Abstract
The Children’s Oncology Group conducted a multi-center Phase III trial for chronic GVHD (cGVHD). The double-blind placebo-controlled randomized study evaluated hydroxychloroquine added to standard therapy for children with newly-diagnosed cGVHD. The study also used a novel grading and response scoring system and evaluated clinical laboratory correlates of cGVHD. The primary endpoint was complete response (CR) after 9 months of therapy. Fifty-four patients (27 on each arm) were enrolled prior to closure due to slow accrual. The CR rate was 28% in the hydroxychloroquine arm vs. 33% in the placebo arm (OR=0.77, 95% CI: 0.20–2.93, p=0.75) for 42 evaluable patients. For 41 patients with severity assessment at enrollment, 20 (49%) were severe and 18 (44%) moderate according to the NIH Consensus Conference global scoring system. The CR rate was 15% for severe cGVHD and 44% for moderate cGVHD (OR=0.24, 95% CI: 0.05–1.06, p = 0.07). Although the study could not resolve the primary question, it provided important information for future cGVHD study design in this population.
Keywords: Chronic graft-versus-host disease, GVHD, randomized, hydroxychloroquine
INTRODUCTION
Chronic GVHD (cGVHD) is the major cause of morbidity and non-relapse mortality after hematopoietic stem cell transplantation (HSCT).1 Historically, complete response rates of cGVHD after 9 months of primary therapy are 33–37%2 suggesting that better therapies are needed. Progress in treating cGVHD has been limited by the clinical complexity of the disease, lack of knowledge about the underlying pathophysiology, and paucity of Phase III clinical trials. A 2005 National Institutes of Health (NIH) Consensus Development Project on criteria for clinical trials of cGVHD provided guidelines for many of the diagnostic, response criteria and supportive care issues.3–7 Most phase III studies of therapy for cGVHD have been conducted at single institutions. Landmark studies include randomized trials of prednisone versus prednisone and azathioprine2 and prednisone versus prednisone and cyclosporine.8 Two trials of thalidomide and one trial of mycophenolate mofetil (MMF) were closed before reaching their target accrual.9–11 Despite these trials, the outcomes for cGVHD have not improved over the last 20 years.12
Hydroxychloroquine (HCQ) is a 4-aminoquinoline antimalarial drug that has activity as salvage therapy for steroid-resistant/dependent cGVHD.13 A multi-institutional phase II trial of HCQ in children and adults with steroid-resistant or steroid-dependent cGVHD utilizing HCQ dosing of 800 mg/day or 12mg/kg/d (if weight < 50kg) demonstrated a response rate of 53% (3 CR and 14 partial responses) in 32 evaluable patients. Responses were most notable in skin, oral, and liver cGVHD and there was no significant toxicity associated with the HCQ.
HCQ interferes with antigen processing and presentation14, decreases production of IL-1, IL-6, and TNF-alpha15, 16, and decreases proliferation and cytotoxicity resulting from allorecognition.17 HCQ also inhibits calcium signaling in T cells.18 HCQ, and the closely related drug chloroquine, is synergistic with cyclosporine and tacrolimus in vitro for suppressing alloreactive responses.17–21
Based on the mechanisms of action of HCQ and the results of the phase II study for cGVHD, we designed a randomized, double-blinded, placebo-controlled study of HCQ added to standard therapy for children with newly-diagnosed extensive cGVHD. The study was designed as a multi-center study because of the low incidence of cGVHD in children22 and to explore the feasibility of conducting multi-center studies of cGVHD. The study also used a novel scoring system for grading cGVHD manifestations and overall severity that is similar to that later proposed by the NIH Consensus Development Project.6 Finally, there was a large research laboratory component to the study with the goal of advancing the understanding of cGVHD pathophysiology.
METHODS
Patient Enrollment
The study was conducted by the Children’s Oncology Group from April 2002 to April 2005. Subjects were less than 30 years of age at time of study entry, had newly-diagnosed extensive cGVHD, and had received a bone marrow, peripheral blood stem cell, or cord blood transplant from a family member or unrelated donor. Confirmation of cGVHD by biopsy was required. Patients could be on steroids at a dose ≤ 2 mg/kg/day of prednisone or equivalent dose of another steroid for the treatment or prophylaxis of acute GVHD (aGVHD), cyclosporine or tacrolimus, and other immunosuppressants for the treatment of aGVHD. Patients were required to have an absolute neutrophil count (ANC) ≥ 1000/mm3 (unless due to cGVHD), adequate renal function, Lansky or Karnofsky performance score of ≥ 50, and a life expectancy of at least 2 months. Patients were not eligible if they had prior systemic treatment for extensive cGVHD, an uncontrolled infection, relapse of malignancy after transplant, lysosomal storage disorder, glucose 6-phosphate dehydrogenase (G6PD) deficiency, psoriasis, or if they were pregnant. Informed consent was obtained from the patient or guardian in accordance with institutional policies and as approved by the U.S. Department of Health and Human Services. The trial was registered at ClinicalTrials.gov with identifier NCT00031824 on May 8, 2002.
