Abstract
Chronic binge alcohol exposure in adult rat models causes neuronal degeneration in the cortex and hippocampus that is not reduced by excitotoxic receptor antagonists, but is prevented by antioxidants. Neuroinflammatory (glial-neuronal) signaling pathways are believed to underlie the oxidative stress and brain damage. Based on our experimental results as well as increased knowledge about the pro-neuroinflammatory potential of glial water channels, we propose that induction of aquaporin-4 can be a critical initiating factor in alcohol’s neurotoxic effects, via the instigation of cellular edema-based neuroinflammatory cascades involving increased phospholipase A2 activities, polyunsaturated fatty acid release/membrane depletion, decreased prosurvival signaling, and oxidative stress. A testable scheme for this pathway is presented that incorporates recent findings in the alcohol-brain literature indicating a role for neuroimmune activation (upregulation of NF-kappaB, proinflammatory cytokines and toll-like receptors). We present the argument that such neuroimmune activation could be associated with or even dependent on increased aquaporin-4 and glial swelling as well.
Keywords: ethanol, neuroimmune, astroglia, brain damage, arachidonic acid, docosahexaenoic acid, AQP4
Chronic alcohol (ethanol) abuse/misuse is a major worldwide cause of morbidity and mortality (Rehm et al. 2009). It is responsible for considerable degrees of dementia (Gupta and Warner 2008) resulting from acquired brain damage—i.e., synaptic degeneration, neuronal loss, and gliosis. In some epidemiological studies, the prevalence of alcoholism-associated dementia approaches vascular dementia (Carlen et al. 1994), and they can also be coexisting conditions. Several decades ago we set out to investigate the neurocellular mechanisms underlying alcohol-induced neuronal degeneration. In the wake of growing appreciation of the role of glutamatergic N-methyl D-aspartate (NMDA) receptor-mediated excitotoxicity in many brain insults, it seemed reasonable to posit that an excitotoxic mechanism producing oxidative stress was essential in binge alcohol-dependent neurodamage in adult brain.
Our hypothesis was first examined with adult male rats subchronically binged with alcohol solutions 3–4 times/d (9–12 g/kg/d i.g.) for 4 days—the so-called Majchrowicz model, developed to study physical dependence behavior (Majchrowicz 1975; Collins et al. 1996). An abstract from those NIH laboratories in the early 1980’s indicated that this model incurred selective neuronal degeneration in the temporal (particularly entorhinal) cortex and the hippocampal dentate gyrus (Switzer et al. 1982). Our results with antagonists of NMDA receptors as well as other linked pathways led to the conclusion that, unlike neuronal damage due to ischemia, trauma and status epilepticus, excitotoxicity was not significant during chronic binge alcohol intoxication (Corso et al. 1998; Neafsey et al. 1989; Zou et al. 1996); subsequent studies in a leading NIH laboratory confirmed this conclusion (Hamelink et al. 2005). Nevertheless, since antioxidants provide significant neuroprotection (Hamelink et al. 2005; Crews et al. 2006), oxidative stress remains an important deleterious event in the binge intoxication model (and as summarized, possibly in other chronic alcohol models (Collins and Neafsey 2011)).
Evidence and role for brain cytotoxic edema
In considering mechanisms other than excitotoxicity that might be responsible for oxidative stress and neuronal degeneration during chronic binge alcohol exposure, we initially focused on brain edema and hydration. In particular, cellular (cytotoxic) edema would involve astroglia, known to be highly susceptible to swelling and, as developed below, ROS generation via phospholipase activation. To that end, our assays of wet-to-dry brain weight ratios in the binged rat neurodegeneration model—modified to once-daily 5 g/kg doses for 5–10 days—revealed modest but statistically significant brain water accumulation. The diuretic, furosemide, in abolishing the brain edema, greatly suppressed the entorhinal cortical and dentate neurodegeneration (Collins et al. 1998). We also found that furosemide and a second diuretic with a different mechanism, acetazolamide (AZA, Figure 1a) (Sripathirathan et al. 2009) were similarly neuroprotective in rat organotypic rat hippocampal-entorhinal cortical (HEC) slice cultures, which we utilized as an in vitro model of alcohol-induced brain damage.
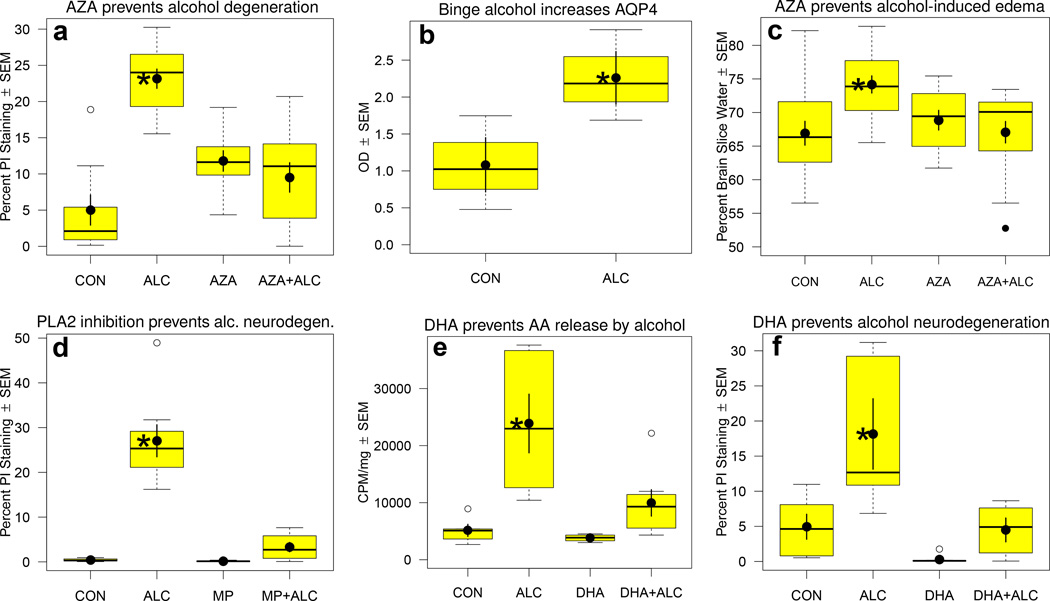
Figure 1.
Results from six sets of experiments with rat organotypic hippocampal-entorhinal cortical (HEC) slice cultures binge-exposed to alcohol (ALC, 100 mM) up to 6 d that show alcohol-induced neuronal degeneration and increased AQP4, the anti-edemic and neuroprotective effects of acetazolamide (AZA), neuroprotection by mepacrine (MP), and prevention of [3H]AA release and neuronal degeneration by docosahexaenoic acid (DHA). Data compiled from Sripathirathan et al. (2009) and Brown et al. (2009), which contain experimental details. a. Chronic binge ALC for 6 d causes significant neuronal degeneration (percent propidium iodide [PI] staining) that is prevented by co-treatment throughout with AZA (1.25 mM). b. Chronic binge ALC over 4 d significantly increases HEC slice levels of AQP4 protein (t-test, *p<0.05). c. Chronic binge ALC for 4 d increases HEC slice water content, which is prevented by co-treatment throughout with AZA (1.25 mM). d. Chronic binge ALC for 6 d causes neurodegeneration (percent PI staining) that is prevented by co-treatment throughout with PLA2 inhibitor, MP (1 uM). e. Chronic binge ALC for 60 hr increases HEC slice release of pre-incorporated 3H-AA during initial withdrawal period, which is prevented by co-treatment with omega-3 fatty acid, DHA (25 uM). f. Chronic binge ALC for 6 d causes neurodegeneration (percent PI staining) that is prevented by co-treatment throughout with DHA (25 uM). Box in boxplot illustrates extent of the middle two quartiles and defines the interquartile range (IQR), along with the median (horizontal line). Solid circle shows the mean with error bars showing SEM. Whiskers extending from box depict maximum range of values within 1.5 IQR, while open circles denote outlier values. One-way ANOVA (a,c,d,e,f) were significant overall; *p <0.05 in Bonferroni t-tests comparing ALC group to Control group. There were no significant differences between AZA + AZA-ALC (a,c), MP and MP + ALC (d), or DHA and DHA + ALC (e,f).
