Abstract
B-1 cells comprise subpopulations of B lymphocytes in mice that display developmental, phenotypic, and functional characteristics that are distinct from that of conventional B cell populations (often referred to as “B-2” cells). Despite the known importance of murine B-1a (CD5+) and B-1b (CD5−) cells in the production of natural antibodies and rapid antigen-specific humoral responses to infection, evidence for B-1 cells in primates, including humans, is very limited. Identifying these cells in humans proves challenging given the limited number of cells that can be obtained from sites expected to harbor increased frequencies of these cells (ie., peritoneal and pleural cavities) and the need to perform functional analyses on these cells, which in the case of B-1b cells must be carried out in vivo. The use of cynomolgus macaques and African Green monkeys has enabled us to bypass these limitations and to identify and extensively analyze primate B cell populations with the phenotypic and functional characteristics of mouse B-1a and B-1b cells. Our results reveal striking similarities between primate and murine B-1 cells, including a conserved functional role for primate B-1b–like cells in immunity to T cell independent type 2 antigens.
Keywords: Non-human primates, B-1a cells, B-1b cells, T cell independent type 2 antigens
B-1b cells - a short history
B-1a and B-1b cells are mouse B cell populations with unique developmental, phenotypic, and functional characteristics1–3. B-1b cells have been studied far less frequently than B-1a cells, and it their role in the immune system has only been recently become appreciated. Nonetheless, murine B-1b cells were described nearly 25 years ago at the first B-1 meeting in 19914. At the time, B-1 cells were noted to be enriched in the serosal cavities and to express high levels of IgM, low levels of IgD and B220, and to uniquely express CD11b (Mac1; complement receptor 3). Although similar to B-1a cells in phenotype and tissue distribution, the B-1b cell population was defined by the lack of CD5 (Ly-1) expression and evidence supporting this population was derived from a distinct developmental lineage. This included data demonstrating the inability of B-1b cells to differentiate into B-1a cells in adoptive transfer experiments (and vice versa) and the differential development of B-1a and B-1b cells from adult Ig− precursors4–6. Thus, although reference is often made to “B-1 cells”, it must be stressed that B-1a and B-1b cells are distinct populations and hence, the behavior and characteristics of B-1b cells do not always parallel that of B-1a cells.
Despite being described in the early 90’s, the functional role of B-1b cells in the immune system did not begin to be appreciated until 2004, when Alugupalli et al. demonstrated a key role for B-1b cells in the protective T cell independent antibody response to Borrelia hermsii7. An important finding of this study was that B-1b cells from convalescent mice functioned in a memory-like capacity in that they were capable of providing superior protection against infection over naïve B-1b cells. Thus, B-1b cells were identified to have a critical role in providing long-lasting protective humoral immunity to this important human pathogen. Shortly thereafter, my colleagues and I demonstrated a central role for B-1b cells in protection against Streptococcus pneumoniae8. While studying the role of the complement receptor, CD21/359, and its signaling partner CD19 in the protective immune response to S. pneumoniae, we made striking observations regarding the effect of CD19 deficiency on protection against pneumococcal infection8. Naïve CD19−/− mice were found to be extremely susceptible to pneumococcal infection following a low dose inoculum. This was explained by the critical role CD19 plays in supporting B-1a cell development and the production of natural antibodies against phosphorylcholine (PC), a key haptenic determinant of S. pneumoniae10. Consistent with these findings, transgenic mice overexpressing human CD19 (hCD19Tg) had increased B-1a cells and PC-reactive natural antibody, and were thus more resistant to infection. Remarkably, however, immunizing CD19−/− mice with either heat-killed bacteria or capsular polysaccharide (a T cell independent type 2 antigen; TI-2 Ag) yielded antibody responses that provided complete protection against a high dose lethal challenge. Conversely, hCD19Tg mice were unable to produce productive anti-capsular antibody responses and were therefore not protected during lethal dose challenge. Phenotypic analysis demonstrated that although CD19−/− mice lacked B-1a cells, they had CD5−CD11b+B220intIgMhi (B-1b) peritoneal B cells (Fig. 1A). In contrast, hCD19Tg mice lacked B-1b cells. The opposing role for CD19 in B-1a versus B-1b cell development provided further evidence for the developmental differences between B-1a and B-1b cells. Adoptive transfers of CD19−/− or wild type B-1b cells into CD19Tg or Rag1−/− mice clearly showed their superior ability to elicit long-lasting adaptive anti-capsular antibody responses relative to other B cell subsets8 (Fig. 1B–D). Moreover, B-1b cells alone could provide significant protection to Rag1−/− mice following lethal systemic pneumococcal challenge (Fig. 1E). Multiple studies provide additional supporting evidence for the key role murine B-1b cells play in antibody responses to TI-2 Ags, including NP-Ficoll11, TNP-Ficoll12, α1–3-dextran13, and Salmonella typhi Vi polysaccharide14, as well as additional pathogen-derived TI Ags7, 15, 16 and the Gal α1–3Galbeta1-4GlcNAc (Gal) carbohydrate epitope involved in transplant rejection17. The role of B-1b cells in immune responses to protective pathogen-derived antigens has been recently reviewed3.
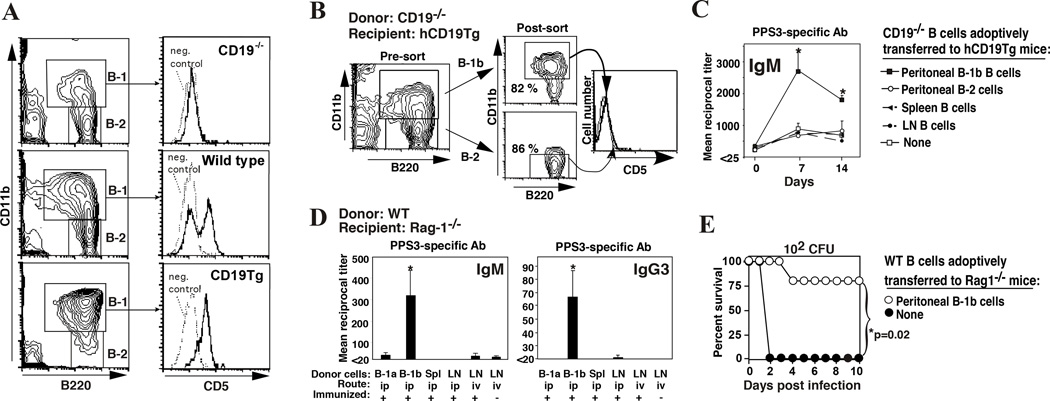
Figure 1. B-1b cells reconstitute protective antibody responses to PPS in B-1b-cell deficient CD19Tg mice and B cell-deficient Rag-1−/− mice.
A) CD19−/− mice are deficient in B-1a cells whereas CD19Tg mice are deficient in B-1b cells. B-1 (B220+CD11b+) and B-2 (B220+CD11b−) lymphocytes are indicated (left column) with histograms showing CD5 expression by peritoneal B-1 (B220+CD11b+-gated) cells (right column). Isotype-matched control antibody staining is indicated by a dotted line. B–C) Reconstituting hCD19Tg mice with peritoneal B-1b cells from CD19−/− mice rescues responsiveness to PPS-3. Peritoneal B-1b or B-2 cells from CD19−/− mice were isolated by FACS (B). FACS-purified peritoneal B cells or enriched spleen and lymph node B cells from CD19−/− mice were transferred i.p. into hCD19Tg mice (105 cells/mouse). Mice were immunized with PPS-3 3 weeks later with PPS-3-specific antibody titers determined by ELISA (C). D–E) Transfer of WT B-1b cells into Rag-1−/− mice reconstitutes PPS3-specific IgM and IgG responses and provides protection against lethal S. pneumoniae infection. D) Purified WT peritoneal B-1a cells, B-1b cells, or unfractionated spleen or LN cells were transferred i.p. or i.v. into Rag-1−/− mice (4 × 105 B cells/mouse; ≥3 mice/group). Mice were immunized with 0.5 µg PPS-3 3 days later, with PPS-3-specific IgM (d7) and IgG3 (d14) antibody levels measured by ELISA. E) Rag-1−/− mice reconstituted with B-1b cells were infected with 102 colony forming units of serotype 3 S. pneumoniae 14 days post-immunization. *Chi-square analysis indicated significant differences in survival. Adapted from Haas et al.8.