Study Design
The study was a randomized, double-blinded, placebo-controlled trial. Sequentially block randomized (block size of 2) assignment was performed electronically at the statistical coordinating center with the result of the randomization transmitted to the study pharmacist who then dispensed study drug (HCQ or placebo in the same tablet form) in a blinded fashion to the treating physician. All patients received a standardized treatment of steroids and cyclosporine or tacrolimus, and were randomized to receive HCQ or matching placebo. Any systemic immunosuppressive therapy other than steroids, cyclosporine, or tacrolimus was discontinued at study entry. The use of topical steroids was permitted. Patients received prednisone 1 mg/kg/day for 2 weeks and then the dose was tapered to 1 mg/kg every other day over the next 6 weeks. Originally, the prednisone dose remained at 1 mg/kg every other day until 9 months after starting therapy. Patients receiving prednisone > 0.5 mg/kg/day at study entry had a slower steroid taper and received methylprednisolone 15 mg/kg IV weekly × 4. Patients receiving prednisone 0.5 – 1 mg/kg/day, received 4 weeks of prednisone 1 mg/kg/day prior to the taper. Patients receiving prednisone > 1 – 2 mg/kg/day remained on the same dose for 4 weeks followed by a taper of 10% weekly until a dose of 1 mg/kg/day was reached. The prednisone dose was then weaned to 1 mg/kg every other day over the next 6 weeks. Patients that had worsening of cGVHD during the steroid taper had the dose increased to the dose given 2 weeks earlier. This dose was continued for two weeks and then the taper was resumed. If the cGVHD progressed, the patient was taken off protocol therapy. An amendment in May 2003 incorporated a second steroid taper starting at 6 months after study entry that decreased the prednisone dose to 0.5 mg/kg every other day over 2.5 months.
Cyclosporine and tacrolimus were given at standard doses with target trough levels of 200–300 ng/ml and 5–15 ng/ml, respectively. The HCQ/placebo dose was 12 mg/kg/day (max 1000 mg), divided into BID dosing. The dose was adjusted for cholestasis (25% and 50% reductions for bilirubin > 6 and > 12× the upper limit of normal, respectively). The dose was also adjusted for decreased renal function (25% and 50% reductions for creatinine > 1.5 and > 2× the upper limit of normal, respectively). The HCQ dosing was the same as that used for the Phase II study.13 HCQ and matching placebo was purchased from Sanofi Pharmaceuticals (New York, NY). HCQ was provided under terms of a Food and Drug Administration Investigational New Drug (IND) application #44,717 issued to one of the authors (A.L.G).
Central Pathology Review
Biopsy specimens were reviewed by George Sale, MD, a GVHD pathology expert, at Fred Hutchinson Cancer Research Center, Seattle, WA. Biopsies were reviewed centrally for retrospective analysis only.
Clinical GVHD Review
A panel of 5 members of the study committee (A.L.G., D.A.W., K.R.S., F.D.G., D.A.J.) reviewed the clinical findings at diagnosis. This included photographs of skin and oral involvement when available.
Required observations
At study entry, patients had complete blood counts, chemistries, liver function tests, quantitative immunoglobulins, direct and indirect Coombs, anti-nuclear antibody (ANA), anti-double-stranded DNA (anti-dsDNA), baseline ophthalmological exam, complete evaluation for cGVHD, and a health-related quality of life (QOL) assessment. Complete evaluations for cGVHD and response were performed after 2, 6, and 9 months of therapy. ANA, anti-dsDNA, and Coombs tests were only repeated if abnormal at entry. Ophthalmological exams with attention to possible HCQ-related retinal toxicity were done at 6 and 12 months.
Response criteria
Responses were evaluated after 2, 6, and 9 months of therapy and categorized as: Complete response: complete clinical resolution of all reversible GVHD manifestations; Partial response: complete clinical resolution in at least one involved site but persistent disease (not progression) in other sites; Stable disease: no clinical improvement in GVHD manifestations and lack of clinical worsening; Progressive disease: clinical worsening of GVHD manifestations. Patients were considered not evaluable for response at 9 months if they terminated protocol therapy prior to this time for reasons not related to progression of cGVHD, toxicity, or relapse.
Improvement and worsening were assessed using a grading system for each involved organ (Table S1, online only). The grades included 0 (not involved), 1 (mild), 2 (moderate), and 3 (severe) and definitions for the severity grading were provided for each organ. Clinical response data were reviewed and adjudicated centrally.
Off protocol therapy criteria
Patients were removed from protocol therapy for (1) progressive cGVHD after 2 months of therapy, (2) life-threatening progression of cGVHD after > 2 weeks, (3) inability to complete steroid taper due to recurrent cGVHD flare, (4) no response (stable disease) after 6 months of protocol therapy (amended to 2 months in June 2004; only 2 patients affected by this change), (5) lack of a complete response after 9 months of therapy, (6) completion of treatment (9 months of protocol therapy and completion of study drug tapering without a flare and completion of 9 months of follow-up) for complete responders, (7) Grade III or IV toxicity not resolving with dose modification or discontinuation of a protocol drug, or any visual impairment attributable to HCQ, (8) intercurrent illness which prevented further administration of treatment, (9) relapse of malignancy, (10) withdrawal of consent, (11) lost to follow-up, or (12) death.