Experimental reports of brain edema in chronically intoxicated animals are few in number, but several studies have pointed out its occurrence (Pushpakiran et al. 2005; Jedrzejewska et al. 1990). Interestingly, it has been known for some time that active chronic alcoholics demonstrate overhydration and expansion of total body water, especially during falling blood alcohol levels (BALs) and/or withdrawal (Beard and Knott 1968). Indeed, "the mere presence or decreasing concentration of [blood] alcohol may be accompanied by water and solute retention," such that diuretic administration (furosemide) improved the physiology of withdrawal recovery (Knott and Beard 1969). Furthermore, brain edema is frequently seen in alcoholics during early withdrawal (Smith et al. 1988; Mander et al. 1988). Lambie suggested that brain overhydration in alcoholics causes neuropathology via cellular routes possibly linked to inappropriate vasopressin secretion (Lambie 1985), a phenomenon later demonstrated in alcoholic withdrawal (Trabert et al. 1992).
Involvement of aquaporin-4 (AQP4) in alcohol-related brain edema
Interested in cellular reasons potentially underlying binge alcohol-induced edema, our attention was drawn to AQP4, the major water channel in the brain that is highly expressed in astroglia (Papadopoulos et al. 2002). Numerous reports indicate that AQP4 is central to brain edema associated with ischemia, trauma and infectious insults (Zador et al. 2009; Papadopoulos et al. 2002). Increases in AQP4 coincide with cytotoxic (cellular) edema development during brain ischemia-reperfusion (Hirt et al. 2009). Also, brain AQP4 expression and cytotoxic edema do not differ between male and female rats subjected to ischemic stroke (Liu et al. 2008). In such models, the anesthetic propofol inhibits rat brain edema while attenuating AQP4 overexpression in the ischemic border zones (Zheng et al. 2008). Furthermore, AQP4 knockout mice show reduced brain cytotoxic edema and neurogical dysfunction after ischemia (Manley et al. 2000), whereas brain AQP4 overexpression in transgenics accelerates ischemia-induced glial swelling (Yang et al. 2008). Anchoring of AQP4 in plasma membranes utilizes α-syntrophin (Amiry-Moghaddam et al. 2004), which is required for dystrophin-AQP4 complex assembly (Bragg et al. 2006). In α-syntrophin-null ischemic mice, AQP4 dyslocalization correlates with delayed brain cellular edema onset (Vajda et al. 2002). However, AQP4 appears to be cytoprotective in vasogenic (extracellular) edema (Papadopoulos et al. 2004; Bloch and Manley 2007; Papadopoulos et al. 2002), indicating that the water channel serves opposite functions depending on type of edema and possibly its location.
Although no literature reports indicated that alcohol perturbs AQP4, our immunoblot assays showed substantially elevated AQP4 protein in extracts of rat HEC slice cultures exposed to repetitive 100 mM binge alcohol exposures (Figure 1b) (Sripathirathan et al. 2009). In those studies, co-exposure with AZA prevented alcohol-induced slice edema (Figure 1c) and neuronal degeneration (previously shown in Figure 1a). AZA diuretic was selected not only because it lacks the antioxidant activity of furosemide which would complicate interpretation of results (Sripathirathan et al. 2009), but equally important, because it is a potent inhibitor of AQP4 activity (Huber et al. 2007; Tanimura et al. 2009). As further support, in vivo results with the rat binge intoxication model were consistent with those in HEC slices in that AZA co-treatment significantly reduced alcohol-dependent brain edema and neuronal degeneration in the entorhinal cortex and hippocampal dentate gyrus (Sripathirathan et al. 2009). Brain AQP4 levels were not measured in the above in vivo experiments; however, in ongoing collaboration with the T.R. Pak laboratory, we have quantitated brain AQP4 in their young adult rat model of repetitive binge-type alcohol exposure (Przybycien-Szymanska et al. 2011). Whether there is neuronal degeneration in these animals is undetermined, but the binge alcohol treatment protocol caused statistically significant elevations (50–65% above control) in hippocampal and entorhinal cortical AQP4 (Collins et al. 2011). Thus, in vitro and in vivo results to date indicate that chronic binge alcohol causes increased brain AQP4, which could eventually underlie neurodamaging cytotoxic edema in those regions.
Accepting that the activity/levels of AQP4 are induced by chronic alcohol exposure, it is worth considering the factor(s) that could promote such changes. Several cellular entities or mediators are reported to upregulate AQP4. In brain glial progenitor cells, AQP4 expression and levels are increased by reactive oxygen species (ROS; e.g., H2O2) (Esposito et al. 2008). Also, both lactate accumulation (Morishima et al. 2008) and elevated proinflammatory IL-1beta (Ito et al. 2006) are reported to increase AQP4 expression. A 4th possible mediator, extracellular glutamate, augments [Ca+2]i via metabotropic receptors, which activates CAM kinase II to phosphorylate AQP4, thus stimulating its function (Gunnarson et al. 2008). ROS may be the most familiar of the above mediators with respect to alcohol; ROS levels can be potentiated by alcohol through multiple routes—among these are oxidation by/induction of cytochrome P450 2E1 (Montoliu et al. 1995), increased glial NADPH oxidase (NOX) and xanthine oxidase activities (Haorah et al. 2008), stimulation of mitochondrial leakage, increased activities of phospholipase A2 (PLA2) (Sun and Sun 2001) and monoamine oxidases (Ou et al. 2010), and depletion of endogenous antioxidants (Guerri and Grisolia 1980). In binge alcohol exposure, early ROS elevations that are not sufficiently neurotoxic might nevertheless induce AQP4 through NF-kappaB and antioxidant response elements. Among the other above mediators of AQP4 that might respond to alcohol exposure, brain or glial interleukin-1beta (IL-1beta) levels/signaling (Blanco and Guerri 2007), lactate (Oyama et al. 2000), and extracellular glutamate (Ward et al. 2009; Salazar et al. 2008) have each been reported increased in alcohol models. Studies are clearly necessary to identify the binge alcohol-dependent factor(s) that augment brain AQP4.
Neuroinflammatory/degenerative pathways linked to AQP4 and glial swelling
Whereas edema in severe brain trauma causes compression neuropathology directly through increased intracranial pressure, the moderate edema engendered by chronic alcohol binging is more likely to cause release of excitatory factors such as amino acids, notably glutamate (Kimelberg and Mongin 1998), and trigger downstream neuroinflammatory signaling. Among these, an appealing possibility is activation of phospholipase A2 (PLA2), which was mentioned above in regard to ROS. In brief, the multiple gene products expressed in glia and neurons that belong to the PLA2 superfamily comprise three structurally different families—intracellular cytosolic Ca+2-dependent cPLA2, intracellular cytosolic Ca+2-independent iPLA2, and relatively small secretory Ca+2-dependent sPLA2 (Sun et al. 2010). The dominant cytosolic activity in rat brain is iPLA2, peaking in young adult brain, whereas sPLA2 is the dominant particulate activity (Yang et al. 1999). (Two related families—platelet-activating factor hydrolases and lysosomal PLA2—will not be considered here). Although the i/c PLA2s are required for membrane remodeling and maintenance, hyperactivation of all three (s/i/c) PLA2 families is differentially implicated in neuronal death pathways (Sun et al. 2007; Farooqui and Horrocks 2006).
