Abstract
Introduction
Alcohol has been shown to increase smoking urges and smoking behavior. However, alcohol’s effects on specific components of smoking behavior for nicotine versus non-nicotine factors and potential sex differences in this response have not been investigated.
Methods
Forty-two young male and female non-dependent, heavy social drinking smokers participated in two double-blind laboratory sessions. They were randomized to either an alcohol (0.8 g/kg; n = 29) or placebo (n = 13) beverage pre-administration group. After beverage consumption, they were assessed for smoking urges and then given the opportunity to smoke cigarettes which were either all nicotinized (0.6 mg/cigarette) or denicotinized (≤0.05 mg/cigarette) over a 3-h period; smoking behavior was quantified by a smoking topography device. Subjects took standardized puffs of the session’s cigarette both before and after beverage administration to provide a reference when making future smoking choices.
Results
Alcohol, compared with placebo beverage, increased both men’s and women’s smoking urge, as well as subjective ratings of smoking reference puffs for either nicotinized or denicotinized cigarettes. In terms of smoking choice behavior, regardless of cigarette type, alcohol (>placebo) increased men’s smoking behavior, including puff count, volume, and duration. In contrast, for women, smoking topography measures did not differ between alcohol and placebo conditions.
Discussion
In summary regardless of nicotine content, in men, alcohol increased smoking urge and behavior, whereas in women, alcohol increased smoking urge but did not increase smoking behavior. These results indicate that the mechanisms underlying co-use of alcohol and tobacco in women may be more complex than in men.
Keywords: Alcohol, Nicotinized and denicotinized cigarettes, Smoking topography, Sex differences, Non-daily smoker
Introduction
Epidemiological and clinical data demonstrate a strong positive relationship between alcohol drinking and cigarette smoking (Ait-Daoud et al. 2005; Bien and Burge 1990; Falk et al. 2006; Istvan and Matarazzo 1984). Heavier drinkers, are more likely to smoke than their moderate or light drinking counterparts (Falk et al. 2006). This positive relationship has been supported by data from controlled laboratory studies showing that acute alcohol, compared with placebo beverage, increases cigarette smoking behavior (Griffiths et al. 1976; Henningfield et al. 1983, 1984). Alcohol also increases urge to smoke, either in the presence of salient smoking cues (Burton and Tiffany 1997; Glautier et al. 1996; Mintz et al. 1985; Sayette et al. 2005) or in the absence of salient cues (Epstein et al. 2007; King and Epstein 2005). In terms of mechanisms, co-use of these substances may relate to positive or negative reinforcement. Recent studies have supported the former, as one study showed that the stimulating and not sedating effects of alcohol partially mediated alcohol’s increase of smoking urges (Epstein et al. 2007), and another study indicated that alcohol may also enhance the rewarding effects of nicotine, including smoking satisfaction and relief of cigarette craving (Rose et al. 2004).
There is a growing body of literature demonstrating sex differences in nicotine responses and drinking–smoking interactions (Drobes 2006; Field and Duka 2004; McClernon et al. 2008; Perkins 1995a; Perkins et al. 1997, 2006). It has been shown that women smokers are less successful and less confident in nicotine discrimination (Perkins 1995b; Perkins et al. 1997), have greater sensitivity to instructional set on ratings of nicotine reward and reinforcement (Perkins et al. 2006), and may have less success in smoking cessation using standardized treatments such as nicotine replacement than men (Bjornson et al. 1995; Perkins and Scott 2008; Royce et al. 1997; Senore et al. 1998; Wetter et al. 1999). Moreover, women may be less sensitive to smoking’s effects on alcohol behaviors, as evidenced by lower alcohol consumption after nicotine administration (Acheson et al. 2006) and less responding for alcohol after ad libitum smoking (Perkins et al. 2000) compared with their male counterparts. Finally, while for both sexes, heavier co-use of cigarettes is associated with heavier alcohol drinking, only in men is there an association between alcohol-related increases in smoking behavior and urge (King et al. 2008).
While alcohol’s effects on smoking urge and behavior have been demonstrated, as well as potential sex differences in their interaction, the mechanisms underlying this relationship remain unknown. More specifically, it is unclear whether alcohol increases smoking urge and behavior for the reinforcing effects of nicotine specifically, or for the associated features of smoking (handling, smoke, airway sensations, etc.). In addition, since the majority of prior studies on alcohol-nicotine interactions have been conducted in regular heavy smokers, it is unclear if results may extend to light/non-daily smokers, who do not show tobacco withdrawal symptoms that may confound other subjective assessments. Non-daily smokers are of interest in examining alcohol-smoking interactions because they may be particularly sensitive to alcohol’s effects on subsequent smoking urge and behavior, smoke proportionately more of their cigarettes in the context of alcohol than their nicotine-dependent counterparts (Shiffman and Paty 2006), and do not experience the confound of withdrawal effects from either substance in a controlled laboratory setting (Shiffman 1989; Shiffman et al. 1995).
Therefore, the goal of the current study was to further elucidate alcohol-smoking interactions by examining alcohol’s effects on heavy social drinkers’ smoking of nicotinized versus denicotinized cigarettes and potential sex differences in these responses. Subjective and objective measures of smoking urge and behavior were obtained during an alcohol challenge session, including quantifiable aspects of smoking behavior assessed via a smoking topography device during the 3-h post-drink period. It was predicted that alcohol, relative to placebo, would increase smoking behavior and that this increase would be preferential to nicotinized cigarettes. Further, based on the theory (Perkins 2001) that women’s smoking (versus men’s) may relate more strongly to exteroceptive factors, it was predicted that women would show less alcohol elicitation of smoking behavior than men.
Methods
Recruitment and screening
Subjects were 42 young adults with concomitant non-dependent, light smoking and non-dependent, heavy social drinking patterns. This group was chosen because of the strong association between alcohol drinking and cigarette smoking evident in heavy drinkers. They were recruited through newspaper and online advertisements in local media and by word-of-mouth referrals. Interested candidates completed both a brief phone interview and more extensive in-person screening to determine study eligibility.
