Abstract
Chronic alcohol consumption disrupts glucocorticoid signaling at multiple physiological levels to interact with several disease-related processes associated with neuroendocrine and psychiatric disorders. Excessive alcohol use produces stress-related neuroadaptations at the level of the hypothalamic-pituitary-adrenal (HPA) axis as well as within central (extra-hypothalamic) neural circuitry, including the central amygdala (CeA) and prefrontal cortex (PFC). Altered glucocorticoid receptor (GR) signaling in these areas following excessive alcohol exposure is postulated to mediate the transition from recreational drinking to dependence, as well as the manifestation of a host of cognitive and neurological deficits. Specifically, a bidirectional regulation of stress systems by glucocorticoids leads to the development of an HPA axis tolerance and a concomitant sensitization of cortical and subcortical circuitries. A greater understanding of how hypothalamic and extra-hypothalamic glucocorticoid systems interact to mediate excessive drinking and related pathologies will lead to more effective therapeutic strategies for alcohol use disorder (AUD) and closely related comorbidities.
Keywords: alcohol-use disorder, amygdala, cognition, corticotropin-releasing factor, glucocorticoids, prefrontal cortex
Introduction
Since their original biological conceptualizations in the 1930s, scientists have intensively focused on the physiological basis of adaptive and maladaptive stress (Le Moal & Koob, 2007; Sapolsky, Romero, & Munck, 2000). Numerous studies have described alterations in the hypothalamic-pituitary-adrenal (HPA) axis in various stress-related disorders such as major depression, post-traumatic stress disorder, and alcohol use disorder (AUD). The discovery of glucocorticoids by Hans Selye and the role of the HPA axis in the integrative stress response fostered the search for hypothalamic-releasing factors (Guillemin, 1978) and the discovery of corticotropin-releasing factor (CRF; Vale, Spiess, Rivier, & Rivier, 1981) as the primary stimulator of adrenocorticotrophic hormone (ACTH) release by the anterior pituitary. Cortisol in humans (or corticosterone in rodents) is the primary glucocorticoid released from the adrenal cortex in response to ACTH. Circulating glucocorticoids produce an array of physiological effects in response to external stressors, and under normal conditions are also responsible for termination of their own actions via negative feedback inhibition at multiple levels of the HPA axis. In the popular media, the concept of stress (and by extension, HPA axis function) has been largely debased from its original conceptualization by Selye as an adaptive response to environmental challenge. However, even Selye observed what he termed a “general adaptive syndrome” and “diseases of adaptation” (Selye, 1950). While HPA axis stimulation and termination provide a valuable mechanism for bodily homeostasis, repetitive activation is hypothesized to contribute to a cumulative load, termed allostatic load (McEwen & Stellar, 1993), onto this system that can tax it to the point of pathology (George, Le Moal, & Koob, 2012). Importantly, the gradual dysregulation of physiological stress mechanisms is postulated to include a functional potentiation of central brain stress circuitry that includes such regions as the central amygdala and prefrontal cortex (Koob et al., 2014; Myers, McKlveen, & Herman, 2014). Dysregulation of neuroendocrine systems has been associated with mental disorders ranging from major depression to drug addiction.
Alcohol-use disorder (AUD) represents a multifaceted psychiatric disease with few effective treatments. The Fifth Edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5, 2013) stratifies AUD into mild, moderate, and severe forms based on the number of criteria met. These specifications include excessive drinking over extended periods, intense craving and desire to consume alcohol, and manifestation of a motivational withdrawal syndrome wherein alcohol is consumed via negative reinforcement processes (i.e., alcohol is consumed to relieve negative affective symptoms produced by abstinence). Importantly, HPA axis stimulation and glucocorticoid actions would appear to play a facilitative role in the development of each of these benchmarks (Becker, 2012), along with driving additional complications associated with excessive alcohol consumption. This review conceptualizes AUD as an unrelenting, relapsing disorder promoted and maintained via persistent alterations in hypothalamic and extra-hypothalamic stress signaling, yet offers hope based on an emerging intersection of preclinical and clinical studies that suggest the promise of effective therapeutic intervention aimed at correcting dysregulated glucocorticoid signaling.
Glucocorticoid regulation of alcohol drinking, craving, and relapse
Alcohol intoxication and withdrawal serve as two distinct activators of the HPA axis to raise circulating corticosterone levels in rodents (Ellis, 1966; Rivier, Bruhn, & Vale, 1984; Tabakoff, Jafee, & Ritzmann, 1978) and cortisol levels in humans (Adinoff, Iranmanesh, Veldhuis, & Fisher, 1998; Adinoff et al., 1990; Adinoff, Ruether, Krebaum, Iranmanesh, & Williams, 2003). Alcohol-induced glucocorticoid release may mediate some of alcohol's reinforcing effects because corticosterone is both reinforcing by itself (Piazza et al., 1993) and also increases alcohol drinking via actions in the ventral striatum (Fahlke & Hansen, 1999). In concert with corticosterone's ability to potentiate mesolimbic activation by excitatory amino acids (Cho & Little, 1999), this mechanism may play a role in the effects of various forms of stress to elevate drinking in non-dependent animals (e.g., Edwards et al., 2013; Little et al., 1999; Logrip & Zorrilla, 2012) via an interaction of stress and reinforcement circuitry. Indeed, Koenig and Olive (2004) found that systemic blockade of glucocorticoid receptors (GRs), but not mineralocorticoid receptors (MRs), significantly reduces alcohol drinking under putatively stressful limited-access conditions. These data indicate that alcohol intake is predominately under the influence of GRs versus MRs, despite the fact that MRs display a greater affinity for corticosterone and, like GRs, are located within limbic reward circuitry (McEwen, 2007; ter Heegde, De Rijk, & Vinkers, 2015).
