Abstract
The complement system plays a key role in many acute injury states as well as chronic autoimmune and inflammatory diseases. Localized complement activation and alternative pathway-mediated amplification on diverse target surfaces promote local recruitment of pro-inflammatory cells and elaboration of other mediators. Despite a general understanding of the architecture of the system, though, many of the mechanisms that underlie site-specific complement activation and amplification in vivo are incompletely understood. In addition, there is no capability yet to measure the level of local tissue site-specific complement activation in patients without performing biopsies to detect products using immunohistochemical techniques. Herein is reviewed emerging evidence obtained through clinical research studies of human rheumatoid arthritis along with translational studies of its disease models which demonstrate that several parallel mechanisms are involved in site-specific amplification of activation of the complement system in vivo. Among these processes are de-regulation of the alternative pathway, effector pathway-catalyzed amplification of proximal complement activation, recognition of injury-associated ligands by components of the lectin pathway, and engagement of pathogenic natural antibodies that recognize a limited set of injury-associated neoepitopes. Studies suggest that each of these inter-related processes can play key roles in amplification of complement-dependent injury on self-tissues in vivo. These findings, in addition to development of an imaging strategy described herein designed to quantitatively measure local complement C3 fixation, have relevance to therapeutic and diagnostic strategies targeting the complement system.
Keywords: Alternative Pathway, Complement Therapeutics, Pathogenesis, Lectin Pathway, Molecular Imaging, Arthritis, Effector Pathways
Introduction
The complement system is a major component of innate immunity (Fearon and Locksley, 1996) and plays central roles in many protective immune processes [reviewed in (Holers, 2001;Holers, Carroll, and Holers, 2005;Ricklin, Hajishengallis, Yang, and Lambris, 2010)]. In contrast to these beneficial roles, inappropriate complement activation that is directed not to foreign pathogens but rather to self-tissues is also believed to underlie the pathogenesis of many human inflammatory and autoimmune diseases (Ricklin and Lambris, 2013). This review will focus on the human autoimmune disease rheumatoid arthritis (RA) and the use of a murine model of this process to identify and characterize specific mechanisms that underlie complement-mediated joint damage. In addition, a new approach to characterizing local complement C3 activation through molecular imaging will be introduced.
The study of RA is particularly relevant in light of new findings that suggest the complement system plays key roles at the initiation of inflammatory arthritis, where the pathophysiology of disease is likely to be different than in the chronic stages (Holers, 2013a). RA is also an especially relevant human disease to study the effects of chronic complement activation, as many previous studies have demonstrated elevated activation fragments in patients with longstanding disease (Linton and Morgan, 1999;Monach, Benoist, and Mathis, 2004;Neumann, Barnum, Tarner, Echols, Fleck, Judex, Kullmann, Mountz, Scholmerich, Gay, and Muller-Ladner, 2002).
New roles of complement in incipient human rheumatoid arthritis
RA is an important human autoimmune disease and exhibits the second highest prevalence of the autoimmune diseases (Silman, 1994;Silman and Hochberg, 1993). RA is a disease that extensively involves both the cartilage and synovium as potential targets of complement-mediated damage. With regard to the human cartilage surface, studies have demonstrated the presence of IgG-containing immune complexes as well as complement C3 activation fragments in >90% of chronic RA patient samples (Cooke, Hurd, Jasin, Bienenstock, and Ziff, 1975). The heavily infiltrated synovium is also a site of extensive complement deposition and synthesis of activation pathway proteins and receptors in patients and animal models (Linton et al., 1999;Neumann et al., 2002). As one potential link to complement activation mechanisms, it is now appreciated that disease-associated autoantibodies such as anti-citrullinated protein/peptide antibodies (ACPA) and rheumatoid factors (RF) are both associated with poor outcomes and are pathogenic when tested in animal models (Klareskog, Ronnelid, Lundberg, Padyukov, and Alfredsson, 2008).
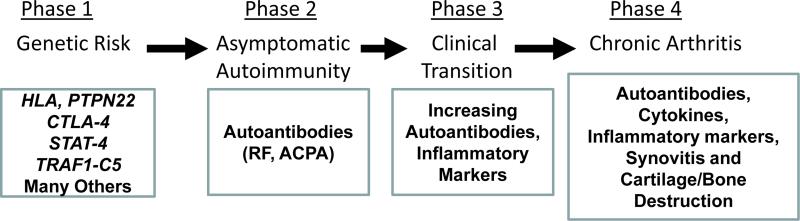
Once well established, chronic human RA appears to be a largely cytokine-driven inflammatory disorder characterized by joint damage and disability (Firestein, 2003). Nevertheless, while this is true of longstanding disease, recent studies that are focused on the very early development of human RA suggests a more complex pathogenic process (Figure 1) (Holers, 2013a;Klareskog et al., 2008). In this regard, it is now apparent that subjects first break immune tolerance at a site peripheral to the joints and develop circulating RA-related autoantibodies which recognize citrullinated epitopes, a post-translational modification on self-proteins. These RA-related ACPA are present for many years in asymptomatic individuals (Deane, Norris, and Holers, 2010) and are focused on a subset of citrullinated autoantigens including fibrinogen, vimentin, enolase and type II collagen (CII) (Klareskog et al., 2008;van Venrooij and Pruijn, 2000;van Venrooij and van de Putte, 2005). Following this period of circulating autoantibodies in the absence of signs or symptoms of arthritis, it appears that when one or more of the citrullinated autoantigens develops in the joint, likely generated through a neutrophil-driven inflammatory process (Khandpur, Carmona-Rivera, Vivekanandan-Giri, Gizinski, Yalavarthi, Knight, Friday, Li, Patel, Subramanian, Thompson, Chen, Fox, Pennathur, and Kaplan, 2013;Klareskog et al., 2008), or when circulating immune complexes deposit in the joint (Sokolove, Zhao, Chandra, and Robinson, 2011), a transition occurs wherein clinically apparent arthritis is now present. Through engagement of effector pathways, these autoantibodies then appear to cause joint damage (Klareskog et al., 2008). Thus, in contrast with chronic well-established RA, the pathogenesis of early arthritis appears now to be very consistent with an immune complex-driven disorder similar to murine passive transfer models (Klareskog et al., 2008;Monach et al., 2004). In that light, studies of the effects of autoantibody disease transfer models are highly relevant, especially as mechanism-based designs for very early treatment and prevention strategies for patients in this phase of disease are being developed (Demoruelle and Deane, 2012;Klareskog, Gregersen, and Huizinga, 2010).
Figure 1.
Current understanding of the step-wise phases of development of RA, starting with the disease-related genotypes, which is followed by an asymptomatic phase of circulating RA-related autoantibodies. This period leads to a clinical transition in which subjects are developing clinically apparent disease, and then finally moving into a chronic arthritis phase.
