Abstract
Objectives
Pessaries are important options for women with pelvic floor disorders, but many pessary users experience bacterial vaginosis (BV). The aim of this study was to evaluate the effect of TrimoSan© gel on BV prevalence among pessary users.
Study Design
Women presenting for a pessary fitting completed questionnaires on vaginal symptoms and hormone therapy (HT) use and underwent a BV® BLUE test and slide collection for BV analysis by Nugent's criteria. Following pessary fitting, women were randomized to either standard pessary care with the use of TrimoSan© placed vaginally twice weekly or to standard pessary care without TrimoSan© gel. Women returned 2 weeks and 3 months later for repeat slide collection for Gram stain, BV® BLUE testing, and completion of questionnaires on vaginal symptoms and desire to continue the pessary.
Results
There were 184 women randomized after successful fitting (92 to the TrimoSan© group), and 147 (79%) presented for 3 month follow up. Mean age was 56 ± 16 years; patients were mostly Caucasian (57%) or Hispanic (23%) and 36% were using HT. The groups did not differ in the prevalence of BV by Nugent's criteria at 2 weeks (20% TrimoSan© vs 26% no gel, p=0.46) or 3 months (24% TrimoSan© vs 23% no gel, p=0.82), nor did they differ in BV by BV® BLUE testing at 2 weeks (0%TrimoSan©vs 4% no gel, p=0.12) or 3 months (3% TrimoSan© vs 0% no gel, p=0.15). The prevalence of at least one vaginal symptom did not differ between groups at 2 weeks (44% TrimoSan© vs 45% no gel, p=0.98) or 3 months (42% TrimoSan© vs 32% no gel, p=0.30). The TrimoSan© group was equally likely to want to continue their pessary use compared to the standard care group at 2 weeks (90% vs 86%, p=0.64) and 3 months (63% vs 60%, p=0.76).
Conclusions
TrimoSan© gel in the first 3 months of pessary use does not decrease the prevalence of BV or vaginal symptoms and does not alter the likelihood of a woman desiring to continue pessary use.
Keywords: pessary, bacterial vaginosis, TrimoSan, discharge, removal
Introduction
Pelvic floor support disorders are common and debilitating to women, resulting in a significant deterioration in quality of life and overall health. Pelvic floor disorders are prevalent, with up to 25% of women having at least one symptomatic pelvic floor disorder.1 Although surgery for these disorders is increasingly common,2 many women cannot undergo surgery or prefer non-surgical treatment options. Many women can be successfully fitted with a pessary, a silicone device placed in the vagina to provide pelvic support for the treatment of pelvic organ prolapse and stress urinary incontinence.3 Although pessaries relieve symptoms in the majority of users, nearly half of women discontinue pessary use within one year due to a variety of troublesome side effects,4 including vaginal discharge and odor. Patients who meet established goals of pessary use are more likely to continue the pessary,5 so clinicians are in need of evidence-based means to maintain pessary satisfaction after fitting.
Bacterial vaginosis (BV), a shift in the ecologic balance of the vagina leading to malodorous vaginal discharge, is common among pessary users and may negatively affect their experience with a pessary. Prior authors have published that up to 30% of women with a pessary experience BV, in contrast to 10% in the general population.6 Many providers attempt to avoid pessary-related BV by counseling patients on hygiene measures and care, but no randomized trials investigate the care of a pessary after fitting.7 TrimoSan© gel, a mildly acidic vaginal lubricant that is dispensed with some pessaries, may lower the pH of the vagina and keep the vaginal ecosystem in balance during pessary use, but no clinical trials examine its effect on BV or on other outcomes.
We conducted a randomized, controlled trial to determine if TrimoSan© gel decreases the prevalence of BV or bothersome vaginal symptoms during the first three months of pessary use. We hypothesized that the Trimo-San© gel would decrease BV and vaginal symptoms compared to women not using the Trimo-San© gel.
Methods
This was a multi-center, randomized, single-blind, controlled trial of patients who were fitted for a pessary for any indication at two tertiary care centers, from 7/1/2010-12/31/2011 at MedStar Washington Hospital Center (Washington, DC) and from 7/1/2012-5/5/2014 at the University of New Mexico Health Sciences Center (Albuquerque, NM). The primary outcome was the determination of BV by Nugent's criteria on Gram stain three months after pessary fitting. The study was approved by the Institutional Review Boards at both clinical sites after ethics committee approval and was registered at ClinicalTrials.gov (ID: NCT01471457).
Potential patients were identified prior to or at the time of a pessary fitting visit, and they were enrolled after providing written, informed consent. We approached all patients who were >18 years of age, suitable for follow up, and undergoing a pessary fitting for any indication. Patients were excluded if they were younger than 18 years of age, had a known allergic or suspected adverse reaction to TrimoSan© gel or any of its components, had an inability to use a pessary or TrimoSan© as indicated, already had used a pessary within the last 12 months, had a history of frequent or chronic BV (>2 episodes per year or symptoms >6 months out of the last 12 months), had a history of active vaginal ulcerative disease (active ulcers from atrophy, herpes, or mesh erosion with >2 episodes of ulcers per year or last ulcer <1 month ago), were using long-term antibiotics for indications not listed above, were unable to speak English, were unable to provide informed consent, or were unable to be fitted with a pessary. If patients were randomized at the time of initial pessary fitting but unable to retain the pessary or be successfully fitted before leaving the office examination and declined a second attempt at pessary fitting, they were excluded after randomization for failure to be fitted with a pessary. Patients who were randomized and fit with a pessary who were planning on wearing their pessary continuously at home and coming to the office for cleaning/removal were still included in the study.