Very relevant is that swelling/edema, particularly cytotoxic (cellular), activates PLA2 isoforms in neuronal and nonneuronal cells (Lehtonen and Kinnunen 1995; Basavappa et al. 1998; Lambert et al. 2006). In turn, PLA2 activation may be a stimulus for glial swelling (Winkler et al. 2000) and further brain edema, for example, in ischemia (Watanabe and Egawa 1994; Bonventre 1997); indeed, mice deficient in cPLA2 are resistant to ischemia-induced brain edema (Bonventre et al. 1997). Furthermore, internal Ca+2 release due to hypo-osmotic swelling is prevented by inhibition of PLA2, and reversed by supplementation with arachidonic acid (AA) (Oike et al. 1994).
Consequences of chronic alcohol-dependent PLA2 activation
PLA2 activity mobilizes polyunsaturated fatty acids; and of particular interest are omega-6 AA and omega-3 docosahexaenoic acid (DHA), mobilized from the 2-position of membrane glycerophospholipids. PLA2 overactivation is considered neuroinflammatory and pro-apoptotic (Farooqui and Horrocks 2006) primarily because of AA and possibly platelet activating factor. The former is linked to inflammation and neurodegeneration via several mechanisms: a) metabolism to a host of eicosanoids as well as ROS through enzymatic oxidation/lipid peroxidation (e.g., cycooxygenases, lipoxygenases, and cytochrome P450's (Sun et al. 2007; Bobba et al. 2008)); b) direct pro-apoptotic activity (Cao et al. 2000; Fang et al. 2008); and c) increased ROS from nonenzymatic auto-oxidation. In addition, released AA, often derived largely from astroglia, can inhibit glial glutamate transporters and potentiate extracellular glutamate (Volterra et al. 1994). It has been reported that chronic alcohol activates various forms of PLA2 in neuroblastoma cells, synaptic membranes and rat brain (John et al. 1985; Basavarajappa et al. 1997; Basavarajappa et al. 1998). In neuronal cultures alcohol also increases glutamate receptor-evoked AA release (Navamani et al. 1997).
In our HEC slice cultures episodically exposed to 100 mM alcohol, increased AA release was apparent during the initial withdrawal episode, and a global PLA2 inhibitor, mepacrine (MP), blocked binge alcohol-induced neurotoxicity (Figure 1d), implicating PLA2 and AA in the neuronal degenerative mechanism (Brown et al. 2009). Indeed, our current neurotoxicity studies using selective PLA2 inhibitors with these cultures point to the involvement of several of the PLA2 families, but this may differ with brain age. Enzyme activities and levels in the alcohol-treated adolescent-age cultures are under study; however, immunoblot assays of PLA2 levels in hippocampal tissue from young adult rats binge-exposed to alcohol in a model previously mentioned (Przybycien-Szymanska et al. 2011) indicate that subchronic alcohol causes elevated levels of cPLA2 and phospho-cPLA2, along with reductions in iPLA2, possibly reflecting neuronal loss (N. Tajuddin, unpublished results).
In concert with AA mobilization, a further outcome of excessive PLA2 activation is DHA release, leading to depletion of the omega-3 fatty acid from neuronal and glial membrane phospholipids. Endogenous DHA is largely esterified at the sn2-position of acidic phospholipids, phosphatidylserine (PS) and phosphatidylethanolamine; it makes up 35–40% of total fatty acid in synaptic membrane PS, but its biosynthesis (from alpha-linolenic acid) is mainly in astrocytes (Kim 2007). Highly enriched in retina and brain, DHA is absolutely essential for neuronal tissue development and maintenance.
Endogenous brain DHA is depleted by alcohol treatment in primates (Pawlosky et al. 2001; Pawlosky and Salem 1995). Similarly, Kim and colleagues showed that alcohol reduced DHA contents in glioma cells and developing rat brain, while inducing a compensatory increase in an omega-6 fatty acid, (22:5) docosapentaenoic acid (DPA) (Kim and Hamilton 2000). Also notable is a rat developmental study in which ~4 wks of chronic alcohol intake by dams during pregnancy and throughout lactation, while perturbing brain phospholipids in offspring, also caused decrements in hippocampal PS, increases in DPA, and a significant reduction in the hippocampal DHA/DPA ratio in the dams (Wen and Kim 2007). Earlier in vivo studies also indicated that chronic alcohol decreases the proportion of DHA in brain PS in adult rats (Gustavsson 1990), mice (Harris et al. 1984) and cats (Pawlosky and Salem 1995), with compensatory DPA increases seen in the cat study. Reasons for DHA loss include inhibition of synthesis of DHA-containing PS species via PS synthase, at least in neuronal cell lines (Kim 2008), and also possibly increased DHA oxidation during alcohol withdrawal (Milne et al. 2006).
Thus, alcohol-induced membrane depletion/loss of membrane PS and a neuroprotective unsaturated fatty acid, DHA, by PLA2 activation could be a factor in conjunction with AA-augmented oxidative stress that together stimulate brain apoptotic and necrotic signaling processes (Akbar et al. 2006; Kim 2008). Supplemented DHA, which is extensively incorporated into acidic phospholipids as well as mobilized largely by iPLA2 (Green et al. 2008), is protective in neurodegenerative situations in vivo (Michael-Titus 2007). Furthermore, as we reported (Brown et al. 2009), DHA pretreatment in our organotypic HEC slice cultures suppresses binge alcohol-induced AA release (shown in Figure 1e) and neuronal damage (Figure 1f). Kim and coworkers have shown that DHA supplementation specifically in neural cells increases DHA-enriched PS, promoting anti-apoptotic, pro-survival pathways (Kim 2008; Kim et al. 2000), in particular, PI3K-Akt and raf-1 (Brunet et al. 2001). Other research indicates that DHA can be directly oxidized by cytochrome P450 isoforms, either when phospholipid-incorporated or free, to form potent “neuroprotectin” molecules (Bazan 2007).
Integration of alcohol-induced neuroimmune signaling events in AQP4 pathway
There is relatively recent recognition of the involvement of neuroimmune activation (e.g., NF-kappaB, pro-inflammatory cytokines, toll-like receptors (TLR), and neuroinflammatory agonists) in brain damage caused by chronic alcohol exposure. A 2011 supplementary issue of Brain, Behavior and Immunity, “Neuroimmune mechanisms of brain function and alcohol-related disorders,” has brought together helpful reviews and articles that, in part, address alcohol-related neuronal degeneration. In that issue, Crews and coworkers summarized that chronic alcohol exposure triggers neuroinflammatory loops involving NF-kappaB, TNF-alpha, interleukins, chemokine MCP-1, and lipopolysaccharide (LPS) (Crews et al. 2011). Additionally, the Bakalkin laboratory expanded the discussion on potential involvement of NF-kappaB-driven neuroinflammation in the brains of alcoholics (Yakovleva et al. 2011). Persidsky and colleagues reviewed aspects of alcohol neurotoxicity research on pro-inflammatory cytokines and oxidative stress (Persidsky et al. 2011), further suggesting therapeutic use of cannabinoid receptor agonists (but overlooking that such an agonist was neuroprotective in the Majchrowicz binge intoxication model (Hamelink et al. 2005)). The Guerri laboratory in Valencia presented important data using TLR-knockout mice that support a key role for TLR in brain damage due to chronic alcohol exposure (Pascual et al. 2011). The Haorah research group in Nebraska clarified alcohol-induced neurodegeneration in the context of the blood-brain barrier, emphasizing the importance of stimulation by ethanol-derived acetaldehyde of NOX, enriched in brain immune cells and microglia, which leads to significant oxidative stress (Alikunju et al. 2011).