Study endpoints
The primary study endpoint was the complete response rate after 9 months of therapy. Secondary endpoints included event-free survival, overall survival, and grade 3 and 4 toxicity.
Statistical considerations
The primary question of treatment effect was assessed by comparing the proportion of CR patients in the HCQ arm to the proportion of CR patients in the placebo arm. The target accrual was 232 patients to have an 80% power at alpha=.05 (one-sided) to detect an odds ratio of 2.0 comparing CR rates in the HCQ arm to the placebo arm. The response odds ratio (OR) was estimated as the cross product from the 2×2 table of response (CR vs not CR) by treatment group with 95% Wald confidence intervals (95% CI) estimated in the usual way. Fisher’s exact test was used for the response rate comparisons.23 A post hoc analysis was performed for CR + PR for the two treatment arms in a similar fashion. Standard chi-square tests were employed to identify significant prognostic factors for response.24 The probability of survival as a function of time since enrollment was calculated using the method of Kaplan and Meier.25 The survivor function was compared across treatment regimens using the log-rank test.26 A post hoc analysis was performed to evaluate the relationship of response rate and degree of severity based on the global scoring system of cGVHD severity proposed by NIH Consensus Development Project, which occurred after the initiation of this study.3 The statistical packages SAS (SAS Institute, Cary, NC) and STATA (Stata Corporation, College Station, Texas) were used for all data management and statistical analysis.
RESULTS
When the study was closed due to slow accrual, 54 patients had been enrolled with 27 assigned to each treatment arm. The participant flow diagram is shown in Figure S1 (online only). None of the patients were lost to follow-up. Patients who were 1) withdrawn from the study at the request of the patient or parent/guardian (n=5) or 2) did not complete therapy because of study closure (n=7), were considered not evaluable for the primary response endpoint.
Patient characteristics
Among 54 enrolled patients, 37 (69%) were male and 17 (31%) were female. The median age was 12 years (range 1 – 21). The stem cell source was bone marrow (n=24), peripheral blood stem cells (n=20), or cord blood (n=10). Donors were related in 43% of cases and unrelated in 57%. Forty-nine patients had received a transplant for malignant disease and 5 patients for non-malignant disease. Thirty-four patients (63%) had a history of acute GVHD and 9 patients (17%) had progressive onset of cGVHD. The median time from transplant to diagnosis of cGVHD was 6 months (range 3–24). Twenty-one patients (39%) were receiving steroids at study entry. Thirty patients (56%) were receiving cyclosporine or tacrolimus and three (6%) were receiving MMF at study entry. Details by study arm are provided in Table 1. There were no significant differences for any of these parameters between the two arms.
Table 1.
Patient characteristics
| By Treatment Arm | Placebo (N=27) |
HCQ (N=27) |
P value |
|---|---|---|---|
| Age (Median, range) | 11 (1,21) | 13 (3,20) | 0.21 |
| Sex | 1.00 | ||
| Male | 19 (70) | 18 (67) | |
| Female | 8 (30) | 9 (33) | |
| Diagnosis | 0.41 | ||
| ALL | 16 (59) | 12 (44) | |
| AML/MDS | 8 (30) | 7 (26) | |
| Chronic Myelogenous Leukemia | 0 (0) | 4 (15) | |
| Other malignant disease | 1 (4) | 1 (4) | |
| Non-malignant disease | 2 (7) | 3 (11) | |
| Donor Type | 1.00 | ||
| Related | 12 (44) | 11 (41) | |
| Unrelated | 15 (56) | 16 (59) | |
| Stem Cell Source | 0.08 | ||
| Bone Marrow | 9 (33) | 15 (56) | |
| PBSC | 14 (52) | 6 (22) | |
| Cord Blood | 4 (15) | 6 (22) | |
| Prior Acute GVHD | 0.88 | ||
| Acute GVHD Grade(I-II) | 12 (44) | 11 (41) | |
| Acute GVHD Grade (III-IV) | 6 (22) | 5 (19) | |
| Immunosuppression at study entry | |||
| Steroids | 11 (41) | 10 (37) | 1.00 |
| Cyclosporine | 11 (41) | 6 (22) | 0.24 |
| Tacrolimus | 5 (19) | 8 (30) | 0.53 |
| Months from transplant to cGVHD (median, range) | 6 (3,24) | 6 (3,14) | 1.00 |
| Onset of cGVHD | 0.76 | ||
| Progressive | 4 (15) | 5 (19) | |
| Quiescent | 13 (48) | 10 (37) | |
| De novo | 10 (37) | 12 (44) | |
| Platelets < 100,000/uL | 7 (26) | 6 (22) | 1.00 |
