broader predator spectrum. Furthermore, male moths biosynthesized the sex pheromone hydroxidanaidal from PAs [69] and passed on PAs to females as a nuptial gift; in turn, females endowed their eggs with this defense [66]. We suggest that the multiple uses of PAs by U. ornatrix may be responsible for the higher sequestration threshold.
To our knowledge, other pure defensive compounds sequestered from larval host plants by insects have not been tested in a dose-dependent manner. The closest example of a dose-dependent mechanism is found in the Asplepias-Danaus systems, where cardiac glycosides are sequestered from host plants of the subfamily Asclepiadoideae to protect Danaus plexippus against predation by birds [6]. Brower et al. [6] showed that the degree of unpalatability of the butterfly was linked to its host plants. However, there was a mismatch in the amount of cardenolides sequestered that caused unpalatability in D. plexippus and the cardenolide concentration in the host plants [68]. The low degree of unpalatability was attributed to the host plant with high cardenolide content, which was a non-native plant, and therefore they did not share a long evolutionary history [68].
The PA profile of adults of U. ornatrix varies in relation to the PA profiles of their larval host plant. The sequestration of unchanged PAs or transformation of plant PAs into insect PAs is also variable, as found for other arctiines [51,52,70,71]. Which PA is present in the adults seems to be unimportant for U. ornatrix chemical defense. Both N-oxides of monocrotaline and senecionine showed similar defensive efficiencies against N. clavipes. When the larvae feed on innocuous PAs such as retronecine, they are able to compensate for the structural inactivity by manufacturing their own PAs from plant precursors. These insect PAs exhibited efficacy comparable to that of monocrotaline- and senecionine-type PAs in protecting the moth against the spider. At high concentrations, analogous to those found in adults whose larvae fed on unripe seeds (3.0 μg/mg), 80–100% of the baits treated with these alkaloids were released by the spider, irrespective of PA type. With a one-tenth concentration (0.3 μg/mg), around 50% of the baits were released when the PAs were monocrotaline and senecionine, and 10% for the insect PAs tested. Siva and Trigo [11], using N. clavipes as the predator, found that a mixture of secionine:integerrimine N-oxide at 1.0 μg/mg led to 100% release of baits treated with this mixture.
Insect PAs constitute an important defensive mechanism. These biosynthesized insect-specific PAs are produced by several arctiine moths, through the esterification of necine bases derived from plant PAs with necic acids of insect origin [16]. These alkaloids have never been reported for U. ornatrix, and we found them in adults fed as larvae on all eight Crotalaria species. Insect PAs are reported as precursors of PA-derived pheromones [6, 53], although both males and females showed these alkaloids. Their role as defensive compounds, however, has received little attention [e.g. 11]. Our study demonstrated that this kind of PA is important for chemical defense of U. ornatrix. For example, those adults fed as larvae on C. vitellina showed high insect PAs and low amounts of retronecine, and were efficiently protected against N. clavipes. An N-oxide mixture of creatonotine B:iso-creatonotine B, in a similar concentration to those found in U. ornatrix fed on C. vitellina and topically applied on a palatable prey, also showed 100% efficiency against N. clavipes. It is known that retronecine is not active against N. clavipes predation, but insect PAs are [11]. Therefore, the transformation of necine bases into insect PAs may have been selected under predation pressure. Similarly to other PA compounds sequestered from plants, these insect-PAs have a dual role: chemical protection against predators, and precursors of sex pheromones in males of specialist lepidopterans.
Finally, the sequestration of defensive PAs by U. ornatrix from Crotalaria species is linked with Crotalaria phylogeny to some extent. Our phylogenetic analysis with the eight Crotalaria species showed one clade with high leaf PA content and another clade with low leaf PA content; both clades showed high PA contents in seeds. Crotalaria phylogeny may affect the chemical defense of U. ornatrix, when larvae feed on leaves, but not on seeds. Therefore, we can expect that if all other traits are equal, larvae would feed on both leaves and seeds of plants from clades with high PA content in the leaves, and would feed only on seeds in species from the clade with low leaf PA concentration. The extent to which the selective pressure of U. ornatrix on Crotalaria species drove the differences in PA concentration and profile among the Crotalaria species remains to be determined. For example, it is unknown if the shared evolutionary history between Crotalaria and U. ornatrix could have coevolved, affecting the chemical defenses in both the plant and the moth. On the other hand, when U. ornatrix or its ancestral line began to use Crotalaria as host plants, the patterns of plant PA defense could have already been established due to other selective forces, e.g. other herbivores.