We propose that the above-identified neuroimmune pathways or players activated or induced by chronic alcohol have mechanistically dependent relationships with AQP4, astroglial swelling/brain edema, and oxidative stress. Key to our argument is the fact that AQP4 activity is directly responsible for pro-inflammatory cytokine generation in brain upon neuroinflammatory LPS insult (Li et al. 2011). These investigators concluded that “[their] results establish a novel role for AQP4 in neuroinflammation, which…at the cellular level involves AQP4-dependent differences in astrocyte water permeability and consequent cell swelling and cytokine release.” An earlier report linking microglial swelling and reactivity, possibly AQP4-dependent, to ROS via activation of NOX is also significant (Reinehr et al. 2007), and moreover, LPS treatment can induce AQP4 in these neuroimmune cells (Tomas-Camardiel et al. 2004). Furthermore, glial AQP4 upregulation due to brain trauma depends on NF-kappaB activity (Rao et al. 2011), and interestingly, pro-inflammatory IL-1beta can activate AQP4 through NF-kappaB (Ito et al. 2006). No studies to date apparently couple TLR activation to increased AQP4, but brain edema arising due to ischemic or hemorrhagic stroke models appears to depend on activation of the TLR4/NF-kappaB pathway (Wang et al. 2011; Chen et al. 2009).
Also, it is important to point out that neuroimmune activation encompassing LPS, TLR and cytokine stimulation is associated with or coupled to increased PLA2 activity—data that serves to strengthen our argument. For example, sPLA2 isoforms are upregulated by LPS treatment in mice (Murakami et al. 2002). Also, it has been known for some time that pro-inflammatory cytokines increase PLA2 activity in glia and other cells (Sun and Hu 1995; Peilot et al. 2000), while anti-inflammatory cytokines antagonize this induction (Touqui and Alaoui-El-Azher 2001). Overlapping and distinct patterns of pro-artherogenic responses are known to occur in monocytic cells between MCP-1, TNFalpha and PLA2 (Fuentes et al. 2002). Additionally, TLR4 stimulation can activate cPLA2 and possibly sPLA2 in macrophages (Grkovich et al. 2009; Ruiperez et al. 2009), as does TLR9 (Lee et al. 2007). In epithelial cells, endotoxin increases levels of iPLA2 via TLR4 activation (Herath et al. 2009). Mast cell studies indicate that TLR activation has a stimulatory effect on cPLA2 that is amplified by released sPLA2 (Kikawada et al. 2007). Interestingly, results with brain glia indicate that AA-derived prostaglandins can be upstream of TLR activation (Yoon et al. 2008).
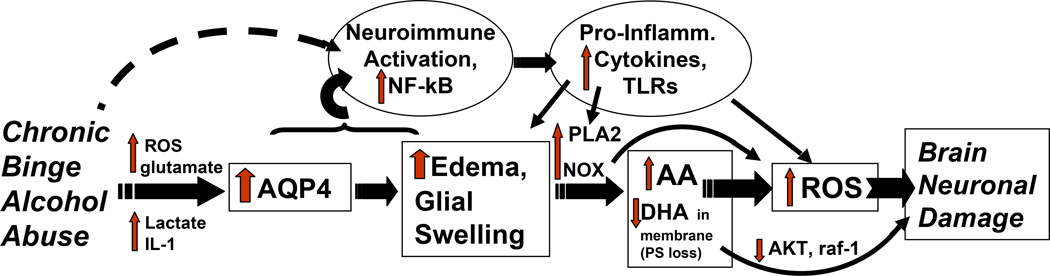
Consequently, Figure 2 integrates certain of these neuroimmune changes within the binge alcohol-activated AQP4/edema/PLA2/oxidative stress scheme. In this diagram, the boxed enzymes, entities or changes are supported through our research or through the H.Y. Kim laboratory at NIAAA. The alcohol-induced neuroimmune activation, pro-inflammatory cytokine increases and TLR stimulation in the ovals are shown linked to AQP4/brain edema as detailed above. However, the dashed arrow indicates that neuroimmune activation by alcohol is also likely to be facilitated via unidentified pathways that are independent of the water channel. Although the mechanistic flow is represented as one-directional, elevated AA and ROS can ostensibly feed back to stimulate swelling (Heo et al. 2005; Chan et al. 1983), thus promoting a “vicious cycle” of brain edema. Also not represented are brain eicosanoid products of AA—e.g., cysteinyl leukotrienes—with receptor-mediated, potent edema-producing actions that involve AQP4 (Wang et al. 2006). Overall, this AQP4-based scheme puts forward testable possibilities that can elucidate neuroimmune mechanistic routes leading to neuroinflammatory brain damage caused by alcohol abuse and alcoholism. Increased knowledge of these routes could facilitate discoveries of improved neuroprotectant analogs for use in conjunction with ongoing therapies.
Figure 2.
Diagram of proposed signaling pathways leading to ROS-dependent neuronal degeneration (brain neuronal damage) due to chronic binge alcohol abuse that integrates NF-kappaB-related neuroimmune activation (increased pro-inflammatory cytokines, chemokines, TLR) with the proposed cascade of AQP4-edema/glial swelling-PLA2-(AA+DHA)-ROS. Refer to text for further description and abbreviations.
Acknowledgements
The laboratory contributions of Dr. Jian Zou, Dr. Kumar Sripathirathan and Dr. James Brown III, and research assistants Mr. Nick Achille and Ms. Nuzhath Tajuddin, are gratefully recognized. Financial support from the NIH (AA011543, AA014436 and AA018279), Loyola University Alcohol Research Program (T32 AA013527) and Loyola University Research Stimulation Fund (LUMC Neuroscience Institute) is responsible for our laboratories’ research funding.
