Abstract
Research with the rhesus macaque population on Cayo Santiago can provide a unique perspective on the costs of sociality and reproduction in primates. Because the Cayo macaques live in unusually large groups and in a predator-free environment, in which their artificial food source lacks seasonal variation in abundance or quality, these monkeys constitute a semi-experimental study of the costs and benefits of group living. Here we review several long- and short-term studies that have focused on female life history and stress physiology. Long-term demographic data have shown that rhesus macaque females of middle- and low-ranking matrilines have lower adult survival probabilities than females of high-ranking matrilines. Costs of reproductive effort are also evident: adult females were more likely to die during the birth than during the mating season and they experienced higher cortisol levels when lactating. Lower-ranking females, in particular, experienced greater relative increase in cortisol production during lactation, in comparison to middle- and high-ranking females. Older high-ranking females had lower plasma cortisol levels than younger ones but cortisol levels were similarly high among young and old middle- and low-ranking females. Higher plasma cortisol levels and/or fecal glucocorticoid concentrations are associated with higher plasma concentrations of some proinflammatory cytokines. High cortisol, in turn, may be associated with chronic inflammation, and perhaps also with immunosuppression. In sum, the studies reviewed here provide multiple lines of evidence that sociality and reproductive effort impose measurable costs on female rhesus macaques. In line with socioecological theory, female dominance rank consistently emerges as an important modulator of variation in female life histories and physiology. The Cayo Santiago macaques are therefore a valuable model for elucidating the mechanisms by which within-group competition and reproduction impact health and survival in nonhuman primates and in humans. Am. J. Primatol.
Keywords: social evolution, competition, reproduction, stress, cortisol, rhesus macaques
Introduction
Measurements of cortisol or glucocorticoid metabolite concentrations in blood, urine, feces, saliva, or hair have been used widely in primatological research, both in captivity and in the wild, to address questions relating to the overall health, energetic status, and psychological wellbeing of individual primates in terms of their social and ecological environments [Abbott et al., 2003; Muehlenbein, 2006; Gesquiere et al., 2011; Jaimez et al., 2011; Foerster et al., 2012; Gómez-Espinosa et al., 2013; Novak et al., 2013; Fürtbauer et al., 2014]. In this review article we discuss how measurements of the physiological stress response from cortisol in the blood plasma and from glucocorticoid metabolites excreted in the feces have informed our understanding of the costs of sociality and reproduction of free-ranging female rhesus macaques on Cayo Santiago. In our review, we first draw on results of several studies that utilized long-term records of the Cayo Santiago colony to examine large-scale patterns of survivorship, mortality and fertility of female rhesus macaques [Hoffman et al., 2008; Blomquist, 2009a; Blomquist et al., 2011]. We then review several shorter-term studies of stress-related physiology of Cayo females conducted by our lab [Maestripieri et al., 2008; Hoffman et al., 2010a; 2011] to elucidate the proximate factors that may account for the patterns observed in the long-term demographic data. Finally, we will extend some of the analyses, published in our previous studies with data on females that have only recently been collected. Our motivation in undertaking this research and in providing its synthesis here was to place inter-individual variation in female stress physiology within the context of primate socio-ecological theory. Specifically, our aim was to furnish a mechanistic understanding of how various costs of group life may act to counter the benefits of sociality, and whether the cost/benefit ratio of group life differs for individuals at different ends of the dominance hierarchy in a despotic female primate. We thus begin with a brief overview of the benefits and costs hypothesized to affect primates living in groups.
Benefits and Costs of Life and Reproduction in Large Primate Groups
Many species of nonhuman primates live in large groups with multiple adult males and females and with immature individuals of various ages. In cercopithecine monkeys such as macaques and baboons females are typically the philopatric sex, while males disperse from their natal group at puberty [Cords, 2012]. As a result of these patterns of sex-biased philopatry and dispersal, groups have a matrilineal structure, consisting of multiple generations of female relatives, while the adult males are typically unrelated to the adult females and to each other. Within these groups, mating is promiscuous and male-male competition is typically intense; females produce one infant at a time and invest heavily in their offspring, often for many years, with little or no help from males.
Ecological explanations for the evolution of primate sociality emphasize the benefits of group-living for protection against predators and/or for the acquisition and the cooperative defense of food [Wrangham, 1980; van Schaik, 1983; Sterck et al., 1997]. As energy availability is a key determinant of female reproductive success [Emery Thompson, 2013], the benefits of group-living for female primates, according to the food-defense hypothesis, entail not only increased survival, but also higher reproductive rates and higher offspring survivorship [Crofoot & Wrangham, 2009]. Being part of a social network within a group in itself can be beneficial to a female. Over the short-term, among rhesus macaques, higher-ranking females had lower glucocorticoid levels during months in which their proximity networks (a measure of spatial association with other monkeys in their group) were smaller and thus more focused [Brent et al., 2011]. Over the long-term, more socially connected female baboons have been shown to experience enhanced offspring survivorship and increased lifespan [Silk et al., 2003; 2009; 2010; Archie et al., 2014].
Ecological theories for the evolution of group-living address not only the benefits of sociality but also its costs. In addition to the increased risk of infectious disease transmission [Altizer et al., 2003; Rifkin et al., 2012], a major cost of group-living is the increased competition for resources among group members, both within and between sexes. When group size becomes too large, the costs of group living may cancel out or even outweigh its benefits [Majolo et al., 2008; Roberts & Cords, 2013]. Females compete with other females for access to food for themselves and their offspring, as well as for access to desirable mating partners who may provide direct (protection, paternal care) or indirect (genetic) benefits [Stockley & Bro-Jørgensen, 2011]. Finally, sexual coercion, which is costly for the targeted females [Smuts & Smuts, 1993; Clutton-Brock & Parker, 1995; Muller et al., 2007], may also be expected to increase in a group setting, as males would be under more persistent pressure to monopolize mating opportunities from multiple competitors [Muller & Wrangham, 2009].
Since continuous fighting among group members would be highly costly to all parties involved, within-group competition in group-living cercopithecine monkeys, as well as other social primates, is often mediated via established differences in social status. Among males, such differences in status are sometimes but not always based on differences in individual fighting ability [van Noordwijk & van Schaik, 2004]. Among female cercopithecines, rank is usually acquired on the basis of hereditary rules [Holekamp & Smale, 1991]. While maintaining social status does involve some agonistic engagement, the existence of various formalized displays of submission serves to limit the use of such aggressive confrontations and thus minimize the potential costs to both the dominant and the subordinate animal [Kaufmann, 1983; de Waal & Luttrell, 1985; de Waal, 1986; Bercovitch, 1991; Maestripieri, 2005]. Clearly, the benefits of an established dominance hierarchy are greater for higher-ranking individuals, who would have priority of access to food and mates, but the reduction of the potential costs of violent confrontations are likely more significant for lower-ranking individuals. Structured dominance hierarchies may have thus evolved to minimize the costs of grouping by reducing risky fighting while allowing individuals to retain the overall benefits of predator protection and advantages in intergroup contest.
From the point of view of the lower-ranking individuals, within-group competition can decrease an individual's fitness in several ways: increase the risk of aggression resulting in injury or death for oneself or one's relatives (e.g. infanticide), reduce access to food (or to high-quality food, in particular), reduce the access to powerful social partners, reduce the access to mating partners in general and/or to specific optimal/preferred partners. Within-group competition, whether through direct aggression or via pre-established dominance relationships, can also generate chronic psychological stress in some individuals, and such stress can impair these individuals' ability to function effectively socially, to reproduce, or to mount an effective immune response against infection[Sapolsky, 2005]. Chronic stress can also directly reduce survival by impairing cardiovascular function or causing brain damage [Sapolsky, 1999; Steptoe & Kivimäki, 2013]. The effects of chronic stress on health, reproduction, and survival are presumably greater later in life, after the negative psychological, neuroendocrine, and cardiovascular consequences of stress have accumulated for many years. Subordinate individuals are not always the ones to suffer such costs disproportionately however; depending on the way in which dominance is established and maintained, the nature and frequency of stressors experienced by subordinates and the availability of social support to them – it can also be the higher-ranking individuals that experience chronic stress more strongly than their subordinates [Abbott et al., 2003; Goymann & Wingfield, 2004; Creel et al., 2012].