In conclusion, the main factor in the chemical defense of U. ornatrix is the amount of PA sequestered or transformed from their host plants. Feeding on plants or parts of plants with high PA contents enhances the protection of U. ornatrix against predators. The presence of a non-active PA, such as retronecine, may not constitute a constraint on U. ornatrix chemical defense. The moth overcomes these limitations by maximizing the production of insect PAs, which have an antipredator role. Another constraint could be non-native Crotalaria host plants that impair both larval and adult performance, increasing the development time and decreasing the pupal weight ([21], J.R. Trigo, personal observation). However, this impairment may not affect the chemical defense of adults when the plants contain enough PAs to be sequestered by larvae. We suggest that the high degree of specialization of U. ornatrix on PAs in Crotalaria led to an efficient uptake of these compounds, independently of other nutritional or toxic constraints on its larval diet.
Acknowledgments
We thank José Carlos da Silva and Danilo Reali for help with Crotalaria cultivation and Nephila bioassays. Monica Kersch-Becker, Daniela Rodrigues, Rodrigo Cogni, Karina Brandão and three anonymous reviewers made valuable comments on the first draft. We also thank Gustavo Romero and Bruna Ramos, who helped with statistical analysis in R; Andreia Flores, who kindly supplied Crotalaria seeds and identified plant species; and Janet W. Reid, who reviewed the English text.
Data Availability
All relevant data are within the paper.
Funding Statement
Financial support was provided by FAPESP to JRT (11/17708-0) and VNS (12/02526-7), and CNPq to JRT (306103/2013-3). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ruxton GD, Sherratt TN, Speed MP. Avoiding attack: the evolutionary ecology of crypsis warning signals and mimicry Oxford: Oxford University Press; 2004. [Google Scholar]
- 2. Trigo JR. The chemistry of antipredator defense by secondary compounds in Neotropical Lepidoptera: facts, perspectives and caveats. J Braz Chem Soc. 2000; 11: 551–561. [Google Scholar]
- 3. Nishida R. Sequestration of defensive substances from plants by Lepidoptera. Annu Rev Entomol. 2002; 47: 57–92. [DOI] [PubMed] [Google Scholar]
- 4. Opitz SEW, Müller C. Plant chemistry and insect sequestration. Chemoecology. 2009; 19: 117–154. [Google Scholar]
- 5. Trigo JR. Effects of pyrrolizidine alkaloids through different trophic levels. Phytochem Rev. 2011; 10: 83–98. [Google Scholar]
- 6. Brower LP, Ryerson WN, Coppinger LL, Glazier SC. Ecological chemistry and the palatability spectrum. Science. 1968; 161: 1349–1350. [DOI] [PubMed] [Google Scholar]
- 7. Ritland DB. Variation in palatability of queen butterflies (Danaus gilippus) and implications regarding mimicry. Ecology. 1994; 75: 732–746. [Google Scholar]
- 8. Bowers MD. Unpalatability as a defense strategy of Euphydryas phaeton (Nymphalidae). Evolution. 1980; 34: 586–600. [DOI] [PubMed] [Google Scholar]
- 9. Bowers MD. Plant allelochemistry and mimicry In: Barbosa P, Letourneau D, editors. Novel aspects of plant-insect interactions, New York: John Wiley; 1988. pp. 273–311. [Google Scholar]
- 10. Hay-Roe MM, Nation J. Spectrum of cyanide toxicity and allocation in Heliconius erato and Passiflora host plants. J Chem Ecol. 2007; 33: 319–329. [DOI] [PubMed] [Google Scholar]
- 11. Silva KL, Trigo JR. Structure-activity relationships of pyrrolizidine alkaloids in insect chemical defense against the orb-weaving spider Nephila clavipes . J Chem Ecol. 2002; 28: 637–648. [DOI] [PubMed] [Google Scholar]
- 12. Hartmann T. Pyrrolizidine alkaloids: The successful adoption of a plant chemical defense In: Conner WE, editor. Tiger moths and woolly bears. Behavior, ecology, and evolution of the Arctiidae. New York: Oxford University Press; 2009. pp. 55–81. [Google Scholar]
- 13. Macel M. Attract and deter: a dual role for pyrrolizidine alkaloids in plant-insect interactions. Phytochem Rev. 2011; 10: 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eisner T, Meinwald J. The chemistry of sexual selection. Proc Natl Acad Sci USA. 1995; 92: 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hartman T, Ober D. Defense by pyrrolizidine alkaloids: Developed by plants and recruited by insects In: Schaller A, editor. Induced plant resistance to herbivory, Springer Science+Business Media BV, 2008. pp. 213–231. [Google Scholar]