The neurophysiological effects of glucocorticoids are complex, and plasma levels (especially in disease conditions) do not necessarily reflect brain levels. Little and colleagues (2008) conducted a comparative measure of plasma and brain corticosterone concentrations following chronic alcohol exposure. While corticosterone levels were similar in both brain and blood during alcohol intoxication, brain levels in specific limbic regions remained significantly elevated into long-term withdrawal (1 day to 2 months), indicating an important distinction between systemic and brain corticosterone synthesis and metabolism. Interestingly, the prefrontal cortex exhibited the greatest prolonged increases in corticosterone levels, and the same study found that nuclear localization of glucocorticoid receptors was increased in the prefrontal cortex (PFC) following chronic alcohol exposure. Such neuroadaptations are hypothesized to drive the transition to alcohol dependence by linking upstream PFC activity with downstream HPA function (Lu & Richardson, 2014). Importantly, individuals suffering from AUD are at a heightened risk of relapse drinking even after extended periods of successful abstinence. Alcohol-associated cues stimulate cortisol release in abstinent alcoholics (Fox, Bergquist, Hong, & Sinha, 2007; Sinha et al., 2009), suggesting that cortisol contributes to a conditioned, appetitive response to promote relapse. Interestingly, the same patients are less responsive to stress cue-induced cortisol induction than healthy controls, further indicative of a blunted HPA stress responsiveness in AUD. Central brain stress systems appear also to be regulated by glucocorticoid signaling during relapse-like behavior. Systemic GR antagonism with mifepristone reduces reinstatement to alcohol seeking produced by the chemical stressor yohimbine, and this effect is recapitulated by microinjection of mifepristone directly into the CeA (Simms, Haass-Koffler, Bito-Onon, Li, & Bartlett, 2012).
Neuroendocrine tolerance to alcohol
The repeated activation of the HPA axis by chronic alcohol drinking appears to produce a type of neuroendocrine tolerance, as glucocorticoid response to alcohol tends to be inversely related to alcohol drinking history. For example, Richardson, Lee, O'Dell, Koob, & Rivier (2008) discovered that low-drinking rats exhibited a higher corticosterone response to alcohol challenge vs. moderate-drinking (but non-dependent) animals, with alcohol-dependent rats displaying a severely dampened corticosterone response. These effects were observed despite all animals reaching similar blood alcohol levels following the alcohol challenge, and were instead attributed to reductions in CRF levels in the paraventricular nucleus (PVN) of the hypothalamus that correlated with levels of alcohol exposure. Importantly, these results suggest that neuroendocrine deficits could be produced by episodes of heavy or binge drinking even before the transition to dependence, and this condition may even interact with low baseline HPA axis function in genetically predisposed individuals with a family history of alcoholism (Gianoulakis, Dai, Thavundayil, & Brown, 2005). Finally, a dampened neuroendocrine state is observed to last well into protracted abstinence in post-dependent animals (Zorrilla, Valdez, & Weiss, 2001), and may either strengthen the drive to escalate alcohol intake to compensate for its attenuated ability to activate the HPA axis or enhance the incentive salience of alcohol-paired cues that continue to stimulate corticosterone release as discussed above.
Glucocorticoid regulation of alcohol dependence
The development of animal models of alcohol dependence has driven the preclinical testing of hypotheses related to AUD development and expression (Gilpin & Koob, 2008; Gilpin, Richardson, Cole, & Koob, 2008). With the use of these preclinical models, multiple novel pharmacological targets have been revealed to target excessive drinking and alleviation of dependence-related conditions (Vendruscolo & Roberts, 2014). Rodents made dependent on alcohol via chronic, intermittent alcohol vapor exposure typically escalate their alcohol intake at withdrawal times associated with a spectrum of somatic and motivational symptoms of dependence (i.e., 6–10 h after the termination of alcohol vapor, when blood alcohol levels are diminished to near zero), whereas moderate levels of alcohol consumption are displayed by their non-dependent littermates. Under most experimental designs, researchers have described a reduction of elevated drinking in postdependent animals at this withdrawal time point via pharmacological intervention, revealing fundamental mechanisms that are responsible for the maintenance of dependent drinking (for review, see Vendruscolo & Roberts, 2014). In comparison, a select few studies have attempted to uncover the neurobiology associated with the development of escalated drinking. For example, Roberto and colleagues (2010) significantly attenuated the otherwise gradual increases in drinking in rats exposed to alcohol vapor via systemic, prophylactic treatment with a CRF receptor 1 (CRF1R) antagonist administered repeatedly before self-administration sessions. This study extended previous findings that established a role for CRF systems in driving alcohol dependence-related symptomatology (Funk, O'Dell, Crawford, & Koob, 2006; Heilig & Koob, 2007; Richardson, Zhao, et al., 2008), while also leaving open the question of which neurobiological mechanisms drive increased CRF expression to promote drinking escalation.