Thus, despite the significant benefits of group-living, life in a multi-male, multi-female primate social group is also costly for individuals, regardless of whether or not they reproduce. Reproduction brings about additional costs, which are usually different for males and females. For males, the most readily identifiable costs of reproduction are often associated with the increased risk of injury, infection and death that may result from aggressive male-male sexual contests. In a less dramatic fashion, but perhaps more pervasively, many male primates incur other costs such as time-allocation trade-offs that involve decreases in energy intake at times when they search for, follow, mate with, and guard estrous females [Emery et al., 2014]. Increases in energy expenditure due to elevated aggression and a resulting reduction of energy balance have also been identified as a important cost of mating effort in some multi-male, multi-female primates groups such as chimpanzees [Georgiev, 2012]. For females, energetic costs are key: attaining a positive energy balance is a pre-requisite for resumption of cycling, increasing the likelihood of conception, and maintaining sufficient investment into their offspring through the energetically costly phases of gestation and lactation [Emery Thompson, 2013].
The Cayo Santiago Rhesus Macaques as an Ideal Population for Investigating the Costs of Sociality and Reproduction
The rhesus macaque population on Cayo Santiago was established in 1938, by importing several hundred monkeys caught in different locations in India [Rawlins & Kessler, 1986], and currently includes approximately 1,300 individuals. There have been no imports of new animals and population growth has been managed through the selective removal of particular age/sex classes of individuals (most often juvenile males). Daily censuses of all individuals are conducted and all births and deaths are recorded. Accurate colony records have been maintained since the late 1950s by the Caribbean Primate Research Center of the University of Puerto Rico so that a large database with demographic and reproductive history information is now available [Hernández-Pacheco et al., 2013; Dubuc et al., 2014]. The monkeys are fed commercial monkey chow every morning and provided water ad libitum but also incorporate small and variable amounts of natural vegetation (leaves, grass, flowers, fruit) into their diet. The island is free of predators rhesus macaques typically encounter in their natural habitats such as large carnivores or raptors. The population was inoculated against tetanus in the mid-1980s [Kessler et al., 2006]. Otherwise, there has been a veterinary policy of nonintervention.
The rhesus macaques on Cayo Santiago live in naturally formed social groups. In recent years, the number of social groups has varied between six and ten, as a few extremely large groups have undergone a process of fission [Ruiz-Lambides et al., 2013]. The demographic composition of the rhesus groups and the typical life history of monkeys in this population is different from those of their wild counterparts, thus limiting the extrapolations that can be made from studies of social behavior and population dynamics in this population [Maestripieri & Hoffman, 2012]. Yet, in many ways, this is an ideal primate population in which to investigate the costs of living and reproducing in large groups and their underlying mechanisms, particularly with regard to stress.
Testing ecological theories for the evolution of group-living in primates requires a great deal of ecological data, particularly with regard to the hypothesized benefits of sociality for predation avoidance and/or for the cooperative acquisition and defense of food resources [Schulke & Ostner, 2012]. These ecological data are best obtained from studies of wild primate populations. Nevertheless, the semi-experimental set-up of the Cayo Santiago colony allows controlling for some ecological factors that in the wild would introduce considerable ‘noise’ in the data, if they were not the primary targets of investigation. Lack of predation and the absence of seasonal variation in food availability (because of daily provisioning with monkey chow) allow research on the costs and benefits of sociality to focus on the specific behavioral or physiological mechanisms through which competition and reproduction in large primate groups impose fitness costs on individuals. A recent example of this approach is a detailed study of feeding competition and dominance dynamics within and between three groups of different sizes (small, medium, and large) that took advantage of the controlled settings in which food and drinking water are available to monkeys on the island [Balasubramaniam et al., 2014]. This study tested some of the predictions of the primate socio-ecological model [Wrangham, 1980; Sterck et al., 1997] and showed that, in line with expectations, females in larger groups experienced more within-group contest competition but less between-group contest; conversely, females in smaller groups experienced higher between-group contest and were at a disadvantage from individuals from larger groups when attempting to access food and water at the provisioning stations [Balasubramaniam et al., 2014]. This study thus suggests that, despite being provisioned, the rhesus macaques of Cayo Santiago are similar to wild primate populations in that not all groups and not all individuals within groups have equal access to food. Group life clearly shapes the costs and benefits that apply to individuals within groups, regardless of whether they experience naturally-determined variation in food availability or such seasonal variation has been removed. Admittedly, the artificial nature of the feeding stations at Cayo Santiago (which can be viewed as the equivalent of super clumped, large fruiting trees, which produce large crops of high-quality ripe fruit every morning, year-round) probably exacerbates the levels of contest feeding competition, in comparison to what rhesus macaques experience in their natural habitat. However, it is precisely because of these ‘exaggerated’ conditions under which groups and individuals exist on Cayo Santiago that questions aiming to understand the benefits and costs of sociality become particularly tractable.
The extent to which agonism features in the daily interactions of monkeys on Cayo Santiago – both at the inter- and intra-group level (pers. obs.) - also reinforces the notion that competition in this population is intense. Cross-species comparisons show that rates of female-female aggression are higher in species with larger group sizes, in which the density of competitors is higher [Wheeler et al., 2013]. This suggests that because rhesus macaque groups are larger on Cayo Santiago, relative to those in the wild, aggression may be much more frequent among them. Groups have linear and rigid dominance hierarchies, and dominance relationships between individuals are generally despotic and intolerant [Maestripieri, 2007]. Acute and chronic stress associated with aggression and subordination can be easily identified behaviorally (e.g. victims of aggression screams and display constant vigilance and avoidance of higher-ranking individuals) and can be effectively measured physiologically, through the collection of blood and fecal samples and the assessment of endocrine or immune function.
Given the availability of food and the lack of predators, mortality is greatly reduced (e.g. life-spans are much longer) and reproductive rates are much higher when compared to wild primate populations. As a result, the chronic physical and psychological stress of subordination could be experienced by some individuals for 20 or more years, while the physical, energetic, and psychological stress associated with mating effort and reproduction can be repeated over many cycles. While it is remarkable that the rhesus macaques on Cayo Santiago live as long as they do and reproduce as much as they do relative to their wild counterparts, human observers cannot escape the impression that social life and reproduction in this population are neither easy nor cheap. This impression is confirmed by the results of many studies investigating inter-individual variation in survivorship, reproduction, behavior, and physiology.
The Costs of Sociality on Cayo Santiago: Matriline Dominance Rank, Female Survivorship, and Lifetime Fitness
Early age at first reproduction, short inter birth intervals, and higher infant survival are three key female traits that contribute to high lifetime reproductive success (LRS). All three traits are characteristic of high-ranking in females in a number of primates, and thus high dominance status is generally a good predictor of high LRS [Pusey, 2012]. Another major predictor of variation in LRS in female primates is longevity: females that live longer produce more offspring [Rhine et al., 2000; Robbins et al., 2011]. In the rhesus population on Cayo Santiago, females begin reproducing at 3–4 years of age and attain full adult body size at approximately 5–6 years of age. Maximum life span for females on Cayo was 31.4 years, while the median life span for mature adult females is 15 years of age [Hoffman et al., 2010b]. The relationship between life span length and lifetime reproductive success for rhesus females on Cayo has been clearly illustrated by Blomquist [Blomquist, 2009b], using the long-term colony database. As shown in Fig. 1a the longer the life span, the higher the number of offspring produced; some females who lived 25 years produced more than 15 infants.
Fig. 1.
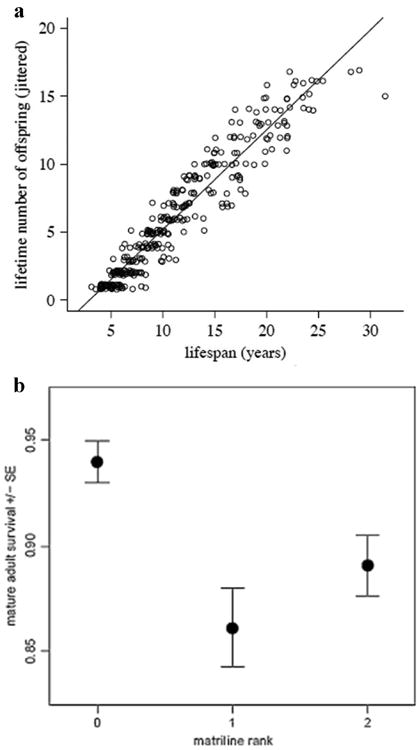
Factors affecting reproductive success and survival of adult female rhesus macaques on Cayo Santiago. (a) Regression of lifetime number of offspring on lifespan for (P< 0.0001, R2 = 0.91). Modified after Fig. 1. in Blomquist [2009]. (b) Mean (+ SE) of individual mature adult survival rates plotted by matriline rank category (0=high, 1=middle, 2=low). Modified after Fig. 4. in Blomquist [2011].