Patients completed baseline questionnaires on their medical history and health, use of hormone therapy (HT) orally and/or vaginally, any vaginal products or medications being used in the vagina, and a questionnaire on vaginal symptoms including vaginal discharge, fishy odor, clear or grey vaginal discharge, “too much” vaginal discharge, vaginal discharge interfering with sex, vaginal itching, vaginal pain, vaginal pain interfering with sex, and vaginal sores. Any hormone therapy use (Any HT) was defined as the use of systemic (oral, transdermal, or vaginal HT), and vaginal hormone therapy use (vaginal HT) was defined as any hormone therapy given via a vaginal route (cream, ring, etc.). Therefore, the group using HT included the women using vaginal HT plus women using other forms of HT. The vaginal HT group included women already using vaginal HT upon entry in to the study and women who initiated vaginal HT at the time of pessary initiation.
Prior to pessary fitting, study patients had a clean, plastic speculum inserted and vaginal secretion swabs were collected on two sterile cotton tip swabs, with care taken to sample from the middle third of the vagina. One swab was used to perform BV® BLUE testing (OSOM® Rapid Tests, Sekisui Diagnostics) at the time of the visit.8 If the patient was found positive for BV by BV® BLUE at this baseline visit, she was treated with 500 mg of oral metronidazole twice daily for 7 days. If the patient had an allergy to this medication, the patient was prescribed 300 mg of oral clindamycin twice daily for 7 days. The second vaginal secretions swab was rolled onto a dry, clean glass microscope slide, air-dried without a cover slip, and transported to the lab and stored at room temperature until the time of Gram staining. After Gram staining, Nugent's criteria was used to define the presence of BV,9 and allowed the laboratory assessor of the Gram stain to be remote from the clinical study site and unaware of the patient's treatment allocation.
Following the completion of vaginal specimen collection and questionnaires, patients were fitted with a pessary that was comfortable and allowed them to adequately void. At the time of pessary fitting, patients were randomized to either proceed with standard pessary hygiene plus the use of TrimoSan© gel (half applicator of gel vaginally twice a week) or to standard pessary care without the use of TrimoSan© gel. The randomization scheme was a blocked randomization sequence with randomly alternating block size (block size ranging from 5-10) with a 1:1 ratio of TrimoSan© gel to no TrimoSan© gel, stratified by women who intended to remove their pessary at least daily and women who intended to remove their pessary less often than daily. The randomization sequence was prepared by a statistician uninvolved in patient recruitment or allocation and was placed in sequentially numbered opaque sealed envelopes (SNOSE) for the two different strata by an investigator who also had no involvement in patient recruitment or treatment allocation. Women stated their intention for pessary removal frequency to the research staff performing the randomization during their pessary fitting, and the investigator opened the next SNOSE in the appropriate strata to assign the patient's randomization group. Patients randomized to the TrimoSan© group were then given TrimoSan© gel and instructions for its use, and patients allocated to the control group were specifically instructed not to use TrimoSan© gel. Patients were not blinded to their treatment allocation.
Women were seen at 2 weeks and 3 months later for examination and completed repeat questionnaires on pessary use, hygiene practices, and vaginal symptoms, including additional questions regarding increase in the four vaginal symptoms of discharge, itching, pain, and sores since pessary fitting. Women were asked how often they removed their pessary (daily or more often, less often than daily but at least once per week, less often than once per week), and how often they wore their pessary (never wear, wear 1-7 days per month, >7 days per month, daily). They were also asked to rank their desire to continue their pessary by 5 point Likert scale. At each examination, a mid-vaginal secretions swab was done to perform the BV® BLUE test in clinic and to prepare the microscopy slide for Gram staining.
For the analysis of the how frequently women removed their pessary, “frequent” pessary removal was defined as daily or more often, while “less often” pessary removal was defined as less often than daily. “Pessary satisfaction” was defined as a reply to the question “How much do you want to keep wearing your pessary in the future?” of “Quite a bit” or “Moderately”. “Excellent pessary satisfaction” was defined as a reply of “Quite a bit” to this question.
We analyzed the prevalence of reported vaginal symptoms individually and also for the presence of at least one reported vaginal symptom. We also analyzed the prevalence of women reporting an increase in one of four vaginal symptoms since pessary fitting (vaginal discharge, itching, pain, sores) and the prevalence of reporting an increase in at least one of these four symptoms since pessary fitting.
The primary outcome was the rate of BV by Gram Stain (Nugent's criteria) at 3 months after pessary fitting. The Nugent's score was determined by following a standardized Gram staining of the microscope slide. BV was defined as a Nugent's score >=7, in accordance with Nugent's criteria.9 Secondary outcomes included the rate of BV at 2 weeks after pessary fitting by Nugent's criteria, the rate of BV at 2 weeks and 3 months after pessary fitting by BV® BLUE test, the prevalence of individual vaginal symptoms or at least one reported vaginal symptom at 2 weeks and 3 months following pessary fitting, and the prevalence of the patient reporting an increase in at least one of the four key vaginal symptoms (discharge, itching, pain, sores) since pessary fitting at 2 weeks or 3 months.