At screening, candidates provided written informed consent and met the following inclusion criteria: age between 21 and 35; body mass index (BMI) between 19 and 30; and non-dependent, lifetime patterns of cigarette smoking and alcohol drinking. Self-reported alcohol and cigarette use patterns were confirmed by daily estimates of each substance as part of a 1-month timeline follow-back interview (TLFB; Sobell et al. 1979; Sobell and Sobell 1995). Smoking criteria included smoking between 1 and 50 total cigarettes per week, with typical daily use of no more than seven cigarettes, while allowing up to 15 cigarettes on heavy drinking days. Alcohol drinking criteria included a predominant adult drinking pattern of ten or more alcoholic drinks weekly with regular “binge” drinking episodes one to five times per week. As in other studies, a “binge” drinking episode was defined as consuming five or more drinks per occasion for men and four or more for women (SAMHSA 2005). Smoking and drinking criteria for inclusion were chosen to be consistent with prior studies in light smokers or tobacco chippers (Epstein et al. 2007; King et al. 2002; King and Epstein 2005; Ray et al. 2007; Sayette et al. 2005).
All candidates underwent a medical examination by a resident physician, completed a diagnostic interview with a trained assessor, provided blood and urine samples, and completed several psychosocial and health history questionnaires. Participants were excluded if they were taking any psychotropic medications, had a major medical or psychiatric condition (including past or current alcohol, nicotine, or other substance dependence), or had a positive urine toxicology screen (morphine, cocaine, methamphetamine, barbiturates, or benzodiazepines). Persons with alcohol and/or nicotine dependence, as determined by structured clinical interview for DSM-IV interview (SCID; First et al. 1995), were excluded to avoid potential withdrawal during the sessions. Finally, women were excluded if they were pregnant or breastfeeding.
Procedures
The study employed a two session, double-blind, double-dummy design that included a 15-min drinking period followed by 3 h of post-drink assessment of subjective effects and smoking behavior. The within-subjects factor was cigarette type (nicotinized versus denicotinized cigarettes), and the between-subjects factor was beverage type (alcohol versus placebo), with randomization to the alcohol or the placebo group at a 2:1 ratio. There were 29 subjects (14 females) in the alcohol administration group and 13 subjects (six females) in the placebo administration group. The rationale for the unequal grouping randomization derived from our prior data indicating that young adult heavy drinking chippers smoke infrequently on non-alcohol drinking days (King et al. 2008) and exhibit little change in subjective smoking urges after consuming a placebo beverage (Epstein et al. 2007; King and Epstein 2005). Therefore, it was assumed that they would not engage in significant choice smoking behavior to placebo relative to an intoxicating alcoholic beverage.
Test sessions began between 2:00 and 5:00 p.m. and were held at the Clinical Addictions Research Laboratory at the University of Chicago. Each session lasted 4 h and were separated by at least 48 h; the average interval between sessions was 6.6 ± 1.0 SEM days. Prior to each session, participants were instructed to abstain from alcohol and medications for 48 h and from caffeine, food, and cigarettes for 3 h. Upon arrival, the subject verified abstinence by providing an expired air carbon monoxide breath test (Smokerlyzer®, Bedfont Scientific, Medford, NJ, USA) of less than 10 ppm and an alcohol breathalyzer reading (Alco-Sensor III, Intoximeter, St. Louis, MO, USA) of less than 0.003 mg%. Subjects also provided a urine sample prior to each session for drug toxicology testing and a pregnancy test (for females). This was followed by consumption of a small snack (20% daily calories) to reduce potential negative effects of hunger on mood and/or nausea while drinking alcohol.
In Fig. 1, the study timetable, procedures, and measures are presented. Thirty minutes prior to beverage consumption, participants completed baseline measures and took their first set of two standardized reference puffs from that session’s randomized cigarette type. The puffs were standardized through an automated computer display prompting the initiation and discontinuation of each puff. They were included to provide a reference by which to make future smoking choices during that session (e.g., see Rukstalis et al. 2005). Following the completion of these procedures, the participant ingested a placebo gel capsule followed by the allocated study beverage (alcohol or placebo) over a 15-min time period (two 5-min periods of drinking separated by 5 min of rest to standardize alcohol intake). Fifteen minutes later, the subject took his/her second set of two standardized reference puffs. This was conducted to examine the effects of the beverage (alcohol or placebo) on subjective responses to the standard reference puffs. Starting at 60-min post-initiation of beverage consumption and at 0.5-h intervals for the next 3 h, the subject was given the option of smoking a cigarette via a smoking topography device (details below). This interval was chosen because smoking urge has been shown to peak approximately 1 h after alcohol consumption, i. e., when blood alcohol contents (BACs) are peaking, and remain elevated for several hours thereafter (Epstein et al. 2007; King and Epstein 2005). Each cigarette was presented to the subject with the following instructions: “At this point, you have the option of having a cigarette. You can smoke as much or as little as you’d like. You’ll have 5 min to decide if you’d like to smoke, and I’ll be back after that time”. After 5 min, the experimenter returned to the room and removed the cigarette if it was not chosen. If the cigarette was chosen, once completed, the experimenter removed the cigarette butt and lighter.
Fig. 1.
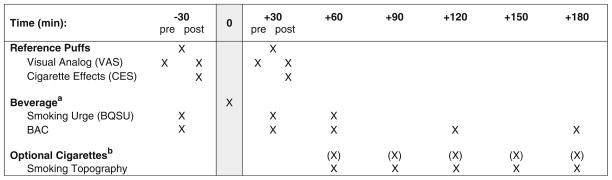
Session timeline of events and study measures. Time measured in relation to initiation of beverage consumption. Superscript a: participants were assigned to either alcohol or placebo beverage administration group for both sessions (between-subjects factors). Superscript b: subjects were given the option of smoking a cigarette via a smoking topography device at 30-min intervals following beverage consumption. Sessions were identical aside from cigarette type (within-subjects factor)
Between time points, participants were allowed to relax in the living room-like subject rooms, watch television or movies, or read. At the end of each session, subjects were transported home by a livery service. Each participant was debriefed and compensated $150 after completing the second study visit. The study was fully approved by the University of Chicago Institutional Review Board.