Whether social status predicts longevity, however, is not as clear. In species where rank increases with age, females who lived longer may have higher rank simply because they have had more time in which to achieve it [Robbins et al., 2011]. Some studies conducted with wild primate populations have provided data that support the prediction that high dominance status has survival benefits for adult females [Pusey, 2012]. Yet, the strongest evidence comes from the rhesus macaque population on Cayo Santiago, both because dominance rank in this species is maternally inherited [Chapais, 2004], and thus relatively independent of age, and because of the large dataset of births and deaths that exists for these macaques.
Using the Cayo Santiago long-term colony database, Blomquist and collaborators [Blomquist et al., 2011] examined the relationship between rank and female survivorship over a period of 40 years (1960–2000). In this study, matriline rank rather than individual rank, was used for data analyses. On Cayo Santiago, each group typically contains different matrilines, and within each matriline, closely-related individuals such as, mothers and daughters or sisters occupy adjacent ranks. All members of one matriline rank above or below all members of another matriline. As with individual rank, matriline rank relationships are transitive, so that if matriline A ranks above matriline B, and matriline B ranks above matriline C, then matriline A also ranks above matriline C. Matriline ranks are assigned after collecting data on aggression and submission in dyadic interactions within the group. Matriline ranks are easier to assign, and are generally more accurate than individual ranks, because they do not require the observation of agonistic interactions between all possible dyads of individuals within a group. Although matriline rank reversals have been frequently observed in captive colonies of rhesus macaques, where group fission is constrained by captivity [Ehardt & Bernstein, 1986; Oates-O'Brien et al., 2010], thus far they have only been documented once on Cayo Santiago [Ruiz-Lambides et al., 2013]; therefore, matriline ranks are generally stable over time and across generations.
In Blomquist et al.'s [2011] study, matriline ranks were considered high, middle, or low depending on whether they fell in the top third, medium third, or bottom third of the group's matriline hierarchy. Survival probabilities were calculated for different age/sex classes of individuals, including infants (< 1 year old), juvenile females (1–2 year old), young adult females (3–5 year old), and mature adult females (≥ 6 year old). Even though total life span length of adult females was not predicted significantly by social status, the use of elasticity path analysis allowed Blomquist et al. [2011] to almost double their sample size by using data from females who were still alive and calculate their annual survival rates.
The results showed that high-ranking mature females, their infants and juvenile offspring had greater survival probabilities, when compared to middle-ranking and low-ranking females (these two groups did not differ significantly from each other). The rank effect was greatest for mature adult females - high-ranking females had mature adult survival rates of 0.94, while middle-ranking had. 0.86, and low-ranking had 0.89 (Fig. 1b). A 6-year old female from a high-ranking matriline was thus expected to live, on average, for an additional 7.8 years, while a middle-ranking and low-ranking 6-year old females – only 3.1 and 4.1 years, respectively. As a result of these rank-related differences in adult survivorship, the high-ranking segments of the rhesus population on Cayo Santiago increased at greater rates than those with lower social status.
In wild primate populations, it may be argued that the effects of rank on adult survivorship or life span arise because higher ranking females benefit disproportionately from the advantages of group-living (e.g. they have better access food, or to high-quality food, and they enjoy better protection from predators due to the spatially central positions they tend to occupy within groups) when compared to lower ranking females. In contrast, in primate populations in which food shortage and predation are not significant causes of adult mortality, the effects of rank on female adult survivorship and life span length presumably reflect differences in the costs of sociality to a greater extent than differences in the benefits of sociality. In other words, lower-ranking females have lower survivorship because they suffer the costs of within-group agonistic competition (e.g. through receiving aggression, experiencing chronic stress associated with social subordination) more so than higher-ranking females. In this view, Blomquist et al.'s [2011] findings represent not only a demonstration that rank affects female lifetime reproductive success, but also a demonstration that group-living is costly and that the fitness costs of sociality are greater for lower-ranking than for higher-ranking females. Blomquist et al.'s study, however, provides no insight into the behavioral or physiological mechanisms through which these costs are expressed. Research in our laboratory has addressed this question and, in addition to the survival costs of group-living, has also examined the survival costs of reproduction.
Survival Costs of Reproduction: Sex Differences in Seasonal Patterns of Mortality
In the wild, rhesus macaques exhibit a distinct pattern of seasonal breeding, with mating concentrated in 4–5 months of the year, and a birth season that begins approximately 6 months after on set of the mating season. The timing of the birth season generally coincides with the period of the year in which fruit and other food resources are most likely to be available, although the onset of the mating season is regulated by changes in photoperiod, temperature, and rainfall [Vandenbergh & Vessey, 1968; Brockman & van Schaik, 2006]. Rhesus macaques maintain their pattern of seasonal breeding after translocation to novel environments, although the months of the year in which mating and births occur can differ across geographic locations [Varley & Vessey, 1977; Hoffman et al., 2008].
Given that rhesus macaques have a social and mating system in which male mating effort is energetically intense [Higham et al., 2011] and is associated with high frequency of wounding [Wilson & Boelkins, 1970] while females invest heavily in gestation and lactation, we hypothesized that if reproduction has any survival costs for the rhesus macaques on Cayo Santiago, these should be different for males and females [Hoffman et al., 2008]. For males, the costs of reproduction should arise mainly as a result of their mating effort, while for females the costs of reproduction should arise from their energetic investment towards gestation and lactation, the risks associated with parturition, and the psychosocial stress associated with motherhood in the early post-partum months. This hypothesis leads to the prediction that for adult males, mortality should be higher during the mating than during the birth season, while adult females should be more likely to die during the birth than during the mating season.
To provide a comprehensive test of this hypothesis, we investigated seasonal patterns of births and adult deaths in the Cayo Santiago rhesus macaque population over the period 1957–2005, using the long-term colony database [Hoffman et al., 2008]. For deaths, only sexually mature females and males that were at least four years old at their time of death were included in the data analyses. Similarities and differences in male and female mortality in the population were assessed by comparing monthly mortality probabilities for males and females, as well as by comparing the total number of male and female deaths in the mating and birth seasons.
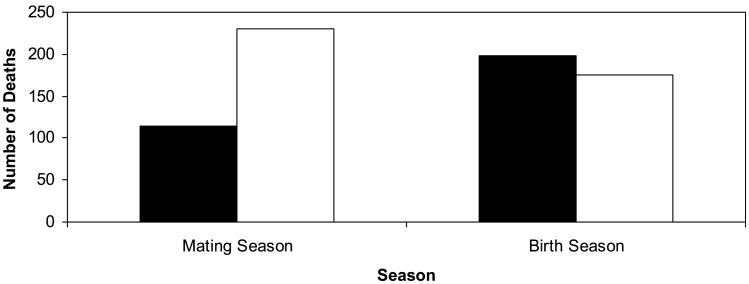
A total of 7,402 live births (males = 3,805; females = 3,597) and 922 deaths (males = 526; females = 396) were included in data analyses. Most births (86%) were concentrated in a five-month period, from November through March, while, on average, the mating season began in mid-May and ended in October. Deaths for both sexes occurred throughout the year, but a higher proportion of males died in August, September, and October, whereas, a higher proportion of females died in February and March, than during the other months of the year. More females died in the birth season than in the mating season, whereas more males died in the mating season than in the birth season (Fig. 2).
Fig. 2.
Total number of male (white) and female (black) deaths in the birth and the mating seasons. Modified after Fig. 2c in Hoffman et al. [2008].
Similar to the study by Blomquist [2009] about rank-related costs of survivorship on Cayo Santiago, our study suggests that reproduction has survival costs for adult males and females even in populations without predators and negligible risk of starvation. Since we had no precise information about the causes of adult mortality we were not able to offer any insight into the mechanisms through which the costs are expressed. It is reasonable to speculate that adult males may be more vulnerable to death from infections during the mating season due to their high rates of aggression and wounding [Wilson & Boelkins, 1970]. In addition, both males and females may be more vulnerable to infections in conjunction with their maximal reproductive effort due to suppression of immune function in particular periods of the year, as a result of reallocation of energy to mating effort (for males), or to gestation and lactation (for females), via the immune-modulating effect of sex steroids, or via a combination of the above [Ellison, 2003; Muehlenbein & Bribiescas, 2005; Harshman & Zera, 2007; Ansar et al., 2009; Prall & Muehlenbein, 2014]. Suppressed immune function can also result from elevated circulating levels of glucocorticoid hormones associated with reproduction-related energetic and social stress in both males and females [Webster et al., 2008; Martin, 2009]. Finally, it is possible that females may be at higher risk of death during the birth season also due to pregnancy or parturition complications. More detailed research into the proximal causes of mortality in this population would be required to resolve this question.
