Abstract
Streptococcus mutans, a Gram positive facultative anaerobe, is one among the approximately seven hundred bacterial species to exist in human buccal cavity and cause dental caries. Quorum sensing (QS) is a cell-density dependent communication process that respond to the inter/intra-species signals and elicit responses to show behavioral changes in the bacteria to an aggressive forms. In accordance to this phenomenon, the S. mutans also harbors a Competing Stimulating Peptide (CSP)-mediated quorum sensing, ComCDE (Two-component regulatory system) to regulate several virulence-associated traits that includes the formation of the oral biofilm (dental plaque), genetic competence and acidogenicity. The QS-mediated response of S. mutans adherence on tooth surface (dental plaque) imparts antibiotic resistance to the bacterium and further progresses to lead a chronic state, known as periodontitis. In recent years, the oral streptococci, S. mutans are not only recognized for its cariogenic potential but also well known to worsen the infective endocarditis due to its inherent ability to colonize and form biofilm on heart valves. The review significantly appreciate the increasing complexity of the CSP-mediated quorum-sensing pathway with a special emphasis to identify the plausible drug targets within the system for the development of anti-quorum drugs to control biofilm formation and associated risks.
Keywords: Streptococcus mutans, Dental biofilms, Quorum Sensing, CSP, ComA, Multi-drug resistance
Quorum Sensing System: Deciphering ‘Dental Talk’
Streptococcus mutans, a Gram positive coccus, is an inhabitant of human buccal cavity and is also a primary causal organism of dental caries. The tooth surface act as an essential ecological niche for S. mutans as it is very difficult to be identified until the teeth is erupted in the buccal cavity and dissipate immediately after the teeth is lost due to infection or old age [1–3]. S. mutans has advanced to rely upon a biofilm “way of life” to survive and persist for longer durations in its characteristic biological community [4–6], dental biofilm, commonly known as plaque. S. mutans with its own or other populations tend to form oral biofilms (dental plaque) via., inter/intra-species communication known as quorum sensing system (QS) [6–8].
QS Pathways in S. mutans
ComCDE QS Pathway
QS in gram-positive bacteria like S. mutans, generally comprise three components: a signal peptide (CSP), a two-component regulatory system (TCTS) with a membrane-bound histidine kinase (HK) sensor and an intracellular response regulator (RR) [9]. Recently, it has been identified that the CSP-mediated QS system in S. mutans up-regulates the genes, cslAB (comAB) and comCDE that exhibits the phenotypic traits like genetic competence, bacteriocin production and biofilm [10, 11]. The genes comC, comD, and comE encodes a precursor of competence-stimulating peptide (CSP), the HK sensor protein, and a cognate RR, respectively [10, 12, 13]. The genes comC and comDE are closely located on the same chromosome and the peptide (CSP) is synthesized as a collective result of their gene products [14, 15]. The genes, cslA and cslB, are divergently mapped on the same chromosome also encodes a CSP-specific maturation and secretion complex factors with an ATP-binding cassette (ABC) transporter (ComA) and an accessory factor (ComB). As these factors, specifically involved in the post-translational processing of the CSP to further secrete it towards the outside of the cell, as a mature signaling peptide [16]. The QS signalling operates optimally when the cells acquire and depend solely and actively on the biofilm lifestyle as the CSP concentration reaches a threshold value [17]. Mature CSP binds to the conserved HK residue (ComD) present in the membrane, resulting in its own phosphorylation and the subsequent relay of this process to its cognate RR protein (ComE) [18]. Thus the cell-density dependent process elicits a cellular response to activate the gene loci such as comA and comB with a simultaneous feed forward circuit for comCDE. Also, the same response was extended to the genes, comR and sigX expression, for which the mechanism is still unknown [2].
ComRS Pathway
Interestingly, S. mutans possess ComCDE as well as ComRS quorum sensing pathways. The ComRS QS system is activated on sensing the extracellular tryptophan signal peptide pheromone, XIP and get internalized to the cells through a membrane-bound oligopeptide ABC transport system, Opp/Ami [2]. Further, XIP binds to a transcriptional regulator, ComR, in turn regulates the sigX, an alternative sigma factor SigX (ComX), to switch on the late competence genes responsible for the genetic transformation. The ComCDE operon regulates the production of anti-microbial peptides, toxins and adherence factors, whereas the functional characterization of the ComRS quorum-sensing circuit show a pre-dominant role in the genetic competence via., the regulator, SigX [19, 20].