Abbreviations
- AA
arachidonic acid
- AQP4
aquaporin-4
- AZA
acetazolamide
- DHA
docosahexaenoic acid
- HEC
hippocampal-entorhinal cortical
- IL
interleukin
- LPS
lipopolysaccharide
- MCP-1
monocyte chemoattractant protein-1
- MP
mepacrine
- NMDA
N-methyl D-aspartate
- NOX
NADPH oxidase
- PLA2
phospholipase A2
- PS
phosphatidylserine
- ROS
reactive oxygen species
- TLR
toll-like receptor(s)
- TNF
tumor necrosis factor
Footnotes
Presented in part at the V International Meeting of the Neurotoxicity Society, Uspallatta, Argentina, April 2011
References
- Akbar M, Baick J, Calderon F, Wen Z, Kim HY. Ethanol promotes neuronal apoptosis by inhibiting phosphatidylserine accumulation. Journal of Neuroscience Research. 2006;83(3):432–440. doi: 10.1002/jnr.20744. [DOI] [PubMed] [Google Scholar]
- Alikunju S, Abdul Muneer PM, Zhang Y, Szlachetka AM, Haorah J. The inflammatory footprints of alcohol-induced oxidative damage in neurovascular components. Brain, Behavior, & Immunity. 2011;25(Suppl 1):S129–S136. doi: 10.1016/j.bbi.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiry-Moghaddam M, Frydenlund DS, Ottersen OP. Anchoring of aquaporin-4 in brain: molecular mechanisms and implications for the physiology and pathophysiology of water transport. Neuroscience. 2004;129(4):999–1010. doi: 10.1016/j.neuroscience.2004.08.049. [DOI] [PubMed] [Google Scholar]
- Basavappa S, Pedersen SF, Jorgensen NK, Ellory JC, Hoffmann EK. Swelling-induced arachidonic acid release via the 85-kDa cPLA2 in human neuroblastoma cells. J Neurophysiol. 1998;79(3):1441–1449. doi: 10.1152/jn.1998.79.3.1441. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Cooper TB, Hungund BL. Effect of chronic ethanol exposure on mouse brain arachidonic acid specific phospholipase A2. Biochemical Pharmacology. 1998;55(4):515–521. doi: 10.1016/s0006-2952(97)00501-7. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Activation of arachidonic acid-specific phospholipase A2 in human neuroblastoma cells after chronic alcohol exposure: prevention by GM1 ganglioside. Alcohol Clin Exp Res. 1997;21(7):1199–1203. [PubMed] [Google Scholar]
- Bazan NG. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr Opin Clin Nutr Metab Care. 2007;10(2):136–141. doi: 10.1097/MCO.0b013e32802b7030. [DOI] [PubMed] [Google Scholar]
- Beard JD, Knott DH. Fluid and electrolyte balance during acute withdrawal in chronic alcoholic patients. Jama. 1968;204(2):135–139. [PubMed] [Google Scholar]
- Blanco AM, Guerri C. Ethanol intake enhances inflammatory mediators in brain: role of glial cells and TLR4/IL-1RI receptors. Front Biosci. 2007;12:2616–2630. doi: 10.2741/2259. [DOI] [PubMed] [Google Scholar]
- Bloch O, Manley GT. The role of aquaporin-4 in cerebral water transport and edema. Neurosurgical Focus. 2007;22(5):E3. doi: 10.3171/foc.2007.22.5.4. [DOI] [PubMed] [Google Scholar]
- Bobba A, Atlante A, Petragallo VA, Marra E. Different sources of reactive oxygen species contribute to low potassium-induced apoptosis in cerebellar granule cells. International Journal of Molecular Medicine. 2008;21(6):737–745. [PubMed] [Google Scholar]
- Bonventre JV. Roles of phospholipases A2 in brain cell and tissue injury associated with ischemia and excitotoxicity. Journal of Lipid Mediators & Cell Signalling. 1997;17(1):71–79. doi: 10.1016/s0929-7855(97)00021-7. [DOI] [PubMed] [Google Scholar]
- Bonventre JV, Huang Z, Taheri MR, O'Leary E, Li E, Moskowitz MA, Sapirstein A. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390(6660):622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- Bragg AD, Amiry-Moghaddam M, Ottersen OP, Adams ME, Froehner SC. Assembly of a perivascular astrocyte protein scaffold at the mammalian blood-brain barrier is dependent on alpha-syntrophin. GLIA. 2006;53(8):879–890. doi: 10.1002/glia.20347. [DOI] [PubMed] [Google Scholar]
- Brown J, 3rd, Achille N, Neafsey EJ, Collins MA. Binge ethanol-induced neurodegeneration in rat organotypic brain slice cultures: effects of PLA2 inhibitor mepacrine and docosahexaenoic acid (DHA) Neurochem Res. 2009;34(2):260–267. doi: 10.1007/s11064-008-9765-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K–Akt signaling pathway. Current Opinion in Neurobiology. 2001;11(3):297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- Cao Y, Pearman AT, Zimmerman GA, McIntyre TM, Prescott SM. Intracellular unesterified arachidonic acid signals apoptosis. Proc Natl Acad Sci USA. 2000;97(21):11280–11285. doi: 10.1073/pnas.200367597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen PL, McAndrews MP, Weiss RT, Dongier M, Hill JM, Menzano E, Farcnik K, Abarbanel J, Eastwood MR. Alcohol-related dementia in the institutionalized elderly. Alcohol Clin Exp Res. 1994;18(6):1330–1334. doi: 10.1111/j.1530-0277.1994.tb01432.x. [DOI] [PubMed] [Google Scholar]
- Chan PH, Fishman RA, Caronna J, Schmidley JW, Prioleau G, Lee J. Induction of brain edema following intracerebral injection of arachidonic acid. Ann Neurol. 1983;13(6):625–632. doi: 10.1002/ana.410130608. [DOI] [PubMed] [Google Scholar]
- Chen G, Zhang S, Shi J, Ai J, Qi M, Hang C, Chen G, Zhang S, Shi J, Ai J, Qi M, Hang C. Simvastatin reduces secondary brain injury caused by cortical contusion in rats: possible involvement of TLR4/NF-kappaB pathway. Exp Neurol. 2009;216(2):398–406. doi: 10.1016/j.expneurol.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Collins MA, Corso TD, Neafsey EJ. Neuronal degeneration in rat cerebrocortical and olfactory regions during subchronic "binge" intoxication with ethanol: possible explanation for olfactory deficits in alcoholics. Alcohol Clin Exp Res. 1996;20(2):284–292. doi: 10.1111/j.1530-0277.1996.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Collins MA, Neafsey EJ. Ethanol and adult CNS neurodamage: oxidative stress, but possibly not excitotoxicity. Front Biosci. 2011;16 doi: 10.2741/465. in press. [DOI] [PubMed] [Google Scholar]
- Collins MA, Tajuddin N, Neafsey EJ, Przybycien-Szymanska MM, Pak TR. Effect of adolescent/adult binge-pattern ethanol exposure on rat brain aquaporin-4 and phospholipase A2 levels. Abstracts of the Society for Neuroscience (256.17) 2011 [Google Scholar]
- Collins MA, Zou JY, Neafsey EJ. Brain damage due to episodic alcohol exposure in vivo and in vitro: furosemide neuroprotection implicates edema-based mechanism. FASEB J. 1998;12(2):221–230. doi: 10.1096/fasebj.12.2.221. [DOI] [PubMed] [Google Scholar]
- Corso TD, Mostafa HM, Collins MA, Neafsey EJ. Brain neuronal degeneration caused by episodic alcohol intoxication in rats: effects of nimodipine, 6,7-dinitro-quinoxaline-2,3-dione, and MK-801. Alcohol Clin Exp Res. 1998;22(1):217–224. [PubMed] [Google Scholar]