Consistent with this model, our own recent studies have confirmed prior findings that subjects with both longstanding chronic RA as well as in subjects who are in the early clinical initiation period demonstrate evidence of complement activation manifest by increases in the activation fragments C3a and sC5b-9 (Holers and Kinslow, unpublished). Thus, complement activation is likely to be involved in the early phases of joint and/or other tissue inflammation in humans. Notably, type II collagen (CII), the target of the pathogenic antibodies utilized the murine studies described below, is also an autoantigen in its native, glycosylated and citrullinated forms in a substantial subset of patients with RA (Backlund, Carlsen, Hoger, Holm, Fugger, Kihlberg, Burkhardt, and Holmdahl, 2002;Uysal, Bockermann, Nandakumar, Sehnert, Bajtner, Engstrom, Serre, Burkhardt, Thunnissen, and Holmdahl, 2009).
Complement Pathogenic Mechanisms in Inflammatory Arthritis Models
Local Activation and Amplification Pathways
Several groups have worked to define the pathogenic role of antibodies and the complement system in animal models of RA (Linton et al., 1999;Monach et al., 2004). Herein the focus of the discussion is upon the CII antibody-induced arthritis (CAIA) model wherein a set of high affinity mAbs directed to epitopes within CII are used to induce disease in susceptible strains (Banda, Thurman, Kraus, Wood, Carroll, Arend, and Holers, 2006). Initial studies of the mechanisms underlying damage demonstrated that, while the classical and lectin pathways are engaged following antibody binding in the joint, the alternative pathway was both necessary and sufficient to develop arthritis through its apparent roles in both the initiation and amplification steps (Banda, Takahashi, Wood, Holers, and Arend, 2007;Banda et al., 2006). Subsequent studies in the model revealed that mannose-binding lectin (MBL)-associated serine proteases MASP-1 and MASP-3 are required to cleave the inactive alternative pathway member pro-factor D (FD) into active FD, which could then function in the alternative pathway C3 convertase, and that this activity was necessary to develop substantial joint damage (Banda, Takahashi, Levitt, Glogowska, Nicholas, Takahashi, Stahl, Fujita, Arend, and Holers, 2010;Banda, Takahashi, Takahashi, Stahl, Hyatt, Glogowska, Wiles, Endo, Fujita, Holers, and Arend, 2011).
With regard to mechanisms of activation within the joint, FD appeared to be produced locally in synovial adipose tissue (SAT), and cultured differentiated 3T3 adipocytes, a surrogate for SAT, produced pro-FD but no mature FD. In contrast, fibroblast-like synoviocytes (FLS), a major cell type centrally involved in the inflamed joint (Bottini and Firestein, 2013), were the main source of MASP-1/3 mRNA and protein (Arend, Mehta, Antonioli, Takahashi, Takahashi, Stahl, Holers, and Banda, 2013). Using cartilage microparticles (CMPs) coated with anti-CII mAb and serum from MASP-1/3(−/−) mice as a source of factor B in the absence of active FD, pro-FD in 3T3 supernatants was cleaved into mature FD by MASP-1/3 in FLS supernatants. These results strongly suggest that pathogenic activation of the alternative pathway can occur in the joint through immune complexes adherent to cartilage, and that local production of alternative pathway proteins by adipocytes and FLS is likely to be sufficient to develop damage.
Additional studies of CAIA have also begun to unravel the role of the lectin pathway in promotion of complement-dependent injury. Despite previously demonstration of no effect on the development of CAIA in mice lacking MBL-A/C expression (Banda et al., 2007;Banda et al., 2006) [there are two MBL encoding genes and proteins in mice (Kjaer, Thiel, and Andersen, 2013)], more recent findings in several other experimental settings have rekindled interest in this pathway. The first was the publication of data demonstrating substantial increases in lectin pathway components in RA patients (Ammitzboll, Thiel, Ellingsen, Deleuran, Jorgensen, Jensenius, and Stengaard-Pedersen, 2012). The second was the emerging understanding of the complexity of the recognition and protease components of the lectin pathway and the consideration that recognition of ligands by other pattern recognition molecules [reviewed in (Kjaer et al., 2013)] could engage MASPs and “bypass” the lack of MBL expression and activate C3 in the MBL-A/C deficient mice that were previously evaluated. And the third was findings in a model of aortic injury wherein IgG antibody activated the alternative pathway amplification loop through MBL-A/C engagement (Zhou, Yana, Bertrama, Hua, Springer, Thompson, Curci, Hourcade, and Pham, 2013), which further suggested a critical in vivo link between the pathways.
The lectin pathway is initiated by a family of pattern recognition molecules, designated the collectins, which includes mannose-binding lectin (MBL) (Ip, Takahashi, Ezekowitz, and Stuart, 2009;Kjaer et al., 2013), Ficolins (Matsushita, 2010;Runza, Schwaeble, and Mannel, 2008) and collectin-K1 (CL-K1, also designated collectin 11) (Hansen, Sehman, Palaniyar, Ziegler, Brandt, Kliem, Jonasson, Skjoedt, Nielsen, Hartshom, Jorgensen, and Holmskov, 2010;Ohtani, Suzuki, and Wakamiya, 2012) that recognize diverse ligands and initiate complement activation. Protective roles of this family include the recognition and clearance of foreign organisms, such as bacteria and viruses (Gadjeva, Takahashi, and Thiel, 2004;Reid and Turner, 1994;Runza et al., 2008) through recognition of certain densely displayed monosaccharides (glucose, mannose) as well as acetylated carbohydrates, such as N-acetyl glucosamine, that are preferentially found on pathogens. In addition to this protective role, however, the lectin pathway is also known to be engaged during tissue injury either through the direct recognition of newly exposed self-ligands for MBL recognition (Collard, Montalto, Reenstra, Buras, and Stahl, 2001) or through IgG autoantibodies containing agalactosyl (G0) carbohydrates that interact with MBL (Malhotra, Wormald, Rudd, Fischer, Dwek, and Sim, 1995). After recognition, it is understood that lectin pathway activation can proceed through the serial catalytic activities of MASP-1 and MASP-2, resulting in cleavage and activation of C4 and C2, and then cleavage of C3 into C3b through the shared classical pathway C3 convertase designated C4b2a. It has also been postulated that there is direct cleavage and activation of C3 through MASP-1, bypassing MASP-2; however, the in vitro rate of activation is slow, and it is not known yet if this process occurs in vivo (Rossi, Cseh, Bally, Thielens, Jensenius, and Arlaud, 2001).
As one means to block activation of the lectin pathway in its entirety, a novel inhibitor designated MAp-44 (also called MAP-1) has been identified. MAp-44 is an alternatively spliced product of the MASP-1/3 gene that contains only the MASP recognition domains for the collectins and does not contain a protease domain (Degn, Hansen, Steffensen, Jacobsen, Jensenius, and Thiel, 2009;Skjoedt, Hummelshoj, Palarasah, Honore, Koch, Skjodt, and Garred, 2010). MAp-44 is normally present at very low circulating levels, < 2 micrograms/ml in serum. MAp-44 was shown in vitro to compete with MASP-1, MASP-2, and MASP-3 for binding to all of the collectins and to act as a dominant composite negative inhibitor of all three pattern recognition mechanisms (Degn, Jensen, Olszowski, Jensenius, and Thiel, 2013;Pavlov, Skjoedt, Tan, Rosbjerg, and Garred, 2012;Skjoedt et al., 2010). Notably, use of recombinant MAp-44 in a murine model of myocardial infarction resulted in decreased injury (Pavlov et al., 2012), although this model is distinct from CAIA in that it is MBL-dependent.