Given the regulation of CRF gene transcription by GRs, Vendruscolo and colleagues (2012) determined the effects of continuous, systemic blockade of GRs with mifepristone during the development of alcohol dependence in rats. Continuous release of subcutaneous mifepristone completely and specifically abolished the escalation of alcohol drinking in dependent animals, indicating a critical role for GR transcriptional mechanisms in the establishment of excessive drinking. Interestingly, alcohol-dependent rats exhibited significant downregulation of GR expression in cortical and limbic regions, a result interpreted as a mechanism to partially counteract excessive HPA axis activation by chronic, intermittent alcohol exposure. Additionally, a rebound effect was observed during protracted abstinence (at least 3 weeks after rats were removed from alcohol exposure). At this later withdrawal time point, GR levels were increased in central brain regions (including the CeA, bed nucleus of the stria terminalis, and nucleus accumbens) that mediate relapse-like behaviors (Edwards & Koob, 2010) in alcohol-dependent rats. Consistent with the previous study, continuous mifepristone delivery normalized excessive drinking observed in dependent rats during protracted abstinence. Overall, these findings suggest that GR signaling is exacerbated during alcohol dependence and that dependent individuals may be more sensitive to glucocorticoid-induced relapse mechanisms even long into abstinence.
Potentiation of brain stress systems by alcohol
The intimate interaction between glucocorticoids and CRF in the regulation of stress signaling is partly mediated by the negative feedback mechanisms at the level of the HPA axis as described above. However, circulating glucocorticoids also appear to sensitize signaling in central stress circuitry, seemingly in opposition to its effects to dampen neuroendocrine mechanisms. For example, in contrast to reducing CRF expression in the PVN, high corticosterone levels increase CRF gene expression in the central amygdala (CeA; Makino, Gold, & Schulkin, 1994; Shepard, Barron, & Myers, 2000). As mentioned above, a host of studies has implicated CeA CRF action in the manifestation of excessive drinking. In addition, CRF signaling regulates a variety of emergent alcohol abuse-related behaviors ranging from relapse to hyperalgesia (Edwards et al., 2012b; Gehlert et al., 2007; Guerrero, Ghoneim, Roberts, & Koob, 2012; Marinelli et al., 2007; Valdez et al., 2002), and many of these behaviors are driven by CRF action within the CeA (Egli, Koob, & Edwards, 2012; Weiss et al., 2001).
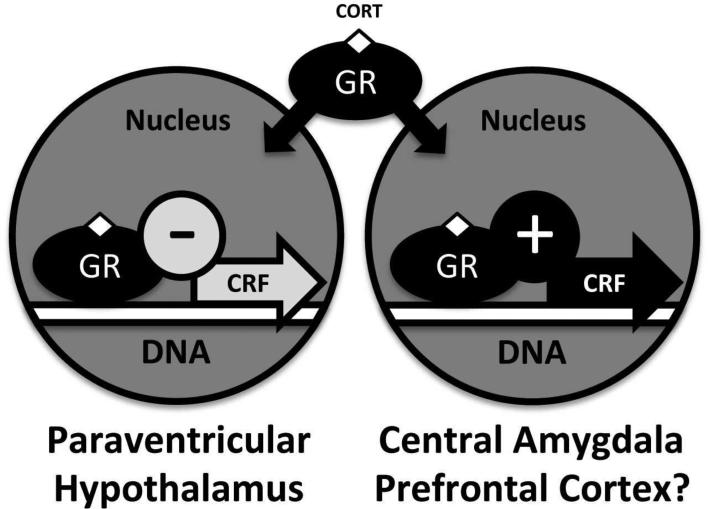
A key question is the way glucocorticoids produce a negative feedback at the HPA level but engender a positive feedback cycle within the CeA. Following glucocorticoid binding, the process of intracellular GR trafficking and nuclear gene expression is regulated by a host of chaperones and functional co-factors that could present a differential regulatory milieu between the hypothalamus and CeA (Fig. 1). One key regulatory molecule for GR efficacy is steroid receptor co-activator 1 (SRC-1), a histone acetyltransferase (Spencer et al., 1997). Interestingly, mice with a genetic deletion of SRC-1 fail to display natural changes in CRF gene expression after administration of dexamethasone (a synthetic glucocorticoid) in either the PVN or CeA (Lachize et al., 2009). That is, both reductions in PVN CRF mRNA and increases in CeA CRF mRNA following GR activation are dependent on SRC-1, suggesting a key role for this epigenetic factor in the differential response to stress (i.e., tolerance vs. sensitization) between the hypothalamic and extra-hypothalamic systems.
Fig. 1.
Distinct regulatory environments exist for glucocorticoid receptor (GR)-mediated gene expression in the hypothalamic paraventricular nucleus and the central amygdala (CeA). Specific epigenetic elements (e.g., steroid receptor co-activator 1, SRC-1) drive corticotropin-releasing factor (CRF) expression in opposite directions within these two stress-regulatory regions, mediating a negative feedback along the hypothalamic-pituitary-adrenal (HPA) axis but a positive feedback in the CeA. In combination with alterations in GR activity within the prefrontal cortex (PFC) that may promote cognitive disruption in heavy drinkers, these neuroadaptations in glucocorticoid signaling are hypothesized to drive the transition from recreational alcohol drinking to alcohol-use disorder (AUD).