- 16. Edgar JA, Boppré M, Kaufmann E. Insect-synthesized retronecine ester alkaloids: precursors of the common arctiine (Lepidoptera) pheromone hydroxydanaidal. J Chem Ecol. 2007; 33: 2266–2280. [DOI] [PubMed] [Google Scholar]
- 17. Conner WE, Jordan AT. From armaments to ornaments: the relationship between chemical defense and sex in tiger moths In: Conner WE, editor. Tiger moths and woolly bears. Behavior, ecology, and evolution of the Arctiidae. New York: Oxford University Press; 2009. pp. 155–152. [Google Scholar]
- 18. Eisner T, Rossini C, González A, Iyengar VK, Seigler MVS, Smedley SR. Paternal investment in egg defense In: Hilker M, Meiners T, editors. Chemoecology of insect eggs and egg deposition. Berlin: Blackwell Verlag; 2002. pp. 91–116. [Google Scholar]
- 19. Conner WE, Iyengar VK. Male pheromones and courtship In: Allison JD, Cardé RT, editors. Pheromone communication in moths: Evolution, behavior and application. California: University of California Press; 2015. In press. [Google Scholar]
- 20. Da Costa MA. Phylogeny of Utetheisa s. str. (Lepidoptera: Noctuidae: Arctinae) with comments on the evolution of colour, hind wing scales and origin of New World species. Invert Syst. 2010; 24: 113–130. [Google Scholar]
- 21. Sourakov A. You are what you eat: native versus exotic Crotalaria species (Fabaceae) as host plants of the Ornate Bella Moth, Utetheisa ornatrix (Lepidoptera: Erebidae: Arctiinae). J Nat Hist. 2015; 49: 2397–2415. [Google Scholar]
- 22. Polhill RM. Crotalaria in Africa and Madagascar. Rotterdam: AA Balkema; 1982. [Google Scholar]
- 23. Eisner T. For love of nature: exploration and discovery at biological field stations. Bioscience 1982; 32: 321–326. [Google Scholar]
- 24. Eisner T, Eisner M. Unpalatability of the pyrrolizidine alkaloid containing moth Utetheisa ornatrix, and its larva. Psyche. 1991; 98: 111–118. [Google Scholar]
- 25. González A, Rossini C, Eisner M, Eisner T. Sexually transmitted chemical defense in a moth (Utetheisa ornatrix). Proc Natl Acad Sci USA. 1999; 96: 5570–5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferro VG, Guimarães PR, Trigo JR. Why do larvae of Utetheisa ornatrix penetrate and feed in pods of Crotalaria species? Larval performance vs. chemical and physical constraints. Entomol Exp Appl. 2006; 121: 23–29. [Google Scholar]
- 27. Hristov N, Conner WE. Effectiveness of tiger moth (Lepidoptera, Arctiidae) chemical defenses against an insectivorous bat (Eptesicus fuscus). Chemoecology. 2005; 15: 105–113. [Google Scholar]
- 28. Cogni R. Resistance to plant invasion? A native specialist herbivore shows preference for and higher fitness on an introduced host. Biotropica. 2010; 42: 188–193. [Google Scholar]
- 29. Flores AS, Tozzi AMGA, Trigo JR. Pyrrolizidine alkaloid profiles in Crotalaria species from Brazil: chemotaxonomic significance. Biochem Syst Ecol. 2009; 37: 459–469. [Google Scholar]
- 30. Felsenstein J. Phylogenies and the comparative method. Am Nat. 1985; 125: 1–15. [Google Scholar]
- 31. Clutton-Brock TH, Harvey PH. Primate ecology and social organization. J Zool. 1977; 183: 1–39. [Google Scholar]
- 32. Agrawal AA, Fishbein M. Plant defense syndromes. Ecology. 2006; 87: S132–S149. [DOI] [PubMed] [Google Scholar]
- 33. Agrawal AA, Salminen J-P, Fishbein M. Phylogenetic trends in phenolic metabolism of milkweeds (Asclepias): evidence for escalation. Evolution. 2008; 63: 663–673. 10.1111/j.1558-5646.2008.00573.x [DOI] [PubMed] [Google Scholar]
- 34. Roque-Albelo L, Garrett SE, Conner WE. Darwin’s moth: Utetheisa in the Galápagos Islands In: Conner WE, editor. Tiger moths and woolly bears. Behavior, ecology, and evolution of the Arctiidae. New York: Oxford University Press; 2009. pp. 207–222. [Google Scholar]
- 35. Roque-Albelo L, Landry B. Two new species of Utetheisa Hübner (Lepidoptera, Noctuidae, Arctiinae) from the Galapagos Islands, Ecuador. ZooKeys. 2009; 21: 55–72. [Google Scholar]
- 36. le Roux MM, Boatwright JS, van Wyk BE. A global infrageneric classification system for the genus Crotalaria (Leguminosae) based on molecular and morphological evidence. Taxon. 2013; 62: 957–971. [Google Scholar]
- 37.Flores AS. Taxonomia, números cromossômicos e química de espécies de Crotalaria L. (Leguminosae-Papilionoideae) no Brasil. Dr. Sc. Thesis, Universidade Estadual de Campinas, Campinas, São Paulo, Brazil. 2004. Available: http://www.bibliotecadigital.unicamp.br/document/?code=vtls000343516.