We performed analyses both by intention-to-treat (primary analysis) and by per-protocol analysis. Women were asked to report their frequency of TrimoSan© use at each follow up visit. Women who used TrimoSan© gel at least once weekly were considered compliant with TrimoSan© use. The per-protocol analysis was performed by comparing those women who were compliant with TrimoSan© gel at least once a week (half of the recommended dosing) to those women who used the gel less frequently or did not use the gel. Power calculation was performed based on a 30% reported prevalence of BV in pessary wearers, in contrast to a prevalence of 10% in the general population.6 For the primary outcome of BV as detected by Gram stain 3 months after pessary fitting, the study sought to achieve a power of 88% at an α of 0.05 to detect a lowering of the prevalence of BV at 3 months in the TrimoSan©-using group from 30% back to the baseline rate of 10%. This translated to a sample size of 62 in each group. To account for at least a 15% dropout rate after randomization, we planned to enroll 75 women in each group. After initial recruitment in the first study site yielded a lower follow up rate than expected, the sample size was expanded to 180 patients, so that a 70% rate of follow up at 3 months would achieve the predetermined power.
The effect of dichotomous variables (such as randomization to TrimoSan©, any HT use, vaginal HT use, and frequent pessary removal) on dichotomous outcomes was determined by Chi-square tests (with Fisher's exact test used where there were significantly less than the expected number of data points), and the effect of dichotomous variables on continuous variables was determined by t-tests. For non-parametric variables (such as parity and number of vaginal deliveries), the Wilcoxon rank-sum test was used to compare between two groups.
We utilized the Mantel-Haenzel method to test the interaction of different frequencies of pessary wearing and pessary removal (as stated by the patient at follow up) with the relationship between the use of TrimoSan© gel and dichotomous outcomes. We also used the Mantel-Haenzel method to test the effect of HT use or vaginal HT use on the relationship between the use of TrimoSan© gel and dichotomous outcomes. We used general linear models to examine the effect of frequency of pessary removal and HT on the relationship between TrimoSan© gel use and continuous outcomes (such as the total Nugent's score).
We used a linear regression model to determine what other patient characteristics had an impact on the relationship between frequent pessary removal or HT and outcomes when significant findings were present in the uncorrected analysis. If certain patient characteristics were found to be impactful on the relationship, we repeated analysis with correction for this variable.
We utilized Chi-Square to analyze if there were significant differences in BV prevalence or vaginal symptoms at 2 weeks or 3 months between different reported frequencies pessary use or pessary removal, again utilizing Fisher's exact test where more appropriate. As noted above, we defined frequent pessary removal as once daily removal or more often, and analyzed the effect of frequent removal by Chi-square on BV and vaginal symptom outcomes.
Results
There were 466 women screened for eligibility during the study period (Figure 1), with 86 women excluded due to study criteria, 85 women declining to participate, and 108 women not presenting for pessary fitting after being screened. The remaining 187 women who consented for participation were randomized, of which 3 additional women were excluded after randomization for inability to be fitted with a pessary at their initial visit and patient declination to attempt another fitting. This left 184 active eligible participants, 92 randomized to TrimoSan© use and 92 randomized to no TrimoSan© use. Patient characteristics are presented in Table 1 and did not vary between the two groups at baseline. The mean age of the patients was 58.6 ± 16.4 years, the mean BMI was 28.3 ± 7.5, the median parity was 3, and the majority of the women were Caucasian (57%), Hispanic (23%), or African American (10%). The prevalence of BV (by Nugent's criteria or by BV® BLUE testing) and the total Nugent's score were similar between the groups at baseline. There were no differences between the groups in the number of women requiring pessary refitting at 2 weeks or 3 months. The two groups also did not differ in the frequency of wearing or removing the pessary reported by the patients at 2 weeks or 3 months, and did not differ in the prevalence of any HT use or vaginal HT use.
Figure 1. Participant Flow Diagram.
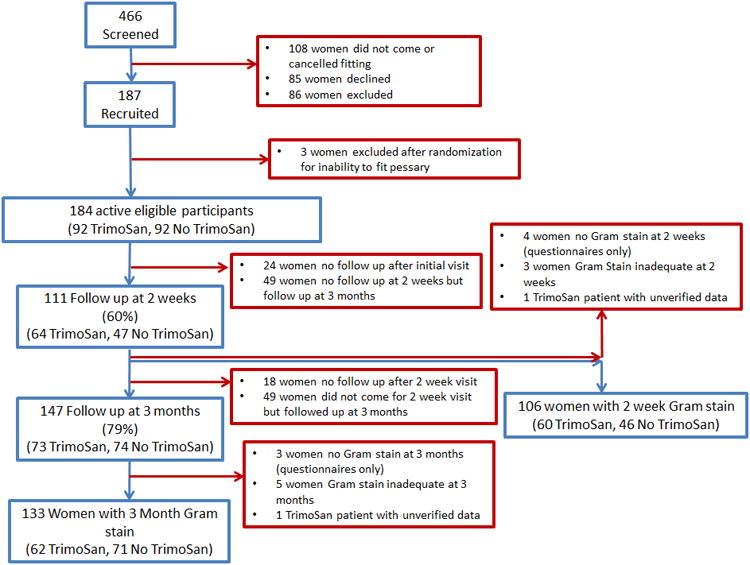
This figure demonstrates the flow of participants through the randomized trial design.
Table 1.