Human B cell responses to TI-2 Ags
Although data supports a central role for murine B-1b cells in T cell independent host defense, the question of whether a similar population exists in other species has received considerable debate, as has been the case for B-1a cells. TI-2 antibody responses in primates, for example, have been proposed to be primarily elicited by marginal zone (MZ) B cells, which may include the controversial IgM+CD27+ “memory” B cell population18–22. This is a controversial population due to the fact that these cells express the CD27 memory marker and exhibit somatic hypermutation and yet, are proposed to be naïve. IgM+CD27+ B cells have been proposed to express mutated antigen receptors due to a process of antigen-independent somatic hypermutation proposed to occur during developmental repertoire diversification in humans20. However, the alternative possibility is that these cells are bona fide IgM+ memory cells that have undergone memory differentiation in response to TI or T cell dependent (TD) antigen stimulation—a process that could potentially occur even in the absence of productive antibody responses. Despite the controversy surrounding the origin, functions, and memory status of IgM+CD27+ “memory” B cells21, recent studies nonetheless support a role for CD27+ B cells in either producing IgM and IgG against PPS19, 23 or increasing in frequency following PPS immunization in humans24. Human IgM+CD27+ memory cells have therefore been proposed to perform the functions of murine B-1 and MZ B cells19, 22. While MZ B cells also contribute to TI antibody responses in mice, their role relative to B-1b cells may be antigen-, dose-, and route-dependent, although our work has shown that the magnitude of splenic TI-2 Ag-specific B- 1b cell responses is similar following intraperitoneal, intravenous, subcutaneous, and intramuscular immunization (ref.12 unpublished data). That CD19−/− and other strains of mice with deficiencies in MZ B cells8, 13, 25, 26 exhibit normal or near-normal antibody responses to TI-2 Ags supports that non-MZ B cell populations such as B-1b cells play an important role in these responses at least in mice. It is clear that human B cells can produce antibodies against the same antigens and pathogens that elicit murine B-1 cell responses7, 8, 23, 27. However, a TI-2 antibody-producing B-1b cell subset has generally not been thought to exist in primates28.
Despite the proposed differences between mouse and human B cell subset responses to TI-2 Ags, remarkable similarities exist between TI-2 antibody responses in rodents and primates. First, neonatal rodents and primates make very poor responses to TI-2 Ags29–31. In both orders, the defective humoral response to polysaccharides can often be overcome by the use of polysaccharide-protein conjugates29–31. Indeed, it was research conducted in mice that lead to the rationale for development of the first polysaccharide-protein conjugate vaccine32. Second, TI-2 Ags largely induce IgM and to a lesser extent, IgG3 (mouse)/IgG2 (human) subclass antibodies. Mouse IgG3 and human IgG2 are typically produced in response to carbohydrate antigens and have the unique capacity to engage in cooperative interactions via noncovalent and covalent interactions, respectively33, 34. This process of IgG oligomerization may promote enhanced antibody effector functions, including complement activation. Third, in both mice and humans, antibody responses to polysaccharide antigens are refractive to boosting. Finally, polysaccharide antigens, including some pneumococcal polysaccharides, have been shown to induce tolerance in both mice and primates35, 36. The above characteristics are unique to polysaccharide antigens, and each represents a significant barrier to developing effective polysaccharide-based vaccines. The practice of using mice to model these conserved features of anti-polysaccharide antibody responses has been long been accepted. However, the notion that primates are lacking the murine counterpart of B-1b cells dedicated to producing antibodies against these antigens seems at odds with these evolutionarily-conserved features of polysaccharide-specific B cell regulation.
Challenges with defining B-1 cells in humans
Evidence for human B cells with the functional and phenotypic characteristics of B-1a or B-1b cells present in tissues typically enriched in B-1 cells in mice (ie., serosal cavities and omentum) is very limited. The identification of B-1a cells in humans in non-serosal tissues has been hampered by the lack of reliable markers that can be used to differentiate these cells from other B cell populations. The use of one of main distinguishing markers for peritoneal B-1 cells, CD11b, is problematic even in mice due to the fact that CD11b expression typically diminishes on B-1 cells outside of the peritoneum and upon differentiation to antibody secreting cells (ASC)1, 17, 37, 38. Recent CD11b+ peritoneal B-1 emigrants as well as CD11b+ B-1b cells participating in TI-2 antibody responses can be detected in spleen, blood, and lymph nodes using CD11b antibodies labeled with bright fluorochromes12, 39, 40. The potential for non-B-1-derived B cells to express CD11b is not completely clear, and thus, adoptive transfers of peritoneal B-1 cells are often used to corroborate findings made for endogenous CD11b-expressing B cells identified to participate in immune responses. For example, we have confirmed that peritoneal B-1b cells from VHB1–8 transgenic (Tg) mice (express a heavy chain that pairs with lambda light chains to confer specificity for NP) maintain CD11b expression in blood, spleen and lymph nodes during the response to NP-Ficoll (unpublished observations). CD5 can also be problematic as a sole marker of B-1a cells in mice, as BCR-activated B-1b cells and B-2 cells12, 41, anergic conventional B cells42, and B10 regulatory B cells can all express low levels of CD543. Another complication is that there is heterogeneity in the expression levels of additional markers among mouse B-1a and B-1b cell populations. For example, peritoneal B-1a (CD11b+CD5+CD19hiIgM+) cells from C57BL/6 mice typically express CD43 and very low levels of CD21/35, CD23, and B220; however, CD19hiCD11b+CD5− B-1b cells can be subdivided into CD21/35int and CD21/35lo/-26, CD23+ and CD23lo/-44, CD43+ and CD43lo/- , as well as B220hi and B220lo populations (Fig. 2A–B). Thus, wild type CD19hiCD11b+CD5− cells generally fall into two major subsets: CD21/35intCD23+CD43lo/-B220hi cells and CD21/35lo/-CD23−CD43+B220lo cells. B-1b cells from CD19−/− mice are predominantly CD21intCD23+CD43lo/-B220hi (Fig. 2B). Importantly, both subsets reconstitute antibody responses to TI-2 antigens (unpublished observations). Thus, a challenge for future studies will be to identify novel reliable markers for B-1a and B-1b cells that can be applied to the identification and further characterization of both mouse and human B cell subsets. In the meantime, comparative studies conducted in non-rodent species must examine multiple phenotypic and functional characteristics of murine B-1a and B-1b cells. For example, Rothstein and colleagues recently probed the existence of human B-1 cells by examining human B cell populations expressing B-1a-like phenotype and functional characteristics. Their work demonstrates a B-1a-like population in human peripheral blood expressing a CD20+CD27+CD43+CD70− phenotype with the capacity for spontaneous IgM secretion, T cell stimulation, heightened tonic intracellular signaling, and typical murine B-1a antigen receptor specificities45. Despite these findings, the existence of B-1a cells in humans has remained controversial22, 46–50.