| Site of cGVHD at Study Entry | |||
| Lichenoid Skin Score | 0.24 | ||
| 1 | 5 (19) | 3 (12) | |
| 2 | 3 (12) | 1 (4) | |
| 3 | 3 (12) | 2 (8) | |
| Sclerodermatous Skin Score | 0.73 | ||
| 1 | 2 (8) | 2 (8) | |
| 2 | 2 (8) | 2 (8) | |
| 3 | 0 (0) | 2 (8) | |
| Oral Score | 0.78 | ||
| 1 | 12 (46) | 5 (19) | |
| 2 | 4 (15) | 8 (31) | |
| 3 | 1 (4) | 2 (8) | |
| Ocular Score | 0.78 | ||
| 1 | 4 (15) | 6 (23) | |
| 2 | 10 (38) | 5 (19) | |
| 3 | 0 (0) | 1 (4) | |
| Bilirubin Score | 0.74 | ||
| 1 | 3 (11) | 4 (15) | |
| 2 | 2 (8) | 2 (8) | |
| 3 | 0 (0) | 1 (4) | |
| Alkaline phosphatase Score | 1.00 | ||
| 1 | 3 (12) | 4 (15) | |
| 2 | 3 (12) | 2 (8) | |
| 3 | 0 (0) | 0 (0) | |
| ALT Score | 0.09 | ||
| 1 | 8 (31) | 4 (15) | |
| 2 | 6 (23) | 2 (8) | |
| 3 | 0 (0) | 1 (4) | |
| Gastrointestinal symptoms | 8 (31) | 15 (58) | 0.09 |
| Diarrhea Score | 0.10 | ||
| 1 | 4 (15) | 7 (27) | |
| 2 | 0 (0) | 3 (11) | |
| 3 | 0 (0) | 0 (0) | |
| Weight Loss Score | 0.14 | ||
| 1 | 5 (19) | 9 (35) | |
| 2 | 1 (4) | 2 (8) | |
| 3 | 0 (0) | 1 (4) | |
| Pulmonary involvement | 5 (19) | 7 (27) | 0.74 |
| Contractures Score | 1.00 | ||
| 1 | 0 (0) | 0 (0) | |
| 2 | 5 (19) | 3 (12) | |
| 3 | 0 (0) | 2 (7) | |
| Performance score < 90 | 15 (58) | 12 (46) | 0.58 |
| Overall cGVHD score (Median, range) | 12 (3, 23) | 11 (4, 22) | 0.77 |
Number in parentheses is percentage; overall cGVHD score calculated by adding individual scores from Table S1. Data was not available for one patient for history of malignancy and data was not available for overall score for two patients.
P values for differences in characteristics scored 0–3 are from Fisher’s exact test of score 1–3 vs score 0. Percentages are from patients with non-missing data.
Clinical manifestations of cGVHD
Data regarding clinical manifestations at study entry were available for 52 patients. The proportion of patients with each manifestation is shown in Table 1. Several factors have been correlated with a worse prognosis for patients with chronic GVHD.27 The proportion of patients with these adverse prognostic factors at study entry was: lichenoid rash involving > 50% of the body surface area (9%), bilirubin > 1.2 mg/dL (23%), progressive onset of chronic GVHD (17%), platelet count < 100K/mL (24%), presence of diarrhea/GI involvement (27%/44%), and weight loss (35%).
Eosinophil counts, IgG levels, and anti-nuclear antibody (ANA) titers were available for most patients at the time of diagnosis of cGVHD. Eosinophilia (absolute eosinophil count > 500/mcl) was present in 40% (18/45) of patients. Hypergammaglobulinemia (IgG level greater than the upper limit of normal) was present in 23% (11/48) of patients. None of these patients was receiving intravenous gammaglobulin. An ANA titer of > 1:80 was present in 28% (12/43) of patients and 9 of these patients had titers > 1:160. At presentation, 62% (30/48) of patients had at least one of these three findings.
Response data
Among 54 enrolled patients, 42 (18 in HCQ arm and 24 in placebo arm) were evaluable for response. Twelve patients were not evaluable for response: 7 patients had not been on study for nine months when the study was closed and 5 patients were withdrawn at parental request. Out of the 42 evaluable patients, 13 (31%) had a CR at nine months and 18 (43%) had a CR or a PR. The rate of CR at nine months was 28% (5/18) in the HCQ arm and 33% (8/24) in the placebo arm (OR=0.77, 95% CI: 0.20–2.93, p=0.75). The rate of CR + PR at nine months was 39% (7/18) in the HCQ arm and 46% (11/24) in the placebo arm (OR=0.75, 95% CI: 0.22–2.60, p=0.76).
Toxicity data
All patients were evaluable for toxicity. Grade 3/4 toxicities which occurred in more than 10% of patients included hypertension (15%), elevated ALT (17%), and infection without neutropenia (18.5%). Grade 3/4 avascular necrosis and hyperglycemia each occurred in 4% of patients. There were no statistically significant differences between the two arms for grade 3/4 toxicities. Toxicities occurring in more than 10% of patients on either arm are shown in Table 2. There were no serious toxicities that were attributed to HCQ. Importantly, although retinal toxicity has been reported with HCQ, it was not seen in the 27 patients treated with HCQ.
Table 2.