To evaluate the effect of inhibiting the lectin pathway using MAp-44, an Adeno Associated Virus (AAV) approach (Bakker, van de Loo, Joosten, Bennink, Arntz, Dmitriev, Kashentsera, Curiel, and van den Berg, 2001) was developed by constructing AAV directing the synthesis of hemagglutinin (HA)-tagged human and mouse MAp-44, along with a control AAV expressing Green Fluorescent Protein (GFP). Treatment of mice undergoing CAIA with AAV directing expression of human and mouse MAp-44 both resulted in marked inhibition of the development of CAIA (Banda, Mehta, Kjaer, Takahashi, Schaack, Morrison, Thiel, Arend, and Holers, 2014). This decrease in clinical disease activity was mirrored by a dramatic decrease in histologic injury and C3 deposition on the cartilage and in synovium, and decreased synovial IL-1beta and TNF-alpha expression in treated mice. These data demonstrated a previously unrecognized but apparently essential role for the lectin pathway in the development of inflammatory arthritis and severe joint damage.
Local Alternative Pathway Regulatory Mechanisms
Given the importance of the alternative pathway in local joint injury, it is also relevant to explore the role of complement regulatory mechanisms that normally would control activation in this site. Prior reviews of the topic have largely focused on the role of membrane regulatory proteins (Linton et al., 1999;Liszewski, Farries, Lublin, Rooney, and Atkinson, 1996), and thus this topic will not be reviewed. With regard to soluble regulatory proteins, though, factor H (FH)-mediated control of complement activation is increasingly understood to be centrally involved in the pathogenesis of human diseases, especially where mutations or highly informative polymorphisms of FH are associated with diseases such as atypical hemolytic uremic syndrome (aHUS) and membranoproliferative glomerulonephritis type II (MPGN Type II) (Jha, Bora, and Bora, 2007;Jokiranta, Zipfel, Fremeaux-Bacchi, Taylor, Goodship, and Noris, 2007;Kavanaugh, Goodship, and Richards, 2006)].
Importantly, FH can function in both the fluid phase and on surfaces, and the major characteristic that controls its surface inhibitory capacity is its relative ability to bind in a tissue-specific manner to glycosaminoglycans (GAG) as well as C3b/C3d molecules that are fixed onto the target surface [reviewed in (Zipfel, Skerka, Hellwage, Jokiranta, Meri, Brade, Kraiczy, Noris, and Remuzzi, 2002)]. FH exhibits in both the fluid phase as well as on surfaces both cofactor activity for factor I mediated cleavage of C3b as well as decay-accelerating activity for the alternative pathway initiation and amplification C3 convertases. FH exhibits a modular structure with the C3 regulatory sites in short consensus repeat (SCR)1-4 and a series of binding sites for ligands in the carboxy-terminal 16 SCRs. Factor H has been suggested to interact with target cells and tissues through several SCR-containing domains, including SCR5-7, which is associated by the “unfurling” of the molecule over the surface, the primary dependence of SCR19-20 interactions with GAGs and C3b/C3d for stable binding, and the elaboration of protective function blocking further C3 activation at the site (Jozsi, Manuelian, Heinen, Opperman, and Zipfel, 2004;Jozsi, Opperman, Lambris, and Zipfel, 2007).
To explore the role of FH in CAIA, its binding to tissues was blocked through administration to wild type mice undergoing disease induction of a recombinant dominant negative inhibitor containing SCRs 19 and 20 (rFH19-20), which impairs FH function and amplifies surface alternative pathway activation in vitro (Ferreira, Herbert, Hocking, Barlow, and Pangburn, 2006). Administration of rFH19-20, but not control rFH3-5, was found to significantly increase clinical disease activity, histopathologic injury and C3 deposition in the synovium and cartilage in both wild-type and FH(+/−) mice undergoing the CAIA model (Banda, Mehta, Ferreira, Cortes, Pickering, Pangburn, Arend, and Holers, 2013). With regard to the mechanism of this effect, in vitro studies demonstrated that rFH19-20 increased complement activation on cartilage extracts treated with anti-CII mAbs as well as injured FLS. These data suggested that endogenous FH makes a significant contribution to inhibition of the alternative pathway and amplification loop in CAIA, likely through localized binding to and decreasing complement activation at sites of immune complex formation and complement activation
Because of this key role for FH revealed through the use of the non-physiologic inhibitor rFH19-20, additional studies are now focusing on control of FH actions by endogenous modulators. One such potential class of modulators is exemplified by the factor H-related (FHR) proteins. FHR proteins are part of the structurally related family that includes the larger FH protein but are encoded by genes that are physically adjacent to the FH gene on chromosome 1 and also contain short consensus repeat (SCR) domains with homology to sub-regions of FH (de Cordoba, Tortajada, Harris, and Morgan, 2012;Jozsi and Zipfel, 2008). Notably, there are an increasing number of identified protective or risk associations of deletions, rare mutations or polymorphic variants of these genes with human diseases (de Cordoba et al., 2012;Jozsi et al., 2008), including age-related macular degeneration where an uncommon deletion of FHR1 and FHR3 is a highly penetrant protective factor (Hughes, Orr, Esfandiary, Diaz-Torres, Goodship, and Chakravarthy, 2006).
What has been uncertain is the role of these key proteins in vivo (Holers, 2013b). Many prior studies of FHRs have reported a role for these proteins in directly regulating complement activation and impairing activation at specific steps of the cascade. For example, FHR2 and FHR4 were reported to demonstrate enhancement of FH cofactor activity, and FHR5 to exhibit weak cofactor and decay accelerating activity [reviewed in (Jozsi et al., 2008)]. Likewise, FHR1 was postulated to inhibit C5 convertase activity and terminal complex formation (Heinen, Hartmann, Lauer, Wiehl, Dahse, Schirmer, Gropp, Enghardt, Wallich, Halbich, Mihlan, Schlotzer-Schrehardt, Zipfel, and Skerka, 2009), and FHR3 to inhibit C3 convertase activity and display anti-inflammatory activity (Fritsche, Lauer, Hartmann, Stippa, Keilhauer, Oppermann, Pandey, Kohl, Zipfel, Weber, and Skerka, 2010). However, as the structure-function relationships of FH has become increasingly understood (Morgan, Schmidt, Guariento, Blaum, Gillespie, Herbert, Kavanagh, Mertens, Svergun, Johansson, Uhrin, Barlow, and Hannan, 2011), it has become more challenging to reconcile regulatory activities of FHRs with the structural determinants, other than through unusual steric hindrance mechanisms, because they do not encode domains of similar structure to FH SCR1-4.