- 38. Pando LA, Carvalho DD, Toyama MH, Ciero LD, Novello JC, Pascholatti SF, et al. Purification and characterization of a lectin from Crotalaria paulina seeds. Protein J. 2004; 23: 437–444. [DOI] [PubMed] [Google Scholar]
- 39. Pilbeam DJ, Bell EA. Free amino acids in Crotalaria seeds. Phytochemistry. 1979; 18: 973–985. [Google Scholar]
- 40. Gomes CEM, Barbosa AEAD, Macedo LLP, Pitanga JCM, Moura FT, Oliveira AS, et al. Effect of trypsin inhibitor from Crotalaria pallida seeds on Callosobruchus maculatus (cowpea weevil) and Ceratitis capitata (fruit fly). Plant Physiol Biochem. 2005; 43: 1095–1102. [DOI] [PubMed] [Google Scholar]
- 41. Guimarães PR, Raimundo LG, Bottcher C, Silva RR, Trigo JR. Extrafloral nectaries as a deterrent mechanism against seed predators in the chemically protected weed Crotalaria pallida (Leguminosae). Austral Ecol. 2006; 31: 776–782. [Google Scholar]
- 42. Franco MS, Cogni R. Common-garden experiments reveal geographical variation in the interaction among Crotalaria pallida (Leguminosae: Papilionideae), Utetheisa ornatrix L. (Lepidoptera: Arctiidae), and extrafloral nectary visiting ants. Neotrop Entomol. 2013; 42: 223–229. 10.1007/s13744-013-0114-8 [DOI] [PubMed] [Google Scholar]
- 43. Pereira MF, Trigo JR. Ants have a negative rather than a positive effect on extrafloral nectaried Crotalaria pallida performance. Acta Oecol. 2013; 51: 49–53. [Google Scholar]
- 44. Robinson MH, Mirick H. The predatory behavior of the golden-web spider Nephila clavipes (Araneae: Araneidae). Psyche. 1971; 78: 123–139. [Google Scholar]
- 45. Travassos L. Contribuição ao conhecimento dos ‘Arctiidae’. XI. Gênero “Utetheisa” Hübner, 1819. Verificação de “U. pulchella” (L., 1758) Kirby, 1892, no Nordeste do Brasil. Rev Bras Biol. 1946; 6: 343–354. [Google Scholar]
- 46. Cogni R, Trigo JR, Futuyma D. A free lunch? No cost for acquiring defensive plant pyrrolizidine alkaloids in a specialist arctiid moth (Utetheisa ornatrix). Mol Ecol. 2012; 21: 6152–6162. 10.1111/mec.12086 [DOI] [PubMed] [Google Scholar]
- 47. Bogner FX. Interspecific advantage results in intraspecific disadvantage: Chemical protection versus cannibalism in Utetheisa ornatrix (Lepidoptera: Arctiidae). J Chem Ecol. 1996; 22: 1439–1451. 10.1007/BF02027723 [DOI] [PubMed] [Google Scholar]
- 48. Vasconcellos-Neto J, Lewinsohn TM. Discrimination and release of unpalatable butterflies by Nephila clavipes, a neotropical orb-weaving spider. Ecol Entomol. 1984; 9: 337–344. [Google Scholar]
- 49. Trigo JR, Witte L, Brown KS, Hartmann T, Barata LES. Pyrrolizidine alkaloids in the arctiid moth Hyalurga syma . J Chem Ecol. 1996; 19: 669–679. [DOI] [PubMed] [Google Scholar]
- 50. Trigo JR, Witte L, Brown KS, Hartmann T, Ernst L, Barata LES. Pyrrolizidine alkaloids: different acquisition and use patterns in Apocynaceae and Solanaceae feeding ithomiine butterflies (Lepidoptera: Nymphalidae). Biol J Linn Soc. 1996; 58: 99–123. [Google Scholar]
- 51. Hartmann T, Theuring C, Beuerle T, Ernst L, Singer MS, Bernays EA. Acquired and partially de novo synthesized pyrrolizidine alkaloids in two polyphagous arctiids and the alkaloid profiles of their larval food-plants. J Chem Ecol. 2004; 30: 229–254. [DOI] [PubMed] [Google Scholar]