Grade 3 and 4 toxicities occurring in more than 10% in either arm
| Toxicity Type | Treatment | |||
|---|---|---|---|---|
| Placebo | HCQ | |||
| Number | Percent | Number | Percent | |
| Hypertension | 4 | 14.8 | 4 | 14.8 |
| Elevated GGT | 3 | 11.1 | 2 | 7.4 |
| Elevated ALT | 7 | 25.9 | 2 | 7.4 |
| Anemia | 3 | 11.1 | 1 | 3.7 |
| Thrombocytopenia | 4 | 14.8 | 0 | 0 |
| Infection without neutropenia | 5 | 18.5 | 5 | 18.5 |
GGT – gamma glutamyl transferase; ALT – alanine aminotransferase; ANC – absolute neutrophil count
Infections
There were 13 grade 3/4 infections in 12 (22%) patients (6 in each treatment arm). Three deaths that occurred during protocol therapy were associated with infection. Two of these were due to fungal infection and the organism was not known for the third.
Central Pathological and Clinical Review
Biopsy specimens were reviewed centrally. Specimens were reviewed for 37/54 (68%) patients enrolled on the study. Biopsies were from skin (n=25), oral/lip (n=9), liver (n=7), GI tract (n=5), lung (n=1), and lacrimal gland (n=1). Biopsies from more than one site were submitted for some patients. There was a high level of concordance between the central and institutional diagnosis of GVHD – 36/37 (97%) patients and 47/48 (98%) biopsies.
Clinical manifestations present at diagnosis and photographs when available were reviewed by a Clinical Review Panel to evaluate the clinical diagnosis of cGVHD. Data were available for 46/54 (85%) patients at the time of the review. Photographs of skin and/or oral findings were available for 19 patients. A clinical diagnosis of cGVHD was confirmed for 45/46 (98%) patients for whom data were available.
Survival data
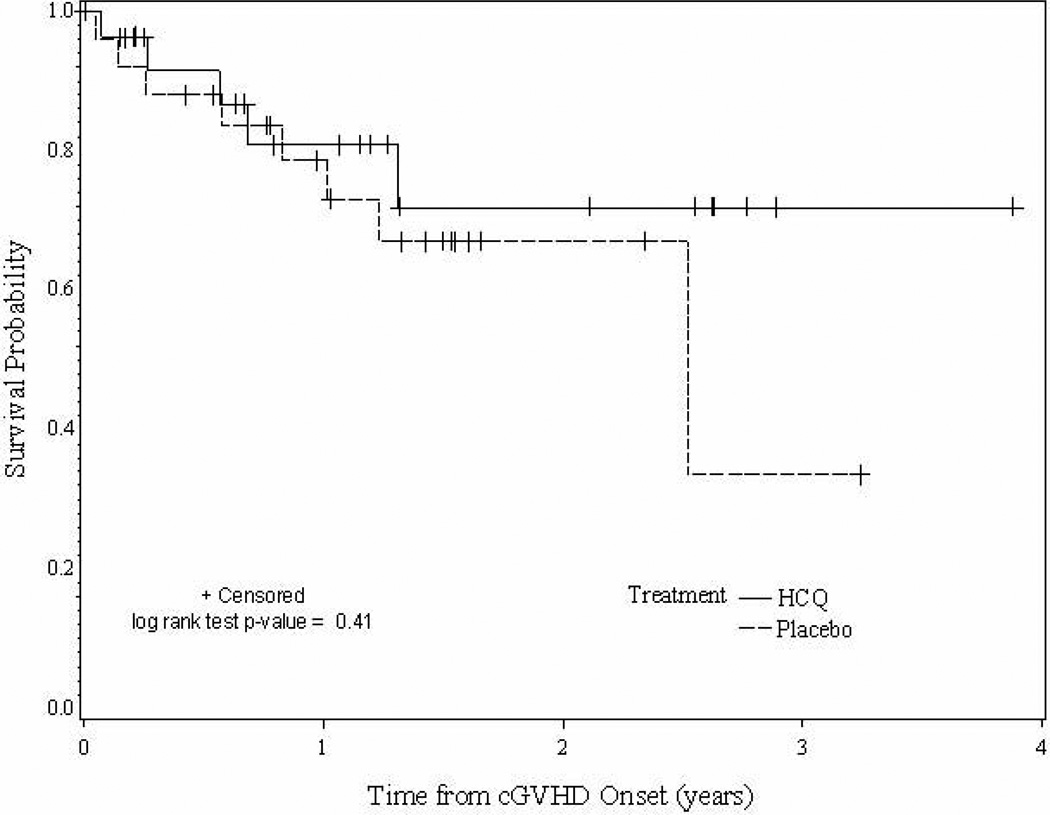
Four of 54 (7%) patients died while on protocol therapy or within one month of discontinuation of therapy. The cause of death included progressive GVHD (n=1), GVHD and infection (n=1), and infection (n=2). The cause of death for 8 patients that died at a later time was relapse of malignancy or complication of treatment of relapse (n=5), GVHD (n=1), GVHD and infection (n=1), pulmonary fibrosis (n=1), and lung disease not otherwise specified (n=1). As seen in Figure 1, there is no evidence of a difference in survival between placebo and HCQ patients (p=0.41).
Figure 1.
Overall survival of patients by treatment group.