Beverages
During each of the two sessions, subjects in the alcohol group consumed a beverage containing a 0.8 g/kg dose of alcohol (equivalent to four to five standard alcoholic drinks), and subjects in the placebo group consumed an identical placebo beverage (1% alcohol by volume added as a taste mask). Beverages were parsed into two equal portions, served in clear, plastic, lidded cups, and consumed through a straw. To reduce alcohol expectancies, two strategies were employed: (a) subjects ingested a small gel capsule containing only dextrose prior to consuming the beverage; and (b) subjects were informed that the session beverage may contain alcohol, a stimulant, a sedative, a combination of these substances, or a placebo. Previous studies by our group have used similar blinding procedures (Conrad et al. 2009), and the gel capsule served to further reduce alcohol expectancies. The validity of this procedure was supported as subjects’ ratings of beverage content revealed that in 59% of alcohol sessions, subjects thought they had received a substance other than alcohol, and in 46% of placebo beverage sessions, subjects thought they had received an active substance. Session beverages were comprised of Kool-Aid (Kraft Foods, Inc, Northfield, IL, USA), Splenda® (McNeil Nutrionals, LLC, Fort Washington, PA, USA), water, and the appropriate dose of 190-proof ethanol based on body weight. Due to body water differences between the sexes, women received an approximate 85% dose as compared with men (Frezza et al. 1990; Sutker et al. 1983). Measures of BAC were obtained at baseline and during two points on both the ascending (+30, +60) and descending (+120, +180) blood alcohol curve (Fig. 1), utilizing an Alco-Sensor IV breathalyzer (Intoximeter, St. Louis, MO, USA).
Subjective measures
Smoking urges were assessed at baseline and at two intervals during the first 1 h following beverage consumption by the ten-item brief questionnaire on smoking urges (BQSU; Cox et al. 2001; see study timetable, Fig. 1). Responses to the standard reference puffs were measured via several visual analog scale (VAS) items and a modified cigarette evaluation scale (CES; Rose et al. 2000). The VAS items have been shown to be sensitive to acute smoking (Mendelson et al. 2008; Perkins et al. 1993, 1994). They were rated from 0 (“none”) to 100 (“most”) and included feeling stimulated, desire to smoke, dizzy, relaxed, head rush, and pleasure from cigarette. Seven CES items were each rated on a Likert scale from 1 (“not at all”) to 7 (“extremely”) and included satisfaction, reduced craving, strength and enjoyment of cigarette sensations in the throat and chest, perceived nicotine content, similarity to the subject’s preferred brand, and enjoyment of cigarette taste. Subjective measures were not included after the optional cigarette choice interval was initiated (i.e., after the 60-min post-drinking time point), due to the potential confound of choice behavior on subjective ratings.
Cigarettes
During each session, the participant received cigarettes (Quest® brand; Vector Tobacco Inc., Morrisville, NC, USA) that were either all nicotinized (0.6 mg nicotine yield per cigarette) or all denicotinized (≤0.05 mg nicotine yield per cigarette). The cigarettes were blinded by a physical ink marking over the brand recognition on the filter.
Objective smoking-related measures
All cigarettes were smoked through a device designed to measure the various smoking topography indices of each individual cigarette puff (Clinical Research Support System Smoking Topography Machine, Plowshare, Baltimore, MD, USA). The device was a small (2.5 × 2.2 × 1.2″), lightweight (3.1 oz), handheld black box with an entry port in which to place a cigarette and a small tube through which to inhale the smoke. Data from each cigarette puff was recorded on the device and later downloaded following each session. The primary measures obtained were puff count (the total number of puffs for each cigarette), puff volume (capacity of each puff in milliliters), and puff duration (length of each puff in milliseconds). The sum of each smoking topography variable across all five intervals at each session was used for analysis in order to examine the cumulative effect of alcohol on smoking behavior.
Statistical analyses
Background and demographic characteristics were compared by sex (men and women) and drinking group (alcohol and placebo) using Student’s t tests and Chi-square tests, where appropriate. Because men were older and had more years of education than women, these variables were used as covariates in analyses of subjective and objective outcomes. The subjective effects of the standardized reference cigarette puffs (i.e., nicotinized versus denicotinized) were analyzed by analyses of variance (ANOVA) with beverage (alcohol and placebo) and sex as the between-subjects factors and cigarette type (nicotinized and denicotinized) as the within-subjects factor. Similar ANOVAs were conducted to examine smoking topography measures. Simple effects tests were used for post hoc examinations of significant main effects and interactions. Pearson product moment partial correlations were also used to examine the relationship between changes in subjective response and subsequent smoking behavior, controlling age, education, sex, and beverage type with behavior as the outcome and changes in subjective response as the independent variable. Three subjects (one male and two females) made no cigarette choices throughout the entire study and therefore were not included in the smoking topography analyses.
Results
Participant characteristics
Background and demographic characteristics were similar between the sexes (see Table 1) with the exception of men being slightly older and having more years of education than women (ps ≤ 0.05). These characteristics were also similar between beverage condition groups, though BMI was slightly higher in the alcohol pre-administration group (p < 0.05). No sex differences were observed in self-reported alcohol drinking and cigarette smoking behaviors or in estimated nicotine content of preferred cigarette brand across sex or beverage group (Table 1). Both men and women’s smoking behaviors increased as a function of drinking day type. There was infrequent use of other substances, with marijuana as the most frequently used (see Table 1).
Table 1.