Recently, studies by Tortajada et al (Tortajada, Yebenes, Abarrategui-Garrido, Anter, Garcia-Fernandez, Martinez-Barricarte, Alba-Dominguez, Malik, Bedoya, Perez, Trascasa, Pickering, Harris, Sanchez-Corral, Llorca, and de Cordoba, 2013) and Goicoechea et al (Goicoechea de Jorge, Caesar, Malik, Paten, Colledge, Johnson, Hakobyan, Morgan, Harris, Pickering, and Lea, 2013) have challenged that model by showing that FHR1 homo- and heterooligomerizes with itself as well as with FHR2 and FHR5, but not with FHR3 or FHR4A/4B. These complexes were shown in vitro to compete for FH binding to C3 fragments, and to impair the ability of FH to block surface complement activation. The conclusion of the study was that FHR1, assembled into multimers either alone or in association with FHR2 and FHR5, primarily acts on surfaces to block FH binding, resulting in complement deregulation and enhanced local activation. Because the CAIA model requires the alternative pathway, is a FH-dependent model and can be strongly enhanced by treatment with recombinant FH SCR19-20 (Banda et al., 2013), it is an excellent experimental means by which the roles of FHRs can be evaluated in vivo in a complex biological environment.
Effector-Mediated Mechanisms of Proximal Amplification
One typically thinks about complement-dependent activation solely in the context of the biochemically defined linear pathway and well characterized amplification loop [reviewed in (Lachmann, 2009)]. However, studies in the CAIA model have also led to the understanding that there are additional effector pathway-catalyzed amplification steps which occur in vivo. These mechanisms are characterized by the engagement of “danger signals” and other pro-inflammatory pathways and uniformly result in more robust deposit of pathogenic antibodies and local complement activation. The conclusion from these studies is that each major complement effector pathway, C5a, C3a and the membrane attack complex (MAC), plays an important and unique role in joint injury and also promotes proximal pathway complement C3 activation (Banda, Hyatt, Antonioli, White, Glogowska, Takahashi, Merkel, Stahl, Mueller-Ortiz, Wetsel, Arend, and Holers, 2012).
In support of this model, it was found that when C3aR(−/−), C5aR(−/−), and C6-deficient (C6-def) mice are studied in the CAIA model, clinical disease activity was decreased by 52, 94, and 65%, respectively, as compared with wild-type mice. Comparable decreases in the levels of histopathologic injury scores as well as IgG and C3 deposition, which paralleled the clinical disease activity scores, were also found. The outcomes in each effector pathway were not identical, as a decrease in the percentage of synovial neutrophils was observed in C3aR(−/−), C5aR(−/−) and C6-def mice, but a decrease in macrophages was only observed in C3aR(−/−) and C5aR(−/−) mice. As a further example of unique aspects of the individual pathway roles, analysis of synovial mRNA obtained by laser capture microdissection revealed a decrease in TNF-α in C5aR(−/−) mice and in IL-1β in both C5aR(−/−) and C6-def mice, whereas C3aR(−/−) mice demonstrated no change in either cytokine. Notably, although there were differential effector mechanisms that were apparent, a decreased proximal joint IgG and C3 deposition in comparison with wild-type mice was common to mice with all three genotypes. In sum, these data suggest the existence of positive-feedback amplification pathways that is downstream of all three effectors and which promotes additional IgG deposition and C3 activation in the joint.
There are several potential mechanisms that can underlie these phenotypes. One is a contribution of bone marrow cell-derived effector pathways, perhaps through polymorphonuclear cell (PMN)-derived C5aR and/or C3aR engagement. In this regard, the alternative pathway amplification loop is believed to be promoted at sites of local injury when inflammatory cells are recruited, either through a mechanism that involves the additional generation of necrotic cells that release proteases which damage cells and result in increased complement fixation, and/or because these infiltrating cells produce C3 and properdin that increase activation specifically at that site. The presence of alternative pathway components carried in neutrophil granules and released following activation in vitro has previously been shown (Schwaeble and Reid, 1999), and this may play an important potential biologic role for this capacity in self tissue injury. In addition, C5a and C3a may play roles in increasing local vascular access in the joints to anti-CII mAbs as well as the engagement of additional innate immune pathways (Binstadt, Patel.P.R., Alencar.H., Nigrovic, Lee, Mahmood, Weisledder, Mathis, and Benoist, 2006;Lee, Friend, Gurish, Benoist, Mathis, and Brenner, 2002).
Another possibility that could underlie this phenotype is that activation of complement leads to changes in the expression of critical neoepitopes that are recognized by an important new class of pathogenic natural antibodies. Rather than being polyreactive, as is true of the great majority of natural antibodies, these natural antibodies recognize a conserved family of neoepitopes which are uniquely expressed on injured tissues and appear to serve an essential role as “danger” signals that are essential to initiate the inflammatory response which follows. Importantly, unlike traditional “autoantibodies” directed to self-antigens, these natural antibodies do not recognize tissues in the absence of injury but only react with neoepitopes that are elaborated as part of the “danger signal” process (Carroll and Holers, 2005;Fleming, 2012). Two major examples of pro-inflammatory IgM natural antibodies have been described through the characterization of highly informative mAbs. The first recognizes non-muscle myosin heavy chain-II (NMHC) (Zhang, Alicot, Chiu, Li, Verna, Vorup-Jensen, Kessler, Shimaoko, Chan, Friend, Mahmood, Weissleder, Moore Jr., and Carroll, 2006;Zhang, Austen Jr., Chiu, Alicot, Hung, Ma, Verna, Xu, Hechtman, Moore Jr., and Carroll, 2004). The second subset recognizes annexin-4 (ANX4) (Kulik, Fleming, Moratz, Reuter, Novikov, Chen, Andrews, Markaryan, Quigg, Silverman, Tsokos, and Holers, 2009). ANX4 belongs to a family of Ca2+ and phospholipid-binding proteins [reviewed in (Rescher and Gerke, 2004)]; and annexins including ANX4 have been shown to be elaborated on the external cell membrane through a poorly understood mechanism (Mayran, Traverso, Maroux, and Massey-Harroche, 1996).
Anti-ANX4 IgM natural antibodies have been shown to play roles in a wide array of injury models, including intestinal ischemia-reperfusion injury (Kulik et al., 2009), choroidal neovascularization following laser injury (Blanche, Maltby, Bennett, Kubes, Lee, and McNagny, 2010), and stroke injury (Elvington, Atkinson, Kulik, Zhu, Yu, Kindy, Holers, and Tomlinson, 2012). These findings suggest the likelihood of a previously under-appreciated role for this “hard-wired” recognition process in disease pathogenesis in reperfusion injury as well as non-vascular models. In addition, the potential ability to therapeutically modulate this type of natural antibody-dependent pro-inflammatory process using experimental approaches in vivo has yielded highly encouraging results. Specifically, the effects of pro-inflammatory natural antibodies in injury have been ameliorated by using peptides or proteins that mimic the tissue neoepitopes, including NMHC (Zhang et al., 2006) and ANX4 (Kulik et al., 2009), thereby blocking pathogenic natural antibody binding to the site through the recognition of neoepitopes.