LuxS Pathway
Previously, it has been reported that S. mutans interact with the other oral flora of the dental plaque to mediate interspecies communication. LuxS is reported to involve in S-adenosylmethionine catabolism and converts ribose homocysteine into homocysteine and 4,5-dihydroxy-2,3-pentanedione that act as a precursor of Autoinducer-2 (AI-2) [21–23]. The LuxS-mediated QS are well characterised to elicit interspecies communication and modulate multiple traits crucial to establish S. mutans pathogenesis. So, the S. mutans flourish in the buccal cavity via., activation of the luxS gene that leads to the production of AI-2 which ensures it survival and virulence expression in multispecies environment. The researchers has shown that the luxS- deficient strains affects the expression of the virulence determinant to a greater extent (>50 %) and in parallel upregulate their acid-adaptive behaviours to increase their survival rate [24]. Also, the luxS gene is highly conserved among the Gram positive and Gram negative bacteria and may operate as a global regulator to be an essential factor for a drug target [22, 23].
S. mutans and Dental Caries
Clarke, in 1924, designated S. mutans, after he could isolate a bacterial species from the carious lesions and looked like a mutant form of a coccus. The relationship of S. mutans with dental caries was not largely perceived until dental practitioners and researchers in the 1960s revived interest in this organism. Since then, various studies confirmed the relationship of S. mutans with dental decay and carious lesions and longitudinal studies followed the predominance of S. mutans on infected sites that ultimately became cariogenic. An experimental study on the mono-infected rats revealed the cariogenic potential of the various plaque species and notably, S. mutans was found to be predominant among cariogens. The research has led to the “specific plaque hypothesis” and stated that the S. mutans, were primarily responsible for the dental decay. With the taxonomical advances, it has become apparent that the S. mutans-like isolates actually accounts for several species and was collectively known as the mutans streptococci (MS).
Like many other diseases, dental caries, also have multiple etiologies. However, the topmost variable for caries occurrence has been sucrose rich diet. Frequent consumption of sucrose has also been implicated in building plaque ecological niche and provide a substrate for MS to synthesize adhesive glucans to promote their own colonization and accumulation on the tooth surface [25]. The acid tolerance capability of the MS further enhances their adhesion and growth as well as the growth of other acid-tolerant species such as the lactobacilli. Due to highly acidogenic nature of these species [26], the fermentable dietary carbohydrate results in a huge drop in plaque pH than normal paving way for demineralization and decalcification of the tooth surface. The scenario also promoted researchers worldwide to focus on the adhesion, acidogenicity, and acid-tolerant properties of the MS [27].
The focus of this review is to primarily describe the quorum sensing–mediated cell–cell communication in S. mutans and its various components. Since most of the virulence properties (Table 1) are shared among the various MS, the review will address on the S. mutans as a paradigm for the virulence of dental caries under the regulation of QS. Also the review discusses the structural and functional aspects of various quorum molecules that would provide an insight to exploit them as drug targets. The application potential of this review would also provoke the scientific community towards target based drug discovery in synthetic biology to effectively control the bacterial biofilms and its associated risks.
Table 1.
QS controlled genes and their phenotypic traits in S. mutans
| QS components | Regulatory gene | Group-derived benefits (Virulence factors) | References |
|---|---|---|---|
| CSP (ComC) | comC/comD/comE | Bacteriocins, Biofilm Formation | [10, 11, 14, 55] |
| XIP (ComS) | comR | Competence | [10, 11] |
| SigX (ComX) | comR | DNA transport, uptake and Recombination | [12, 36] |
| ComAB | nlmAB (comAB) | Mutacin IV (bacteriocins), Maturation and export of CSP outside the cell | [16, 49] |
| ComD | comC/comD/comE (TCSTS) | Class II bacteriocins and HK receptor for CSP signal | [14, 22] |
| immA and immB (VicRK) | comC | Self- immunity proteins for protection from bacteriocins, acid tolerance | [55] |
| Lux S | luxS | Interspecies communication | [39, 40] |
Plausible Drug Targets: Hindering ‘Bacterial Chat’
Signals
CSP