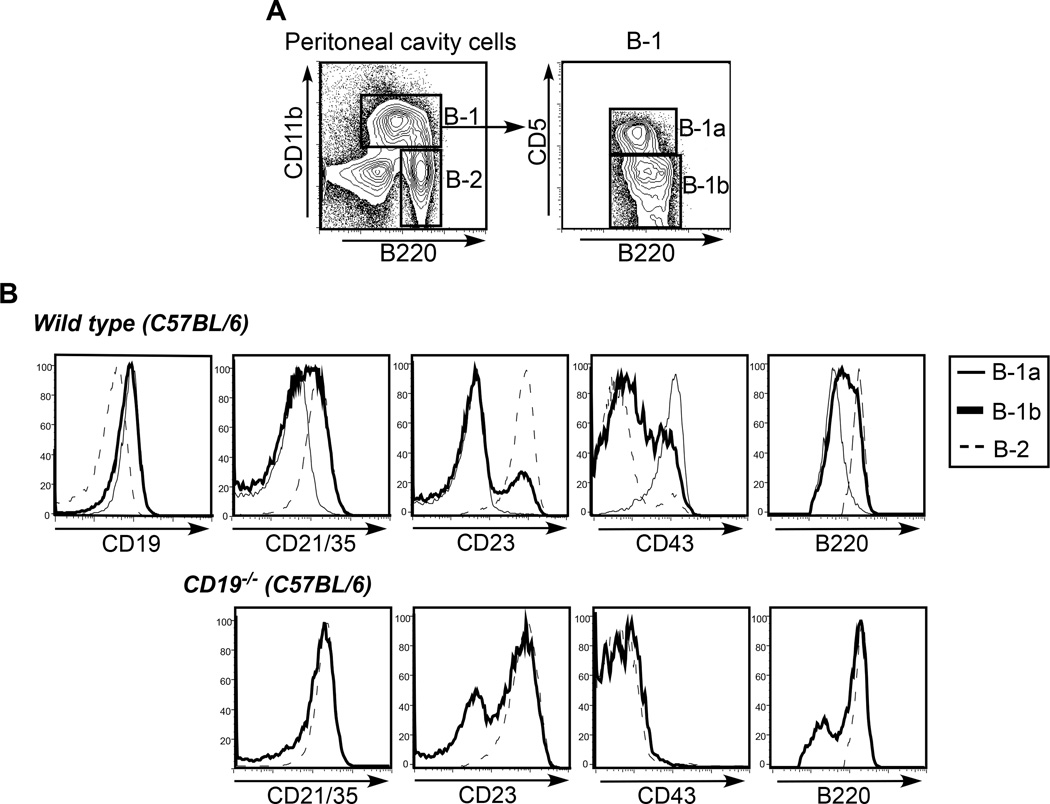
Figure 2. Heterogeneity in expression of surface markers on B-1b cells.
A) Gating strategy to identify B-1a, B-1b, and B-2 cells in lymphocytes from peritoneal cavity lavages of wild type C57BL/6 mice. B) CD19, CD21/35, CD23, CD43, and B220 expression levels on peritoneal cavity B-1a, B-1b, and B-2 B cells in wild type and CD19−/− mice on a C57BL/6 background.
Identification of B-1b-like cells in humans is more problematic than B-1a cells, since in contrast to B-1a cells for which human counterparts can be identified by fixed functional characteristics, B-1b cells are best identified by their participation in adaptive responses to TI Ags. This has been achieved in mice using strong TI-2 Ags as immunogens and then tracking Ag-specific B-1b cell responses in blood, spleen, lymph nodes, and serosal tissues by multicolor flow cytometry12, 13, 26, 40, 51. Nonetheless, this may be difficult to achieve in humans given that: 1) they may have pre-existing immunity to vaccine antigens or cross-reactive polysaccharide antigens known to induce B-1b cell responses in mice (such as type 3 pneumococcal polysaccharide) and 2) B cell subset analysis is largely limited to peripheral blood cells. The latter poses a challenge to obtaining sufficient numbers of Ag-specific B cells for analysis, since polysaccharide antigen vaccines typically elicit weak responses due to the lack of T cell help as well as a lack of suitable adjuvants that can be administered to augment responses. Thus, investigating whether humans have a B-1b cell population is not a trivial undertaking.
Evidence for B-1 cells in non-human primates
We rationalized that the identification of B-1 cell populations in primates might be best achieved using non-human primate (NHP) models since tissues were readily obtainable through our primate facility and the same model TI-2 Ags we used in wild type mice to measure antigen-specific B-1b cell responses12, 40 could be used in NHP. We therefore sought to determine whether NHP harbored B cell subpopulations exhibiting the phenotype, tissue localization, and functional characteristics of murine B-1a and B-1b cells.
We approached the question of whether primates have B-1-like cells by first examining the tissues and cavities in which these cells are found enriched in mice. B cells were present in both omental tissue and peritoneal lavages of both African green monkeys (AGM) and cynomolgus macaques52. However, peritoneal cavity cell yields were low relative to that which can be obtained from C57BL/6 mice (>5 ×106). Cell yields obtained from NHP peritoneal lavages and processed omentum were typically ~1–2 ×106 cells/animal, with B cells typically comprising <10% of the population. Healthy patients have been reported to have similar leukocyte numbers recovered in peritoneal lavages (~3×106), with ~8% CD19+ B cells 53. In NHP, roughly ~25–40% of peritoneal and omental B cells express the CD11b marker used to phenotypically define peritoneal B-1 cells in mice (Fig. 3A). Interestingly, the frequency of peritoneal B cells expressing CD11b significantly increases during the first 3 years of primate life, despite unchanging total peritoneal B cell frequencies throughout this period of development52. CD11b is present on a fraction of blood B cells in NHP, as in humans54, although expression levels are lower than that found for peritoneal and omental B cells. CD11b is only expressed by a small fraction of primate splenic B cells (<10%), as observed in mice.
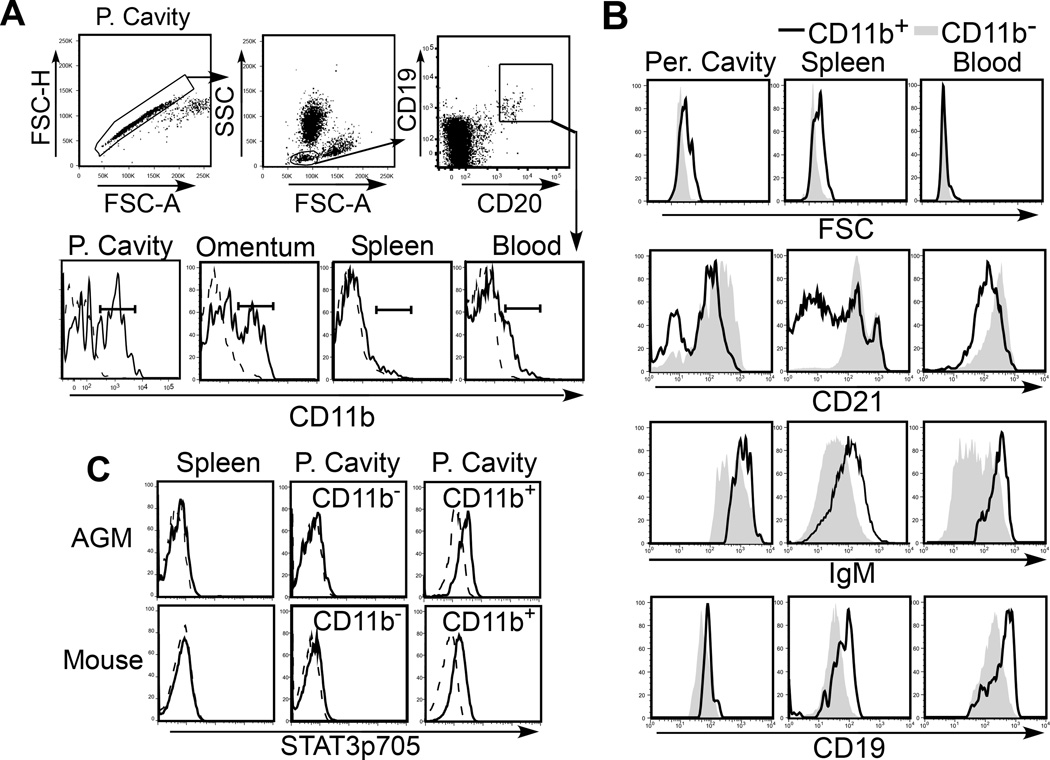
Figure 3. B-1–like cells are present in higher primates.