Glucocorticoid regulation of alcohol's effects on cognition and neurodegeneration
In addition to the development of pathological motivational states that drive the urge to drink, repeated binge or excessive alcohol use produces substantial and debilitating cognitive deficits (Hunt, 1993; Persidsky et al., 2011; Riege, Holloway, & Kaplan, 1981; Zorumski, Mennerick, & Izumi, 2014). Although some of these effects are due to alcohol-related thiamine deficiency (Korsakoff's syndrome), evidence also points to a causative role for brain glucocorticoids in the exacerbation of neurocognitive deficits in alcoholism (Prendergast & Little, 2007; Rose, Shaw, Prendergast, & Little, 2010). Importantly, high cortisol levels during alcohol withdrawal correlated with the severity of cognitive problems in withdrawn alcoholics (Errico, King, Lovallo, & Parsons, 2002), while treatment with the GR antagonist mifepristone during acute withdrawal persistently reduced memory deficits observed in mice during later abstinence (Jacquot et al., 2008). Similar to its role in driving excessive alcohol drinking, a potentiation of CRF systems by GR signaling may underlie cognitive impairment during alcohol withdrawal (George, Sanders, et al., 2012).
In accordance with its deleterious effects on cognition, heavy alcohol use is also well known to produce direct neuronal damage, particularly within regions susceptible to excitotoxicity such as the hippocampus and prefrontal cortex. These regions also exhibit significant neurogenesis and gliogenesis (respectively) that is reduced by excessive alcohol exposure (Nixon & Crews, 2002; Richardson et al., 2009; Taffe et al., 2010). Corticosterone facilitates the effects of alcohol withdrawal to damage hippocampal slices (Mulholland et al., 2005), likely via enhancement of glutamatergic signaling (Abrahám, Harkany, Horvath, & Luiten, 2001; Butler, Berry, Sharrett-Field, Pauly, & Prendergast, 2013) that is already potentiated via chronic and intermittent alcohol exposure (Becker, Veatch, & Diaz-Granados, 1998). Importantly, treatment with mifepristone confers neuroprotection in the hippocampal dentate gyrus of binge alcohol-treated animals (Cippitelli et al., 2014), a brain region that displays robust neurogenesis (Mandyam, 2013). Together with mifepristone's demonstrated ability to reduce somatic symptoms of alcohol withdrawal in rats (Sharrett-Field, Butler, Berry, Reynolds, & Prendergast, 2013), these data suggest that GR antagonism would be capable of treating a full range of AUD symptoms.
Conclusions and future directions
In response to excessive drinking, a dysregulation of glucocorticoid function promotes neurocognitive deficits while locking individuals into dependent states by incentivizing the pursuit of alcohol to alleviate the negative motivational symptoms of withdrawal. The myriad effects of glucocorticoids on brain and HPA axis function contribute to AUD being a quintessential neurodegenerative and psychiatric disease.
As a transcription factor, dysregulation of GR activity would be expected to modulate a wide variety of neurotransmitter systems that regulate stress and alcohol use (Koob, 2009; Silberman et al., 2009). For example, it is noteworthy that in addition to CRF, the role of GR in the modulation of vasopressin gene expression and function may explain some of the effects of glucocorticoid dysregulation on stress responsiveness and alcohol dependence (Daubert, Looney, Clifton, Cho, & Scheuer, 2014; Kim, Summer, Wood, & Schrier, 2001; Rabadan-Diehl & Aguilera, 1998; Zhou & Kreek, 2014). Along with CRF, vasopressin is present in parvocellular PVN neurons and acts as a co-regulator of the HPA axis (Sawchenko, Swanson, & Vale, 1984; Whitnall, Mezey, & Gainer, 1985) through its actions on V1b receptors. Glucocorticoids exert a negative feedback on PVN vasopressin release (Liu, Wang, & Chen, 1995), while central brain vasopressin circuitry promotes a host of anxiety- and depression-like behaviors (Salomé, Stemmellin, Cohen, & Griebel, 2006; Stemmelin, Lukovic, Salome, & Griebel, 2005). Importantly, vasopressin acting through V1b receptors has been demonstrated to participate in the manifestation of excessive drinking in alcohol-dependent and alcohol-preferring rats (Edwards et al., 2012a; Zhou et al., 2011).
AUD is frequently associated with the use of commonly abused drugs such as nicotine (Little, 2000; Leão et al., 2015). Like virtually all drugs of abuse, nicotine both stimulates the HPA axis (Armario, 2010) and potentiates CRF systems in the CeA during dependence (Cohen et al., 2013; George et al., 2007). The restoration of adaptive glucocorticoid signaling may therefore represent a simple and comprehensive treatment strategy for drug and alcohol co-dependence, along with a host of psychiatric conditions co-morbid with AUD (Pettinati, O'Brien, & Dundon, 2013). In this regard, the demonstrated efficacy of the GR antagonist mifepristone in psychiatric disease and neurocognitive disorders (DeBattista & Belanoff, 2006) warrants additional investigation into its beneficial use in AUD populations that may suffer from these devastating conditions.