Study patient characteristics in the TrimoSan© gel and no TrimoSan© gel groups.
| TrimoSan© (n (%) or mean ± standard deviation* or median (variance)**) n=92 | No TrimoSan© (n (%) or mean ± standard deviation*or median (variance)**) n=92 | Total Patients n=184 | |
|---|---|---|---|
| Age (years)* | 58 ± 15 | 60 ± 17 | 59 ± 16 |
| Body Mass Index (kg/m2)* | 29 ± 7.6 | 28 ± 7.4 | 28 ± 7.5 |
| Race | |||
| Caucasian | 56 (61) | 48 (52) | 105 (57) |
| African American | 6 (7) | 13 (14) | 19 (10) |
| Hispanic | 23 (25) | 20 (22) | 43 (23) |
| Asian | 1 (1) | 3 (3) | 4 (2) |
| Other | 5 (5) | 8 (9) | 13 (7) |
| Parity** | 3 (2.6) | ||
| Vaginal deliveries** | 2 (2.4) | ||
| Indication for pessary use | |||
| Prolapse | 41 (44) | 45 (49) | 86 (47) |
| Urinary incontinence | 35 (38) | 30 (33) | 65 (35) |
| Both prolapse and urinary incontinence | 13 (14) | 10 (11) | 23 (13) |
| Smoking | |||
| Current | 8 (9) | 6 (7) | 14 (8) |
| Past | 26 (28) | 28 (30) | 54 (29) |
| Charleston Comorbidity Index* | 0.77 ± 1.1 | 0.89 ± 1.8 | 0.82 ± 1.5 |
| Insurance type | |||
| Private | 36 (39) | 38 (41) | 74 (40) |
| Public | 27 (29) | 34 (37) | 61 (33) |
| None | 7 (8) | 6 (7) | 13 (7) |
| Education Level | |||
| Graduate | 25 (27) | 28 (30) | 53 (29) |
| College | 37 (40) | 29 (32) | 66 (36) |
| High school | 20 (22) | 28 (30) | 48 (26) |
| Less than high school diploma | 3 (3) | 4 (4) | 7 (4) |
| Job Activity Level | |||
| Sedentary work | 16 (17) | 16 (17) | 32 (17) |
| Homemaker | 18 (20) | 18 (20) | 36 (20) |
| Light labor | 20 (22) | 26 (28) | 46 (25) |
| Heavy labor | 8 (9) | 5 (5) | 13 (7) |
| Unemployed or retired | 26 (28) | 19 (21) | 45 (24) |
| Prior pelvic surgery | 41 (49) | 39 (42) | 80 (43) |
| Prior pessary use | 9 (10) | 4 (4) | 13 (7) |
| Vaginal Infection History | |||
| Vaginal infection last 12 months | 10 (11) | 7 (8) | 17 (9) |
| HT | |||
| Using hormone therapy (HT) at time of recruitment | 22 (24) | 24 (26) | 46 (25) |
| Vaginal HT at time of recruitment | 19 (21) | 19 (21) | 38 (21) |
| HT prescribed at time of recruitment | 13 (14) | 14 (15) | 27 (15) |
| Pessary Refitting | |||
| Required refitting at 2 weeks | 13 (14) | 12 (13) | 25 (14) |
| Required refitting at 3 months | 15 (16) | 12 (13) | 27 (15) |
| BV Measures | |||
| BV by Nugent's criteria at baseline | 23 (25) | 15 (16) | 38 (21) |
| Total Nugent's score at baseline* | 4.5 ± 2.0 | 4.8 ± 2.4 | 4.6 ± 2.2 |
| BV by BV® BLUE at baseline | 1 (1) | 2 (2) | 3 (2) |
There were 160 women of 184 eligible study patients (87%) who presented for either 2 week or 3 month follow up, with 147 (79%) women presenting at 3 months for follow up and 133 of them (72%) qualifying for the primary outcome measure with an interpretable 3 month Gram stain. Women who presented for some type of follow up (n=160) were more likely to have had a vaginal infection within the last year (16% vs. 6%, p=0.04), but were otherwise similar in characteristics to women that never presented for follow up after the initial visit (n=24).
The prevalence of BV did not differ significantly between groups at 2 weeks or 3 months (Table 2). Pessary satisfaction was not different between the groups at 2 weeks or 3 months, and excellent pessary satisfaction was also similar between the groups at both time points (p>0.05). Pessary satisfaction was substantially lower at 3 months than at 2 weeks in both groups and overall (88% at 2 weeks vs 62% at 3 months, p<0.01). Individual and composite vaginal symptoms also did not differ between the groups at 2 weeks or 3 months, although the prevalence of at least one reported vaginal symptom was high (>30% in both groups at 2 weeks and >40% in both groups at 3 months, Table 2). Also, the prevalence of an increase in at least one vaginal symptom since pessary fitting was high (>30% in both groups at 2 weeks and 3 months, Table 2), but did not differ between the groups.
Table 2.