To gather a better understanding of the physiological mechanisms underlying the costs of sociality and reproduction in the Cayo Santiago population, in the past 5–10 years the Maestripieri Lab has conducted two parallel lines of research, one focusing on the energetic, hormonal, and immune correlates of sociality and reproduction in males, and the other focusing on stress hormones and immune function in females in relation to their age, dominance rank, and reproduction. The research on the costs of reproduction in males has been reviewed elsewhere [Higham & Maestripieri, 2014]. The following sections therefore synthesize the work on adult females, some of which has also already been reviewed [Hoffman & Maestripieri, 2011].
Rank-Related Differences in the Cost of Lactation
In this section we review two studies conducted in 2007 and 2008 [Maestripieri et al., 2008; Hoffman et al., 2010a], which collectively provided evidence that cortisol is higher when females are lactating than when they are nonpregnant and nonlactating (NPNL); in addition, the lactation-related elevation in cortisol levels is higher in lower-ranking than in higher-ranking females.
The 2007 study. Between January and March 2007, we measured plasma cortisol levels in 53 multiparous females from six social groups who were between 15 and 26 years of age. Subjects were classified as high-, middle-, or low-ranking depending on whether their rank fell within the top, middle, or bottom third of the dominance hierarchy within their social group. At the time of capture, 25 females were lactating and had a live infant, three of them had had an infant within 6 months but the infant had died for unknown reasons, two of them were pregnant, and 23 of them were NPNL. One blood sample was collected shortly after capture and another one the following morning. Cortisol levels were higher for lactating than for NPNL females across the two days (Fig. 3) and lactation status was a significant predictor of variation in cortisol levels, after controlling for female age, group of origin, dominance rank, and sampling time.
Fig. 3.
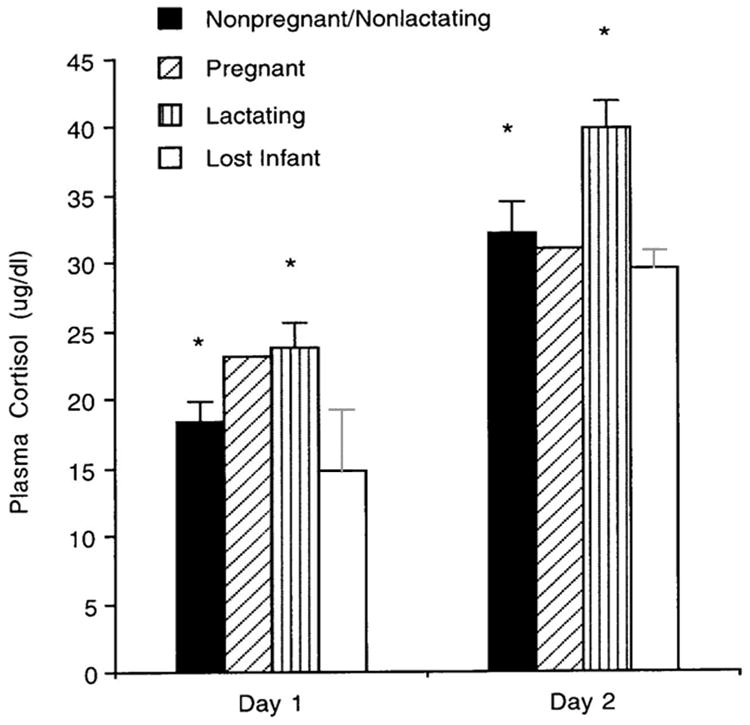
Mean (+ SEM) concentrations of plasma cortisol on Day 1 and Day 2 in females who were neither pregnant nor lactating (NPNL, N = 23), pregnant females (N = 2), lactating females (N = 25), and females who had given birth but their infant had died within 6 months prior to this study (N = 3). Cortisol levels were significantly higher in lactating than in NPNL females. * P≤ 0.0001. Modified after Fig. 1. in Maestripieri et al. [2008].
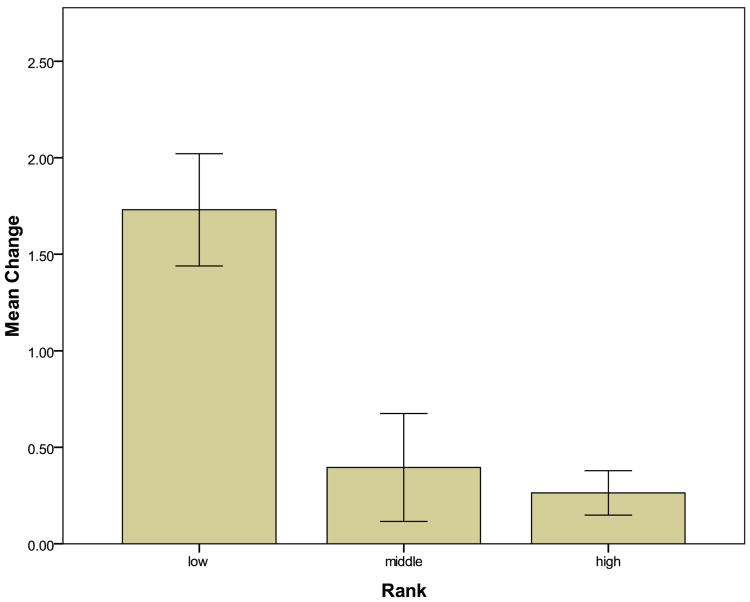
The 2008 study. Between January and February 2008, we measured plasma cortisol levels from 40 adult females who were between 6 and 26 years of age. 26 of the females were lactating and had a live infant, one of them had given birth to an infant within 6 months but the infant had died for unknown reasons, and 13 were NPNL. Of these 40 females, 22 of them had already been caught the previous year. Of the 22 females caught both years, six were lactating in 2007 and NPNL in 2008 six were NPNL in 2007 and lactating in 2008 2 were pregnant in 2007 and NPNL in 2008 six were lactating both years; and two were NPNL both years. We found that individual differences in cortisol levels were generally stable across the two years, that cortisol was significantly higher when females were lactating than when they were NPNL, and that low-ranking females had greater increases in cortisol from the NPNL to the lactating condition than did middle- or high-ranking females (Fig. 4).
Fig. 4.
Mean (± SEM) change in cortisol levels from the NPNL condition to the lactating condition (expressed as z-score values). The difference in cortisol between the lactating and the NPNL condition is significantly greater for low-ranking females than for middle-ranking and high-ranking females. Modified after Fig. 2. in Hoffman et al. [2010].
It may be argued that given that lactation is energetically costly [Prentice & Prentice, 1988; Wade & Schneider, 1992; Emery et al., 2012], higher levels of cortisol among mothers may be associated with the increased glucose production during lactation [Bell & Bauman, 1997]. However, we found no relationships between reproductive condition and body mass or between cortisol and body mass; therefore, it is unlikely that the metabolic costs associated with lactation accounted for the observed differences in cortisol levels. The higher cortisol responses to stress exhibited by lactating females may alternatively have been the result of concerns about infant safety [Otali & Gilchrist, 2006; Emery et al., 2010]. This explanation is consistent with the finding that the increase in cortisol responses to stress occurring during lactation was higher in low-ranking than in middle-and high-ranking females. Low-ranking mothers may perceive their infants to be at risk from other group members to a greater extent than middle-and high-ranking females and experience greater constraints in their ability to provide protection for offspring [Maestripieri, 1995]. Infants born to low-ranking females have a lower probability of surviving their first year than infants born to high-ranking females [Drickamer, 1974]. This implies that motherhood may be particularly challenging for low-ranking females. Nevertheless, a more accurate measure of energy balance may help further elucidate the proximal cause of elevated cortisol in lactating low-ranking females.
Thus, in addition to the energetic demands of pregnancy and lactation, the psychosocial stress of motherhood can contribute to the overall cost of reproduction for rhesus macaque females. Reproducing females probably have elevated concentrations of cortisol 9–10 months per year, and females who give birth every year probably have elevated glucocorticoid hormones most of their life. This should be especially true for low-ranking females. The findings reviewed in the next section show that this is indeed the case.
Rank Differences in Age-Related Changes in Cortisol Levels
Following the glucocorticoid-cascade hypothesis [Sapolsky et al., 1986], if stress and elevated cortisol resulting from within-group competition andfrequent reproduction contribute to reduced survivorship, their effects should increase with age. The psychosocial stress of subordination can be chronic for lower-ranking females, while the stress of reproduction can accumulate over time due to repeated breeding events over the lifetime of a female. Thus, it is important to examine the patterns of cortisol excretion over the life-span of a female in relation to reproduction and dominance rank.