Correlation of response and cGVHD severity
Patients were retrospectively scored as having mild, moderate, or severe cGVHD based on the global scoring system recommended by the NIH Consensus Development Project on cGVHD.3 There are small differences in some of the organ-specific grades between our grading table (Table S1, online only) and the one proposed by the Consensus. However, this did not affect the overall grading category for any patient.
For the 41 evaluable patients for whom grading data were available, 20 (49%) were graded as severe, 18 (44%) as moderate, and 3 (7%) as mild. The CR rate was 44% for patients with moderate cGVHD and 15% for patients with severe cGVHD (OR=0.24, 95% CI: 0.05–1.06, p = 0.07). The CR + PR response rate was 56% for patients with moderate cGVHD and 30% for patients with severe cGVHD (OR=0.39, 95% CI: 0.11–1.41, p = 0.18).
DISCUSSION
We report the first phase III trial of cGVHD conducted solely in children. The study was designed to evaluate a therapeutic question, but also was the first study to explore the feasibility of conducting a multicenter Phase III trial for cGVHD. The study incorporated a grading system and central pathology and clinical review to address this issue. The study performed extensive immunological testing to evaluate the pathophysiology of cGVHD and these results have been published.28–30 Despite not achieving the primary endpoint, the study provided data including response rates with standard therapy and the correlation of local and central pathology that will be useful for the design of future studies of cGVHD.
The primary aim of the study was to determine if the addition of HCQ to standard therapy including prednisone and a calcineurin inhibitor could improve the complete response rate of cGVHD after 9 months of therapy. This endpoint, which had been used for most Phase III studies prior to the initiation of our study, was chosen to allow sufficient time for maximal clinical response and for steroid tapering. The primary aim was not able to be addressed with adequate statistical power due to the limited patient accrual, but there was no suggestion of any significant difference between the two treatment arms. Several factors may have contributed to the suboptimal accrual. The eligibility criteria were complex and strict. The participating investigators struggled with differentiating the frequently insidious onset of cGVHD from persistence or exacerbation of acute GVHD. This resulted in steroid pretreatment that made potential study subjects ineligible for the trial. In an attempt to isolate the specific impact of HCQ and to ensure that the arms were similar, the treatment plan rigidly controlled steroid dosing and tapering. The participating centers found this study requirement challenging because of competing reasons for which steroid dosing is adjusted (eg. toxicity, risk of relapse). Of note, subsequent multi-center trials for cGVHD have also struggled with accrual.
The study provided the only data available for response rates of children with newly-diagnosed extensive cGVHD treated with standard therapy on a multi-center trial. The central clinical and pathology review to confirm the diagnosis of cGVHD showed excellent correlation between the local institution and central review. The results support multi-center studies of cGVHD and the use of institutional pathology for these trials.
The study used a unique scoring system for grading cGVHD manifestations similar to the one later proposed by the NIH Consensus Development Project.3 There are small differences in some of the organ-specific grades between the scoring systems but this did not affect the overall grading category for any patient. We were able to use the information gathered in our scoring system to analyze the patients according to the NIH Consensus global scoring system (mild, moderate, and severe) of cGVHD severity.3 Our data suggest that the NIH Consensus global scoring system for cGVHD severity for patients with severe or moderate cGVHD correlates with the likelihood of response after 9 months of therapy. There were too few patients with mild disease to evaluate this group. Another study retrospectively evaluated the correlation between disease severity according to this scoring system and the ability to discontinue immunosuppression, which is an alternative endpoint to response to therapy. Patients with more severe disease were significantly less likely to be able to discontinue immunosuppression.31 Additional studies correlating response rates and cGVHD severity are warranted. The ability to identify patients unlikely to respond to standard therapy is important since it would support these patients being considered as candidates for studies of novel therapies at the time of diagnosis.
There is a paucity of Phase III studies of therapy for newly-diagnosed cGVHD. A summary of these studies is shown in Table 3. Comparison between these studies is difficult because of differences in the proportion of patients with extensive cGVHD, sites of cGVHD involvement, severity and type of onset of cGVHD, donor types, and other prognostic factors. There are also differences in response criteria and study endpoints, and most of the studies are single center studies. For example, a study reported by Sullivan et al. in 1988, evaluated the addition of azathioprine to prednisone.2 Thirty-nine percent of the patients had subclinical cGVHD (GVHD on blind biopsy without clinical evidence of cGVHD) when therapy was started. The response definition was stricter than other studies, with a CR defined as clinically inactive cGVHD and a negative biopsy and a PR defined as clinically inactive cGVHD but biopsies showing active GVHD. The CR rate at 9 months was 33% for prednisone and 37% for prednisone/azathioprine. Some studies have used discontinuation of immunosuppression as an endpoint, so response data are not available.8,11
Table 3.
Phase III studies of chronic GVHD therapy
| Number of patients | HLA-identical sibling donor |
Progressive onset of cGVHD |
Platelet count <100,000/ul |
CR at 9 mo | CR + PR at 9 mo | |
|---|---|---|---|---|---|---|
| This report | 54 | 61% | 17% | 28% | 33% | 44% |
| Sullivan (2) | 126 | 94% | 18% | None | 35% | 63% |
| Koc (8) | 287 | 68% | 16% | None | N/A | N/A |
| Arora (10) | 54 | 63% | 17% | 37% | 52% at 1 yr | 79% at 1 yr |
| Martin (11) | 151 | 51% | 10% | 22% | N/A | N/A |
Number in parentheses is the reference number; HLA – human leukocyte antigen; CR – complete response; CR + PR – complete or partial response; N/A – not available.