Background characteristics and substance use in men and women
| Alcohol
|
Placebo
|
|||
|---|---|---|---|---|
| Men (n=15) | Women (n=14) | Men (n=7) | Women (n=6) | |
| General characteristics | ||||
| Age (years) | 26.1 (0.9)* | 23.7 (0.6) | 24.7 (1.5)* | 22.5 (0.7) |
| Race (Caucasian) | 11 (73%) | 9 (64%) | 5 (71%) | 4 (67%) |
| BMI (kg/m2) | 25.2 (0.7)* | 24.3 (0.9)* | 23.5 (0.7) | 22.5 (0.7) |
| Education (years) | 16.1 (0.5)* | 14.8 (0.4) | 15.8 (0.3)* | 15.0 (0.5) |
| Smoking behavior | ||||
| Frequency (days/month) | 13.9 (1.9) | 15.8 (1.9) | 12.3 (1.8) | 14.7 (2.1) |
| Quantity (number of cigarettes/smoking day) | 4.3 (0.6) | 4.2 (0.4) | 3.0 (0.5) | 3.9 (0.8) |
| Estimated nicotine content of preferred brand of cigarettes (mg) | 0.91 (0.1) | 0.87 (0.0) | 0.97 (0.1) | 0.95 (0.1) |
| Drinking behavior | ||||
| Frequency (days/month) | 12.5 (1.5) | 12.1 (1.2) | 11.4 (1.6) | 11.3 (1.9) |
| Quantity (number of drinks/drinking day) | 6.6 (0.7) | 5.1 (0.4) | 5.8 (0.7) | 4.9 (0.8) |
| Heavy drinking frequency (number of heavy drinking days/month)a | 8.2 (1.0) | 8.1 (0.9) | 6.3 (0.7) | 8.2 (2.1) |
| Lifetime alcohol abuse | 12 (80%) | 11 (78%) | 7 (100%) | 4 (67%) |
| Smoking and drinking behavior | ||||
| Average number of cigarettes smoked on: | ||||
| Non-drinking days | 1.1 (0.4) | 1.5 (0.3) | 1.4 (0.3) | 1.4 (0.8) |
| Light drinking daysb | 1.6 (0.4) | 2.5 (0.5) | 2.0 (1.0) | 2.0 (1.1) |
| Heavy drinking daysc | 5.0 (0.9) | 5.8 (0.8) | 5.0 (1.3) | 4.8 (0.9) |
| Other substance use | ||||
| Marijuana (% using once/week or more) | 4 (27)% | 2 (14%) | 4 (57%) | 2 (33%) |
Data are mean (±SEM) or n (%)
Smoking and drinking data presented are obtained from a timeline follow-back interview
p ≤ 0.05 (age and education: men>women; body mass index: alcohol>placebo)
Heavy drinking=5+ drinks/occasion for men, 4+ for women
light drinking days=days in which one to four drinks reported for men, one to three for women;
heavy drinking days=days in which 5+ drinks reported for men, 4+ for women
Subjective measures: reference puffs
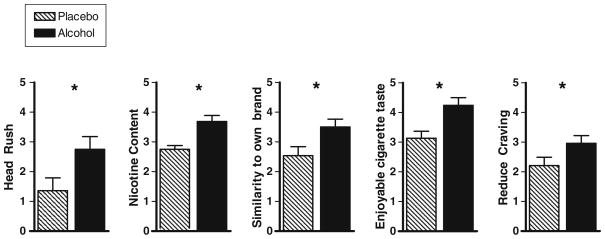
The first set of reference puffs had no effect on smoking urge, subjective effects, or evaluation of cigarettes (p ≥ 0.17). In contrast, the second set of reference puffs, i.e., conducted 15 min after beverage completion, did produce several differential effects by sex and cigarette type. First, after either alcohol or placebo beverage, the nicotinized puffs increased women’s but not men’s ratings of “feeling stimulated” (cigarette type×sex; F(1,35) = 6.69, p = 0.01; women: nic>denic, p < 0.001). Second, for both sexes, after consumption of either alcohol or placebo, the nicotinized puffs increased ratings of desire to smoke, satisfaction, enjoyable sensation, and enjoyable taste (cigarette type; F(1,35) = 5.91, p < 0.05). Finally, alcohol (versus placebo) increased ratings of head rush, nicotine content, similarity to own brand, enjoyable cigarette taste, and reduced cigarette craving (beverage group; F(1,35) = 4.04, p ≤ 0.05), regardless of the nicotine content of the puff (see Fig. 2).
Fig. 2.
Subjective responses (mean, SEM) to the second set of reference puffs (collapsed on cigarette type) in the alcohol and placebo conditions. Head rush measured on a visual analog scale (1–10 cm). Nicotine content, similarity to own brand, enjoyable cigarette taste, and reduce craving measured on a Likert scale (1–7). *p < 0.05
Subjective measures: smoking urge
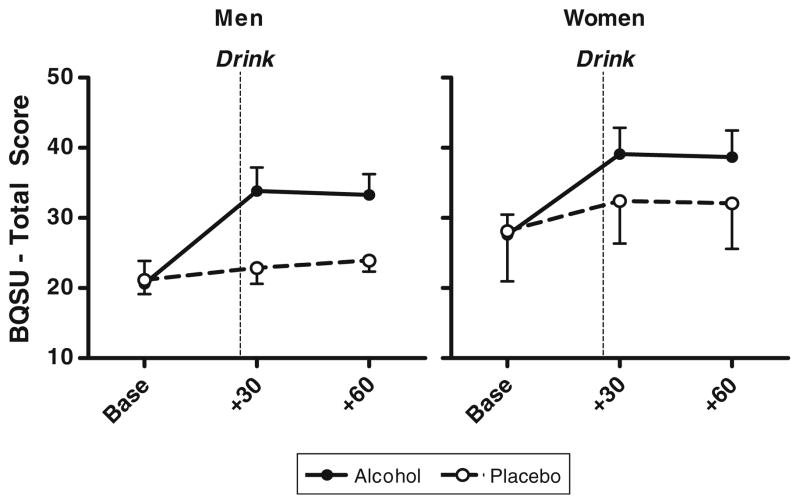
For both sexes, alcohol, compared with placebo beverage, significantly increased smoking urge (beverage group×time; F(4,35) = 5.16, p < 0.01). The pre-drink baseline BQSU scores were comparable between beverage conditions and directionally but non-significantly higher in women. Within 30 min of alcohol consumption, BQSU scores increased for both sexes (men: baseline 20.0 ± 3.2 versus post-beverage 32.4 ± 3.7; women: baseline 26.3 ± 3.0 versus post-beverage 37.2 ± 3.9) (see Fig. 3) and remained elevated at 60 min.
Fig. 3.
Smoking urge (BQSU total mean, SEM) in men and women. Time measured in relation to initiation of beverage consumption. p < 0.001, smoking urge was higher at 30 min post-beverage consumption as compared with baseline ratings in both men and women
Objective measures
As expected, alcohol increased BAC levels steadily in the first hour (30 min post-drink: 0.084 ± 0.004; 45 min post-drink: 0.097 ± 0.003; 60 min post-drink: 0.096 ± 0.002) which declined during the elimination phase (120 min post-drink: 0.075 ± 0.002; 180 min post-drink: 0.057 ± 0.002). The BAC levels did not differ between men and women (p = 0.24).