In 2012, we captured a new set of 48 lactating females of ages ranging between 5 and 20 years. Cortisol data from these females were pooled together with those obtained in 2007 and 2008, using in all cases cortisol measures from morning samples. Lactating females that were captured twice contributed only one cortisol value to the dataset. A total dataset of 98 lactating females was obtained, along with data for 43 NPNL females. Pearson correlations were used to assess an association between the ages of the females at the time of capture and their cortisol levels. Data for lactating and NPNL females were analyzed separately. Cortisol was significantly negatively correlated with age among lactating females (N = 95; r = -0.45; P < 0.0001; Fig 5a) and NPNL females (N = 41; r = -0.31; P = 0.04; Fig. 5b).
Fig. 5.
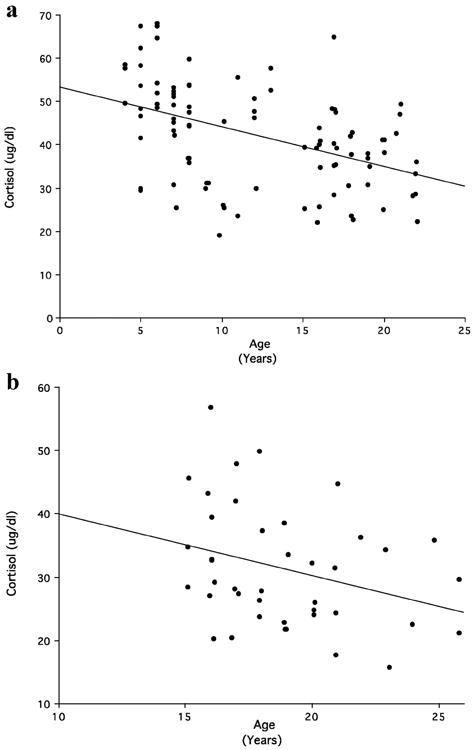
Correlation between age and plasma cortisol levels among (a) lactating females and (b) NPNL females. [Maestripieri et al., unpublished data].
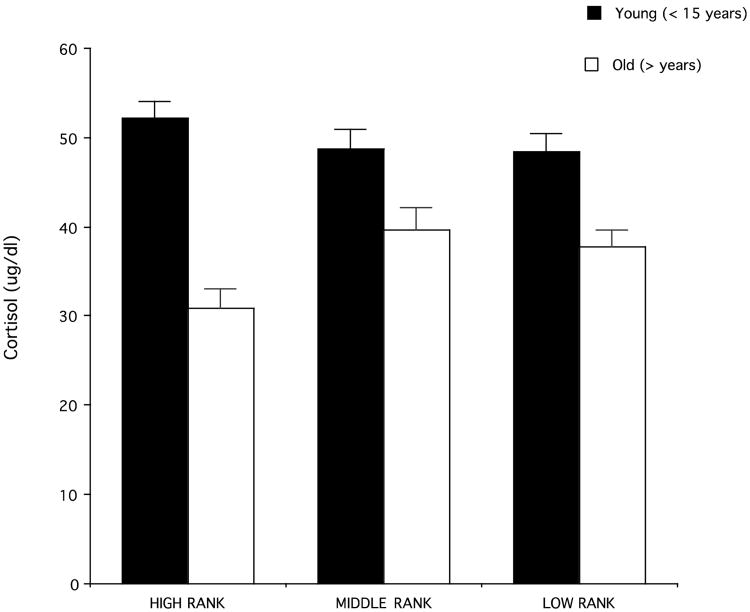
For the females for which information about rank (high, middle, or low) was available, we investigated whether the age-related difference in cortisol varied in relation to rank. Females were classified as younger (<15 years of age) or older (>15 years). This analysis was limited to lactating females, for which sample size was larger. A two-way ANOVA revealed that there was no main effect of rank on cortisol levels (F2,73 = 0.50, P = 0.60) but a significant interaction between rank and age such that high-ranking females had a significantly higher drop in cortisol in older age, when compared to middle-ranking and low-ranking females, for which cortisol levels remained relatively elevated in older age (F2,73 = 3.15; P < 0.05) (Fig. 6). Among older lactating females, high-ranking females (N = 9) had significantly lower cortisol levels than middle- and low-ranking females combined (N = 27) (t = 2.38; df = 34; P = 0.02) (Fig. 6).
Fig. 6.
Plasma cortisol levels in younger (< 15 years old) and older (> 15 years old) high-ranking (young: N = 14; old: N = 9), middle-ranking (young: N = 15; old: N = 9), and low-ranking females (young: N = 14; old: N = 18). All cortisol data are from plasma samples obtained when females were lactating [Maestripieri et al., unpublished data].
Plasma Cortisol in Response to Stress, Glucocorticoid Levels in Fecal Samples, and Immune Function
Chronic stress, whether social or energetic, causes the repeated or continuous activation of compensatory allostatic responses. These responses have an immediate benefit but also a cost. The wear and tear of the body resulting from chronic allostatic activation is referred to as allostatic load [Juster et al., 2010]. Catecholamines, cortisol, and cytokines are considered primary mediators of allostatic load. These primary mediators have direct effects on cellular activities as well as activate secondary mediators, i.e. metabolic, cardiovascular, and immune parameters that change their ranges of action to maintain chemical, tissue, and organ function. Chronic stress and allostasis result in a permanent shift of physiological parameters away from their normal homeostatic ranges and toward abnormal values. The chronic activation of primary and secondary mediators of allostatic load results in tertiary outcomes, such as, permanent physiological dysregulation, brain changes (synaptic and dendritic remodeling, suppressed neurogenesis, structural atrophy/hypertrophy), accelerated aging, disease, or death [McEwen, 2007]. The brain is a major target of allostatic load and damaging effects of chronic stress and elevated cortisol on the hippocampus, and in some cases also the amygdala and prefrontal cortex have been reported in rodents, monkeys, and humans [Uno et al., 1989; Sapolsky et al., 1990; McEwen, 2007; Lupien et al., 2009].
Of the many potential pathways and mechanisms by which chronically elevated cortisol levels can impair health and reduce survival, in our work with female rhesus macaques we selected to investigate the interaction between the HPA axis and the immune system (in our research with adult males, we are currently investigating both immune function and oxidative stress: Georgiev et al. unpublished data). The HPA axis and the immune system generally protect the body from stress and illness by exerting reciprocal regulatory influences. Sustained hyperactivation of the HPA axis can impair immune function and, therefore, contribute to increased vulnerability to infection. Research reviewed in this section suggests that chronic inflammation, along with immunosuppression, may be one of the outcomes of chronic stress associated with sociality and reproduction among female rhesus macaques on Cayo Santiago.
To assess the relation between HPA and immune function, we conducted a pilot study in which we used proinflammatory cytokines as a marker of immune function. Proinflammatory cytokines, such as, interleukin 1 (IL-1), IL-6 and IL-8, activate the HPA axis and increase production of cortisol during acute stress such as injury. Cortisol and proinflammatory cytokines can both be chronically elevated, and such elevation may indicate a damaged cytokine-glucocorticoid feedback loop, which impairs healing and leads to pathological conditions. In humans, upregulation of proinflammatory cytokines due to chronic stress or chronic inflammation is known to accelerate the aging process and is associated with aging-related diseases [McEwen, 2007]. In our study we measured plasma levels of IL-1ra (we examinedIL-1ra as a proxy for IL-1 because concentrations of IL-1 and IL-1ra are positively correlated, and concentrations of the receptor antagonist are higher), IL-6 and IL-8. HPA function was assessed with both plasma cortisol and fecal glucocorticoid metabolites. Cortisol plasma samples provide a measure of HPA reactivity to acute stress while fecal glucocorticoid (FGC) metabolites can be collected noninvasively and averaged over long periods of time, thus providing a “summary” measure of HPA axis activity, under both baseline and stressed conditions.
We collected fecal samples from 44 adult females between April 2007 and December 2007. Of these 44 females, we had blood samples from 22 in 2007, and from 36 in 2008. These females differed in reproductive condition (NPNL, pregnant, or lactating) but not in age [Hoffman et al., 2011]. Similar to what we found with plasma cortisol levels, we found that FGC concentrations varied significantly with respect to female reproductive state and that females had higher FGC concentrations when lactating than when NPNL. FGC concentrations were correlated with plasma cortisol concentrations in NPNL but not in lactating females.