Comparison of outcomes between the TrimoSan© gel and no TrimoSan© gel groups (intent-to-treat analysis).
| TrimoSan© gel (n (%) or mean ± standard deviation*) | No TrimoSan© gel (n (%) or mean ± standard deviation*) | p-value | |
|---|---|---|---|
| BV by Nugent's criteria | |||
| 2 weeks (n=106, 60 TrimoSan) | 12 (20) | 12 (26) | 0.46 |
| 3 months (n=133, 62 TrimoSan) | 15 (24) | 16 (23) | 0.82 |
| Total Nugent's score | |||
| 2 weeks* (n=106, 60 TrimoSan) | 4.5 ± 2.1 | 4.7 ± 2.3 | 0.75 |
| 3 months* (n=133, 62 TrimoSan) | 4.7 ± 2.3 | 4.6 ± 2.3 | 0.83 |
| BV by BV® BLUE test | |||
| 2 weeks (n=116, 63 TrimoSan) | 0 (0) | 2 (4) | 0.12 |
| 3 months (n=137, 67 TrimoSan) | 2 (3) | 0 (0) | 0.15 |
| Pessary satisfaction | |||
| 2 weeks (n=86, 49 TrimoSan) | 44 (90) | 32 (86) | 0.64 |
| 3 months (n=115, 57 TrimoSan) | 36 (63) | 35 (60) | 0.76 |
| Excellent pessary satisfaction | |||
| 2 weeks (n=86, 49 TrimoSan) | 26 (53) | 21 (57) | 0.73 |
| 3 months (n=115, 57 TrimoSan) | 23 (40) | 27 (47) | 0.50 |
| Vaginal Symptoms | |||
| 2 weeks | |||
| At Least One Vaginal Symptom Reported (n=110, 63 TrimoSan) | 28 (44) | 21 (45) | 0.98 |
| Vaginal discharge (n=110, 63 TrimoSan) | 14 (22) | 10 (21) | 0.91 |
| Vaginal itching (n=108, 60 TrimoSan) | 11 (18) | 8 (17) | 0.82 |
| Vaginal pain (n=109, 61 TrimoSan) | 6 (10) | 3 (6) | 0.50 |
| Vaginal cuts/sores (n=55, 33 TrimoSan) | 4 (12) | 1 (5) | 0.34 |
| ≥1 vaginal symptom reported as increased since pessary fitting (n=63, 37 TrimoSan) | 15 (41) | 9 (35) | 0.83 |
| 3 months | |||
| ≥1 vaginal symptom reported (n=147, 72 TrimoSan) | 30 (42) | 24 (32) | 0.30 |
| Vaginal discharge (n=147, 72 TrimoSan) | 17 (24) | 12 (16) | 0.25 |
| Vaginal itching (n=147, 71 TrimoSan) | 8 (11) | 12 (16) | 0.42 |
| Vaginal pain (n=144, 71 TrimoSan) | 6 (8) | 6 (8) | 0.96 |
| Vaginal cuts/sores (n=78, 41 TrimoSan) | 4 (10) | 3 (8) | 0.80 |
| ≥1 vaginal symptom reported as increased since pessary fitting (n=81, 42 TrimoSan) | 18 (43) | 13 (33) | 0.41 |
In the TrimoSan© group presenting for follow up, 53 out of 64 women (83%) were compliant with TrimoSan© use at 2 weeks and only 39 out of 62 women (63%) were compliant at 3 months. The per-protocol analysis, where the group of women compliant with TrimoSan© gel use once a week or more were compared to women who used the gel less often or did not use the gel, did not substantially change results.
The reported frequency of wearing the pessary or removing the pessary did not significantly affect any of the relationships between the randomization to TrimoSan© gel use and BV or vaginal symptoms at 2 weeks or 3 months (all p>0.05). Comparisons between women with different frequencies of wearing or removing the pessary did not impact the prevalence of BV at 2 weeks or 3 months or the presence of at least one reported vaginal symptom at 2 weeks or 3 months (all p>0.05).
There were 34 out of 136 women reporting pessary removal frequency (25%) who reported frequent (at least once a day) pessary removal. Women reporting frequent pessary removal were younger than women who removed their pessary less often (53 years vs. 63 years, p<0.01). These women also had a lower Charleston comorbidity index (0.03 vs. 1.03, p<0.01), were less likely to have HT prescribed at their fitting visit (3% vs. 20%, p=0.03), tended to have higher levels of education (p=0.03 all-groups comparison for education level), and were more likely to be sexually active (85% vs. 49%, p<0.01) than women who removed the pessary less often. Women who removed their pessary frequently during the study had a decreased prevalence of BV by Nugent's criteria at their initial baseline visit (6% vs. 30%, p<0.01). In the logistic regression model, only age significantly affected the relationship between frequent pessary removal and BV at baseline. Correcting for age did not remove the significant relationship between frequent pessary removal and BV at baseline (corrected p=0.03), although correcting for either sexual activity at baseline (corrected p=0.13) or HT prescribed at pessary fitting (corrected p=0.06) made this relationship no longer significant. Frequent pessary removal was not associated with a significant difference in BV prevalence at 2 weeks (32% frequent vs 20% non-frequent, p=0.24) or 3 months (29% frequent vs 22% non-frequent, p=0.44), and the prevalence of individual or composite vaginal symptoms was similar between frequent and non-frequent pessary removal at 2 weeks and 3 months (all p>0.05).