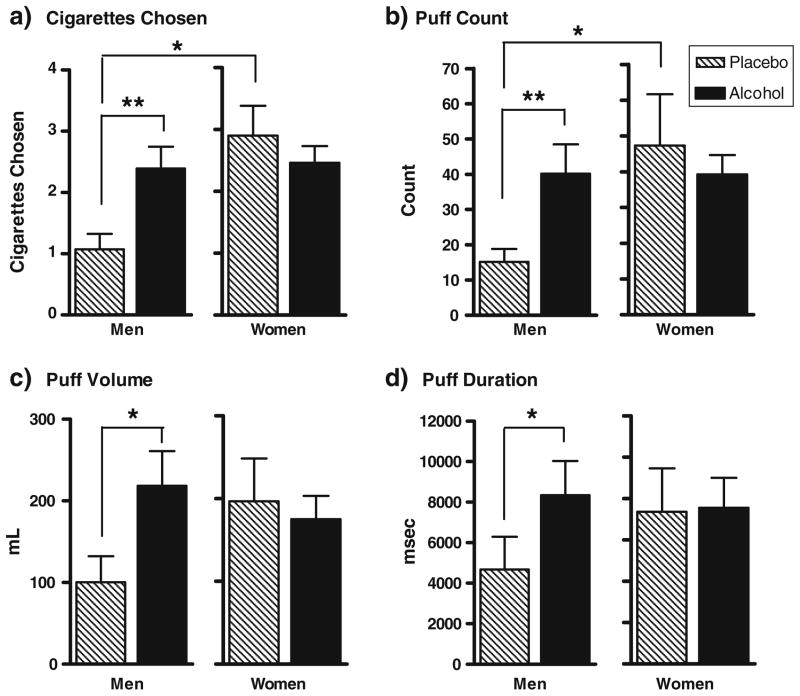
There were no differences in cigarette choice for the nicotinized versus denicotinized cigarettes (cigarette type: F(1,77) = 1.84, p=ns); therefore, all subsequent analyses examining beverage and sex effects were conducted collapsing on cigarette type. Examination of smoking choice behavior over the entire smoking choice interval, however, revealed several sex differences as a function of beverage type. These sex differences were corroborated by measures of smoking topography, including puff count, volume, and duration. Figure 4 depicts smoking choice behavior as well as smoking topography indices by beverage type and sex. In men, but not in women, alcohol (versus placebo) significantly increased smoking choice (beverage type×sex: F(1, 35) = 6.58, p < 0.05; simple effects men: alcohol>placebo, p < 0.01) and all three smoking topography measures, including puff count (beverage×sex; F(1, 35) = 7.46, p = 0.01; simple effects, men: alcohol>placebo, p < 0.01), puff volume (beverage× sex; F(1, 35) = 4.99, p < 0.05; simple effects, men: alcohol>placebo, p = 0.01), and puff duration (beverage× sex; F(1, 35) = 4.36, p < 0.05; simple effects, men: alcohol>placebo, p = 0.01). Calculation of partial Eta-squared revealed a medium effect size of the beverage type×sex interaction (cigarettes chosen: hp2 = 0.14; puff count: hp2 = 0.18; puff volume: hp2 = 0.13; puff duration: hp2 = 0.12; Cohen 1973). For women, there were no differences in smoking choice or topography measures between the alcohol and placebo conditions; in either beverage session, cigarettes chosen, puff count, volume, and duration for women were similar to those observed in men in the alcohol condition (see Fig. 4). In other words, alcohol selectively increased men’s smoking behavior but women’s smoking behavior did not differ in the alcohol and placebo conditions.
Fig. 4.
Smoking choice and topography measures (mean, SEM) for optional cigarettes smoked by men and women. Data collapsed on cigarette type as there were no significant differences between nicotinized and denicotinized cigarettes for choice or topography indices. Cigarettes chosen were averaged across nicotinized and denicotinized sessions. a *p < 0.05, women showed more cigarettes chosen than men; women showed more cigarettes chosen in the placebo condition than men. b **p ≤ 0.01, alcohol increased puff count in men; *p < 0.05, women showed higher puff count in the placebo condition than men. c *p < 0.05, alcohol increased puff volume in men. d *p < 0.05, alcohol increased puff duration in men
Relationship between subjective and objective measures
Smoking urge during the 60 min after beverage consumption was significantly correlated with subsequent smoking behavior, including both puff volume (r(38)=+0.36, p < 0.05) and puff duration (r(38)=+0.38, p < 0.05). These effects were apparent even after controlling for sex and beverage type, as well as age and education, as increases in smoking urge showed moderate relationships to smoking uptake measures, including puff duration (p < .05), and puff volume and count (p ≤ 0.08). However, other subjective measures taken during the initial post-beverage interval, such as feeling stimulated and cigarette pleasure, were not associated with subsequent smoking topography measures.
Discussion
In this study, we have further elucidated differences in alcohol-smoking interactions between men and women non-dependent drinker-smokers. First, we replicated prior findings by our group and others that under well-controlled laboratory conditions, an intoxicating dose of alcohol significantly increases smoking urge (Epstein et al. 2007; King and Epstein 2005; Ray et al. 2007; Sayette et al. 2005), and both men and women are sensitive to this effect (King et al. 2008). Second, we extended this finding to show that both men and women are sensitive to alcohol’s effects on numerous subjective ratings (i.e., head rush, enjoyable cigarette taste) after two brief cigarette puffs. Third, we found that in men, but not in women, alcohol significantly increased smoking behavior, as measured by cigarette choice and corroborated by puff count, volume, and duration. So, while women self-reported greater smoking urges after drinking alcohol compared with placebo, their smoking choice behavior and topography did not differ after consuming either beverage, and this smoking level was comparable to men’s smoking behavior observed only after alcohol drinking. Fourth, as mentioned earlier, while subjective responses to the nicotinized (0.6 mg yield) reference puffs indicated greater satisfaction and enjoyment compared with the denicotinized (≤0.05 mg yield) reference puffs, subsequent smoking choice in either beverage condition was not preferential to nicotinized versus denicotinized cigarettes.
Taking all the results together, it appears that smoking-drinking interactions are more complex in women than in men. Our results may extend Perkins’ (2001) theory that women, compared with men, smoke less to interoceptive factors and more to associated stimuli and psychophysical sensations of smoking (Benowitz and Hatsukami 1998; Perkins et al. 1999). It is unclear why women smoked in the laboratory at similar rates between the alcohol and placebo conditions. The moderate overall relationship observed in this study between smoking urge and behavior, which is similar to other studies, supports the notion that substance use behavior may be influenced by a number of factors beyond craving (For review, see Sayette et al. 2000). For instance, women may be more sensitive than men to the contextual and social factors involved in frequent cigarette choice offerings by the research assistant during the 2-h choice interval, regardless of beverage content. Compared with men, women have been shown to be more sensitive to instructional set stating accurate nicotine content of cigarettes on subsequent smoking reinforcement (Perkins et al. 2006) and to show a lack of association of alcohol-induced smoking urge in the laboratory with real-world co-use of alcohol and cigarettes (King et al. 2008). However, while smoking choice did not differ by beverage group in women, the similarity of topography between groups suggests that this explanation may not fully account for smoking behavior. Another possibility is that women were less sensitive than men to alcohol’s effects on respiration and bronchodilation (Sisson 2007), which may affect smoking behavior. Future studies comparing prompted smoking choice versus ad libitum smoking, and also discerning physiological mechanisms, after alcohol or placebo consumption may help determine the contribution of these factors to alcohol-smoking interactions between the sexes.