Finally, the interaction of glucocorticoids in so many areas of stress signaling has also been suggested to play a role in resilience factors against psychiatric disease (Srinivasan, Shariff, & Bartlett, 2013). For example, differential cortisol levels and responsiveness contribute to individual vulnerability in the development of PTSD (Whitaker, Gilpin, & Edwards, 2014), while GR-associated cofactors such as FKBP51 appear to influence affective disorder development and even medication responsiveness in individuals (Galigniana et al., 2012; Wilker et al., 2014). Animal models of PTSD have demonstrated both the central role of GR signaling in the delineation of resilient vs. susceptible individuals (Daskalakis, Cohen, Cai, Buxbaum, & Yehuda, 2014), as well as the heightened propensity for escalation of alcohol drinking in traumatic stress-sensitive populations (Edwards et al., 2013). Further study into the role of individual differences in glucocorticoid signaling in relation to AUD may provide better insight into individualized therapies (e.g., pharmacogenetic strategies) and also help return us to the original conceptualizations of adaptive versus maladaptive stress in health and disease as intended by Selye and colleagues.
Acknowledgments
Preparation of this review was supported by the National Institute on Alcohol Abuse and Alcoholism (AA020839, SE) and the National Institute on Drug Abuse, Intramural Research Program (LFV). We thank Dr. Marisa Roberto and all of the individuals who allowed us to share our work at the 2014 Alcoholism and Stress Conference in Volterra, Italy (supported by AA017581).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahám IM, Harkany T, Horvath KM, Luiten PG. Action of glucocorticoids on survival of nerve cells: promoting neurodegeneration or neuroprotection? Journal of Neuroendocrinology. 2001;13:749–760. doi: 10.1046/j.1365-2826.2001.00705.x. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Iranmanesh A, Veldhuis J, Fisher L. Disturbances of the stress response: the role of the HPA axis during alcohol withdrawal and abstinence. Alcohol Health and Research World. 1998;22:67–72. [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Martin PR, Bone GH, Eckardt MJ, Roehrich L, George DT, et al. Hypothalamic-pituitary-adrenal axis functioning and cerebrospinal fluid corticotropin releasing hormone and corticotropin levels in alcoholics after recent and long-term abstinence. Archives of General Psychiatry. 1990;47:325–330. doi: 10.1001/archpsyc.1990.01810160025004. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Ruether K, Krebaum S, Iranmanesh A, Williams MJ. Increased salivary cortisol concentrations during chronic alcohol intoxication in a naturalistic clinical sample of men. Alcoholism: Clinical and Experimental Research. 2003;27:1420–1427. doi: 10.1097/01.ALC.0000087581.13912.64. [DOI] [PubMed] [Google Scholar]
- Armario A. Activation of the hypothalamic-pituitary-adrenal axis by addictive drugs: different pathways, common outcome. Trends in Pharmacological Sciences. 2010;31:318–325. doi: 10.1016/j.tips.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Becker HC. Effects of alcohol dependence and withdrawal on stress responsiveness and alcohol consumption. Alcohol Research. 2012;34:448–458. [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Veatch LM, Diaz-Granados JL. Repeated ethanol withdrawal experience selectively alters sensitivity to different chemoconvulsant drugs in mice. Psychopharmacology (Berl) 1998;139:145–153. doi: 10.1007/s002130050699. [DOI] [PubMed] [Google Scholar]
- Butler TR, Berry JN, Sharrett-Field LJ, Pauly JR, Prendergast MA. Long-term ethanol and corticosterone co-exposure sensitize the hippocampal ca1 region pyramidal cells to insult during ethanol withdrawal in an NMDA GluN2B subunit-dependent manner. Alcoholism: Clinical and Experimental Research. 2013;37:2066–2073. doi: 10.1111/acer.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K, Little HJ. Effects of corticosterone on excitatory amino acid responses in dopamine-sensitive neurons in the ventral tegmental area. Neuroscience. 1999;88:837–845. doi: 10.1016/s0306-4522(98)00264-4. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Hamelink C, Brunnquell M, Thorsell A, Heilig M, et al. Binge-like ethanol consumption increases corticosterone levels and neurodegeneration whereas occupancy of type II glucocorticoid receptors with mifepristone is neuroprotective. Addiction Biology. 2014;19:27–36. doi: 10.1111/j.1369-1600.2012.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Treweek J, Edwards S, Leão RM, Schulteis G, Koob GF, et al. Extended access to nicotine leads to a CRF1 receptor dependent increase in anxiety-like behavior and hyperalgesia in rats. Addiction Biology. 2013;20:56–68. doi: 10.1111/adb.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis NP, Cohen H, Cai G, Buxbaum JD, Yehuda R. Expression profiling associates blood and brain glucocorticoid receptor signaling with trauma-related individual differences in both sexes. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:13529–13534. doi: 10.1073/pnas.1401660111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubert DL, Looney BM, Clifton RR, Cho JN, Scheuer DA. Elevated corticosterone in the dorsal hindbrain increases plasma norepinephrine and neuropeptide Y, and recruits a vasopressin response to stress. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2014;307:R212–224. doi: 10.1152/ajpregu.00326.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBattista C, Belanoff J. The use of mifepristone in the treatment of neuropsychiatric disorders. Trends in Endocrinology and Metabolism. 2006;17:117–121. doi: 10.1016/j.tem.2006.02.006. [DOI] [PubMed] [Google Scholar]