There were 66 women out of 184 (36%) using any HT during the study, 38 (21%) of which reported using vaginal HT. Any HT or vaginal HT did not significantly affect the analysis of the relationship between TrimoSan© gel randomization and the study outcomes. Women using any HT were older than women who were not (67 vs. 54 years, p<0.01), and similar in other patient characteristics. Women who were using any HT at baseline were more likely to have BV by Nugent's criteria at baseline (33% vs. 18%, p=0.03), but women on any HT and women without any HT did not differ in BV at baseline when correcting for age (corrected p=0.33). Women on any HT had similar rates of BV at 2 weeks (19% vs 24%, p=0.57) and 3 months (26% vs 21%, p=0.55) compared to women not using HT. Interestingly, women using any HT were more likely to report “too much” vaginal discharge at 2 weeks (31% vs 4%, p<0.01), report an increase of vaginal sores at 2 weeks (19% vs 0%, p<0.01), and were less likely to be satisfied with their pessary at 2 weeks (21% vs 37%, p=0.02). Correcting for age, the relationship of any HT use to a reported increase in vaginal sores (corrected p=0.01) and to decreased satisfaction (corrected p=0.03) remained significant.
Women using vaginal HT were similar in age to women not using vaginal HT (65 vs. 60 years, p=0.33), but women using vaginal HT at baseline had a higher BMI (29 vs. 24 kg/m2, p=0.04). Women using vaginal HT had a similar rate of BV by Nugent's criteria at baseline (24% vs 43%, p=0.29) compared to women not using vaginal HT, but had a decreased risk of BV at 2 weeks (7% vs 50%, p=0.04). At 3 months, the rate of BV was again similar between women using vaginal HT and not using vaginal HT (19% vs 13%, p=0.70). The decreased risk of BV at 2 weeks for women using vaginal HT did not remain significant when correcting for age (corrected p=0.10) and BMI (corrected p=0.24). Women using vaginal HT had similar rates of vaginal symptoms at 2 weeks and 3 months (all p>0.05). There was no significant differences in BV prevalence or vaginal symptoms between women initiating vaginal HT at pessary fitting and women who were already using vaginal HT prior to entry in to the study (all p>0.05).
Discussion
In this randomized, controlled, multi-center trial, the use of TrimoSan© gel in the first three months after initiation of pessary use did not decrease the prevalence of bacterial vaginosis or other outcomes such as bothersome vaginal symptoms or desire to continue pessary use. Our data indicates that the presence of at least one vaginal symptom is prevalent in pessary users (>30% just 2 weeks after pessary fitting and >40% at 3 months), but that TrimoSan© gel use does not decrease these symptoms.
Most women have improvement in pelvic floor function and many meet their goals after pessary insertion.4,10,11 Unfortunately, no interventions or hygiene practices after pessary fitting have been shown in prospective, clinical trials to improve pessary care and satisfaction.7 Our findings are consistent with those in a previous study indicating that BV occurs in 30% of pessary users;6 BV was found in approximately 24% of our patients. This trial clinically tests a pessary hygiene intervention meant to prevent adverse pessary outcomes. Unfortunately, we did not find that TrimoSan© gel has a substantial influence on BV or other relevant pessary outcomes studied. It appears that women using a pessary cannot be protected from BV by acidification of the vagina in this manner. One hypothesis is that women who develop this issue are physiologically different than women who do not, either in their inherent microbiome characteristics or in their immunologic response to a vaginal foreign body. It is possible that other patient-initiated interventions may affect the rate of BV in this population.
While this study did not yield evidence that TrimoSan© gel use significantly affected women's vaginal symptoms, these data do indicate that other behavioral factors may affect the prevalence of BV. We found an association between a decreased prevalence of BV before pessary use and frequent pessary removal after the pessary was initiated. This indicates that women who go on to remove their pessary frequently are inherently at less risk for BV than women who do not based on certain patient characteristics, like sexual activity and HT use. Furthermore, correction for other factors such as sexual activity and HT prescribed at the pessary fitting did remove the significance of this association. This implies that sexually active women without vaginal atrophy are likely to remove their pessary more frequently during the first months of use. However, these women who remove their pessary frequently do not have a decreased prevalence of BV or an improved profile of any other vaginal symptoms. Women may be removing their pessary frequently for any number of lifestyle reasons, including for sexual activity, but it does not seem that these hygiene measures translate into different outcomes. As there are insufficient data to guide patients in this area,7 patients can be reassured that a removal frequency of their choice does not seem to put them at great risk for BV or vaginal symptoms. However, while we are not aware of any present interventional trials investigating the optimal frequency of pessary removal, this study was also not designed or powered to investigate this particular question.
While these data demonstrated that HT did not affect the relationship between TrimoSan© and the study outcomes, HT does appear to be a factor in vaginal symptoms and pessary satisfaction. It is known that a postmenopausal state increases vaginal pH, and prevalence of BV increases with increasing age and further into the menopausal transition.12,13 In this study, women using HT were more likely to report an increase in vaginal sores and be less satisfied with the pessary 2 weeks into use, even correcting for the patient age. This was not seen in women using vaginal HT. This may be due to the fact that women using any form of HT probably represent a mostly postmenopausal group with atrophic vaginal tissue, and 43% of the group using any HT was not using HT vaginally. Non-vaginal HT may not be adequate to protect the vaginal tissue from ulceration in the setting of a foreign body and prevent pessary dissatisfaction associated with this issue.