A) Sample gating strategy to identify B cells in NHP tissues. B cells were defined as either CD19+CD20+ cells present within the lymphocyte gate. CD11b expression (solid line) by B cells in AGM peritoneal cavity (P. cavity), omentum, spleen, and blood is shown in histograms in the lower panel. B) FSC, IgM, CD21, and CD19 expression by CD11b+ (solid line) and CD11b− B cells (gray shading), gated as shown in (A). C) Constitutive active Stat3(p705) expression (solid line) by CD19+ spleen B and peritoneal CD11b− and CD11b+ B cells in AGMs (top histograms) and mice (bottom histograms). Histograms with dashed lines indicate intracellular mAb isotype control staining. Adapted from Yammani and Haas52.
Similar to mouse B-1 cells, primate peritoneal and omental CD11b+ B cells express higher FSC, IgM, and CD19, and lower levels of CD21 relative to CD11b− B cells (Fig. 3B). A fraction of CD11b+, but not CD11b−, peritoneal B cells express CD80 and CD5. CD27 is only expressed on a fraction of CD11b+ B cells (<50%) in the peritoneal cavity, suggesting that this marker alone cannot be used to define primate B-1 cells. CD5 expression also did not correlate with CD11b expression on peritoneal cavity or omental B cells, and was found on a high percentage of spleen and blood cells and therefore cannot be used as reliable marker for defining the B-1a subset in NHP, as is true in other species55. However, CD11b+ peritoneal B cells exhibit increased specificity for phosphorylcholine (PC)52 and express constitutively active Stat3 (Fig. 3C), in contrast to CD11b− peritoneal B cells and spleen B cells. These characteristics are unique to murine B-1a cells55, 56. Thus, primate CD11b+ B cells exhibit a distribution pattern and surface phenotype that is similar to murine B-1 cells (FSChiSSChiCD11b+CD19hiIgMhi CD21lo/-CD80+/− ) and a subset of these peritoneal B cells constitutively express active Stat3 and exhibit enhanced reactivity with PC, similar to B-1a cells. Collectively, these data strongly support the existence of a functional B-1a-like population in primates.
While our results clearly indicate NHP have a population of B cells with defining characteristics of mouse B-1a cells, there are some challenges to comparing our results with characteristics reported for human B-1-like cells45. For example, we have not been able to identify human CD43 antibodies that cross-react with AGM or cynomolgus macaque CD43 and are therefore unable to make comparisons between the NHP peritoneal B-1a-like cells and the human CD20+CD27+CD43+CD70− blood B-1 population described by Rothstein and colleagues45. Circulating CD11b+ cells compose only ~10% of the human B-1-like CD20+CD27+CD43+CD70− blood population57. How this human B cell population relates to the CD11b+ blood and spleen B cells found in NHP is not yet clear, although a fraction of NHP CD11b+ B cells clearly express CD27. Future work is necessary to further characterize human serosal B cells53 and to determine their relationship to the human CD20+CD27+CD43+CD70− blood B-1 cells that have been described45. Identification of additional surface markers and defined transcriptional and epigenetic signatures for mouse, NHP, and human B cell subsets will certainly aid in future studies examining the relationships among these populations. Progress in this area will help to improve upon mouse and NHP models of B cell regulation that are critically important for biomedical advances in vaccine development and therapies for autoimmunity and B cell malignancies.
Evidence for functional B-1b-like cells in primates
To determine whether NHP B-1-like cells perform similar functions attributed murine B-1b cells, we examined NHP Ag-specific B cell responses to TNP-Ficoll, a classical synthetic TI-2 Ag which induces murine Ag-specific B-1b cells to expand, differentiate, and produce TNP-specific antibody, regardless of whether Ag is delivered i.p. or i.v.12 or whether MZ B cells are present26. We have validated the use of this model system to track Ag-specific B cell responses to TNP-Ficoll in mice12, 26. TNP-binding B cells are found within all murine B cell subsets; however, B-1b cells predominantly respond to TNP-Ficoll immunization12, similar to what has been reported for NP-Ficoll11. TNP-Ficoll immunization significantly increased the number of Ag(TNP)-specific blood B cells in AGM and cynomolgus macaques52. As we also observe in mice12, immunization induced a significant increase in CD11b+ TNP-specific B cells in NHP with increased FSC, SSC, CD19, PD-1, and CD86 expression and decreased CD21 expression (Fig. 4A–B and data not shown). The CD20loCD21loCD19+CD11b+ Ag-specific B cell population was present in blood between 5 and 21 days post immunization52. Additional phenotypic markers were also shared between the murine and primate Ag-specific B cells responding to TNP-Ficoll immunization, including CD80 and CD44. As shown in Table I, the surface marker profiles expressed by Ag-specific B cells following TNP-Ficoll immunization are indeed remarkably similar between mice and NHP. Importantly, we have observed similar phenotypic changes in mice and primates following Pneumovax23 immunization, with significant increases in PPS3-specific blood and spleen B cells expressing CD11b, PD-1, and CD86, 5 days following immunization (ref.40 and unpublished observations).
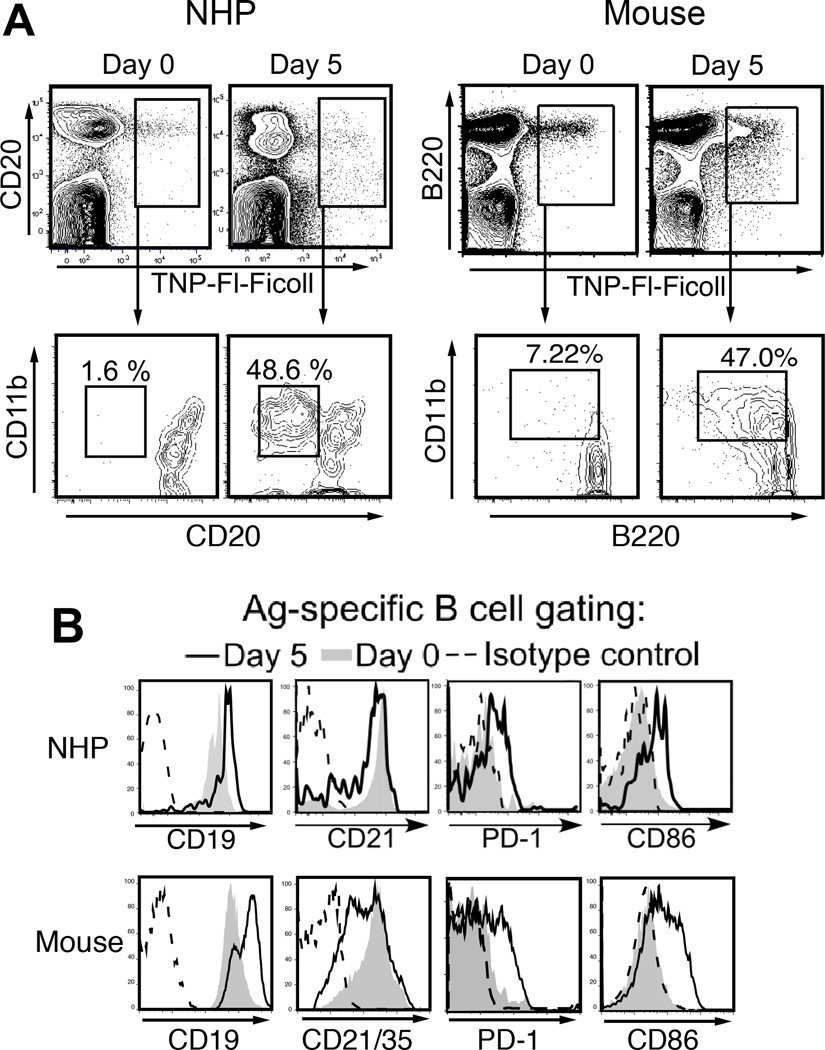
Figure 4. Ag-specific B cell responses to TNP-Ficoll are similar between mice and non-human primates (NHP).