Molecular imaging of tissue-fixed complement C3 activation fragments
During complement activation the C3 protein is cleaved, and C3 activation fragments are covalently attached to tissues and can serve as a durable biomarker of tissue inflammation and damge. These fragments have been exploited as addressable binding ligands for targeted therapeutics utilizing complement receptor type 2 (CR2)-linked inhibitors that direct inhibitors to the brain, intestine, eye, joints and other tissues (Holers, Rohrer, and Tomlinson, 2013). Recently, novel cross-reactive murine monoclonal antibodies to human and mouse C3d have also been generated which bind to the iC3b, C3dg, and C3d fragments in solution and on surfaces, but do not bind to intact C3 or C3b (Thurman, Kulik, Orth.H., Wong, Renner, Sargsyan, Mitchell, Hourcade, Hannan, Kovacs, Coughlin, Woodell, Pickering, Rohrer, and Holers, 2013). The same mAbs bind C3d from human, murine and cynomolgous sources also bind to tissue-bound C3 activation fragments in mice when injected systemically.
Using mouse models of renal and ocular disease, it has shown that following systemic injection the antibodies accumulate at sites of C3 fragment deposition within the glomerulus, the renal tubular interstitium, and the posterior pole of the eye. To detect antibodies bound within the eye, optical imaging of FITC-bound mAb was used, which demonstrated accumulation of the antibodies within retinal lesions in a model of choroidal neovascularization (CNV) (Thurman et al., 2013). Such imaging methods that utilize these antibodies may provide a sensitive means of detecting and monitoring complement activation-associated tissue inflammation.
Informative mAbs to tissue-bound C3 fragments could also have other biomedical uses. For example, similarly to CR2, they could be used as in vivo targeting methods for therapeutic agents. In addition, they could potentially modulate the biologic functions of the C3 fragments.
Conclusions and Next Steps
This review has laid out several new directions of research in the complement field that are focused on the actions which occur in the micro-environments on surfaces that lie within sites of tissue injury. These processes include are effector pathway-catalyzed amplification of proximal complement activation, de-regulation of the alternative pathway through interference with FH, recognition of injury-associated ligands by components of the lectin pathway, and engagement of pathogenic natural antibodies that recognize a limited set of injury-associated neoepitopes. One way to consider these processes on the cartilage and within the synovium is outlined in Figure 2. Finally, each of these concepts has potential therapeutic relevance, either as a pathway that should be blocked, a process that could be utilized to direct inhibitors to the correct sites, or as a potential biomarker that can be detected in live subjects through imaging approaches. One particular example of imaging sites of injury through detection of tissue-fixed C3 fragment deposition with monoclonal antibodies has already been demonstrated through pre-clinical studies.
Figure 2.
Model of initiation and propagation of joint inflammation and damage in CAIA following infusion of anti-CII mAbs. Explored in this review are the importance of lectin pathway activation, the role of FHRs in controlling alternative pathway activation, and the deconstruction of the pathogenic mechanisms which are engaged following the generation of effector molecules (C5a, C3a and the MAC). These processes promote alternative pathway engagement and the amplification loop, resulting in enhanced anti-CII and natural antibody access and/or deposition followed by C3 activation. Note also the proposed role for local production of indicated factors from fibroblast-like synovicytes & and adipocytes*. CP: classical pathway; LP, lectin pathway.
Highlights.
The complement alternative pathway and amplification loop are major mediators of injury in vivo
These pathways act on tissue surfaces and are controlled by both positive and negative factors
Distal effector mechanisms of complement exert a positive feedback on local activation
Injury-induced ligand recognition by the lectin pathway plays a critical role in tissue injury
Local pro-inflammatory mechanisms engaged by complement include pathogenic natural antibodies
Acknowledgements
Studies described herein that have been undertaken by the author have been funded by NIH R01 AR51749, the Rheumatology Research Foundation, the Arnold and Mabel Beckman Foundation, and the Beckman Initiative for Macular Research. The author also acknowledges the many longstanding conversations and research activities with collaborators Josh Thurman, Steve Tomlinson and Barb Rohrer that have helped to shape the content of this review. Dr. Holers receives royalties from Taligen/Alexion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ammitzboll CG, Thiel S, Ellingsen T, Deleuran B, Jorgensen A, Jensenius JC, Stengaard-Pedersen K. Levels of lectin pathway proteins in plasma and synovial fluid of rheumatoid arthritis and osteoarthritis. Rheumatol. Intl. 2012;32:1457–1463. doi: 10.1007/s00296-011-1879-x. [DOI] [PubMed] [Google Scholar]
- 2.Arend WP, Mehta G, Antonioli AH, Takahashi M, Takahashi K, Stahl GL, Holers VM, Banda NK. Roles of adipocytes and fibroblasts in activation of the alternative pathway of complement in inflammatory arthritis in mice. J. Immunol. 2013;190:6423–6433. doi: 10.4049/jimmunol.1300580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backlund J, Carlsen S, Hoger T, Holm B, Fugger L, Kihlberg J, Burkhardt H, Holmdahl R. Predominant selection of T cells specific for the glycosylated collagen type II epitope (263-270) in humanized transgenic mice and in rheumatoid arthritis. PNAS. 2002;99:9960–9965. doi: 10.1073/pnas.132254199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakker AC, van de Loo FA, Joosten LA, Bennink MB, Arntz OJ, Dmitriev P, Kashentsera EA, Curiel DT, van den Berg WB. A tropism-modified adenoviral vector increased the effectiveness of gene therapy for arthritis. Gene Ther. 2001;8:1785–1793. doi: 10.1038/sj.gt.3301612. [DOI] [PubMed] [Google Scholar]