- DSM-5 . American psychiatric association: diagnostic and statistical manual of mental disorders Fifth edition. American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- Edwards S, Baynes BB, Carmichael CY, Zamora-Martinez ER, Barrus M, Koob GF, et al. Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Translational Psychiatry. 2013;3:e296. doi: 10.1038/tp.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Guerrero M, Ghoneim OM, Roberts E, Koob GF. Evidence that vasopressin V1b receptors mediate the transition to excessive drinking in ethanol-dependent rats. Addiction Biology. 2012a;17:76–85. doi: 10.1111/j.1369-1600.2010.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Koob GF. Neurobiology of dysregulated motivational systems in drug addiction. Future Neurology. 2010;5:393–401. doi: 10.2217/fnl.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE, et al. Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: alleviation by CRF1 receptor antagonism. Neuropharmacology. 2012b;62:1142–1151. doi: 10.1016/j.neuropharm.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M, Koob GF, Edwards S. Alcohol dependence as a chronic pain disorder. Neuroscience and Biobehavioral Reviews. 2012;36:2179–2192. doi: 10.1016/j.neubiorev.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis FW. Effect of ethanol on plasma corticosterone levels. The Journal of Pharmacology and Experimental Therapeutics. 1966;153:121–127. [PubMed] [Google Scholar]
- Errico AL, King AC, Lovallo WR, Parsons OA. Cortisol dysregulation and cognitive impairment in abstinent male alcoholics. Alcoholism: Clinical and Experimental Research. 2002;26:1198–1204. doi: 10.1097/01.ALC.0000025885.23192.FF. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Hansen S. Effect of local intracerebral corticosterone implants on alcohol intake in the rat. Alcohol and Alcoholism. 1999;34:851–861. doi: 10.1093/alcalc/34.6.851. [DOI] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcoholism: Clinical and Experimental Research. 2007;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Funk CK, O'Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. The Journal of Neuroscience. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galigniana NM, Ballmer LT, Toneatto J, Erlejman AG, Lagadari M, Galigniana MD. Regulation of the glucocorticoid response to stress-related disorders by the Hsp90-binding immunophilin FKBP51. Journal of Neurochemistry. 2012;122:4–18. doi: 10.1111/j.1471-4159.2012.07775.x. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Lê AD, Hipskind PA, Hamdouchi C, et al. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl imidazo[1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. The Journal of Neuroscience. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, et al. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Le Moal M, Koob GF. Allostasis and addiction: role of the dopamine and corticotropin-releasing factor systems. Physiology & Behavior. 2012;106:58–64. doi: 10.1016/j.physbeh.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, et al. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:18156–18161. doi: 10.1073/pnas.1116523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianoulakis C, Dai X, Thavundayil J, Brown T. Levels and circadian rhythmicity of plasma ACTH, cortisol, and beta-endorphin as a function of family history of alcoholism. Psychopharmacology (Berl) 2005;181:437–444. doi: 10.1007/s00213-005-0129-x. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Koob GF. Neurobiology of alcohol dependence: focus on motivational mechanisms. Alcohol Research & Health. 2008;31:185–195. [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF. Vapor inhalation of alcohol in rats. Current Protocols in Neuroscience. 2008 doi: 10.1002/0471142301.ns0929s44. Chapter 9, Unit 9.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin R. Peptides in the brain: the new endocrinology of the neuron. Science. 1978;202:390–402. doi: 10.1126/science.212832. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends in Neurosciences. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt WA. Are binge drinkers more at risk of developing brain damage? Alcohol. 1993;10:559–561. doi: 10.1016/0741-8329(93)90083-z. [DOI] [PubMed] [Google Scholar]
- Jacquot C, Croft AP, Prendergast MA, Mulholland P, Shaw SG, Little HJ. Effects of the glucocorticoid antagonist, mifepristone, on the consequences of withdrawal from long term alcohol consumption. Alcoholism: Clinical and Experimental Research. 2008;32:2107–2116. doi: 10.1111/j.1530-0277.2008.00799.x. [DOI] [PubMed] [Google Scholar]
- Kim JK, Summer SN, Wood WM, Schrier RW. Role of glucocorticoid hormones in arginine vasopressin gene regulation. Biochemical and Biophysical Research Communications. 2001;289:1252–1256. doi: 10.1006/bbrc.2001.6114. [DOI] [PubMed] [Google Scholar]
- Koenig HN, Olive MF. The glucocorticoid receptor antagonist mifepristone reduces ethanol intake in rats under limited access conditions. Psychoneuroendocrinology. 2004;29:999–1003. doi: 10.1016/j.psyneuen.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56(Suppl 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, et al. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76(Pt B):370–382. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachize S, Apostolakis EM, van der Laan S, Tijssen AM, Xu J, de Kloet ER, et al. Steroid receptor coactivator-1 is necessary for regulation of corticotropin-releasing hormone by chronic stress and glucocorticoids. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8038–8042. doi: 10.1073/pnas.0812062106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leão RM, Cruz FC, Vendruscolo LF, de Guglielmo G, Logrip ML, Planeta CS, et al. Chronic nicotine activates stress/reward-related brain regions and facilitates the transition to compulsive alcohol drinking. The Journal of Neuroscience. 2015;35:6241–6253. doi: 10.1523/JNEUROSCI.3302-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moal M, Koob GF. Drug addiction: pathways to the disease and pathophysiological perspectives. European Neuropsychopharmacology. 2007;17:377–393. doi: 10.1016/j.euroneuro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Little HJ. Behavioral mechanisms underlying the link between smoking and drinking. Alcohol Research & Health. 2000;24:215–224. [PMC free article] [PubMed] [Google Scholar]