For individual monkeys with data for both years, there were significant positive correlations between plasma concentrations of IL-1ra and IL-8, but not for IL-6, measured in 2007 and 2008. Thus, individual differences in IL-1ra and IL-8 concentrations were stable across the two years. IL-1ra values were related to IL-6 values but IL-8 values were not associated with the other cytokines. There were no significant effects of female reproductive condition on plasma cytokine concentrations, or significant associations between age and IL-6 or IL-8, but the relationship between age and IL-1ra was significant.
There was a significant positive correlation between FGC and IL-8 values for both NPNL and lactating females while neither the IL-1ra nor the IL-6 values were significantly associated with FGC or plasma cortisol values in either NPNL or lactating females.
Taken together, these results provided some evidence for the effects of pregnancy and lactation on measures of HPA function, and for positive associations between glucocorticoid and cytokine (particularly IL-8) concentrations. These results are consistent with research in humans showing that chronically elevated glucocorticoid concentrations increase vulnerability to viral infections and decrease antibody production. Female age was positively associated with IL-1ra concentrations, suggesting that older females experience greater inflammation and immune system activity. There was no significant effect of rank or a significant interaction between rank and age on cytokines.
We found stable individual differences in body weight and BMI, plasma cortisol responses to stress, and plasma cytokine responses to stress in two consecutive years. These differences are consistent with the hypothesis that there are strong differences in chronic stress among individuals, and that chronic stress affects many aspects of the aging process. Body condition and cytokine concentrations were affected by age, while plasma cortisol concentrations were affected by rank. However, there was a correlation between plasma cortisol and one of the cytokines, suggesting an interaction between psychosocial stress, HPA function, and immune function in aging females. Such long-term hyperactivation of the HPA axis can impact allostatic load and survival. Furthermore, since, chronic hyperactivation of the HPA axis increases susceptibility to illness, chronically elevated glucocorticoids observed during pregnancy and lactation might at least partially explain why female mortality rates in this population are highest during the birth season.
Conclusions
In many highly social organisms, living and reproducing in large groups can be costly to all individuals (and within a group, to some more than others), despite the various ecological and social benefits derived from within-group cooperation. The costs of sociality and reproduction can be particularly significant in species of nonhuman primates with long lifespans, long periods of gestation, lactation, and parental investment, and intense within-group competition for food, safety, or mates, leading to high rates of aggression or despotic dominance systems. Sapolsky [2005] has argued that low dominance rank is associated with high chronic stress in despotic primate species in which high-ranking individuals maintain dominance through threats and other forms of psychological intimidation. This is particularly true in groups in which dominance hierarchies are stable, rank is socially inherited from mothers and difficult to change, and low ranking individuals are subjected to high rates of harassment, so that their daily lives are characterized by lack of control and predictability. The female rhesus macaques on Cayo Santiago meet all of these characteristics. The rhesus macaque population on Cayo Santiago, therefore, is an ideal model for studying the costs of sociality associated with agonistic competition for resources.
In rhesus macaque groups, female-female agonistic competition occurs between members of different matrilines, but also between members of the same matriline, whether these individuals are distantly-related (e.g. distant cousins) or closely-related (e.g. mother-daughter or sister-sister pairs). Higher-ranking females, however, receive less aggression and intimidation from other group members than lower-ranking females; moreover, higher-ranking females have access to larger social support networks with which they buffer the stressful effects of agonistic competition. Thus, one of the benefits of high rank among female rhesus macaques is that the costs of living in groups are lower for higher-ranking than for lower-ranking individuals. On Cayo Santiago, where social groups are much larger than in the wild, these benefits of high rank seem to be restricted to the members of the top-ranking matrilines. This is apparent both from data on adult survivorship in relation to matriline rank and from data on cortisol levels in relation to age. Middle-ranking females, therefore, are probably just as chronically stressed as low-ranking females. With regard to the psychosocial stress associated with lactation, however, low-ranking females, however, seem to be worse off than middle-ranking females. In fact, low-ranking females had greater lactation-related increases in cortisol than did middle- or high-ranking females. Therefore, while social life in a large group may be equally stressful for middle- and low-ranking females, reproduction and parental investment may be costlier for low-ranking than for middle-ranking females.
In humans, female reproduction is associated with increased risk of mortality [Penn & Smith, 2007], while in mice, females who experience frequently repeated reproductive stress during their lifetime exhibit signs of accelerated biological aging [Kotrschal et al., 2007]. Our research on Cayo Santiago suggests that reproduction is associated with significant survival and stress-related physiological costs also among female rhesus macaques. As detailed above, the costs of reproduction may be greater for lower-ranking matrilines and individuals. Some of the psychosocial and physiological mechanisms through which social and reproductive stress affects health and survival, particularly in older ages, may be similar (e.g. both low status and motherhood can result in high anxiety, activation of HPA axis hormones, and suppression of immune function), while others may be different (e.g. metabolic stress and its molecular consequences may play a greater role in reproductive stress than in social stress). Clearly, research on how the stress of social life and reproduction affects health and survival is just beginning and our current understanding of these processes is very limited. The rhesus macaque population on Cayo Santiago has proven so far to be an invaluable model for research on the costs of sociality and reproduction and future research with this population can continue to make significant contributions to our understanding of social life and social evolution in nonhuman primates and in humans.
Acknowledgments
We thank all the CPRC staff members and all of our research collaborators, students, and research assistants who have helped us with our research on Cayo Santiago over the years. This research was supported by NIH grant R21-AG029862 and R01-HD067175. The Caribbean Primate Research Center is supported by grant number 8 P40 OD012217-25 from the National Center for Research Resources (NCRR) and the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health to the CPRC of the University of Puerto Rico. The research reported here was conducted in compliance with the American Society of Primatologists' Principles for the ethical treatment of primates. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Contract grant sponsor: NIH; contract grant numbers: R21-AG029862, R01-HD067175; contract grant sponsor: National Center for Research Resources (NCRR); contract grant number: 8 P40 OD012217-25.
Footnotes
Conflicts of interest: None.
References
- Abbott DH, Keverne EB, Bercovitch FB, et al. Are subordinates always stressed? a comparative analysis of rank differences in cortisol levels among primates. Hormones and Behavior. 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Altizer S, Nunn CL, Thrall PH, et al. Social organization and parasite risk in mammals: integrating theory and empirical studies. Annual Review of Ecology. Evolution and Systematics. 2003;34:517–547. [Google Scholar]
- Ansar Ahmed S, Karpuzoglu E, Khan D. Effects of sex steroids on innate and adaptive immunity In sex hormones and immunity to infection. Berlin, Heidelberg: Springer; 2009. pp. 19–51. [Google Scholar]
- Archie EA, Tung J, Clark M, Altmann J, Alberts SC. Social affiliation matters: both same-sex and opposite-sex relationships predict survival in wild female baboons. Proceedings of the Royal Society B: Biological Sciences. 2014;281:20141261–20141261. doi: 10.1098/rspb.2014.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramaniam KN, Dunayer ES, Gilhooly LJ, Rosenfield KA, Berman CM. Group size, contest competition, and social structure. Cayo Santiago Rhesus Macaques. 2014 doi: 10.1163/1568539X-00003216. [DOI] [Google Scholar]