- Crews F, Nixon K, Kim D, Joseph J, Shukitt-Hale B, Qin L, Zou J. BHT blocks NF-kappaB activation and ethanol-induced brain damage. Alcohol Clin Exp Res. 2006;30(11):1938–1949. doi: 10.1111/j.1530-0277.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- Crews FT, Zou J, Qin L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain, Behavior, &. Immunity. 2011;25(Suppl 1):S4–S12. doi: 10.1016/j.bbi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Imitola J, Lu J, De Filippis D, Scuderi C, Ganesh VS, Folkerth R, Hecht J, Shin S, Iuvone T, Chesnut J, Steardo L, Sheen V. Genomic and functional profiling of human Down syndrome neural progenitors implicates S100B and aquaporin 4 in cell injury. Human Molecular Genetics. 2008;17(3):440–457. doi: 10.1093/hmg/ddm322. [DOI] [PubMed] [Google Scholar]
- Fang KM, Chang WL, Wang SM, Su MJ, Wu ML. Arachidonic acid induces both Na+ and Ca2+ entry resulting in apoptosis. J Neurochem. 2008;104(5):1177–1189. doi: 10.1111/j.1471-4159.2007.05022.x. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Horrocks LA. Phospholipase A2-generated lipid mediators in the brain: the good, the bad, and the ugly. Neuroscientist. 2006;12(3):245–260. doi: 10.1177/1073858405285923. [DOI] [PubMed] [Google Scholar]
- Fuentes L, Hernandez M, Fernandez-Aviles FJ, Crespo MS, Nieto ML. Cooperation between secretory phospholipase A2 and TNF-receptor superfamily signaling: implications for the inflammatory response in atherogenesis. Circulation Research. 2002;91(8):681–688. doi: 10.1161/01.res.0000038341.34243.64. [DOI] [PubMed] [Google Scholar]
- Green JT, Orr SK, Bazinet RP. The emerging role of group VI calcium-independent phospholipase A2 in releasing docosahexaenoic acid from brain phospholipids. Journal of Lipid Research. 2008;49(5):939–944. doi: 10.1194/jlr.R700017-JLR200. [DOI] [PubMed] [Google Scholar]
- Grkovich A, Armando A, Quehenberger O, Dennis EA, Grkovich A, Armando A, Quehenberger O, Dennis EA. TLR-4 mediated group IVA phospholipase A(2) activation is phosphatidic acid phosphohydrolase 1 and protein kinase C dependent. Biochim Biophys Acta. 2009;1791(10):975–982. doi: 10.1016/j.bbalip.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerri C, Grisolia S. Changes in glutathione in acute and chronic alcohol intoxication. Pharmacology, Biochemistry & Behavior. 1980;13(Suppl 1):53–61. doi: 10.1016/s0091-3057(80)80009-8. [DOI] [PubMed] [Google Scholar]
- Gunnarson E, Zelenina M, Axehult G, Song Y, Bondar A, Krieger P, Brismar H, Zelenin S, Aperia A. Identification of a molecular target for glutamate regulation of astrocyte water permeability. GLIA. 2008;56(6):587–596. doi: 10.1002/glia.20627. [DOI] [PubMed] [Google Scholar]
- Gupta S, Warner J. Alcohol-related dementia: a 21st-century silent epidemic? British Journal of Psychiatry. 2008;193(5):351–353. doi: 10.1192/bjp.bp.108.051425. [DOI] [PubMed] [Google Scholar]
- Gustavsson L. Brain lipid changes after ethanol exposure. Ups J Med Sci. 1990;(Suppl 48):245–266. [PubMed] [Google Scholar]
- Hamelink C, Hampson A, Wink DA, Eiden LE, Eskay RL. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. J Pharmacol Exp Therap. 2005;314(2):780–788. doi: 10.1124/jpet.105.085779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haorah J, Ramirez SH, Floreani N, Gorantla S, Morsey B, Persidsky Y. Mechanism of alcohol-induced oxidative stress and neuronal injury. Free Radic Biol Med. 2008;45(11):1542–1550. doi: 10.1016/j.freeradbiomed.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RA, Baxter DM, Mitchell MA, Hitzemann RJ. Physical properties and lipid composition of brain membranes from ethanol tolerant-dependent mice. Molecular Pharmacology. 1984;25(3):401–409. [PubMed] [Google Scholar]
- Heo JH, Han SW, Lee SK. Free radicals as triggers of brain edema formation after stroke. Free Radic Biol Med. 2005;39(1):51–70. doi: 10.1016/j.freeradbiomed.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Herath S, Lilly ST, Fischer DP, Williams EJ, Dobson H, Bryant CE, Sheldon IM. Bacterial lipopolysaccharide induces an endocrine switch from prostaglandin F2alpha to prostaglandin E2 in bovine endometrium. Endocrinology. 2009;150(4):1912–1920. doi: 10.1210/en.2008-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt L, Ternon B, Price M, Mastour N, Brunet JF, Badaut J. Protective role of early aquaporin 4 induction against postischemic edema formation. J Cereb Blood Flow Metab. 2009;29(2):423–433. doi: 10.1038/jcbfm.2008.133. [DOI] [PubMed] [Google Scholar]
- Huber VJ, Tsujita M, Yamazaki M, Sakimura K, Nakada T. Identification of arylsulfonamides as Aquaporin 4 inhibitors. Bioorg Med Chem Lett. 2007;17(5):1270–1273. doi: 10.1016/j.bmcl.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Ito H, Yamamoto N, Arima H, Hirate H, Morishima T, Umenishi F, Tada T, Asai K, Katsuya H, Sobue K. Interleukin-1beta induces the expression of aquaporin-4 through a nuclear factor-kappaB pathway in rat astrocytes. J Neurochem. 2006;99(1):107–118. doi: 10.1111/j.1471-4159.2006.04036.x. [DOI] [PubMed] [Google Scholar]
- Jedrzejewska A, Staff-Zielinska E, Wierzba-Bobrowicz T, Poszwinska Z, Olejniczak P. [Histology of the central nervous system in rats after intensive chronic ethanol intoxication] Neuropatologia Polska. 1990;28(1–2):93–99. [PubMed] [Google Scholar]
- John GR, Littleton JM, Nhamburo PT. Increased activity of Ca2+-dependent enzymes of membrane lipid metabolism in synaptosomal preparations from ethanol-dependent rats. J Neurochem. 1985;44(4):1235–1241. doi: 10.1111/j.1471-4159.1985.tb08749.x. [DOI] [PubMed] [Google Scholar]
- Kikawada E, Bonventre JV, Arm JP. Group V secretory PLA2 regulates TLR2-dependent eicosanoid generation in mouse mast cells through amplification of ERK and cPLA2alpha activation. Blood. 2007;110(2):561–567. doi: 10.1182/blood-2006-10-052258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY. Novel metabolism of docosahexaenoic acid in neural cells. J Biol Chem. 2007;282(26):18661–18665. doi: 10.1074/jbc.R700015200. [DOI] [PubMed] [Google Scholar]
- Kim HY. Biochemical and biological functions of docosahexaenoic acid in the nervous system: modulation by ethanol. Chemistry & Physics of Lipids. 2008;153(1):34–46. doi: 10.1016/j.chemphyslip.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Akbar M, Lau A, Edsall L. Inhibition of neuronal apoptosis by docosahexaenoic acid (22:6n-3). Role of phosphatidylserine in antiapoptotic effect. J Biol Chem. 2000;275(45):35215–35223. doi: 10.1074/jbc.M004446200. [DOI] [PubMed] [Google Scholar]
- Kim HY, Hamilton J. Accumulation of docosahexaenoic acid in phosphatidylserine is selectively inhibited by chronic ethanol exposure in C-6 glioma cells. Lipids. 2000;35(2):187–195. doi: 10.1007/BF02664769. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Mongin AA. Swelling-activated release of excitatory amino acids in the brain: relevance for pathophysiology. Contrib Nephrol. 1998;123:240–257. doi: 10.1159/000059916. [DOI] [PubMed] [Google Scholar]
- Knott DH, Beard JD. A diuretic approach to acute withdrawal from alcohol. Southern Medical Journal. 1969;62(4):485–488. doi: 10.1097/00007611-196904000-00028. [DOI] [PubMed] [Google Scholar]
- Lambert IH, Pedersen SF, Poulsen KA. Activation of PLA2 isoforms by cell swelling and ischaemia/hypoxia. Acta Physiol (Oxf) 2006;187(1–2):75–85. doi: 10.1111/j.1748-1716.2006.01557.x. [DOI] [PubMed] [Google Scholar]