A) Ag-specific B cells (upper panels) and their CD11b expression levels (lower panels) in mice and AGM prior to and 5 days following TNP-Ficoll immunization. B220 and CD19 were used to identify splenic mouse B cells and CD20 and CD19 were used to identify primate blood B cells. Representative CD11b+ Ag-specific B cell frequencies before and following (d5) immunization are indicated. B) CD19, CD21, PD-1, and CD86 expression levels by gated Ag-specific B cells on d0 (thin line) and 5 days post immunization (thick line). Shaded histograms indicate expression by non-Ag-specific B cells in immune animals. Dotted lines indicate isotype control staining for Ag-specific B cells. Adapted from Yammani and Haas52 and Haas12.
Table I.
Markers expressed by mouse and primate TNP-specific B cells before and after immunizationa
| Phenotypic Marker | Naive | Immune (day 5) | ||
|---|---|---|---|---|
| Mice | Primates | Mice | Primates | |
| Forward scatter (FSC) | Low | Low | High | High |
| Side scatter (SSC) | Low | Low | High | High |
| CD11b (Mac-1/CR3) | − | − | + | + |
| CD19 | + | + | High | High |
| CD21 | + | + | Low | Low |
| CD20 | + | + | ? | Low |
| B220 | + | ? | Low | ? |
| PD-1 | − | − | + | + |
| CD86 | − | − | + | + |
| CD80 | − | − | + | + |
| CD44 | − | − | + | + |
| CD43 | − | ? | + | ? |
| CD23 | + | ? | − | ? |
| CD27 | − | − | − | + |
Data is derived from references 12 and 52 and represent the phenotype of mouse spleen B cells or primate blood B cells before and 5 to 7 days after immunization with TNP-Ficoll (immunization was intraperitoneal for mice and intraveneous for African Green monkeys and cynomolgus macaques). It should be noted that not all TNP-specific B cells express changes in markers following immunization, but that these alterations are due to the presence of cells expressing CD11b and increased size (FSChi).
A large fraction of Ag-specific CD11b+CD19+ blood B cells in TNP-Ficoll-immunized NHP express low levels of CD20 (Fig. 4A), whereas CD20loCD11b+CD21loCD19+ B cells represent only ~1 % of the total CD20+ blood B cell pool in naïve AGM. The CD19+CD20lo phenotype is consistent with that of plasmablasts. Indeed, ELISPOT analysis of the CD11b+ B blood cells indicated that TNP-specific antibody-secreting B cells were enriched (30-fold) in the CD11b+ B cell pool52. That CD11b-expressing B cells were found to be superior to CD11b− cells in producing antibody against this model TI-2 Ag is consistent with observations made in mice. Adoptive transfer experiments of CD11b+ B-1b cells from normal mice optimally reconstitute antibody responses to haptenated Ficoll and PPS38, 11 and Ag-specific CD11b-expressing CD138+ plasmablasts are found in spleens of normal mice following TNP-Ficoll immunization12. Moreover, results from adoptive transfer experiments performed in our lab indicate an enhanced ability of VHB1-8 Tg CD11b+ peritoneal B-1b cells versus other subsets to differentiate into ASC following NP-Ficoll immunization (unpublished observations). Thus, B-1b cells in mice and B-1b-like CD11b+ cells in NHP optimally differentiate into ASC following immunization with haptenated-Ficoll.
The long-term fate of the CD11b+CD20lo blood plasmablasts elicited in response to TI-2 Ag in NHP is not presently clear. However, as we have reported in mice12, TNP-specific ASC are found in both the spleen and bone marrow of NHP 6 weeks post TNP-Ficoll immunization52. Whether these cells have the potential to compete for the bone marrow niche and/or fully differentiate into plasma cells has yet to be explored. Based on studies in mice, the bone marrow ASC elicited in response to TI-2 Ags have lower antibody output than that found for plasma cells elicited in response to protein-polysaccharide conjugates58. TI-2 Ag-elicited ASC may therefore be more representative of plasmablasts59. The extent to which their numbers are maintained by constant renewal due to persisting Ag, as seems to be the case for splenic TI-2 Ag-elicited plasmablasts11, 60, is unclear. Importantly, when TI-2 Ags are associated with infections or adjuvants, the resulting bone marrow ASC may be more differentiated and in fact, non-dividing long-lived ASC58, 60. The pathways regulating the differentiation of TI Ag-activated B-1b cells into long-lived bone marrow ASC versus self-renewing memory-like splenic plasmablasts or quiescent memory cells are not fully elucidated7, 11, 13, 60. Nonetheless, this is clearly an important area of investigation, since progress in this area would inform improved vaccine strategies for this unique class of Ag.
Given the proposed role of CD27+ “memory” B cells in human immune responses to polysaccharide Ags22, 24, we examined CD27 expression on Ag-specific blood B cells before and following TNP-Ficoll immunization. In most monkeys, CD27-expressing TNP-specific IgM+ blood and spleen B cells were below the limit of detection. In contrast, CD27-expressing non-Ag-specific blood B cells represented ~25–50% of the population. Notably, this has not been our experience with PPS3-specific B cells from naïve NHP. In many cases, monkey B cells that bind to PPS3 express CD27 prior to immunization (manuscript in preparation). This may reflect previous exposure to serotype 3 S. pneumoniae or a polysaccharide antigen with similar structure. Although CD27 was undetectable on naïve TNP-specific B cells, CD27 expression was present on activated Ag-specific blood and spleen B cells following TNP-Ficoll immunization. This finding lends support to the argument that CD27 is induced on naïve B cells during the primate response to some TI-2 Ags and in fact, maintained as a marker of memory, as discussed in the next section.
B-1b-like memory cells in mice and primates
Evidence suggests that B-1b cells activated by isolated TI-2 Ags or bacteria support long-lived antibody responses as either dividing plasmablasts within the spleen11, 13, 60–62 or as non-dividing long-lived BM ASC (ref.60 and unpublished observations). Hapten-specific splenic ASC raised against TNP-Ficoll clearly persist in normal mice and NHP12, 52. However, these cells lose CD11b expression during the differentiation process to ASC, making identification of B-1-derived CD138+ cells (using CD11b as a defining marker) in intact mice after the peak of the response impossible. Thus, adoptive transfer experiments have been performed by our laboratory to confirm that B-1b cells contribute to the maintenance of this splenic ASC pool in mice (unpublished observations). MacLennan and colleagues11 used immunohistochemistry to demonstrate that NP-reactive B cells can persist in splenic tissue of Rag-1−/− mice as either plasma cells or plasmablasts for >2 months in the response to NP-Ficoll, which is known to involve B-1b cells11, 51. Thus, the long-lived humoral response to NP-Ficoll in mice is, in part supported by a dividing splenic plasmablast pool. Recent work by Kearney and Foote indicates that splenic plasmablasts elicited in response to alpha1-->3-dextran are maintained for long periods by continuous Ag-driven formation of short-lived plasmablasts60. Importantly, this population may further expand during secondary bacterial challenge7, 60. Therefore, splenic B-1b plasmablasts provide an unconventional type of memory that enables rapid increases in antibody levels upon secondary exposure to infectious agents.