- 5.Banda NK, Hyatt S, Antonioli AH, White JT, Glogowska M, Takahashi K, Merkel TJ, Stahl GL, Mueller-Ortiz SL, Wetsel RA, Arend WP, Holers VM. Role of C3a receptors, C5a receptors, and complement protein C6 deficiency in collagen antibody-induced arthritis in mice. J. Immunol. 2012;188:1469–1478. doi: 10.4049/jimmunol.1102310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banda NK, Mehta G, Ferreira VP, Cortes C, Pickering MC, Pangburn MK, Arend WP, Holers VM. Essential role of surface-bound complement factor H in controlling immune complex-induced arthritis. J. Immunol. 2013;190:3560–3569. doi: 10.4049/jimmunol.1203271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banda NK, Mehta G, Kjaer TR, Takahashi M, Schaack J, Morrison TE, Thiel S, Arend WP, Holers VM. Essential role for the lectin pathway in collagen antibody-induced arthritis revealed through use of adenovirus programming complement inhibitor MAp44 expression. J. Immunol. 2014;193:2455–2468. doi: 10.4049/jimmunol.1400752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banda NK, Takahashi K, Wood AK, Holers VM, Arend WP. Pathogenic complement activation in collagen antibody-induced arthritis in mice requires amplification by the alternative pathway. J. Immunol. 2007;179:4101–4109. doi: 10.4049/jimmunol.179.6.4101. [DOI] [PubMed] [Google Scholar]
- 9.Banda NK, Takahashi M, Levitt B, Glogowska M, Nicholas J, Takahashi K, Stahl GL, Fujita T, Arend WP, Holers VM. Essential role of complement mannose-binding lectin-associated serine proteases-1/3 in the murine collagen antibody-induced model of inflammatory arthritis. J. Immunol. 2010;185:5598–5606. doi: 10.4049/jimmunol.1001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banda NK, Takahashi M, Takahashi K, Stahl GL, Hyatt S, Glogowska M, Wiles TA, Endo Y, Fujita T, Holers VM, Arend WP. Mechanisms of mannose-binding lectin-associated serine proteases-1/3 activation of the alternative pathway of complement. Mol. Immunol. 2011;49:281–289. doi: 10.1016/j.molimm.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banda NK, Thurman JM, Kraus D, Wood A, Carroll MC, Arend WP, Holers VM. Alternative complement pathway activation is essential for inflammation and joint destruction in the passive transfer model of collagen-induced arthritis. J. Immunol. 2006;177:1904–1912. doi: 10.4049/jimmunol.177.3.1904. [DOI] [PubMed] [Google Scholar]
- 12.Binstadt BA, Patel PR, Alencar H, Nigrovic PA, Lee DM, Mahmood U, Weisledder R, Mathis D, Benoist C. Particularities of the vasculature can promote the organ specificity of autoimmune attack. Nature Immunol. 2006;7:284–292. doi: 10.1038/ni1306. [DOI] [PubMed] [Google Scholar]
- 13.Blanche M-RGM, Maltby S, Bennett JPB, Kubes P, Lee DM, McNagny KM. Loss of CD34 leads to exacerbates autoimmune arthritis through increased vascular permeability. J. Immunol. 2010;184:1292–1299. doi: 10.4049/jimmunol.0900808. [DOI] [PubMed] [Google Scholar]
- 14.Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nature Rev. Rheum. 2013;9:24–33. doi: 10.1038/nrrheum.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll MC, Holers VM. Innate autoimmunity. Adv. Immunol. 2005;86:137–157. doi: 10.1016/S0065-2776(04)86004-8. [DOI] [PubMed] [Google Scholar]
- 16.Collard CD, Montalto MC, Reenstra WR, Buras JA, Stahl GL. Endothelial oxidative stress activates the lectin complement pathway: role of cytokeratin 1. Am. J. Pathol. 2001;159:1045–1054. doi: 10.1016/S0002-9440(10)61779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooke TD, Hurd ER, Jasin HE, Bienenstock H, Ziff M. Identification of immunoglobulins and complement in rheumatoid articular collagenous tissues. Arth. Rheum. 1975;18:541–551. doi: 10.1002/art.1780180603. [DOI] [PubMed] [Google Scholar]
- 18.de Cordoba SR, Tortajada A, Harris CL, Morgan BP. Complement dysregulation and disease: from genes and proteins to diagnostics and drugs. Immunobiology. 2012;217:1034–1046. doi: 10.1016/j.imbio.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 19.Deane KD, Norris JM, Holers VM. Pre-clinical rheumatoid arthritis: identification, evaluation and future directions for investigation. Rheum. Dis. Clin. North Am. 2010:236–241. doi: 10.1016/j.rdc.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Degn SE, Hansen AG, Steffensen R, Jacobsen C, Jensenius JC, Thiel S. MAp-44, a human protein associated with patter recognition molecules of the complement system and regulating the lectin pathway of complement activation. J. Immunol. 2009;183:7371–7378. doi: 10.4049/jimmunol.0902388. [DOI] [PubMed] [Google Scholar]
- 21.Degn SE, Jensen L, Olszowski T, Jensenius JC, Thiel S. Co-complexes of MASP-1 and MASP-2 associated with the soluble pattern-recognition molecules drive lectin pathway activation in a manner inhibitable by Map44. J. Immunol. 2013;191:1334–1345. doi: 10.4049/jimmunol.1300780. [DOI] [PubMed] [Google Scholar]
- 22.Demoruelle MK, Deane KD. Treatment strategies in early rheumatoid arthritis and prevention of rheumatoid arthritis. Curr. Rheumatol. Rep. 2012;14:472–480. doi: 10.1007/s11926-012-0275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elvington A, Atkinson C, Kulik L, Zhu H, Yu J, Kindy MS, Holers VM, Tomlinson S. Pathogenic natural antibodies propagate cerebral injury following ischemic stroke in mice. J. Immunol. 2012;188:1460–1468. doi: 10.4049/jimmunol.1102132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–54. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 25.Ferreira VP, Herbert AP, Hocking HG, Barlow PN, Pangburn MK. Critical role of the C-terminal domains of factor H in regulating complement activation at cell surfaces. J. Immunol. 2006;177:6308–6316. doi: 10.4049/jimmunol.177.9.6308. [DOI] [PubMed] [Google Scholar]
- 26.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 27.Fleming SD. Naturally occurring autoantibodies mediate ischemia/reperfusion-induced tissue injury. Adv. Exp. Med. Biol. 2012;750:174–185. doi: 10.1007/978-1-4614-3461-0_13. [DOI] [PubMed] [Google Scholar]
- 28.Fritsche LG, Lauer N, Hartmann A, Stippa S, Keilhauer CN, Oppermann M, Pandey MK, Kohl J, Zipfel PF, Weber BHF, Skerka C. An imbalance of human complement regulatory proteins CFHR1, CFHR3 and factor H influences risk for age-related macular degeneration. Human Molecular Genetics. 2010;19:4694–4704. doi: 10.1093/hmg/ddq399. [DOI] [PubMed] [Google Scholar]