For our study, the CR and CR + PR rates after 9 months of therapy for all patients (both treatment arms) were 31% and 43%, respectively, for 42 evaluable patients. The CR rate is comparable to a study by Sullivan et al7, but the CR + PR rate is lower. Both the CR and CR + PR rates are much lower than those in a study reported by Arora et al.10 This may be due to differences in study populations and the single versus multi-institutional setting. Of note, 49% of the evaluable patients for whom data were evaluable in our study had severe disease according to the NIH Consensus criteria. Additional support for the fact that our patient population was skewed towards more severely affected patients is provided by a comparison to a report of a large cohort of children with cGVHD.22 GI and lung involvement were seen in 44% and 20% of our patients and in 24% and 11% of that cohort, respectively.
Studying cGVHD at the time of initial diagnosis is a challenge. The initial presentation can be insidious and often develops at the time of taper of planned GVHD prophylaxis or acute GVHD treatment. The early symptom complex at presentation can overlap with features of acute GVHD and other post transplant complications (e.g. infection, malabsorption). Subsequent trials might benefit greatly from the development of biomarkers specific to cGVHD that confirm the diagnosis at onset and possibly as surrogate indicators of response. A pretreatment window that allows a short course of steroids could make more patients eligible for future ‘frontline’ studies and allow a less rushed study entry. A less rigid and complex steroid taper would provide investigators with flexibility needed for individual patients, better reflecting current clinical practice.
The results of studies2, 10, 11 in which a drug with activity in a Phase II salvage study fails to add benefit in a Phase III upfront treatment study suggest that a better approach may be needed. One approach is to perform a randomized Phase II study in the upfront setting prior to committing to a large Phase III trial. The caution is to avoid the assumption that agents active in the salvage setting will be active at time of initial diagnosis. Our study provides response rates for cGVHD in children in a multi-center trial setting which can serve as a baseline for such trials. The results with the addition of a study drug would have to be substantially better than the baseline to warrant a Phase III trial. Finally, our data suggest that risk stratification based on the NIH consensus staging is likely to be useful for study design by identifying patients with severe cGVHD who have little chance of a complete response with standard therapy and for whom novel therapies need to be developed and tested.
Supplementary Material
Acknowledgements
We would like to thank Stephanie Lee, MD for her critical review of the manuscript. The research was supported by the following grants from the National Cancer Institute, National Institutes of Health, Bethesda, MD, USA - R01 CA 84137, U10 CA98543, and U10 CA98413.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure Statement: All authors wish to disclose they do not have any existing or potential conflicts of interest.
REFERENCES
- 1.Gilman AL, Serody J. Diagnosis and Treatment of chronic GVHD. Semin Hematol. 2006;43:70–80. doi: 10.1053/j.seminhematol.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan KM, Witherspoon RP, Storb R, et al. Prednisone and azathioprine compared with prednisone and placebo for treatment of chronic graft-v-host disease: prognostic influence of prolonged thrombocytopenia after allogeneic marrow transplantation. Blood. 1988;72:546–554. [PubMed] [Google Scholar]
- 3.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 2005;11:945–955. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Shulman HM, Kleiner D, Lee SJ, et al. Histopathologic Diagnosis of Chronic Graft-versus-Host Disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant. 2006;12:31–47. doi: 10.1016/j.bbmt.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 5.Schultz KR, Miklos DB, Fowler D, et al. Toward Biomarkers for Chronic Graft-versus-Host Disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: III. Biomarkers Working Group Report. Biol Blood Marrow Transplant. 2006;12:126–137. doi: 10.1016/j.bbmt.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Pavletic S, Martin P, Lee SJ, et al. Measuring Therapeutic Response in Chronic Graft-versus-Host Disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group Report. Biol Blood Marrow Transplant. 2006;12:252–266. doi: 10.1016/j.bbmt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Couriel D, Carpenter PA, Cutler C, et al. Ancillary Therapy Supportive Care of Chronic Graft-versus-Host Disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. 2006;12:375–396. doi: 10.1016/j.bbmt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Koc S, Leisenring W, Flowers ME, et al. Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood. 2002;100:48–51. doi: 10.1182/blood.v100.1.48. [DOI] [PubMed] [Google Scholar]
- 9.Koc S, Leisenring W, Flowers ME, et al. Thalidomide for treatment of patients with chronic graft-versus-host disease. Blood. 2000;96:3995–3996. [PubMed] [Google Scholar]