- Lambie DG. Alcoholic brain damage and neurological symptoms of alcohol withdrawal--manifestations of overhydration. Med Hypotheses. 1985;16(4):377–388. doi: 10.1016/0306-9877(85)90058-1. [DOI] [PubMed] [Google Scholar]
- Lee SH, Lee JG, Kim JR, Baek SH. Toll-like receptor 9-mediated cytosolic phospholipase A2 activation regulates expression of inducible nitric oxide synthase. Biochem Biophys Res Commun. 2007;364(4):996–1001. doi: 10.1016/j.bbrc.2007.10.111. [DOI] [PubMed] [Google Scholar]
- Lehtonen JY, Kinnunen PK. Phospholipase A2 as a mechanosensor. Biophys J. 1995;68(5):1888–1894. doi: 10.1016/S0006-3495(95)80366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhang H, Varrin-Doyer M, Zamvil SS, Verkman AS. Proinflammatory role of aquaporin-4 in autoimmune neuroinflammation. FASEB J. 2011;25(5):1556–1566. doi: 10.1096/fj.10-177279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang W, Alkayed NJ, Froehner SC, Adams ME, Amiry-Moghaddam M, Ottersen OP, Hurn PD, Bhardwaj A. Lack of sex-linked differences in cerebral edema and aquaporin-4 expression after experimental stroke. J Cereb Blood Flow Metab. 2008;28(12):1898–1906. doi: 10.1038/jcbfm.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43(3):245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Mander AJ, Weppner GJ, Chick JD, Morton JJ, Best JJ. An NMR study of cerebral oedema and its biological correlates during withdrawal from alcohol. Alcohol & Alcoholism. 1988;23(2):97–102. [PubMed] [Google Scholar]
- Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nature Medicine. 2000;6(2):159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- Michael-Titus AT. Omega-3 fatty acids and neurological injury. Prostaglandins Leukotrienes & Essential Fatty Acids. 2007;77(5–6):295–300. doi: 10.1016/j.plefa.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Milne GL, Morrow JD, Picklo MJ., Sr Elevated oxidation of docosahexaenoic acid, 22:6 (n-3), in brain regions of rats undergoing ethanol withdrawal. Neuroscience Letters. 2006;405(3):172–174. doi: 10.1016/j.neulet.2006.06.058. [DOI] [PubMed] [Google Scholar]
- Montoliu C, Sancho-Tello M, Azorin I, Burgal M, Valles S, Renau-Piqueras J, Guerri C. Ethanol increases cytochrome P4502E1 and induces oxidative stress in astrocytes. J Neurochem. 1995;65(6):2561–2570. doi: 10.1046/j.1471-4159.1995.65062561.x. [DOI] [PubMed] [Google Scholar]
- Morishima T, Aoyama M, Iida Y, Yamamoto N, Hirate H, Arima H, Fujita Y, Sasano H, Tsuda T, Katsuya H, Asai K, Sobue K. Lactic acid increases aquaporin 4 expression on the cell membrane of cultured rat astrocytes. Neuroscience Research. 2008;61(1):18–26. doi: 10.1016/j.neures.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Murakami M, Yoshihara K, Shimbara S, Lambeau G, Singer A, Gelb MH, Sawada M, Inagaki N, Nagai H, Kudo I. Arachidonate release and eicosanoid generation by group IIE phospholipase A(2) Biochem Biophys Res Commun. 2002;292(3):689–696. doi: 10.1006/bbrc.2002.6716. [DOI] [PubMed] [Google Scholar]
- Navamani M, Morgan M, Williams RJ. Ethanol modulates N-methyl-D-aspartate-evoked arachidonic acid release from neurones. European Journal of Pharmacology. 1997;340(1):27–34. doi: 10.1016/s0014-2999(97)01396-4. [DOI] [PubMed] [Google Scholar]
- Neafsey EJ, Mostafa MHA, Collins MA. MK801 fails to block ethanol induced cortical damage in rats. Transactions of the American Society of Neurochemistry. 1989;20:109. [Google Scholar]
- Oike M, Droogmans G, Nilius B. Mechanosensitive Ca2+ transients in endothelial cells from human umbilical vein. Proc Natl Acad Sci USA. 1994;91(8):2940–2944. doi: 10.1073/pnas.91.8.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou XM, Stockmeier CA, Meltzer HY, Overholser JC, Jurjus GJ, Dieter L, Chen K, Lu D, Johnson C, Youdim MB, Austin MC, Luo J, Sawa A, May W, Shih JC. A novel role for glyceraldehyde-3-phosphate dehydrogenase and monoamine oxidase B cascade in ethanol-induced cellular damage. Biological Psychiatry. 2010;67(9):855–863. doi: 10.1016/j.biopsych.2009.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama LM, Couto RC, Couto GE, Damaso AR, Oller do Nascimento CM. Ethanol intake during lactation. II. Effects On pups’ liver and brain metabolism. Alcohol. 2000;21(3):201–206. doi: 10.1016/s0741-8329(00)00074-4. [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Krishna S, Verkman AS. Aquaporin water channels and brain edema. Mount Sinai Journal of Medicine. 2002;69(4):242–248. [PubMed] [Google Scholar]
- Papadopoulos MC, Manley GT, Krishna S, Verkman AS. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004;18(11):1291–1293. doi: 10.1096/fj.04-1723fje. [DOI] [PubMed] [Google Scholar]
- Pascual M, Balino P, Alfonso-Loeches S, Aragon CM, Guerri C. Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain, Behavior, & Immunity 25 Suppl. 2011;1:S80–S91. doi: 10.1016/j.bbi.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Pawlosky RJ, Bacher J, Salem N., Jr Ethanol consumption alters electroretinograms and depletes neural tissues of docosahexaenoic acid in rhesus monkeys: nutritional consequences of a low n-3 fatty acid diet. Alcohol Clin Exp Res. 2001;25(12):1758–1765. [PubMed] [Google Scholar]
- Pawlosky RJ, Salem N., Jr Ethanol exposure causes a decrease in docosahexaenoic acid and an increase in docosapentaenoic acid in feline brains and retinas. Am J Clin Nutr. 1995;61(6):1284–1289. doi: 10.1093/ajcn/61.6.1284. [DOI] [PubMed] [Google Scholar]
- Peilot H, Rosengren B, Bondjers G, Hurt-Camejo E. Interferon-gamma induces secretory group IIA phospholipase A2 in human arterial smooth muscle cells. Involvement of cell differentiation, STAT-3 activation, and modulation by other cytokines. J Biol Chem. 2000;275(30):22895–22904. doi: 10.1074/jbc.M002783200. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Ho W, Ramirez SH, Potula R, Abood ME, Unterwald E, Tuma R. HIV-1 infection and alcohol abuse: neurocognitive impairment, mechanisms of neurodegeneration and therapeutic interventions. Brain, Behavior, & Immunity 25 Suppl. 2011;1:S61–S70. doi: 10.1016/j.bbi.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybycien-Szymanska MM, Mott NN, Paul CR, Gillespie RA, Pak TR. Binge-pattern alcohol exposure during puberty induces long-term changes in HPA axis reactivity. PLoS ONE. 2011;6(4):e18350. doi: 10.1371/journal.pone.0018350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushpakiran G, Mahalakshmi K, Viswanathan P, Anuradha CV. Taurine prevents ethanol-induced alterations in lipids and ATPases in rat tissues. Pharmacol Rep. 2005;57(5):578–587. [PubMed] [Google Scholar]
- Rao KV, Reddy PV, Curtis KM, Norenberg MD. Aquaporin-4 expression in cultured astrocytes after fluid percussion injury. J Neurotrauma. 2011;28(3):371–381. doi: 10.1089/neu.2010.1705. [DOI] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373(9682):2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Reinehr R, Gorg B, Becker S, Qvartskhava N, Bidmon HJ, Selbach O, Haas HL, Schliess F, Haussinger D. Hypoosmotic swelling and ammonia increase oxidative stress by NADPH oxidase in cultured astrocytes and vital brain slices. GLIA. 2007;55(7):758–771. doi: 10.1002/glia.20504. [DOI] [PubMed] [Google Scholar]