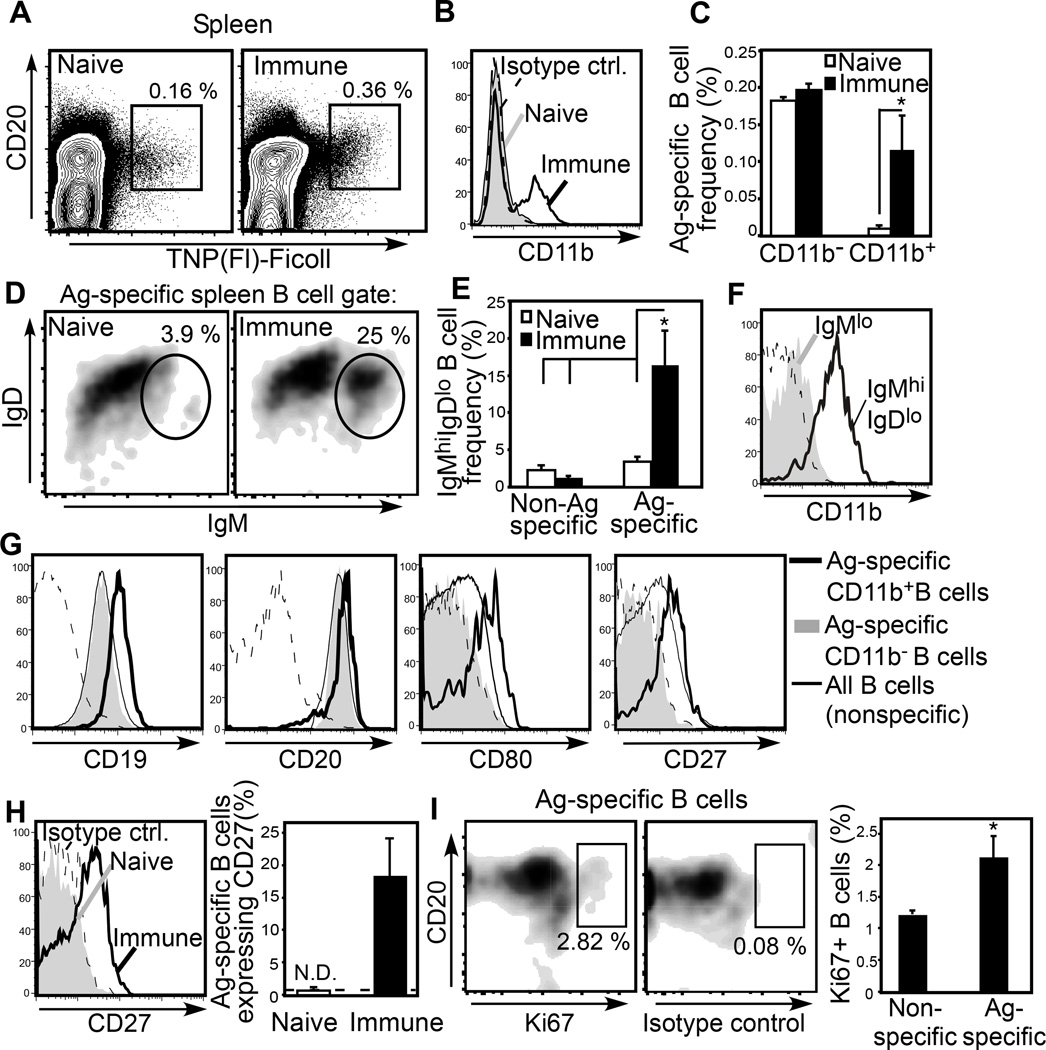
In addition to plasmablasts, a population of CD11bloCD138− Ag-specific B cells persists for > 6 weeks in the spleen and peritoneal cavity following haptenated-Ficoll immunization in mice12. CFSE-labeled B-1b cells from VHB1–8 transgenic mice similarly establish this population in normal mice following NP-Ficoll immunization (unpublished observations). The lack of CD138 expression by these cells along with IgG expression, resting size on forward scatter analysis, and loss of CFSE indicate that they are non-antibody secreting memory cells. Although suppressive mechanisms limit recall responses to TI-2 Ags, memory B cells are clearly formed in response to these Ags63. As in mice, six weeks post TNP-Ficoll immunization, splenic Ag-specific B cell frequencies are also significantly increased in NHP (Fig. 5A), and this is largely due to a ~10-fold increase in CD11b+ IgMhiIgDint Ag-specific cells (Fig. 5B–F). These Ag-specific splenic B cells are CD19hi, coexpress CD80, and in contrast to the CD20lo plasmablast phenotype found in blood, express high levels of CD20 (Fig. 5G). Only a small frequency (~5%) of these cells express Ki67 (Fig. 5I), and hence, most are non-dividing. Importantly, we did not detect TNP-specific CD27+ B cells in naïve cynomolgus spleens, but these cells were present at 6 weeks post immunization (Fig. 5H) and belonged to the CD11b+CD20hi population (Fig. 5G). Thus, in NHP, the majority of CD11b+ Ag-specific splenic B cells 6 weeks post TNP-Ficoll immunization exhibit a memory-like phenotype, as in mice. Our findings support the likelihood that CD27 may be a bone fide marker of memory for IgM+ B cells that have participated in a TI-2 Ag-specific immune response. Further work is necessary to determine the functional characteristics of these putative memory cells in mice and NHP. Indeed, work in this area may lead to a better understanding of the regulatory pathways suppressing boosting of secondary humoral responses to polysaccharide Ags—a major obstacle to effective polysaccharide-based vaccines.
Figure 5. Ag-specific B-1 cells are significantly increased in NHP spleens 6 weeks following TNP-Ficoll immunization and display an IgMhiIgDloCD80+CD27+ phenotype.
(A–I) Flow cytometric analysis of Ag-specific B cells in spleens of naive and immune cynomolgus macaques 6 weeks post TNP-Ficoll immunization. A) Representative flow cytometric analysis of Ag-specific splenic B cells (gated). B) CD11b expression on Ag-specific B cells from naive (shaded histogram) and immune (solid line) animals. C) Frequencies of CD11b+ and CD11b− Ag-specific B cells among splenocytes. D) IgM and IgD expression by Ag-specific B cells, with representative IgMhiIgDlo population-gating shown. E) Frequencies of Ag-specific and non–Ag-specific B cells expressing an IgMhiIgDlo B cell phenotype. F) Representative CD11b expression by Ag-specific IgMhiIgDlo (solid line) and IgMlo (shaded histogram) B cells in immune animals. G) CD19, CD20, CD80, and CD27 expression by CD11b+ (thick solid line) and CD11b− (shaded histogram) Ag-specific B cells and non–Ag-specific B cells (thin line) in immune animals. H) CD27 expression by Ag-specific B cells in naive and immune spleen (left panel) and frequencies of Ag-specific B cells expressing CD27. N.D., “none detected”, indicates the frequency of Ag-specific B cells staining positive for CD27 was not significantly increased over the frequencies of these cells binding to isotype-matched control antibody (indicated by horizontal dashed line). I) Frequencies of Ki67+ B cells in immune animals. For all analyses, n = 3 cynomolgus macaques per group. Histograms with dashed horizontal lines depict isotype control staining by Ag-specific B cells from immune animals. Significant differences in mean (±SEM) frequencies are indicated, *p < 0.05. Adapted from Yammani and Haas52.