- 29.Gadjeva M, Takahashi K, Thiel S. Mannan-binding lectin--a soluble pattern recognition molecule. Mol. Immunol. 2004;41:113–121. doi: 10.1016/j.molimm.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 30.Goicoechea de Jorge E, Caesar JJE, Malik TH, Paten M, Colledge M, Johnson S, Hakobyan S, Morgan BP, Harris CL, Pickering MC, Lea SM. Dimerization of complement factor H-related proteins modulates complement activation in vivo. PNAS. 2013;110:4685–4690. doi: 10.1073/pnas.1219260110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansen S, Sehman L, Palaniyar N, Ziegler K, Brandt J, Kliem A, Jonasson M, Skjoedt M-O, Nielsen O, Hartshom K, Jorgensen TJ, Holmskov U. Collectin 11 (CL-11, CL-K1) is a MASP-1/3 assciated plasma collectin with microbial-binding activity. J. Immunol. 2010;185:6096–6104. doi: 10.4049/jimmunol.1002185. [DOI] [PubMed] [Google Scholar]
- 32.Heinen S, Hartmann A, Lauer N, Wiehl U, Dahse H-M, Schirmer S, Gropp K, Enghardt T, Wallich R, Halbich S, Mihlan M, Schlotzer-Schrehardt U, Zipfel PF, Skerka C. Factor H-related protein 1 (CFHR-1) inhibits complement C5 convertase activity and terminal complex formation. Blood. 2009;114:2439–2447. doi: 10.1182/blood-2009-02-205641. [DOI] [PubMed] [Google Scholar]
- 33.Holers VM. Complement. In: Rich R, editor. Principles and Practices of Clinical Immunology Mosby. St. Louis, MO: 2001. pp. 21.1–21.8. [Google Scholar]
- 34.Holers VM. Autoimmunity to citrullinated proteins and the initiation of rheumatoid arthritis. Curr. Opin. Immunol. 2013a;25:728–735. doi: 10.1016/j.coi.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holers VM. Human C3 glomerulopathy provides unique insights into complement factor H-related protein function. J. Clin. Invest. 2013b;123:2357–2360. doi: 10.1172/JCI69684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holers VM, Carroll MC, Holers Innate Autoimmunity. Adv. Immunol. 2005;86:137–157. doi: 10.1016/S0065-2776(04)86004-8. [DOI] [PubMed] [Google Scholar]
- 37.Holers VM, Rohrer B, Tomlinson S. CR2-mediated targeting of complement inhibitors: bench-to-bedside using a novel strategy for site-specific complement modulation. Adv. Exp. Med. Biol. 2013;735:137–154. doi: 10.1007/978-1-4614-4118-2_9. [DOI] [PubMed] [Google Scholar]
- 38.Hughes AE, Orr N, Esfandiary H, Diaz-Torres M, Goodship T, Chakravarthy U. A common CFH haplotype,with deletion of CFHR1 and CFHR3,is associated with lower risk of age-related macular degeneration. Nat. Genetics. 2006;38:1173–1177. doi: 10.1038/ng1890. [DOI] [PubMed] [Google Scholar]
- 39.Ip WK, Takahashi K, Ezekowitz RA, Stuart LM. Mannose-binding lectin and innate immunity. Immunol. Rev. 2009;230:9–21. doi: 10.1111/j.1600-065X.2009.00789.x. [DOI] [PubMed] [Google Scholar]
- 40.Jha P, Bora PS, Bora NS. The role of the complement system in ocular diseases including uveitis and macular degeneration. Mol. Immunol. 2007;44:3901–3908. doi: 10.1016/j.molimm.2007.06.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jokiranta ST, Zipfel PF, Fremeaux-Bacchi V, Taylor CM, Goodship TJH, Noris M. Where next with atypical hemolytic uremic syndrome? Mol. Immunol. 2007;44:3889–3900. doi: 10.1016/j.molimm.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Jozsi M, Manuelian T, Heinen S, Opperman M, Zipfel PF. Attachment of the soluble complement regulator factor H to cell and tissue surfaces: relevance for pathology. Hist. Histopath. 2004;19:251–258. doi: 10.14670/HH-19.251. [DOI] [PubMed] [Google Scholar]
- 43.Jozsi M, Opperman M, Lambris JD, Zipfel PF. The C-terminus of complement factor H is essential for host cell protection. Mol. Immunol. 2007;44:2697–2706. doi: 10.1016/j.molimm.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jozsi M, Zipfel PF. Factor H family proteins and human diseases. Trends Immunol. 2008;29:380–387. doi: 10.1016/j.it.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Kavanaugh D, Goodship THJ, Richards A. Atypical haemolytic uraemic syndrome. British Med. Bulletin. 2006;77:5–22. doi: 10.1093/bmb/ldl004. [DOI] [PubMed] [Google Scholar]
- 46.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski AM, Yalavarthi S, Knight JS, Friday S, Li S, Patel RM, Subramanian V, Thompson P, Chen P, Fox DA, Pennathur S, Kaplan MJ. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Trans. Med. 2013 Mar 27;5(178):178ra40. doi: 10.1126/scitranslmed.3005580. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kjaer TR, Thiel S, Andersen GR. Toward a structure-based comprehension of the lectin pathway of complement. Mol. Immunol. 2013;56:413–422. doi: 10.1016/j.molimm.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 48.Klareskog L, Gregersen PK, Huizinga TW. Prevention of autoimmune rheumatic disease: state of the art and future perspectives. Ann. Rheum. Dis. 2010;69:2062–2069. doi: 10.1136/ard.2010.142109. [DOI] [PubMed] [Google Scholar]
- 49.Klareskog L, Ronnelid J, Lundberg K, Padyukov L, Alfredsson L. Immunity to citrullinated proteins in rheumatoid arthritis. Ann. Rev. Immunol. 2008;26:651–675. doi: 10.1146/annurev.immunol.26.021607.090244. [DOI] [PubMed] [Google Scholar]
- 50.Kulik L, Fleming SD, Moratz C, Reuter JW, Novikov A, Chen K, Andrews KA, Markaryan A, Quigg RJ, Silverman GJ, Tsokos GJ, Holers VM. Pathogenic natural antibodies recognizing annexin IV are required to develop intestinal ischemia-reperfusion injury. J. Immunol. 2009;182:5363–5373. doi: 10.4049/jimmunol.0803980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lachmann PJ. The amplification loop of the complement pathways. Adv. Immunol. 2009;104:115–149. doi: 10.1016/S0065-2776(08)04004-2. [DOI] [PubMed] [Google Scholar]
- 52.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;197:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 53.Linton SM, Morgan BP. Complement activation and inhibition in experimental models of arthritis. Mol. Immunol. 1999;36:905–914. doi: 10.1016/s0161-5890(99)00113-3. [DOI] [PubMed] [Google Scholar]
- 54.Liszewski MK, Farries TC, Lublin DM, Rooney IA, Atkinson JP. Control of the complement system. Adv. Immunol. 1996;61:201–283. doi: 10.1016/s0065-2776(08)60868-8. [DOI] [PubMed] [Google Scholar]
- 55.Malhotra R, Wormald MR, Rudd PM, Fischer PB, Dwek RA, Sim RB. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat. Med. 1995;1:237–243. doi: 10.1038/nm0395-237. [DOI] [PubMed] [Google Scholar]
- 56.Matsushita M. Ficolins: complement-activating lectins involved in innate immunity. J. Innate Imm. 2010;2:24–32. doi: 10.1159/000228160. [DOI] [PubMed] [Google Scholar]
- 57.Mayran N, Traverso V, Maroux S, Massey-Harroche D. Cellular and subcellular localization of annexins I, IV, and V in lung epithelia. Am. J. Physiol. 1996;270:L863–L871. doi: 10.1152/ajplung.1996.270.5.L863. [DOI] [PubMed] [Google Scholar]