In this population, vaginal HT appeared to be protective for BV at 2 weeks on the uncorrected analysis, consistent with the principle that higher estrogen levels increase glycogen production and decrease vaginal pH.14,15 However, this significant relationship was no longer seen when the analysis was corrected for patient age, and we did not observe a difference in BV at the 3 month follow up between women using and not using vaginal HT. We also did not note any significant relationship of vaginal HT to adverse symptoms or pessary satisfaction. As noted prior, this furthers the hypothesis that women who develop BV with a pessary are inherently different than women who do not, and that BV in pessary users cannot be attributable solely to age and lack of estrogen. The lack of significant protection associated with vaginal HT in this study may also be attributable to the fact that our study was not powered for this analysis, or to the fact that the pessary may mitigate the protection offered by vaginal hormones.
Strengths of this trial include the randomized, controlled design of adequate power to detect a difference in BV. The population was representative of most populations of women who use pessaries and for a variety of indications, making it generalizable to most populations seeking pessary care. The study patients did have the rate of BV expected at the trial's design, similar to rates seen in one prior study in a pessary population,6 and is further evidence that adequate power was attained to detect so a relationship between TrimoSan© if such an association existed. We also performed stratification by the intended frequency of pessary removal with the intent to minimize bias that might be caused by the variable, and verified in the analysis that actual patient practice in pessary removal frequency did not affect the relationship between our intervention and our main outcomes. Also, the trial outcomes included two different measures of BV, one that could be performed by a blinded assessor and one whose results were immediately available in the clinical setting, making the results further applicable to most pessary providers. Lastly, important covariates for the vaginal environment, such as pessary removal frequency and HT use, were collected and considered in the analysis.
There are several limitations to this study. First, although the assessor of the primary outcomes was blinded to the patient's allocation, there was no blinding of the patients and no placebo use in this trial. As any substance applied to the vagina may also affect the vaginal microbiome, there is no placebo substance that is truly known to be “inactive” in this patient population. Given the lack of literature to guide the selection of a veritable placebo, we believed that a comparison to no gel (rather than a placebo gel) was the most appropriate. Second, exposure to TrimoSan© gel, or the per-protocol analysis, was done by patient report. If women were using TrimoSan© less compliantly than reported, the relationship between TrimoSan© gel use and the outcomes would be overstated in our data, which did not find a significant difference in outcomes. Also, as only 63% (39/62) of patients used TrimoSan once a week at 3 months, we cannot state whether or not TrimoSan© may be shown effective if more patients had been compliant with gel use. Third, while we reported and analyzed the effect of HT on this trial's outcomes, we did not intervene on the patient's use of hormone therapy. Given that there is limited evidence on the effect of estrogen use on BV in this particular population, the authors wished to be able to investigate this exposure without it being controlled. While bias from this factor should be minimized by our randomized design and our analysis indicated no significant interactions from HT between TrimoSan© gel and the study outcomes, we cannot rule out the fact that this affects the trial results.
Fourth, although the BV® BLUE test has correlated well with Gram stain results in past studies,16 BV® BLUE has not been tested in a pessary-using patient population and, as stated by the manufacturer, is not validated in women using vaginal medications or foreign bodies.8,17 Many of the women in this study who had BV by Nugent's criteria did not have an BV® BLUE positive test, so we concur that this test is likely not accurate in pessary users, and women who had BV at the initial visit would not have been treated if they had a negative BV® BLUE test at their clinical visit. Although the groups had a similar prevalence of baseline BV by Nugent's criteria, we cannot exclude that this affected the results. Lastly, we did not use any other clinical measures of BV (such as Amsel's criteria) as outcomes measures in this trial to avoid potential bias introduced by a non-blinded clinician, and therefore cannot extend these results to BV prevalence as detected by these other outcome measures. Also, as noted in a recent cross-sectional study on the microenvironment in pessary users,18 shifts that increase the Nugent score in pessary users are not necessarily related to bothersome vaginal discharge, so Nugent's criteria may not be accurately measuring the process that actually troubles this population.
Overall, this trial provides data that TrimoSan© gel does not affect the prevalence of BV in new pessary wearers, and also does not affect vaginal symptoms or desire to continue pessary use. However, these data indicate that other hygiene measures besides TrimoSan©, such as the frequency of removing the pessary or HT use, may play a role in pessary outcomes and merit further investigation. Findings from this trial will aid clinicians in the counseling and management of women newly fitted with a pessary.
Acknowledgments
We extend thanks to Sekisui Chemical Co., Ltd, which provided discounted BV® BLUE test kits for use in this study. We would also like to thank Ms. Joanna Peterson, RN of MedStar Washington Hospital Center and the Pessary Clinic staff of the University of New Mexico, including Ms. Marsha Grey, Ms. Martha Rode, Ms. Laura Migliaccio, and Ms. Abigail Reese, for their help with this study.
Trial Support: This study was funded by the University of New Mexico Clinical Translational Science Center (CTSC) grant by Pilot Award (Grant 3A302A) and by the MedStar Washington Hospital Center funding for trainee investigator research.
Footnotes
Location of study: Albuquerque, New Mexico, USA and Washington, DC, USA
Conflict of Interest Disclosure Statement: RG Rogers is a Chair DSMB for the Transform trial sponsored by American Medical Systems and receives royalties from Up-to-Date and McGraw Hill. The remaining authors report no conflicts of interest.
Paper Presentation Information: These data will be presented as an oral poster at the 41st Annual Scientific Meeting, Society of Gynecologic Surgeons, Orlando, FL (March 23, 2015) under the title “The effect of TrimoSan© gel on the rate of pessary-associated bacterial vaginosis: a multicenter, randomized, controlled trial.”