- 10.Arora M, Wagner JE, Davies SM, et al. Randomized clinical trial of thalidomide, cyclosporine, and prednisone versus cyclosporine and prednisone as initial therapy for chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2001;7:265–273. doi: 10.1053/bbmt.2001.v7.pm11400948. [DOI] [PubMed] [Google Scholar]
- 11.Martin PJ, Storer BE, Rowley SD, et al. Evaluation of mycophenolate mofetil for initial treatment of chronic graft-versus-host disease. Blood. 2009;113:5074–5082. doi: 10.1182/blood-2009-02-202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin PJ, Gilman AL. Front Line Treatment of Chronic Graft versus Host Disease. In: Vogelsang GB, Pavletic SZ, editors. Chronic Graft versus Host Disease: Interdisciplinary Management. New York, NY: Cambridge University Press; 2009. pp. 124–133. [Google Scholar]
- 13.Gilman AL, Chan KW, Mogul M, et al. Hydroxychloroquine for the treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2000;6:327–334. doi: 10.1016/s1083-8791(00)70058-9. [DOI] [PubMed] [Google Scholar]
- 14.Ziegler HK, Unanue ER. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A. 1982;79:175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ertel W, Morrison MH, Ayala A, Chaudry IH. Chloroquine attenuates hemorrhagic shock-induced suppression of Kupffer cell antigen presentation and major histocompatibility complex class II antigen expression through blockade of tumor necrosis factor and prostaglandin release. Blood. 1991;78:1781–1788. [PubMed] [Google Scholar]
- 16.Sperber K, Quraishi H, Kalb TH, Panja A, Stecher V, Mayer L. Selective regulation of cytokine secretion by hydroxychloroquine: inhibition of interleukin 1 alpha (IL-1-alpha) and IL-6 in human monocytes and T cells. J Rheumatol. 1993;20:803–808. [PubMed] [Google Scholar]
- 17.Gilman AL, Beams F, Tefft M, Mazumder A. The effect of hydroxychloroquine on alloreactivity and its potential use for graft-versus-host disease. Bone Marrow Transplant. 1996;17:1069–1075. [PubMed] [Google Scholar]
- 18.Goldman FD, Gilman AL, Hollenback C, Kato RM, Premack RA, Rawlings DJ. Hydroxychloroquine inhibitis calcium signals in T cells: a new mechanism to explain its immunomodulatory properties. Blood. 2000;95:3460–3466. [PubMed] [Google Scholar]
- 19.Schultz KR, Nelson D, Bader S. Synergy between lysosomotropic amines and cyclosporin A on human T cell responses to an exogenous protein antigen, tetanus toxoid. Bone Marrow Transplant. 1996;18:625–631. [PubMed] [Google Scholar]
- 20.Schultz KR, Bader S, Nelson D, Wang MD, HayGlass KT. Immune suppression by lysosomotropic amines and cyclosporine on T-cell responses to minor and major histocompatibility antigens: does synergy exist? Transplantation. 1997;64:1055–1065. doi: 10.1097/00007890-199710150-00019. [DOI] [PubMed] [Google Scholar]
- 21.Hsiao CC, Su WN, Forooghian F, Bader S, Rempel J, HayGlass KT, Gilman A, Schultz KR. Evaluation for synergistic suppression of T cell responses to minor histocompatibility antigens by chloroquine in combination with tacrolimus and a rapamycin derivative, SDZ-RAD. Bone Marrow Transplant. 2002;30:905–913. doi: 10.1038/sj.bmt.1703727. [DOI] [PubMed] [Google Scholar]
- 22.Zecca M, Prete A, Rondelli R, et al. Chronic graft-versus-host disease in children: Incidence, risk factors, and impact on outcome. Blood. 2002;100:1192–1200. doi: 10.1182/blood-2001-11-0059. [DOI] [PubMed] [Google Scholar]
- 23.Fisher RA. On the interpretation of X2 from contingency tables, and the calculation of P. Journal of the Royal Statistical Society B. 1922;85:87–94. [Google Scholar]
- 24.Fleiss JL, Levin B, Paik MC, et al. Statistical Methods for Rates and Proportions. New York: Wiley & Sons, Inc; 2003. [Google Scholar]
- 25.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. Journal of American Statistical Association. 1958;53:457–481. [Google Scholar]
- 26.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemotherapy Reports. 1966;50:163–170. [PubMed] [Google Scholar]
- 27.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:215–233. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 28.She K, Gilman AL, Aslanian S, et al. Altered TLR9 Responses in Circulating B cells at the Onset of Chronic GVHD. Biology of Blood and Marrow Transplantation. 2007;13:386–397. doi: 10.1016/j.bbmt.2006.12.441. [DOI] [PubMed] [Google Scholar]
- 29.Fujii H, Cuvelier G, She K, et al. Biomarkers in Newly Diagnosed Pediatric Extensive Chronic Graft-versus-Host Disease: A report from the Children’s Oncology Group. Blood. 2008;111:3276–3285. doi: 10.1182/blood-2007-08-106286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuvelier GD, Kariminia A, Fujii H, et al. Anti-CD13 Abs in children with extensive chronic GVHD and their relation to soluble CD13 after allogeneic blood and marrow transplantation from a Children's Oncology Group Study, ASCT0031. Bone Marrow Transplant. 2010;45:1653–1657. doi: 10.1038/bmt.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho BS, Min CK, Eom KS, et al. Feasibility of NIH consensus criteria for chronic graft-versus-host disease. Leukemia. 2009;23:78–84. doi: 10.1038/leu.2008.276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.