- 58.Monach PA, Benoist C, Mathis D. The role of antibodies in mouse models of rheumatoid arthritis, and relevance to human disease. Adv. Immunol. 2004;82:217–248. doi: 10.1016/S0065-2776(04)82005-4. [DOI] [PubMed] [Google Scholar]
- 59.Morgan HP, Schmidt CQ, Guariento M, Blaum BS, Gillespie D, Herbert AP, Kavanagh D, Mertens HD, Svergun DI, Johansson CM, Uhrin D, Barlow PN, Hannan JP. Structural basis for engagement by complement factor H of C3b on a self surface. Nature Struct. Mol. Biol. 2011;18:463–470. doi: 10.1038/nsmb.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neumann E, Barnum SR, Tarner IH, Echols J, Fleck M, Judex M, Kullmann F, Mountz JD, Scholmerich J, Gay S, Muller-Ladner U. Local production of complement proteins in rheumatoid arthritis synovium. Arth. Rheum. 2002;46:934–945. doi: 10.1002/art.10183. [DOI] [PubMed] [Google Scholar]
- 61.Ohtani K, Suzuki Y, Wakamiya N. Biological functions of the novel collectins CL-L1, CL-K1, and CL-P1. J. Biomed. Biotech. 20122012:493945. doi: 10.1155/2012/493945. doi: 10.1155/2012/493945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pavlov VI, Skjoedt M-O, Tan YS, Rosbjerg A, Garred PSGL. Endogenous and natural complement inhibitor attenuates myocardial injury and arterial thrombogenesis. Circulation. 2012;126:2227–2235. doi: 10.1161/CIRCULATIONAHA.112.123968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reid KBM, Turner MW. Mammalian lectins in activation and clearance mechanisms involving the complement system. Springer Semin. Immunopathol. 1994;15:307–325. doi: 10.1007/BF01837363. [DOI] [PubMed] [Google Scholar]
- 64.Rescher U, Gerke V. Annexins - unique membrane binding proteins with diverse functions. J. Cell Sci. 2004;117:2631–2639. doi: 10.1242/jcs.01245. [DOI] [PubMed] [Google Scholar]
- 65.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ricklin D, Lambris JD. Progress and trends in complement therapeutics. Adv. Exp. Med. Biol. 2013;735:1–22. doi: 10.1007/978-1-4614-4118-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rossi V, Cseh S, Bally I, Thielens NM, Jensenius JC, Arlaud GJ. Substrate specificities of recombinant mannan-binding lectin-associated serine proteases-1 and -2. J. Biol. Chem. 2001;276:40880–40887. doi: 10.1074/jbc.M105934200. [DOI] [PubMed] [Google Scholar]
- 68.Runza VL, Schwaeble W, Mannel DN. Ficolins: novel pattern recognition molecules of the innate immune response. Immunobiology. 2008;213:297–306. doi: 10.1016/j.imbio.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 69.Schwaeble WJ, Reid KBM. Does properdin crosstalk the cellular and the humoral immune response? Immunol. Today. 1999;20:17–21. doi: 10.1016/s0167-5699(98)01376-0. [DOI] [PubMed] [Google Scholar]
- 70.Silman AJ. Epidemiology of rheumatoid arthritis. APMIS. 1994;102:721–728. doi: 10.1111/j.1699-0463.1994.tb05226.x. [DOI] [PubMed] [Google Scholar]
- 71.Silman AJ, Hochberg MC. Rheumatoid Arthritis. In: Silman AJ, Hochberg MC, editors. Epidemiology of the Rheumatic Diseases. Oxford University Press; New York: 1993. pp. 7–68. [Google Scholar]
- 72.Skjoedt M-O, Hummelshoj T, Palarasah Y, Honore C, Koch C, Skjodt K, Garred P. A novel mannose-binding lectin/ficolin-associated protein is highly expressed in heart and skeletal muscle tissues and inhibits complement activation. J. Biol. Chem. 2010;285:32913–32921. doi: 10.1074/jbc.M109.065805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sokolove J, Zhao X, Chandra PE, Robinson WH. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fc-gamme receptor. Arth. Rheum. 2011;63:53–62. doi: 10.1002/art.30081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thurman JM, Kulik L, Orth H, Wong M, Renner B, Sargsyan SA, Mitchell LM, Hourcade DE, Hannan JP, Kovacs JM, Coughlin B, Woodell AS, Pickering MC, Rohrer B, Holers VM. Detection of complement activation using monoclonal antibodies against C3d. J. Clin. Invest. 2013;123:2218–2230. doi: 10.1172/JCI65861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tortajada A, Yebenes H, Abarrategui-Garrido C, Anter J, Garcia-Fernandez JM, Martinez-Barricarte R, Alba-Dominguez M, Malik TH, Bedoya R, Perez RC, Trascasa ML, Pickering MC, Harris CL, Sanchez-Corral P, Llorca O, de Cordoba SR. C3 glomerulopathy-associated CFHR1 mutation alters FHR oligomerization and complement regulation. J. Clin. Invest. 2013;123:2434–2446. doi: 10.1172/JCI68280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Uysal H, Bockermann R, Nandakumar KS, Sehnert B, Bajtner E, Engstrom A, Serre G, Burkhardt H, Thunnissen MMGM, Holmdahl R. Structure and pathogenicity of antibodies specific for citrullinated collagen type II in experimental arthritis. J. Exp. Med. 2009;206:449–462. doi: 10.1084/jem.20081862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Venrooij WJ, Pruijn GJM. Citrullination: a small change for a protein with great consequences for rheumatoid arthritis. Arthritis Research. 2000;2:249–251. doi: 10.1186/ar95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van Venrooij WJ, van de Putte LBA. Is assaying autoantibodies useful for diagnosing rheumatoid arthritis. Nature Clin Practice Rheum. 2005;1:4–5. doi: 10.1038/ncprheum0018. [DOI] [PubMed] [Google Scholar]
- 79.Zhang M, Alicot EM, Chiu I, Li J, Verna N, Vorup-Jensen T, Kessler B, Shimaoko M, Chan R, Friend D, Mahmood U, Weissleder R, Moore FD, Jr., Carroll MC. Identification of the target self antigens in reperfusion injury. J. Exp. Med. 2006;203:141–152. doi: 10.1084/jem.20050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang M, Austen WG, Jr., Chiu I, Alicot EM, Hung R, Ma M, Verna N, Xu M, Hechtman HB, Moore FD, Jr., Carroll MC. Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Pro. Natl. Acad. Sci. 2004;101:3886–3891. doi: 10.1073/pnas.0400347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou H, Yana H, Bertrama P, Hua Y, Springer LE, Thompson RW, Curci JA, Hourcade DE, Pham CTN. Fibrinogen-specific antibody induces abdominal aortic aneurysm in mice through complement lectin pathway activation. PNAS. 2013;110:4335–4344. doi: 10.1073/pnas.1315512110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zipfel PF, Skerka C, Hellwage J, Jokiranta ST, Meri S, Brade V, Kraiczy P, Noris M, Remuzzi G. Factor H family proteins: on complement, microbes and human diseases. Biochem. Soc. Trans. 2002;30:971–978. doi: 10.1042/bst0300971. [DOI] [PubMed] [Google Scholar]