- Little HJ, Croft AP, O'Callaghan MJ, Brooks SP, Wang G, Shaw SG. Selective increases in regional brain glucocorticoid: a novel effect of chronic alcohol. Neuroscience. 2008;156:1017–1027. doi: 10.1016/j.neuroscience.2008.08.029. [DOI] [PubMed] [Google Scholar]
- Little HJ, O'Callaghan MJ, Butterworth AR, Wilson J, Cole J, Watson WP. Low alcohol preference among the “high alcohol preference” C57 strain of mice; preference increased by saline injections. Psychopharmacology (Berl) 1999;147:182–189. doi: 10.1007/s002130051159. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang CA, Chen YZ. Nongenomic effect of glucocorticoid on the release of arginine vasopressin from hypothalamic slices in rats. Neuroendocrinology. 1995;62:628–633. doi: 10.1159/000127059. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Zorrilla EP. Stress history increases alcohol intake in relapse: relation to phosphodiesterase 10A. Addiction Biology. 2012;17:920–933. doi: 10.1111/j.1369-1600.2012.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YL, Richardson HN. Alcohol, stress hormones, and the prefrontal cortex: a proposed pathway to the dark side of addiction. Neuroscience. 2014;277:139–151. doi: 10.1016/j.neuroscience.2014.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Research. 1994;640:105–112. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- Mandyam CD. The Interplay between the Hippocampus and Amygdala in Regulating Aberrant Hippocampal Neurogenesis during Protracted Abstinence from Alcohol Dependence. Frontiers in Psychiatry. 2013;4:61. doi: 10.3389/fpsyt.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, et al. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2007;195:345–355. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological Reviews. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Archives of Internal Medicine. 1993;153:2093–2101. [PubMed] [Google Scholar]
- Mulholland PJ, Self RL, Harris BR, Little HJ, Littleton JM, Prendergast MA. Corticosterone increases damage and cytosolic calcium accumulation associated with ethanol withdrawal in rat hippocampal slice cultures. Alcoholism: Clinical and Experimental Research. 2005;29:871–881. doi: 10.1097/01.alc.0000163509.27577.da. [DOI] [PubMed] [Google Scholar]
- Myers B, McKlveen JM, Herman JP. Glucocorticoid actions on synapses, circuits, and behavior: implications for the energetics of stress. Frontiers in Neuroendocrinology. 2014;35:180–196. doi: 10.1016/j.yfrne.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. Journal of Neurochemistry. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Ho W, Ramirez SH, Potula R, Abood ME, Unterwald E, et al. HIV-1 infection and alcohol abuse: neurocognitive impairment, mechanisms of neurodegeneration and therapeutic interventions. Brain, Behavior and Immunity. 2011;25(Suppl 1):S61–70. doi: 10.1016/j.bbi.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinati HM, O'Brien CP, Dundon WD. Current status of co-occurring mood and substance use disorders: a new therapeutic target. The American Journal of Psychiatry. 2013;170:23–30. doi: 10.1176/appi.ajp.2012.12010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deroche V, Deminière JM, Maccari S, Le Moal M, Simon H. Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:11738–11742. doi: 10.1073/pnas.90.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast MA, Little HJ. Adolescence, glucocorticoids and alcohol. Pharmacology, Biochemistry, and Behavior. 2007;86:234–245. doi: 10.1016/j.pbb.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Rabadan-Diehl C, Aguilera G. Glucocorticoids increase vasopressin V1b receptor coupling to phospholipase C. Endocrinology. 1998;139:3220–3226. doi: 10.1210/endo.139.7.6121. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Chan SH, Crawford EF, Lee YK, Funk CK, Koob GF, et al. Permanent impairment of birth and survival of cortical and hippocampal proliferating cells following excessive drinking during alcohol dependence. Neurobiology of Disease. 2009;36:1–10. doi: 10.1016/j.nbd.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O'Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. The European Journal of Neuroscience. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Zhao Y, Fekete EM, Funk CK, Wirsching P, Janda KD, et al. MPZP: a novel small molecule corticotropin-releasing factor type 1 receptor (CRF1) antagonist. Pharmacology, Biochemistry, and Behavior. 2008;88:497–510. doi: 10.1016/j.pbb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riege WH, Holloway JA, Kaplan DW. Specific memory deficits associated with prolonged alcohol abuse. Alcoholism: Clinical and Experimental Research. 1981;5:378–385. doi: 10.1111/j.1530-0277.1981.tb04920.x. [DOI] [PubMed] [Google Scholar]