Summary
In conclusion, our findings demonstrate that: 1) primate B cells in serosal tissues exhibit striking phenotypic and functional similarities with murine B-1 cells, 2) there is remarkable similarity between mouse and primate Ag-specific B cell responses to TNP-Ficoll, with primate Ag-specific B-1-like cells playing a dominant role in the humoral immune response to this model TI-2 Ag, and 3) Ag-specific primate B-1 cells enter the IgM+IgDloCD27+ compartment upon TI-2 Ag exposure, thereby linking the mouse B-1b cell population with the previously described human/primate IgM+IgD+CD27+ “memory” population52. Although the populations contributing to TI-2 Ag responses in mice and humans have been proposed to differ, our results with NHP raise the possibility that B-1b-like cells also contribute to these responses in humans. Future studies examining the relationships between mouse and primate B-1a and B-1b cells, including details of their developmental lineage and ontogeny, major functions in the immune system, and the pathways regulating their participation in host defense, autoimmunity, allergy, and malignancy are certainly warranted as they are expected to lead to advances in vaccine development and the treatment of human diseases.
Material and Methods
Flow cytometry
Staining and flow cytometric analysis of B cell surface marker expression for data shown in Figure 1 and Figures 3–5 have been previously described as indicated in the figure legends. For the data shown in Figure 2, cells were harvested from the peritoneal cavities of adult C57BL/6 wild type (Jackson Laboratories) or CD19−/− mice8. Briefly, 10 ml phosphate buffered saline (PBS), pH=7.4, was injected into the peritoneal cavity and harvested. Recovered cells were adjusted to a concentration of 1×107/ml in PBS containing 2% fetal calf serum (PBS-FCS), blocked with Fc Block (BD Biosciences) and then stained with fluorochrome-labeled antibodies against mouse CD19 (1D3), CD5 (53–7.3), B220 (RA3–6B2), and CD21/35 (7E9) from Biolegend, CD11b (M1/70) from eBioscience, and CD43 (S7) and CD23 (B3B4) from BD Biosciences. Cells were washed in PBS-FCS and analyzed using a CantoII flow cyotometer (BD Biosciences) and Flowjo software (Treestar).
Acknowledgements
This work was supported by NIH/NIAID grants R21AI095800 and R56AI108654-01, an American Cancer Society research scholar grant (RSG-12-170-01), and shared resources grant NIH/NCI P30CA012197.
References
- 1.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 2.Montecino-Rodriguez E, Dorshkind K. B-1 B cell development in the fetus and adult. Immunity. 2012;36:13–21. doi: 10.1016/j.immuni.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham AF, et al. B1b cells recognize protective antigens after natural infection and vaccination. Frontiers in Immunology. 2014;5:535. doi: 10.3389/fimmu.2014.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stall AM, et al. Characteristics and development of the murine B-1b (Ly-1 B sister) cell population. Ann. N. Y. Acad. Sci. 1992;651:33–43. doi: 10.1111/j.1749-6632.1992.tb24591.x. [DOI] [PubMed] [Google Scholar]
- 5.Kantor AB, et al. Differential development of progenitor activity for three B-cell lineages. Proc. Natl. Acad. Sci. 1992;89:3320–3324. doi: 10.1073/pnas.89.8.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lalor PA, et al. Feedback regulation of murine Ly-1 B cell development. Eur. J. Immunol. 1989;19:507–513. doi: 10.1002/eji.1830190315. [DOI] [PubMed] [Google Scholar]
- 7.Alugupalli KR, et al. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Haas KM, et al. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Haas KM, et al. Complement receptors CD21/35 link innate and protective immunity during Streptococcus pneumoniae infection by regulating IgG3 antibody responses. Immunity. 2002;17:713–723. doi: 10.1016/s1074-7613(02)00483-1. [DOI] [PubMed] [Google Scholar]
- 10.Briles DE, et al. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J. Exp. Med. 1982;156:1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu MC, et al. B cell clones that sustain long-term plasmablast growth in T-independent extrafollicular antibody responses. Proc. Natl. Acad. Sci. USA. 2006;103:5905–5910. doi: 10.1073/pnas.0601502103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haas KM. Programmed cell death 1 suppresses B-1b cell expansion and long-lived IgG production in response to T cell-independent type 2 antigens. J. Immunol. 2011;187:5183–5195. doi: 10.4049/jimmunol.1101990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foote JB, Kearney JF. Generation of B cell memory to the bacterial polysaccharide alpha-1,3 dextran. J. Immunol. 2009;183:6359–6368. doi: 10.4049/jimmunol.0902473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall JL, et al. The capsular polysaccharide Vi from Salmonella typhi is a B1b antigen. J. Immunol. 2012;189:5527–5532. doi: 10.4049/jimmunol.1103166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colombo MJ, Alugupalli KR. Complement factor H-binding protein, a putative virulence determinant of Borrelia hermsii, is an antigenic target for protective B1b lymphocytes. J. Immunol. 2008;180:4858–4864. doi: 10.4049/jimmunol.180.7.4858. [DOI] [PubMed] [Google Scholar]
- 16.Gil-Cruz C, et al. The porin OmpD from nontyphoidal Salmonella is a key target for a protective B1b cell antibody response. Proc. Natl. Acad. Sci. 2009;106:9803–9808. doi: 10.1073/pnas.0812431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohdan H, et al. Mac-1-negative B-1b phenotype of natural antibody-producing cells, including those responding to Gal alpha 1,3Gal epitopes in alpha 1,3-galactosyltransferase-deficient mice. J. Immunol. 2000;165:5518–5129. doi: 10.4049/jimmunol.165.10.5518. [DOI] [PubMed] [Google Scholar]
- 18.Weller S, et al. Somatic diversification in the absence of antigen-driven responses is the hallmark of the IgM+ IgD+ CD27+ B cell repertoire in infants. J. Exp. Med. 2008;205:1331–1342. doi: 10.1084/jem.20071555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruetzmann S, Rosado MMW, Germing H, Tournilhac U, Peter O, Berner HH, Peters R, Boehm A, Plebani T, Quinti A, Carsetti IR. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J. Exp. Med. 2003;197:939–945. doi: 10.1084/jem.20022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weller S, et al. Human blood IgM "memory" B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tangye SG, Good KL. Human IgM+CD27+ B cells: memory B cells or "memory" B cells? J. Immunol. 2007;179:13–19. doi: 10.4049/jimmunol.179.1.13. [DOI] [PubMed] [Google Scholar]
- 22.Reynaud CA, et al. IgM memory B cells: a mouse/human paradox. Cell. Mol. Life Sci. 2012;69:1625–1634. doi: 10.1007/s00018-012-0971-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moens L, et al. Human memory B lymphocyte subsets fulfill distinct roles in the antipolysaccharide and anti-protein immune response. J. Immunol. 2008;181:5306–5312. doi: 10.4049/jimmunol.181.8.5306. [DOI] [PubMed] [Google Scholar]
- 24.Khaskhely N, et al. Phenotypic analysis of pneumococcal polysaccharide-specific B cells. J. Immunol. 2012;188:2455–2463. doi: 10.4049/jimmunol.1102809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samanta DN, et al. B cell hyperresponsiveness and expansion of mature follicular B cells but not of marginal zone B cells in NFATc2/c3 double-deficient mice. J. Immunol. 2005;174:4797–4802. doi: 10.4049/jimmunol.174.8.4797. [DOI] [PubMed] [Google Scholar]