- Bell AW, Bauman DE. Adaptations of glucose metabolism during pregnancy and lactation. Journal of Mammary Gland Biology and Neoplasia. 1997;2:265–278. doi: 10.1023/a:1026336505343. [DOI] [PubMed] [Google Scholar]
- Bercovitch FB. Social stratification, social strategies, and reproductive success in primates. Ethology and Sociobiology. 1991;12:315–333. [Google Scholar]
- Blomquist GE, Sade DS, Berard JD. Rank-related fitness differences and their demographic pathways in semi-free-ranging rhesus macaques (Macaca mulatta) International Journal of Primatology. 2011;32:193–208. [Google Scholar]
- Blomquist GE. Environmental and genetic causes of maturational differences among rhesus macaque matrilines. Behavioral Ecology and Sociobiology. 2009a;63:1345–1352. [Google Scholar]
- Blomquist GE. Trade-off between age of first reproduction and survival in a female primate. Biology Letters. 2009b;5:339–342. doi: 10.1098/rsbl.2009.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent LJN, Semple S, Dubuc C, Heistermann M, MacLarnon A. Social capital and physiological stress levels in free-ranging adult female rhesus macaques. Physiology & Behavior. 2011;102:76–83. doi: 10.1016/j.physbeh.2010.09.022. [DOI] [PubMed] [Google Scholar]
- Brockman DK, van Schaik CP. Seasonality and reproductive function. In: Brockman DK, van Schaik CP, editors. Seasonality in Primates. Cambridge: Cambridge University Press; 2006. pp. 269–305. [Google Scholar]
- Chapais B. How kinship generates dominance structures: a comparative perspective. In: Thierry B, Singh M, Kaumanns W, editors. Macaque Societies: A Model for the Study of Social Organization. Cambridge: Cambridge University Press; 2004. pp. 186–204. [Google Scholar]
- Clutton-Brock TH, Parker GA. Sexual coercion in animal societies. Animal Behaviour. 1995;49:1345–1365. [Google Scholar]
- Cords M. The behavior, ecology, and social evolution of cercopithecine monkeys. In: Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB, editors. The Evolution of Primate Societies. Chicago: The University of Chicago Press; 2012. pp. 91–112. [Google Scholar]
- Creel S, Dantzer B, Goymann W, Rubenstein DR. The ecology of stress: effects of the social environment. Functional Ecology. 2012;27:66–80. [Google Scholar]
- Crofoot MC, Wrangham RW. Intergroup aggression in primates and humans: the Case for a Unified Theory. In: Kappeler PM, Silk JB, editors. Mind The Gap. Berlin Heidelberg: Springer; 2009. pp. 171–197. [Google Scholar]
- de Waal FBM. The integration of dominance and social bonding in primates. Quarterly Review of Biology. 1986;61:459–479. doi: 10.1086/415144. [DOI] [PubMed] [Google Scholar]
- de Waal FBM, Luttrell LM. The formal hierarchy of rhesus macaques: an investigation of the bared-teeth display. American Journal of Primatology. 1985;9:73–85. doi: 10.1002/ajp.1350090202. [DOI] [PubMed] [Google Scholar]
- Drickamer LC. A ten-year summary of reproductive data for free-ranging macaca mulatta. Folia Primatologica. 1974;21:61–80. doi: 10.1159/000155596. [DOI] [PubMed] [Google Scholar]
- Dubuc C, Ruiz-Lambides AV, Widdig A. Variance in male lifetime reproductive success and estimation of the degree of polygyny in a primate. Behavioral Ecology. 2014 doi: 10.1093/beheco/aru052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehardt CL, Bernstein IS. Matrilineal overthrows in rhesus monkey groups. International Journal of Primatology. 1986;7:157–181. [Google Scholar]
- Ellison PT. Energetics and reproductive effort. American Journal of Human Biology. 2003;15:342–351. doi: 10.1002/ajhb.10152. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, Georgiev AV. The high price of success: costs of mating effort in male primates. International Journal of Primatology. 2014;35:609–627. [Google Scholar]
- Emery Thompson M, Muller MN, Kahlenberg SM, Wrangham RW. Dynamics of social and energetic stress in wild female chimpanzees. Hormones and Behavior. 2010;58:440–449. doi: 10.1016/j.yhbeh.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery Thompson M, Muller MN, Wrangham RW. The energetics of lactation and the return to fecundity in wild chimpanzees. Behavioral Ecology. 2012;23:1234–1241. [Google Scholar]
- Emery Thompson M. Comparative reproductive energetics of human and nonhuman primates. Annual Review of Anthropology. 2013;42:287–304. [Google Scholar]
- Foerster S, Cords M, Monfort SL. Seasonal energetic stress in a tropical forest primate: proximate causes and evolutionary implications. PLoS ONE. 2012;7:e50108. doi: 10.1371/journal.pone.0050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürtbauer I, Heistermann M, Schülke O, Ostner J. Low female stress hormone levels are predicted by same- or opposite-sex sociality depending on season in wild Assamese macaques. Psychoneuroendocrinology. 2014;48:19–28. doi: 10.1016/j.psyneuen.2014.05.022. [DOI] [PubMed] [Google Scholar]
- Georgiev AV. PhD dissertation. Harvard University; Cambridge MA: 2012. Energetic Costs of Reproductive Effort in Male Chimpanzees. [Google Scholar]
- Gesquiere LR, Learn NH, Simao MCM, et al. Life at the top: rank and stress in wild male baboons. Science. 2011;333:357–360. doi: 10.1126/science.1207120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goymann W, Wingfield JC. Allostatic load, social status and stress hormones: the costs of social status matter. Animal Behaviour. 2004;67:591–602. [Google Scholar]
- Gómez-Espinosa E, Rangel-Negrín A, Chavira R, Canales-Espinosa D, Dias PAD. The effect of energetic and psychosocial stressors on glucocorticoids in mantled howler monkeys (Alouatta palliata) American Journal of Primatology. 2013;76:362–373. doi: 10.1002/ajp.22240. [DOI] [PubMed] [Google Scholar]
- Harshman LG, Zera AJ. The cost of reproduction: the devil in the details. Trends in Ecology & Evolution. 2007;22:80–86. doi: 10.1016/j.tree.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Hernández-Pacheco R, Rawlins RG, Kessler MJ, et al. Demographic variability and density-dependent dynamics of a free-ranging rhesus macaque population. American Journal of Primatology. 2013;75:1152–1164. doi: 10.1002/ajp.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham JP, Heistermann M, Maestripieri D. The energetics of male-male endurance rivalry in free-ranging rhesus macaques, Macaca mulatta. Animal Behaviour. 2011;81:1001–1007. [Google Scholar]
- Higham JP, Maestripieri D. The costs of reproductive success in male rhesus macaques (Macaca mulatta) on Cayo Santiago. International Journal of Primatology. 2014;35:661–676. [Google Scholar]
- Hoffman CL, Ayala JE, Mas-Rivera A. Effects of reproductive condition and dominance rank on cortisol responsiveness to stress in free-ranging female rhesus macaques. American journal of Primatology. 2010a;72:559–565. doi: 10.1002/ajp.20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman CL, Higham JP, Heistermann M, et al. Immune function and HPA axis activity in free-ranging rhesus macaques. Physiology & Behavior. 2011;104:507–514. doi: 10.1016/j.physbeh.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman CL, Higham JP, Mas-Rivera A, Ayala JE, Maestripieri D. Terminal investment and senescence in rhesus macaques (Macaca mulatta) on Cayo Santiago. Behavioral Ecology. 2010b;21:972–978. doi: 10.1093/beheco/arq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman CL, Maestripieri D. Costs of reproduction among rhesus macaque females on cayo santiago. In: Wang Q, editor. Bones, Genetics, and Behavior of Rhesus Macaques. Berlin: Springer; 2011. pp. 209–226. [Google Scholar]
- Hoffman CL, Ruiz-Lambides AV, Davila E, et al. Sex differences in survival costs of reproduction in a promiscuous primate. Behavioral Ecology and Sociobiology. 2008;62:1711–1718. doi: 10.1007/s00265-008-0599-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holekamp KE, Smale L. Dominance acquisition during mammalian social development: the “inheritance” of maternal rank. Integrative and Comparative Biology. 1991;31:306–317. [Google Scholar]
- Jaimez NA, Bribiescas RG, Aronsen GP, Anestis SA, Watts DP. Urinary cortisol levels of gray-cheeked mangabeys are higher in disturbed compared to undisturbed forest areas in Kibale National Park, Uganda. Animal Conservation. 2011;15:242–247. [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and Biobehavioral Reviews. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kaufmann JH. On the definitions and functions of dominance and territoriality. Biological Reviews. 1983;58:1–20. [Google Scholar]
- Kessler MJ, Berard JD, Rawlins RG, et al. Tetanus antibody titers and duration of immunity to clinical tetanus infections in free-ranging rhesus monkeys (Macaca mulatta) American Journal of Primatology. 2006;68:725–731. doi: 10.1002/ajp.20262. [DOI] [PubMed] [Google Scholar]