In S. mutanscomC encodes a precursor signal peptide, a 21-amino acid Competence Stimulating Peptide (CSP) processed and exported out of the cell to elicit its response via., the ComCDE QS pathway. The formation of biofilm and virulence traits in S. mutans such as acidogenicity, bacteriocin production, genetic competence solely depends on the signal, CSP-mediated QS [22]. S. mutans has evolved various mechanisms to allow the survival and persistence of the species in a broad range of adapting to frequent environmental pressure such as acidic environment that act as a constant stress in the oral cavity to enhance the production of CSP [28]. The stress-inducible activation of CSP induces the cell death of a sub-population accounting for the altruistic nature of bacteria to be beneficial for its own population. Similarly, the native CSP of Steptococcus pneumonia was known to cause chronic diseases in humans such as pneumococcal meningitis and pneumonia [13]. Earlier reports shows that the S. mutans JH1005 (deletion of three amino acid residues in the C- terminal domain of the peptide, CSP) has overexpressed bacteriocin and resulted in 600-fold reduction in the competence and drive a greater significance of those residues in QS signalling pathway [29]. The CSP has been reported to exist as a factor to be structurally random coiled and highly soluble nature to increase its diffusion rate in the aqueous environment [10, 30]. The randomly coiled CSP undergoes conformational change and folding upon binding to the membrane bound Histidine Kinase (HK) receptor resulting in the formation of an amphipathic α-helix with a hydrophobic face. The change in the conformation enables the CSP to show a stronger interface of its interaction with the HK receptor binding pocket via., hydrophobic interactions [29, 31]. Till date, approximately 700 species has been reported in the buccal cavity and a few cariogenic pathogens, including S. mutans are well characterized to mediate dental caries. So as to reduce or completely eliminate dental caries, alternate strategies has to be prioritized as a one-step forward to prevent the S. mutans adherence on the tooth surface without affecting the normal oral flora. In recent years, antimicrobial peptides (AMPs) have received attention of the researchers worldwide, as a novel class of antimicrobial agents because of their ability to kill a wide range of pathogenic species, including bacteria, fungi, and viruses, through various mechanism of action [9]. The AMPs are also reported to be effective against resistant pathogens with their ability to disrupt the functions of the cellular membranes and nucleic acids directly and moreover the rate to which AMP-resistant strains appears, is very low or almost negligible [32].
Similarly, a new class of pathogen-specific antimicrobials, STAMPs (selectively targeted antimicrobial peptides) were discovered against the dental pathogen, S. mutans. The peptides were designed to have a targeted domain (8-aminoacid region of the CSP) fused with an antimicrobial peptide domain to show a robust species specific activity and eliminate the S. mutans from multi-species biofilm without affecting the normal (non-cariogenic) streptococci [33]. Syvitski et al. [29] reported that the C-terminal truncated CSP peptides could competitively affect the QS activity and the structural motif in the C-terminal domain restores the activation of the QS signal transduction pathway [34, 35]. Further the mutational studies (Point deletion) revealed the loss of function of CSP in promoting genetic competence without affecting the binding of peptide to the receptor [34–37].
XIP
XIP (Sig X Inducing Peptide) modulates the expression of σx (ComX) via., ComRS signaling pathway. The comS encodes the XIP precursor is processed to mature and internalize the cells through the transporter, Opp/Ami. The mature signal in-turn interacts with the comR and activate the gene comX to express the factor, σx [36, 37]. Recent data has shown a drastic reduction of XIP levels in the comX (σx) deficient S. mutans strains, as it evidence the positive feed-back role of ComX in ComRS/XIP expression where its binding sites were localized either within or upstream of ComRS [38]. Earlier reports have also demonstrated that the increase in the concentration (2–4 μM) of CSP mediated cell-killing and growth arrest of S. mutans [39, 40]. Likewise, the S. mutans on exposure to 10 μM of synthetic XIP (sXIP) has shown the killing effect, naturally score the phenomena of exploiting the pheromones (XIP) as a potent effector molecules to treat dental caries [41].
Autoinducer-2 (AI-2)
The previous studies had established that in dental plaque, the oral streptococci and other oral bacterial species interact with each other and form an ideal system to mediate interspecies signalling and biofilm formation in a particular niche [42]. The AI-2 mediated interspecies QS response is a well-characterized cross-talk strategy, a known co-operative synergy exists in both gram-positive and gram-negative pathogens [43]. As it is evidenced from the fact that they all have a highly conserved luxS gene encoding autoinducer-2 (AI-2) serve the function of optimization of the virulence gene expression in a cell density dependent manner in a particular biological niche. A study carried out by Merritt et al. [44], Yoshida et al. [42] demonstrated that the mutation in the luxS gene of S. mutans leads to an altered biofilm structure and also affected the production of the bacteriocin and mutacin I. In Escherichia coli and Serratia marcescens the mutacin production was lexA and recA dependent whereas in S.mutans, it was solely dependent on a Lex-A like factor, IrvR [45]. Rickard et al. [43] and McNab et al. [46] have also shown the involvement of AI-2 multispecies biofilm communities and thus, an approach leading to the suppression of luxS gene expression could lead an altered interspecies behavior in the oral microbial community leading to hindrance in the QS system.