Another interesting and somewhat surprising finding in the present study is the lack of clear preference, in either alcohol or placebo conditions, for choosing to smoke nicotinized compared with denicotinized cigarettes. While both men and women demonstrated sensitivity to the post-beverage reference puffs for nicotinized cigarettes, including higher smoking desire, satisfaction, and enjoyment, subsequent smoking topography was similar between cigarette types. This finding is in contrast to a prior study showing that lower nicotine dependence may relate to greater reward from nicotine versus non-nicotine cigarettes (Brauer et al. 2001). However, a more recent study demonstrated that the converse relationship, i.e., smoking’s impact on subsequent alcohol self-administration, was observed after subjects smoked nicotinized versus denicotinized cigarettes (Barrett et al. 2006). It is has been posited that smoking reinforcement relates to both the sensory aspects of smoking as well as the direct central nervous system effects of nicotine (Rose et al. 2000). In this study, we utilized the Quest® brand of cigarettes because of the similarity in taste and appearance of the nicotinized and denicotinized cigarettes. However, the highest level of nicotine yield from this brand is 0.6 mg, which is lower than the yield of most marketed brands of cigarettes (FTC 2007); therefore, it cannot be ruled out that this lower level of nicotine may have impacted smoking uptake behavior due to inability to reach the threshold of nicotine necessary to activate brain reward pathways and motivational salience (Kalivas and Volkow 2005). While beyond the scope of the present study, future research is needed to discern the potential interaction of taste discrimination, sensory aspects of smoking, and the neurobiological effects of varying levels of nicotine content on social smoking-drinking behaviors.
Although there are many strengths in the present study, including a well-controlled laboratory paradigm with a placebo control, a priori investigation of sex differences, and smoking uptake measurement using a smoking topography device, there are several limitations worth noting. First, the sample size of the placebo group was relatively small. This was chosen because prior studies have shown little to no smoking urge changes after placebo consumption (Epstein et al. 2007; King and Epstein 2005) and infrequent cigarette smoking on non-drinking days in similar samples of non-dependent drinker-smokers (King et al. 2008). Given the relatively small sample size in the placebo group, caution should be taken to avoid over-interpreting results and replication in larger sample sizes is warranted. Because women’s smoking pattern may reflect a more complex interplay between alcohol and smoking behaviors, it would be important for future studies to fully examine factors potentially underlying sex differences, such as social influences and instructional set (Perkins et al. 2006; Rose et al. 1996; Wilson et al. 1995), gonadal hormones, and menstrual cycle effects (Allen et al. 1999; Hamilton and Yonkers 1996), and laboratory versus natural environment assessment of co-use via ecological momentary assessment and other advanced technological tools. Second, we studied only heavy drinking, non-daily smokers, and it is unclear if results may generalize to other drinking and smoking subgroups. Heavy drinkers were of interest in the current study as epidemiological data shows that most non-daily smoking occurs in the context of alcohol use, especially in young adults (Harrison et al. 2008). Other survey-based research has also demonstrated this association, as experimenting and non-daily smokers will delay smoking until after consuming larger quantities of alcohol as compared with their regular smoker counterparts (Harrison et al. 2009). Preliminary data by our group indicates that light/moderate social drinkers may also be sensitive to alcohol’s elicitation of smoking urge, although not to the extent of heavier social drinkers (King et al. 2009). Larger studies of alcohol-related smoking urge and behavior within a wider range of drinkers would enable us to understand if the current study findings are specific to heavier drinkers or if they generalize to both lighter drinkers and/or to alcohol-dependent drinkers. Finally, this study measured smoking behavior at maximum BAC levels, when smoking urge has been shown to peak; ascending BAC limb effects of alcohol on smoking behavior were not assessed. Studies of alcohol’s effects on smoking behavior throughout the BAC curve would provide more information on the temporal effects of alcohol on smoking choice.
In summary, the present study showed that alcohol and cigarette co-use may involve more complex factors in women compared with men. For men, consuming an intoxicating dose of alcohol, versus a placebo beverage, produced increases in smoking urge and subsequent measures of smoking behavior. In women, while alcohol also increased smoking urge, subsequent smoking behaviors were similar regardless of whether the beverage contained an intoxicating dose of alcohol or a placebo. Interestingly, in both sexes, smoking behavior in the laboratory was not specific to nicotine-containing cigarettes. Future studies with larger sample sizes and more detailed assessment of the complex interplay and development of comorbid alcohol drinking and cigarette smoking will provide valuable information to guide prevention and treatment efforts for the sexes.
Acknowledgments
This research was supported by NIH/NIAAA (#R03-AA015337, #R01-DA016834), a University of Chicago Cancer Research Center Grant (#P30-CA14599), and a General Clinical Research Center Grant (#M01-RR00055). The authors would like to thank Dr. Royce Lee for performing medical screenings and for medical oversight of the study and Lauren Kemp McNamara for conducting experimental sessions. The experiments in this study comply with the current US laws and were in compliance with the Declaration of Helsinki for human subjects.
Footnotes
Presented in part at the 2008 Society for Research on Nicotine and Tobacco Conference, Portland, OR, USA.
Work was performed at the Clinical Addictions Research Laboratory, University of Chicago, Chicago, IL, USA.
Contributor Information
Andrea King, Email: aking@bsd.uchicago.edu, Department of Psychiatry, Pritzker School of Medicine, University of Chicago, Chicago, IL, USA. Department of Psychiatry and Behavioral Neuroscience, University of Chicago, 5841 S. Maryland Avenue (MC-3077), Chicago, IL 60637, USA.
Patrick McNamara, Department of Psychiatry, Pritzker School of Medicine, University of Chicago, Chicago, IL, USA.
Megan Conrad, Department of Psychiatry, Pritzker School of Medicine, University of Chicago, Chicago, IL, USA.
Dingcai Cao, Department of Surgery, University of Chicago, Chicago, IL, USA.