- Ruiperez V, Astudillo AM, Balboa MA, Balsinde J. Coordinate regulation of TLR-mediated arachidonic acid mobilization in macrophages by group IVA and group V phospholipase A2s. Journal of Immunology. 2009;182(6):3877–3883. doi: 10.4049/jimmunol.0804003. [DOI] [PubMed] [Google Scholar]
- Salazar M, Pariente JA, Salido GM, Gonzalez A. Ethanol induces glutamate secretion by Ca2+ mobilization and ROS generation in rat hippocampal astrocytes. Neurochemistry International. 2008;52(6):1061–1067. doi: 10.1016/j.neuint.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Smith MA, Chick JD, Engleman HM, Kean DM, Mander AJ, Douglas RH, Best JJ. Brain hydration during alcohol withdrawal in alcoholics measured by magnetic resonance imaging. Drug & Alcohol Dependence. 1988;21(1):25–28. doi: 10.1016/0376-8716(88)90006-3. [DOI] [PubMed] [Google Scholar]
- Sripathirathan K, Brown J, Neafsey EJ, Collins MA. Linking binge alcohol-induced neurodamage to brain edema and potential aquaporin-4 upregulation: evidence in rat organotypic brain slice cultures and in vivo. J Neurotrauma. 2009;26(2):261–273. doi: 10.1089/neu.2008.0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun AY, Sun GY. Ethanol and oxidative mechanisms in the brain. Journal of Biomedical Science. 2001;8(1):37–43. doi: 10.1007/BF02255969. [DOI] [PubMed] [Google Scholar]
- Sun GY, Horrocks LA, Farooqui AA. The roles of NADPH oxidase and phospholipases A2 in oxidative and inflammatory responses in neurodegenerative diseases. J Neurochem. 2007;103(1):1–16. doi: 10.1111/j.1471-4159.2007.04670.x. [DOI] [PubMed] [Google Scholar]
- Sun GY, Hu ZY. Stimulation of phospholipase A2 expression in rat cultured astrocytes by LPS, TNF alpha and IL-1 beta. Progress in Brain Research. 1995;105:231–238. [PubMed] [Google Scholar]
- Sun GY, Shelat PB, Jensen MB, He Y, Sun AY, Simonyi A. Phospholipases A2 and inflammatory responses in the central nervous system. NeuroMolecular Medicine. 2010;12(2):133–148. doi: 10.1007/s12017-009-8092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer RC, Majchrowicz E, Weight F. Ethanol-induced argyrophilia in entorhinal cortex of rat. Anat Rec. 1982;202:186A. [Google Scholar]
- Tanimura Y, Hiroaki Y, Fujiyoshi Y. Acetazolamide reversibly inhibits water conduction by aquaporin-4. Journal of Structural Biology. 2009;166(1):16–21. doi: 10.1016/j.jsb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Tomas-Camardiel M, Venero JL, de Pablos RM, Rite I, Machado A, Cano J. In vivo expression of aquaporin-4 by reactive microglia. J Neurochem. 2004;91(4):891–899. doi: 10.1111/j.1471-4159.2004.02759.x. [DOI] [PubMed] [Google Scholar]
- Touqui L, Alaoui-El-Azher M. Mammalian secreted phospholipases A2 and their pathophysiological significance in inflammatory diseases. Current Molecular Medicine. 2001;1(6):739–754. doi: 10.2174/1566524013363258. [DOI] [PubMed] [Google Scholar]
- Trabert W, Caspari D, Bernhard P, Biro G. Inappropriate vasopressin secretion in severe alcohol withdrawal. Acta Psychiatrica Scandinavica. 1992;85(5):376–379. doi: 10.1111/j.1600-0447.1992.tb10322.x. [DOI] [PubMed] [Google Scholar]
- Vajda Z, Pedersen M, Fuchtbauer EM, Wertz K, Stodkilde-Jorgensen H, Sulyok E, Doczi T, Neely JD, Agre P, Frokiaer J, Nielsen S. Delayed onset of brain edema and mislocalization of aquaporin-4 in dystrophin-null transgenic mice. Proc Natl Acad Sci USA. 2002;99(20):13131–13136. doi: 10.1073/pnas.192457099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volterra A, Trotti D, Racagni G. Glutamate uptake is inhibited by arachidonic acid and oxygen radicals via two distinct and additive mechanisms. Molecular Pharmacology. 1994;46(5):986–992. [PubMed] [Google Scholar]
- Wang ML, Huang XJ, Fang SH, Yuan YM, Zhang WP, Lu YB, Ding Q, Wei EQ. Leukotriene D4 induces brain edema and enhances CysLT2 receptor-mediated aquaporin 4 expression. Biochem Biophys Res Commun. 2006;350(2):399–404. doi: 10.1016/j.bbrc.2006.09.057. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zuo G, Shi XY, Zhang J, Fang Q, Chen G. Progesterone administration modulates cortical TLR4/NF-kappaB signaling pathway after subarachnoid hemorrhage in male rats. Mediators of Inflammation. 2011;2011:848309. doi: 10.1155/2011/848309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RJ, Lallemand F, de Witte P. Biochemical and neurotransmitter changes implicated in alcohol-induced brain damage in chronic or ‘binge drinking’ alcohol abuse. Alcohol & Alcoholism. 2009;44(2):128–135. doi: 10.1093/alcalc/agn100. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Egawa M. Effects of an antistroke agent MCl-186 on cerebral arachidonate cascade. J Pharmacol Exp Therap. 1994;271(3):1624–1629. [PubMed] [Google Scholar]
- Wen Z, Kim HY. Inhibition of phosphatidylserine biosynthesis in developing rat brain by maternal exposure to ethanol. Journal of Neuroscience Research. 2007;85(7):1568–1578. doi: 10.1002/jnr.21263. [DOI] [PubMed] [Google Scholar]
- Winkler AS, Baethmann A, Peters J, Kempski O, Staub F. Mechanisms of arachidonic acid induced glial swelling. Brain Res Mol Brain Res. 2000;76(2):419–423. doi: 10.1016/s0169-328x(00)00017-6. [DOI] [PubMed] [Google Scholar]
- Yakovleva T, Bazov I, Watanabe H, Hauser KF, Bakalkin G. Transcriptional control of maladaptive and protective responses in alcoholics: a role of the NF-kappaB system. Brain, Behavior, &. Immunity. 2011;25(Suppl 1):S29–S38. doi: 10.1016/j.bbi.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Zador Z, Verkman AS. Glial cell aquaporin-4 overexpression in transgenic mice accelerates cytotoxic brain swelling. J Biol Chem. 2008;283(22):15280–15286. doi: 10.1074/jbc.M801425200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HC, Mosior M, Ni B, Dennis EA. Regional distribution, ontogeny, purification, and characterization of the Ca2+-independent phospholipase A2 from rat brain. J Neurochem. 1999;73(3):1278–1287. doi: 10.1046/j.1471-4159.1999.0731278.x. [DOI] [PubMed] [Google Scholar]
- Yoon HJ, Jeon SB, Kim IH, Park EJ. Regulation of TLR2 expression by prostaglandins in brain glia. Journal of Immunology. 2008;180(12):8400–8409. doi: 10.4049/jimmunol.180.12.8400. [DOI] [PubMed] [Google Scholar]
- Zador Z, Stiver S, Wang V, Manley GT. Role of aquaporin-4 in cerebral edema and stroke. Handbook of Experimental Pharmacology. 2009;190:159–170. doi: 10.1007/978-3-540-79885-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YY, Lan YP, Tang HF, Zhu SM. Propofol pretreatment attenuates aquaporin-4 over-expression and alleviates cerebral edema after transient focal brain ischemia reperfusion in rats. Anesthesia & Analgesia. 2008;107(6):2009–2016. doi: 10.1213/ane.0b013e318187c313. [DOI] [PubMed] [Google Scholar]
- Zou JY, Martinez DB, Neafsey EJ, Collins MA. Binge ethanol-induced brain damage in rats: effect of inhibitors of nitric oxide synthase. Alcohol Clin Exp Res. 1996;20(8):1406–1411. doi: 10.1111/j.1530-0277.1996.tb01141.x. [DOI] [PubMed] [Google Scholar]