- Rivier C, Bruhn T, Vale W. Effect of ethanol on the hypothalamic-pituitary-adrenal axis in the rat: role of corticotropin-releasing factor (CRF). The Journal of Pharmacology and Experimental Therapeutics. 1984;229:127–131. [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, et al. Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biological Psychiatry. 2010;67:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AK, Shaw SG, Prendergast MA, Little HJ. The importance of glucocorticoids in alcohol dependence and neurotoxicity. Alcoholism: Clinical and Experimental Research. 2010;34:2011–2018. doi: 10.1111/j.1530-0277.2010.01298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomé N, Stemmelin J, Cohen C, Griebel G. Differential roles of amygdaloid nuclei in the anxiolytic- and antidepressant-like effects of the V1b receptor antagonist, SSR149415, in rats. Psychopharmacology (Berl) 2006;187:237–244. doi: 10.1007/s00213-006-0424-1. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, Vale WW. Co-expression of corticotropin-releasing factor and vasopressin immunoreactivity in parvocellular neurosecretory neurons of the adrenalectomized rat. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:1883–1887. doi: 10.1073/pnas.81.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye H. Stress and the general adaptation syndrome. British Medical Journal. 1950;1:1383–1392. doi: 10.1136/bmj.1.4667.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrett-Field L, Butler TR, Berry JN, Reynolds AR, Prendergast MA. Mifepristone pretreatment reduces ethanol withdrawal severity in vivo. Alcoholism: Clinical and Experimental Research. 2013;37:1417–1423. doi: 10.1111/acer.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Research. 2000;861:288–295. doi: 10.1016/s0006-8993(00)02019-9. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Bajo M, Chappell AM, Christian DT, Cruz M, Diaz MR, et al. Neurobiological mechanisms contributing to alcohol-stress-anxiety interactions. Alcohol. 2009;43:509–519. doi: 10.1016/j.alcohol.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Haass-Koffler CL, Bito-Onon J, Li R, Bartlett SE. Mifepristone in the central nucleus of the amygdala reduces yohimbine stress-induced reinstatement of ethanol-seeking. Neuropsychopharmacology. 2012;37:906–918. doi: 10.1038/npp.2011.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou J, Mizzen CA, et al. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Shariff M, Bartlett SE. The role of the glucocorticoids in developing resilience to stress and addiction. Frontiers in Psychiatry. 2013;4:68. doi: 10.3389/fpsyt.2013.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmelin J, Lukovic L, Salome N, Griebel G. Evidence that the lateral septum is involved in the antidepressant-like effects of the vasopressin V1b receptor antagonist, SSR149415. Neuropsychopharmacology. 2005;30:35–42. doi: 10.1038/sj.npp.1300562. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Jafee RC, Ritzmann RF. Corticosterone concentrations in mice during ethanol drinking and withdrawal. The Journal of Pharmacy and Pharmacology. 1978;30:371–374. doi: 10.1111/j.2042-7158.1978.tb13259.x. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Kotzebue RW, Crean RD, Crawford EF, Edwards S, Mandyam CD. Long-lasting reduction in hippocampal neurogenesis by alcohol consumption in adolescent nonhuman primates. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11104–11109. doi: 10.1073/pnas.0912810107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Heegde F, De Rijk RH, Vinkers CH. The brain mineralocorticoid receptor and stress resilience. Psychoneuroendocrinology. 2015;52:92–110. doi: 10.1016/j.psyneuen.2014.10.022. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, et al. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcoholism: Clinical and Experimental Research. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr., Logrip ML, et al. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. The Journal of Neuroscience. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Roberts AJ. Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol. 2014;48:277–286. doi: 10.1016/j.alcohol.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, et al. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Annals of the New York Academy of Sciences. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Whitaker AM, Gilpin NW, Edwards S. Animal models of post-traumatic stress disorder and recent neurobiological insights. Behavioural Pharmacology. 2014;25:398–409. doi: 10.1097/FBP.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitnall MH, Mezey E, Gainer H. Co-localization of corticotropin-releasing factor and vasopressin in median eminence neurosecretory vesicles. Nature. 1985;317:248–250. doi: 10.1038/317248a0. [DOI] [PubMed] [Google Scholar]
- Wilker S, Pfeiffer A, Kolassa S, Elbert T, Lingenfelder B, Ovuga E, et al. The role of FKBP5 genotype in moderating long-term effectiveness of exposure-based psychotherapy for posttraumatic stress disorder. Translational Psychiatry. 2014;4:e403. doi: 10.1038/tp.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Colombo G, Carai MA, Ho A, Gessa GL, Kreek MJ. Involvement of arginine vasopressin and V1b receptor in alcohol drinking in Sardinian alcohol-preferring rats. Alcoholism: Clinical and Experimental Research. 2011;35:1876–1883. doi: 10.1111/j.1530-0277.2011.01532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Kreek MJ. Alcohol: a stimulant activating brain stress responsive systems with persistent neuroadaptation. Neuropharmacology. 2014;87:51–58. doi: 10.1016/j.neuropharm.2014.05.044. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Mennerick S, Izumi Y. Acute and chronic effects of ethanol on learning-related synaptic plasticity. Alcohol. 2014;48:1–17. doi: 10.1016/j.alcohol.2013.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]