- 52. Hartmann T, Theuring C, Beuerle T, Klewer N, Schulz S, Singer MS, et al. Specific recognition, detoxification and metabolism of pyrrolizidine alkaloids by the polyphagous arctiid Estigmene acrea . Insect Biochem Mol Biol. 2005; 35: 391–411. [DOI] [PubMed] [Google Scholar]
- 53. Craig JC, Puroshothaman KK. An improved preparation of tertiary amine N-oxides. J Org Chem. 1970; 35: 1721–1722. [DOI] [PubMed] [Google Scholar]
- 54. Avise JC. Evolutionary pathways in nature. A phylogenetic approach New York: Cambridge University Press; 2006. [Google Scholar]
- 55. Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987; 19: 11–15. [Google Scholar]
- 56. White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics In: Innis MA, Gelfand MA, Sninsky JJ, White TJ, editors. PCR protocols. New York: Academic Press; 1990. pp. 315–322. [Google Scholar]
- 57. Sun Y, Skinner DZ, Liang GH, Hulbert SH. Phylogenetic analysis of sorghum and related taxa using internal transcribed spacers of nuclear ribosomal DNA. Theor Appl Genet. 1994; 89: 26–32. 10.1007/BF00226978 [DOI] [PubMed] [Google Scholar]
- 58. Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004; 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary genetics analysis. Version 6.0. Mol Biol Evol. 2013; 30: 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol. 2012; 29: 1969–1973. 10.1093/molbev/mss075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007; 7: 214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rambaut A, Suchard MA, Xie D, Drummond AJ. Tracer v1.6. 2014. Available: http://beast.bio.ed.ac.uk/Tracer.
- 63. Crawley MJ. The R book, 2nd edition Chichester: Wiley; 2012. [Google Scholar]
- 64. R Development Core Team. R: A language and environment for statistical computing Vienna: R Foundation for Statistical Computing; 2012. [Google Scholar]
- 65. Gotelli NJ, Ellison AM. A primer of ecological statistics Massachusetts: Sinauer Associates, Inc; 2004. [Google Scholar]
- 66. Dussourd DE, Ubik K, Harvis C, Resch J, Meinwald J, Eisner T. Biparental defensive endowment of eggs with acquired plant alkaloid in the moth Utetheisa ornatrix . Proc Natl Acad Sci USA. 1988; 85: 5992–5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hoina A, Martins CHZ, Trigo JR, Cogni R. Preference for high concentrations of plant pyrrolizidine alkaloids in the specialist arctiid moth Utetheisa ornatrix depends on previous experience. Arthropod Plant Interact. 2013; 7: 169–175. [Google Scholar]
- 68. Malcolm SB. Milkweeds, monarch butterflies and the ecological significance of cardenolides. Chemoecology. 1994/1995; 3/4/5/6: 101–117. [Google Scholar]
- 69. Conner WE, Eisner T, Vander Meer RK, Guerrero A, Meinwald J. Precopulatory sexual interaction in an arctiid moth (Utetheisa ornatrix): Role of a pheromone derived from dietary alkaloids. Behav Ecol Sociobiol. 1981; 9: 227–235. [Google Scholar]
- 70. Hartmann T, Theuring C, Beuerle T, Bernays EA. Phenological fate of plant-acquired pyrrolizidine alkaloids in the polyphagous arctiid Estigmene acrea . Chemoecology. 2004; 14: 207–216. [Google Scholar]
- 71. Hartmann T, Theuring C. Beuerle T, Bernays EA, Singer MS. Acquisition, transformation and maintenance of plant pyrrolizidine alkaloids by the polyphagous arctiid Grammia geneura . Insect Biochem Mol Biol. 2005; 35: 1083–1099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.