- Kotrschal A, Ilmonen P, Penn DJ. Stress impacts telomere dynamics. Biology Letters. 2007;3:128–130. doi: 10.1098/rsbl.2006.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Hoffman CL, Fulks R, Gerald MS. Plasma cortisol responses to stress in lactating and non-lactating female rhesus macaques. Hormones and Behavior. 2008;53:170–176. doi: 10.1016/j.yhbeh.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D, Hoffman CL. Behavior and social dynamics of rhesus macaques on Cayo Santiago. In: Wang Q, editor. Bones, Genetics, and Behavior of Rhesus Macaques. Berlin: Springer; 2012. pp. 247–262. [Google Scholar]
- Maestripieri D. First steps in the macaque world: do rhesus mothers encourage their infants' independent locomotion? Animal Behaviour. 1995;49:1541–1549. [Google Scholar]
- Maestripieri D. Gestural communication in three species of macaques (Macaca mulatta, M. nemestrina, M. arctoides): use of signals in relation to dominance and social context. Gesture. 2005;5:57–73. [Google Scholar]
- Majolo B, de Bortoli Vizioli, Schino A. Costs and benefits of group living in primates: group size effects on behaviour and demography. Animal Behaviour. 2008;76:1235–1247. [Google Scholar]
- Martin LB. Stress and immunity in wild vertebrates: timing is everything. General and Comparative Endocrinology. 2009;163:70–76. doi: 10.1016/j.ygcen.2009.03.008. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological Reviews. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Muehlenbein MP, Bribiescas RG. Testosterone-mediated immune functions and male life histories. American Journal of Human Biology. 2005;17:527–558. doi: 10.1002/ajhb.20419. [DOI] [PubMed] [Google Scholar]
- Muehlenbein MP. Intestinal parasite infections and fecal steroid levels in wild chimpanzees. American Journal of Physical Anthropology. 2006;130:546–550. doi: 10.1002/ajpa.20391. [DOI] [PubMed] [Google Scholar]
- Muller MN, Kahlenberg SM, Emery Thompson, Wrangham M. Male coercion and the costs of promiscuous mating for female chimpanzees. Proceedings of the Royal Society B: Biological Sciences. 2007;274:1009–1014. doi: 10.1098/rspb.2006.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MN, Wrangham RW. Sexual coercion in primates and humans: an evolutionary perspective on male aggression against females. Cambridge, MA: Harvard University Press; 2009. [Google Scholar]
- Novak MA, Hamel AF, Kelly BJ, Dettmer AM, Meyer JS. Stress, the HPA axis, and nonhuman primate well-being: a review. Applied Animal Behaviour Science. 2013;143:135–149. doi: 10.1016/j.applanim.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates-O'Brien RS, Farver TB, Anderson-Vicino KC, McCowan B, Lerche NW. Predictors of matrilineal overthrows in large captive breeding groups of rhesus macaques (Macaca mulatta) Journal of the American Association for Laboratory Animal Science. 2010;49:196–201. [PMC free article] [PubMed] [Google Scholar]
- Otali E, Gilchrist JS. Why chimpanzee (Pan troglodytes schweinfurthii) mothers are less gregarious than non-mothers and males: the infant safety hypothesis. Behavioral Ecology and Sociobiology. 2006;59:561–570. [Google Scholar]
- Penn DJ, Smith KR. Differential fitness costs of reproduction between the sexes. Proceedings of the National Academy of Sciences USA. 2007;104:553–558. doi: 10.1073/pnas.0609301103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prall SP, Muehlenbein MP. Testosterone and immune function in primates: a brief summary with methodological considerations. International Journal of Primatology. 2014;35:805–824. [Google Scholar]
- Prentice AM, Prentice A. Energy costs of lactation. Annual Review of Nutrition. 1988;8:63–79. doi: 10.1146/annurev.nu.08.070188.000431. [DOI] [PubMed] [Google Scholar]
- Pusey A. Magnitude and sources of variation in female reproductive performance. In: Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB, editors. The Evolution of Primate Societies. Chicago: The University of Chicago Press; 2012. pp. 343–366. [Google Scholar]
- Rawlins RG, Kessler MJ. The history of the Cayo Santiago colony. In: Rawlins RG, Kessler MJ, editors. The Cayo Santiago Macaques. Albany: SUNY Press; 1986. pp. 13–45. [Google Scholar]
- Rhine RJ, Norton GW, Wasser SK. Lifetime reproductive success, longevity, and reproductive life history of female yellow baboons (Papio cynocephalus) of Mikumi National Park, Tanzania. American Journal of Primatology. 2000;51:229–241. doi: 10.1002/1098-2345(200008)51:4<229::AID-AJP2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Rifkin JL, Nunn CL, Garamszegi LZ. Do animals living in larger groups experience greater parasitism? A meta-analysis. The American Naturalist. 2012;180:70–82. doi: 10.1086/666081. [DOI] [PubMed] [Google Scholar]
- Robbins AM, Stoinski T, Fawcett K, Robbins MM. Lifetime reproductive success of female mountain gorillas. Yearbook of Physical Anthropology. 2011;146:582–593. doi: 10.1002/ajpa.21605. [DOI] [PubMed] [Google Scholar]
- Roberts SJ, Cords M. Group size but not dominance rank predicts the probability of conception in a frugivorous primate. Behavioral Ecology and Sociobiology. 2013;67:1995–2009. [Google Scholar]
- Ruiz-Lambides AV, Aure B, Caraballo G, Platt ML, Brent LJN. Matrilineal overthrow followed by high mortality levels in free-ranging rhesus macaques. American Journal of Primatology. 2013;75S1:98. [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocrine Reviews. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. The Journal of Neuroscience. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Experimental Gerontology. 1999;34:721–732. doi: 10.1016/s0531-5565(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Schulke O, Ostner J. Ecological and social influences on sociality. In: Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB, editors. The Evolution of Primate Societies. Chicago: The University of Chicago Press; 2012. pp. 193–219. [Google Scholar]
- Silk JB, Alberts SC, Altmann J. Social bonds of female baboons enhance infant survival. Science. 2003;302:1231–1234. doi: 10.1126/science.1088580. [DOI] [PubMed] [Google Scholar]
- Silk JB, Beehner JC, Bergman TJ, et al. The benefits of social capital: close social bonds among female baboons enhance offspring survival. Proceedings of the Royal Society B: Biological Sciences. 2009;276:3099–3104. doi: 10.1098/rspb.2009.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JB, Beehner JC, Bergman TJ, et al. Strong and consistent social bonds enhance the longevity of female baboons. Current Biology. 2010;20:1359–1361. doi: 10.1016/j.cub.2010.05.067. [DOI] [PubMed] [Google Scholar]
- Smuts BB, Smuts RW. Male aggression and sexual coercion of females in nonhuman primates and other mammals: evidence and theoretical implications. Advances in the Study of Behavior. 1993;22(22):1–63. [Google Scholar]
- Steptoe A, Kivimäki M. Stress and csrdiovascular disease: an update on current knowledge. Annual Review of Public Health. 2013;34:337–354. doi: 10.1146/annurev-publhealth-031912-114452. [DOI] [PubMed] [Google Scholar]
- Sterck E, Watts DP, van Schaik CP. The evolution of female social relationships in nonhuman primates. Behavioral Ecology and Sociobiology. 1997;41:291–309. [Google Scholar]
- Stockley P, Bro-Jørgensen J. Female competition and its evolutionary consequences in mammals. Biological Reviews. 2011;86:341–366. doi: 10.1111/j.1469-185X.2010.00149.x. [DOI] [PubMed] [Google Scholar]
- Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM. Hippocampal damage associated with prolonged and fatal stress in primates. The Journal of Neuroscience. 1989;9:1705–1711. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noordwijk MA, van Schaik CP. Sexual selection and the careers of primate males: paternity concentration, dominance-acquisition tactics and transfer decisions. In: Kappeler PM, van Schaik CP, editors. Sexual Selection in Primates: New and Comparative Perspectives. Cambridge: Cambridge University Press; 2004. pp. 208–229. [Google Scholar]
- van Schaik CP. Why are diurnal primates living in groups? Behaviour. 1983;87:120–144. [Google Scholar]
- Vandenbergh JG, Vessey S. Seasonal breeding of free-ranging rhesus monkeys and related ecological factors. Journal of Reproduction and Fertility. 1968;15:71–79. doi: 10.1530/jrf.0.0150071. [DOI] [PubMed] [Google Scholar]
- Varley MA, Vessey SH. Effects of geographic transfer on the timing of seasonal breeding of rhesus monkeys. Folia Primatologica. 1977;28:52–59. doi: 10.1159/000155798. [DOI] [PubMed] [Google Scholar]
- Wade GN, Schneider JE. Metabolic fuels and reproduction in female mammals. Neuroscience & Biobehavioral Reviews. 1992;16:235–272. doi: 10.1016/s0149-7634(05)80183-6. [DOI] [PubMed] [Google Scholar]
- Webster Marketon, Glaser JI. Stress hormones and immune function. Cellular Immunology. 2008;252:16–26. doi: 10.1016/j.cellimm.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Wheeler BC, Scarry CJ, Koenig A. Rates of agonism among female primates: a cross-taxon perspective. Behavioral Ecology. 2013;24:1369–1380. doi: 10.1093/beheco/art076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AP, Boelkins RC. Evidence for seasonal variation in aggressive behaviour by Macaca mulatta. Animal Behaviour. 1970;18:719–724. doi: 10.1016/0003-3472(70)90017-5. [DOI] [PubMed] [Google Scholar]
- Wrangham RW. An ecological model of female-bonded primate groups. Behaviour. 1980;75:262–300. [Google Scholar]