References
- Acheson A, Mahler SV, Chi H, de Wit H. Differential effects of nicotine on alcohol consumption in men and women. Psycho-pharmacology. 2006;186:54–63. doi: 10.1007/s00213-006-0338-y. [DOI] [PubMed] [Google Scholar]
- Ait-Daoud N, Wiesbeck GA, Bienkowski P, Li MD, Pfützer RH, Singer MV, Lesch OM, Johnson BA. Comorbid alcohol and nicotine dependence: from the biomolecular basis to clinical consequences. Alcohol Clin Exp Res. 2005;29:1541–1549. doi: 10.1097/01.alc.0000174692.20933.49. [DOI] [PubMed] [Google Scholar]
- Allen SA, Hatsukami DK, Christianson D, Nelson D. Withdrawal and pre-menstrual symptomatology during the menstrual cycle in short-term smoking abstinence: effects of menstrual cycle on smoking abstinence. Nicotine Tob Res. 1999;1:129–142. doi: 10.1080/14622299050011241. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Tichauer M, Leyton M, Pihl RO. Nicotine increase alcohol self-administration in non-dependent male smokers. Drug Alcohol Depend. 2006;81:197–204. doi: 10.1016/j.drugalcdep.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Hatsukami D. Gender differences in the pharmacology of nicotine addiction. Addict Biol. 1998;3:383–404. doi: 10.1080/13556219871930. [DOI] [PubMed] [Google Scholar]
- Bien TH, Burge R. Smoking and drinking: a review of the literature. Int J Addict. 1990;25:1429–1454. doi: 10.3109/10826089009056229. [DOI] [PubMed] [Google Scholar]
- Bjornson W, Rand C, Connett JE, Lindgren P, Nides M, Pope F, Buist AS, Hoppe-Ryan C, O’Hara P. Gender differences in smoking cessation after 3 years in the lung health study. Am J Public Health. 1995;85:223–230. doi: 10.2105/ajph.85.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer LH, Behm FM, Lane JD, Westman EC, Perkins C, Rose JE. Individual differences in smoking reward from de-nicotinized cigarettes. Nicotine Tob Res. 2001;3:101–109. doi: 10.1080/14622200123249. [DOI] [PubMed] [Google Scholar]
- Burton SM, Tiffany ST. The effect of alcohol consumption on craving to smoke. Addiction. 1997;92:15–26. [PubMed] [Google Scholar]
- Cohen J. Eta-squared and partial eta-squared in fixed factor ANOVA designs. Educ Psychol Meas. 1973;33:107–112. [Google Scholar]
- Conrad MF, McNamara PM, King AC. Effects of blinding procedures on beverage content and subjective alcohol response. Paper presented at the 32nd Annual Conference of the Research Society on Alcoholism; San Diego, CA. 2009. [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Drobes DJ. Craving reactivity to smoking and alcohol cues in alcoholic and nonalcoholic smokers. Alcohol-nicotine interactions: are women like men?. Symposium conducted at the annual meeting of the Society for Research on Nicotine and Tobacco; Orlando, FL. 2006. [Google Scholar]
- Epstein AM, Sher TG, Young MA, King AC. Tobacco chippers show robust increases in smoking urge after alcohol consumption. Psychopharmacology. 2007;190:321–329. doi: 10.1007/s00213-006-0438-8. [DOI] [PubMed] [Google Scholar]
- Falk DE, Yi H, Hiller-Sturmhöfel S. An epidemiologic analysis of co-ocurring alcohol and tobacco use disorders. Alcohol Res Health. 2006;29:162–171. [PMC free article] [PubMed] [Google Scholar]
- Field M, Duka T. Cue reactivity in smokers: the effects of perceived cigarette availability and gender. Pharmacol Biochem Behav. 2004;78:647–652. doi: 10.1016/j.pbb.2004.03.026. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders—patient edition (SCID I/P, Version 2.0) Biometrics Research Department; New York, NY: 1995. [Google Scholar]
- Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990;322:95–99. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- FTC. Federal Trade Commission Cigarette Report for 2004 and 2005. Federal Trade Commission; Washington, D.C: 2007. [Google Scholar]
- Glautier S, Clements K, White JAW, Taylor C, Stolerman IP. Alcohol and the reward value of cigarette smoking. Behav Pharmacol. 1996;7:144–154. [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Liebson I. Facilitation of human tobacco self-administration by ethanol: a behavioral analysis. J Exp Anal Behav. 1976;25:279–292. doi: 10.1901/jeab.1976.25-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JA, Yonkers KA. Sex differences in pharmacokinetics of psychotropic medications: part I: physiological basis for effects. In: Jensvold M, Halbreich U, Hamilton JA, editors. Psychopharmacology of women: sex, gender, and hormonal considerations. American Psychiatric Press; Washington DC: 1996. pp. 11–41. [Google Scholar]
- Harrison ELR, Desai RA, MccKee SA. Nondaily smoking and alcohol use, hazardous drinking, and alcohol diagnoses among young adults: findings from the NESARC. Alcohol Clin Exp Res. 2008;32:2081–2087. doi: 10.1111/j.1530-0277.2008.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison ELR, Hinson RE, McKee SA. Experimenting and daily smokers: episodic patterns of alcohol and cigarette use. Addict Behav. 2009;34:484–486. doi: 10.1016/j.addbeh.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Chait LD, Griffiths RR. Cigarette smoking and subjective response in alcoholics: effects of pentobarbital. Clin Pharmacol Ther. 1983;33:806–812. doi: 10.1038/clpt.1983.110. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Chait LD, Griffiths RR. Effects of ethanol on cigarette smoking by volunteers without histories of alcoholism. Psychopharmacology. 1984;82:1–5. doi: 10.1007/BF00426371. [DOI] [PubMed] [Google Scholar]
- Istvan J, Matarazzo JD. Tobacco, alcohol, and caffeine use: a review of their interrelationships. Psychol Bull. 1984;95:301–326. [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- King AC, Epstein AM. Alcohol dose-dependent increases in smoking urge in light smokers. Alcohol Clin Exp Res. 2005;29:547–552. doi: 10.1097/01.alc.0000158839.65251.fe. [DOI] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response in heavy versus light drinkers. Alcohol Clin Exp Res. 2002;26:827–835. [PubMed] [Google Scholar]
- King A, Epstein A, Conrad M, McNamara P, Cao D. Sex differences in the relationship between alcohol drinking and smoking behavior: a pilot study. Am J Addict. 2008;17:347–353. doi: 10.1080/10550490802268140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, McNamara P, Cao D. Laboratory and longitudinal examination of co-occurring drinking and smoking behaviors in heavy and light drinkers in their twenties. Poster presented at the 2009 Joint Conference of SRNT and SRNT-Europe; Dublin, Ireland. 2009. [Google Scholar]
- McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsycho-pharmacology. 2008;33:2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Goletiani N, Sholar MB, Siegel AJ, Mello NK. Effects of smoking successive low-and high-nicotine cigarettes on hypothalamic-pituitary-adrenal axis hormones and mood in men. Neuropsychopharmacology. 2008;33:749–760. doi: 10.1038/sj.npp.1301455. [DOI] [PubMed] [Google Scholar]
- Mintz J, Boyd G, Rose JE, Charuvastra VC, Jarvik ME. Alcohol increases cigarette smoking: a laboratory demonstration. Addict Behav. 1985;10:203–207. doi: 10.1016/0306-4603(85)90001-2. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Individual variability in responses to nicotine. Behav Genet. 1995a;25:119–132. doi: 10.1007/BF02196922. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Nicotine discrimination in men and women. Pharmacol Biochem Behav. 1995b;64:295–299. doi: 10.1016/s0091-3057(99)00085-4. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Smoking cessation in women: special considerations. CNS Drugs. 2001;15:391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res. 2008;10:1245–1251. doi: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Epstein LH, Caggiula A, Stiller RL, Jacob RG. Chronic and acute tolerance to subjective effects of nicotine. Pharmacol Biochem Behav. 1993;45:375–381. doi: 10.1016/0091-3057(93)90254-q. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Sexton JE, Stiller RL, Fonte C, DiMarco A, Goettler J, Scierka A. Subjective and cardiovascular responses to nicotine combined with caffeine during rest and casual activity. Psychopharmacology. 1994;113:438–444. doi: 10.1007/BF02245220. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Sanders M, D’Amico D, Wilson A. Nicotine discrimination and self-administration as a function of smoking status. Psychopharmacology. 1997;131:361–370. doi: 10.1007/s002130050304. [DOI] [PubMed] [Google Scholar]
- Perkins K, Donny E, Caggiula AR. Sex difference in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999;1:301–315. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Fonte C, Grobe JE. Sex differences in the acute effects of cigarette smoking on the reinforcing value of alcohol. Behav Pharmacol. 2000;11:63–70. doi: 10.1097/00008877-200002000-00007. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Doyle T, Ciccocioppo M, Conklin C, Sayette M, Caggiula A. Sex differences in the influence of nicotine dose instructions on the reinforcing and self-reported rewarding effects of smoking. Psychopharmacology. 2006;184:600–607. doi: 10.1007/s00213-005-0103-7. [DOI] [PubMed] [Google Scholar]
- Ray LA, Miranda R, Kahler CW, Leventhal AM, Monti PM, Swift R, Hutchison KE. Pharmacological effects of naltrexone and intravenous alcohol on craving for cigarettes among light smokers: a pilot study. Psychopharmacology. 2007;193:449–456. doi: 10.1007/s00213-007-0794-z. [DOI] [PubMed] [Google Scholar]
- Rose JS, Chassin L, Presson CC, Sherman SJ. Demographic factors in adult smoking status: mediating and moderating influences. Psychol Addict Behav. 1996;10:28–37. [Google Scholar]
- Rose JE, Behm FM, Westman EC, Johnson M. Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacol Biochem Behav. 2000;67:71–81. doi: 10.1016/s0091-3057(00)00301-4. [DOI] [PubMed] [Google Scholar]
- Rose JE, Brauer LH, Behm FM, Cramblett M, Calkins K, Lawhon D. Psychopharmacological interactions between nicotine and ethanol. Nicotine Tob Res. 2004;6:133–144. doi: 10.1080/14622200310001656957. [DOI] [PubMed] [Google Scholar]
- Royce JM, Corbett K, Sorensen G, Ockene J. Gender, social pressure, and smoking cessations: the Community Intervention Trial for Smoking Cessation (COMMIT) at baseline. Soc Sci Med. 1997;44:359–370. doi: 10.1016/s0277-9536(96)00149-9. [DOI] [PubMed] [Google Scholar]
- Rukstalis M, Jepson C, Strasser A, Lynch KG, Perkins K, Patterson F, Lerman C. Naltrexone reduces the relative reinforcing value of nicotine in a cigarette smoking choice paradigm. Psychopharmacology. 2005;180:41–48. doi: 10.1007/s00213-004-2136-8. [DOI] [PubMed] [Google Scholar]
- SAMHSA. National Survey on Drug Use and Health. Office of Applied Studies; Bethesda, MD: 2005. [Google Scholar]
- Sayette MA, Shiffman S, Tiffany ST, Niaura RS, Martin CS, Shadel WG. The measurement of drug craving. Addiction. 2000;95(Suppl 2):S189–S210. doi: 10.1080/09652140050111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Perrott MA, Peters AR. The effects of alcohol on cigarette craving in heavy smokers and tobacco chippers. Psychol Addict Behav. 2005;19:263–270. doi: 10.1037/0893-164X.19.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senore C, Battista RN, Shapiro SH, Segnan N, Ponti A, Rosso S, Aimar D. Predictors of smoking cessation following physicians’ counseling. Prev Med. 1998;27:412–421. doi: 10.1006/pmed.1998.0286. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Tobacco “chippers”—individual differences in tobacco dependence. Psychopharmacology. 1989;97:539–547. doi: 10.1007/BF00439561. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty J. Smoking patterns and dependence: contrasting chippers and heavy smokers. J Abnorm Psychology. 2006;115:509–523. doi: 10.1037/0021-843X.115.3.509. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JD, Elash C. Nicotine withdrawal in chippers and regular smokers: subjective and cognitive effects. Health Psychol. 1995;14:301–309. doi: 10.1037//0278-6133.14.4.301. [DOI] [PubMed] [Google Scholar]
- Sisson JH. Alcohol and airways function in health and disease. Alcohol. 2007;41:293–307. doi: 10.1016/j.alcohol.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Alcohol timeline follow-back users’ manual. Addiction Research Foundation; Toronto, Canada: 1995. [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers’ self-reports of drinking behavior. Behav Res Ther. 1979;17:157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Sutker PB, Tabakoff B, Goist KC, Randall CL. Acute alcohol intoxication, mood states and alcohol metabolism in women and men. Pharmacol Biochem Behav. 1983;18(suppl):349–354. doi: 10.1016/0091-3057(83)90198-3. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. J Consult Clin Psychol. 1999;67:555–562. doi: 10.1037//0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]
- Wilson D, Taylor A, Roberts L. Can we target smoking groups more effectively? A study of male and female heavy smokers. Prev Med. 1995;24:363–368. doi: 10.1006/pmed.1995.1059. [DOI] [PubMed] [Google Scholar]