- 26.Haas KM, Poe JC, Tedder TF. CD21/35 promotes protective immunity to Streptococcus pneumoniae through a complement-independent but CD19-dependent pathway that regulates PD-1 expression. J. Immunol. 2009;183:3661–3671. doi: 10.4049/jimmunol.0901218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vuyyuru R, et al. Characteristics of Borrelia hermsii infection in human hematopoietic stem cell-engrafted mice mirror those of human relapsing fever. Proc. Natl. Acad. Sci. 2011;108:20707–20712. doi: 10.1073/pnas.1108776109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat. Rev. Immunol. 2009;9:767–777. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- 29.Klein Klouwenberg P, Bont L. Neonatal and infantile immune responses to encapsulated bacteria and conjugate vaccines. Clin. Dev. Immunol. 2008;2008:628963. doi: 10.1155/2008/628963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas AH, Apicella MA, Taylor CE. Carbohydrate moieties as vaccine candidates. Clin. Infect. Dis. 2005;41:705–712. doi: 10.1086/432582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez-Fernandez A, Faro J, Fernandez C. Immune responses to polysaccharides: lessons from humans and mice. Vaccine. 2008;26:292–300. doi: 10.1016/j.vaccine.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 32.Schneerson R, et al. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J. Exp. Med. 1980;152:361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenspan NS, Cooper LJ. Cooperative binding by mouse IgG3 antibodies: implications for functional affinity, effector function, and isotype restriction. Springer Semin. Immunopathol. 1993;15:275–291. doi: 10.1007/BF00201107. [DOI] [PubMed] [Google Scholar]
- 34.Yoo EM, et al. Human IgG2 can form covalent dimers. J. Immunol. 2003;170:3134–3138. doi: 10.4049/jimmunol.170.6.3134. [DOI] [PubMed] [Google Scholar]
- 35.McMaster PR, et al. Tolerance to type 3 pneumococcal polysaccharide in monkeys. Immunol. Commun. 1973;2:361–370. doi: 10.3109/08820137309022807. [DOI] [PubMed] [Google Scholar]
- 36.Poolman J, Borrow R. Hyporesponsiveness and its clinical implications after vaccination with polysaccharide or glycoconjugate vaccines. Expert Rev. Vaccines. 2011;10:307–322. doi: 10.1586/erv.11.8. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, et al. Division and differentiation of natural antibody-producing cells in mouse spleen. Proc. Natl. Acad. Sci. 2007;104:4542–4546. doi: 10.1073/pnas.0700001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawahara T, et al. Peritoneal cavity B cells are precursors of splenic IgM natural antibody-producing cells. J. Immunol. 2003;171:5406–5414. doi: 10.4049/jimmunol.171.10.5406. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, et al. Division and differentiation of natural antibody-producing cells in mouse spleen. Proc. Natl. Acad. Sci. 2007;104:4542–4546. doi: 10.1073/pnas.0700001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haas KM, et al. Aging Promotes B-1b Cell Responses to Native, but Not Protein-Conjugated, Pneumococcal Polysaccharides: Implications for Vaccine Protection in Older Adults. J. Infect. Dis. 2014;209:87–97. doi: 10.1093/infdis/jit442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cong T, Rabin E, Wortis H. Treatment of CD5− B cells with anti-Ig but not LPS, induces surface CD5: two B cell activation pathways. Int. Immunol. 1991;3:467. doi: 10.1093/intimm/3.5.467. [DOI] [PubMed] [Google Scholar]
- 42.Hippen KL, Tze LE, Behrens TW. CD5 maintains tolerance in anergic B cells. J. Exp. Med. 2000;191:883–889. doi: 10.1084/jem.191.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yanaba K, et al. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 44.Hansell CA, et al. Universal expression and dual function of the atypical chemokine receptor D6 on innate-like B cells in mice. Blood. 2011;117:5413–5424. doi: 10.1182/blood-2010-11-317115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J. Exp. Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reynaud CA, Weill JC. Gene profiling of CD11b(+) and CD11b(-) B1 cell subsets reveals potential cell sorting artifacts. J. Exp. Med. 2012;209:433–434. doi: 10.1084/jem.20120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Descatoire M, et al. A human equivalent of mouse B-1 cells? J. Exp. Med. 2011;208:2563–2564. doi: 10.1084/jem.20112232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perez-Andres M, et al. The nature of circulating CD27+CD43+ B cells. J. Exp. Med. 2011;208:2565–2566. doi: 10.1084/jem.20112203. author reply 2566-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Covens K, et al. Characterization of proposed human B-1 cells reveals pre-plasmablast phenotype. Blood. 2013;121:5176–5183. doi: 10.1182/blood-2012-12-471953. [DOI] [PubMed] [Google Scholar]
- 50.Li W, Batliwalla F, Rothstein TL. Human B-1 cells are not preplasmablasts: analysis of microarray data and other issues. Blood. 2013;122:3691–3693. doi: 10.1182/blood-2013-08-520031. [DOI] [PubMed] [Google Scholar]
- 51.Shriner AK, et al. IL-7-dependent B lymphocytes are essential for the anti-polysaccharide response and protective immunity to Streptococcus pneumoniae. J. Immunol. 2010;185:525–531. doi: 10.4049/jimmunol.0902841. [DOI] [PubMed] [Google Scholar]
- 52.Yammani RD, Haas KM. Primate B-1 cells generate antigen-specific B cell responses to T cell-independent type 2 antigens. J. Immunol. 2013;190:3100–3108. doi: 10.4049/jimmunol.1203058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Donze HH, et al. Human peritoneal B-1 cells and the influence of continuous ambulatory peritoneal dialysis on peritoneal and peripheral blood mononuclear cell (PBMC) composition and immunoglobulin levels. Clin. Exp. Immunol. 1997;109:356–361. doi: 10.1046/j.1365-2249.1997.4541352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawai K, et al. CD11b-mediated migratory property of peripheral blood B cells. J. Allergy Clin. Immunol. 2005;116:192–197. doi: 10.1016/j.jaci.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 55.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annual review of immunology. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 56.Rothstein TL, et al. STAT3 activation, chemokine receptor expression, and cyclin-Cdk function in B-1 cells. Curr. Top. Microbiol. Immunol. 2000;252:121–130. doi: 10.1007/978-3-642-57284-5_13. [DOI] [PubMed] [Google Scholar]
- 57.Griffin DO, Rothstein TL. A small CD11b(+) human B1 cell subpopulation stimulates T cells and is expanded in lupus. J. Exp. Med. 2011;208:2591–2598. doi: 10.1084/jem.20110978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taillardet M, et al. Toll-like receptor agonists allow generation of long-lasting antipneumococcal humoral immunity in response to a plain polysaccharidic vaccine. J. Infect. Dis. 2010;202:470–479. doi: 10.1086/653739. [DOI] [PubMed] [Google Scholar]
- 59.Racine R, et al. IgM production by bone marrow plasmablasts contributes to long-term protection against intracellular bacterial infection. J. Immunol. 2011;186:1011–1021. doi: 10.4049/jimmunol.1002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Foote JB, et al. Long-Term Maintenance of Polysaccharide-Specific Antibodies by IgM-Secreting Cells. J. Immunol. 2012;188:57–67. doi: 10.4049/jimmunol.1100783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alugupalli KR. A distinct role for B1b lymphocytes in T cell-independent immunity. Curr. Top. Microbiol. Immunol. 2008;319:105–130. doi: 10.1007/978-3-540-73900-5_5. [DOI] [PubMed] [Google Scholar]
- 62.Racine R, Chatterjee M, Winslow GM. CD11c expression identifies a population of extrafollicular antigen-specific splenic plasmablasts responsible for CD4 T-independent antibody responses during intracellular bacterial infection. J. Immunol. 2008;181:1375–1385. doi: 10.4049/jimmunol.181.2.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Obukhanych TV, Nussenzweig MC. T-independent type II immune responses generate memory B cells. J. Exp. Med. 2006;203:305–310. doi: 10.1084/jem.20052036. [DOI] [PMC free article] [PubMed] [Google Scholar]