Trial Registration: This study was registered with ClinicalTrials.gov under the ID#NCT01471457.
References
- 1.Wu JM, Vaughan CP, Goode PS, Redden DT, Burgio KL, Richter HE, Markland AD. Prevalence and trends of symptomatic pelvic floor disorders in U.S. women. Obstet Gynecol. 2014 Jan;123(1):141–8. doi: 10.1097/AOG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 3.Robert M, Schulz JA, Harvey MA, Urogynaecology Committee. Lovatsis D, Walter JE, Chou Q, Easton WA, Epp A, Farrell SA, Geoffrion R, Girouard L, Gupta CK, Harvey MA, Larochelle A, Maslow KD, Neustaeder G, Pascali D, Pierce M, Robert M, Ross S, Schachter J, Schulz JA, Senikas V, Wilkie DH. Technical update on pessary use. J Obstet Gynaecol Can. 2013 Jul;35(7):664–74. doi: 10.1016/S1701-2163(15)30888-4. [DOI] [PubMed] [Google Scholar]
- 4.Komesu YM, Rogers RG, Rode MA, Craig EC, Gallegos KA, Montoya AR, Swartz CD. Pelvic floor symptom changes in pessary users. Am J Obstet Gynecol. 2007 Dec;197(6):620.e1–6. doi: 10.1016/j.ajog.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Komesu YM, Rogers RG, Rode MA, Craig EC, Schrader RM, Gallegos KA, Villareal B. Patient-selected goal attainment for pessary wearers: what is the clinical relevance? Am J Obstet Gynecol. 2008 May;198(5):577.e1–5. doi: 10.1016/j.ajog.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alnaif B, Drutz HP. BV increases in pessary users. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11(4):219–22. doi: 10.1007/pl00004026. discussion 222-3. [DOI] [PubMed] [Google Scholar]
- 7.Adams E, Hagen S, Maher C, Thomsson A. Cochrane Review, Issue 2. Oxford: Cochrane Library; 2009. Mechanical devices for pelvic organ prolapse in women. [DOI] [PubMed] [Google Scholar]
- 8.Myziuk L, Romanowsky B, Johnson SC. BV® BLUE test for diagnosis of bacterial vaginosis. J Clin Microbiol. 2003 May;41(5):1925–8. doi: 10.1128/JCM.41.5.1925-1928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991 Feb;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clemons JL, Aguilar VC, Tillinghast TA, Jackson ND, Myers DL. Patient satisfaction and changes in prolapse and urinary symptoms in women who are fitted successfully with a pessary for pelvic organ prolapse. AJOG. 2004;190:1025–9. doi: 10.1016/j.ajog.2003.10.711. [DOI] [PubMed] [Google Scholar]
- 11.Patel M, Mellen C, O'Sullivan DM, LaSala CA. Impact of pessary use of prolapse symptoms, quality of life, and body image. Female Pelvic Med Reconstructive Surgery. 2011;17:298. doi: 10.1097/SPV.0b013e31823a8186. [DOI] [PubMed] [Google Scholar]
- 12.García-Closas M, Herrero R, Bratti C, Hildesheim A, Sherman ME, Morera LA, Schiffman M. Epidemiologic determinants of vaginal pH. Am J Obstet Gynecol. 1999 May;180(5):1060–6. doi: 10.1016/s0002-9378(99)70595-8. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann JN, You HM, Hedberg EC, Jordan JA, McClintock MK. Prevalence of Bacterial Vaginosis and Candida among Postmenopausal Women in the United States. J Gerontol B Psychol Sci Soc Sci. 2014 Nov;69(Suppl 2):S205–14. doi: 10.1093/geronb/gbu105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van de Wijgert JH, Verwijs MC, Turner AN, Morrison CS. Hormonal contraception decreases bacterial vaginosis but oral contraception may increase candidiasis: implications for HIV transmission. AIDS. 2013 Aug 24;27(13):2141–53. doi: 10.1097/QAD.0b013e32836290b6. [DOI] [PubMed] [Google Scholar]
- 15.Spinillo A, Bernuzzi AM, Cevini C, Gulminetti R, Luzi S, De Santolo A. The relationship of bacterial vaginosis, Candida and Trichomonas infection to symptomatic vaginitis in postmenopausal women attending a vaginitis clinic. Maturitas. 1997 Jul;27(3):253–60. doi: 10.1016/s0378-5122(97)00038-8. [DOI] [PubMed] [Google Scholar]
- 16.Kampan NC, Suffian SS, Ithnin NS, Muhammad M, Zakaria SZ, Jamil MA. Evaluation of BV® Blue Test Kit for the diagnosis of bacterial vaginosis. Sex Reprod Healthc. 2011 Jan;2(1):1–5. doi: 10.1016/j.srhc.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 17.OSOM BV® Blue package insert, CPT Code 87905QW, Sekisui Diagnostics, Copyright 2014 © Sekisui Chemical Co., Ltd. http://www.sekisuidiagnostics.com/writable/product_documents/files/osom-bvblue_183_pi.pdf
- 18.Collins S, Beigi R, Mellen C, O'Sullivan D, Tulikangas P. The effect of pessaries on the vaginal microenvironment. Am J Obstet Gynecol. 2015 Jan;212(1):60.e1–6. doi: 10.1016/j.ajog.2014.07.024. [DOI] [PubMed] [Google Scholar]