Receptor and Response Regulator
ComDE
The two signal transduction system, ComDE are proven to elicit CSP-mediated responses to the neighbouring cells via., a phospho-relay mechanism and activate its target genes, comCDE,comAB and comX to establish biofilm, stress response and bacteriocin production. These virulent traits show an anti-bacterial effect to the other commensal species, so that their DNA released in the growth surroundings, would provide an ecological advantage to S.mutans to compete the natural ecosystem i.e. multi-species dental biofilms in oral cavity. Recent studies had revealed the bifunctional nature of the ComED system showing a dual response as an activator of nlmC and repressor of comC. The researchers also reveal the puzzle behind the dual opposing characteristics of ComED as nlmC share an intergenic region (IGSA499) with the divergently transcribed comC [47, 48]. Further, experiments show that the nlmC expression in comD mutant show >40-fold fold and >fourfold reduction in comparison with the wild-type under CSP-induced or un-induced state respectively [49]. Also, the ComE share higher sequence homology to the DNA binding domains of AlgR, AlgA, LytR family members and provide an insight to be exploited for developing broad-spectrum anti-biofilm compounds. Li et al. suggest that those compound would also block the quorum sensing dependent virulence properties and reduce the cariogenic properties of S. mutans, regardless of the fact that the organism is still active in oral biofilms [50, 51].
ABC Transporters
ComA
The gene comAB encodes ComA, to act in the initial step of the QS pathway of Streptococcus, consists of bacteriocin-associated ATP-binding cassette (ABC) transporters, that employs ComB as an accessory protein for specific processing and maturation of the precursor (AMS family of ABC transporters involved in maturation and secretion of molecules) CSP [16]. The ComA has three domains (1) N-terminal Peptidase domain comprising of 150-amino acid (2) a six membrane-spanning segments of transmembrane domain (3) an ATP-binding C-terminal motif localized on the cytoplasmic face of the membrane. The functional aspects of the peptidase domain was understood specifically to cleave their cognate propeptides after the consensus Gly–Gly motif [52].
The ATP-binding cassette (ABC) transporters are the largest well characterized protein super-family from various organisms to participate in a wide array of physiological functions, that includes virulence, antigen presentation in pathogenic bacteria, multi-drug resistance and regulation of ion channels across the bacterial membranes [53]. The core structural organization of the ABC transporter consists of a two transmembrane domains (TMD) and two cytosolic nucleotide-binding domains (NBD) [53]. The sequence variation in the several membrane-spanning α- helices that form the transport pathway for TMDs reflects its complement sequence variation in their ligands for the ABC transporters and the highly conserved NBDs provide energy on ATP hydrolysis for the substrate transport [54].
In ABC exporters, a linker region with a 20 amino acids adjoins the last α-helix of TMD and the first β-strand of NBD. The highly conserved glycine residue is located near the C-terminal end of the linker and 11 amino-acid residues proceeds the adenine stacking the tyrosine residue of the NBDs [53, 55, 56]. To date, the molecular characterization of the three-dimensional structures of the four full-length ABC exporters are known to be Sav1866 from Staphylococcus aureus, MsbA from Salmonella typhimurium, and Thermotoga maritima TM287/288 [5, 54–57]. Whereas, the protein sequence alignment of ComA with the ABC exporters evidence that the residue Gly526 occupy a significant position in the ABC exporters [53, 58, 59].
Since, ABC transporters have been reported to play a vital role in the maturation and secretion of CSP, anti-quorum compounds targeting the vital glycine residue and blocking the ABC transporter (ComA) in S. mutans may pave a way to novel drug design [52]. Moreover, the family of the bacteriocin- associated ABC transporters has so far been found only in prokaryotes, the PEP domains of ComAs would be an ideal target for the development of drugs that inhibit the biofilm formation of Streptococcus [52] (Fig. 1). Mutations at the active site of PEP domain has resulted in the complete loss of the catalytic activity of PEP domain [16]. Based on the observation and substrate specificity of peptidase domain of ComA, we could hypothesize that the therapeutic inhibition of PEP domain of ComA will halt the maturation and secretion of CSP from S. mutans and thus hindering the virulence expression associated with a quorum sensing circuit of S. mutans.
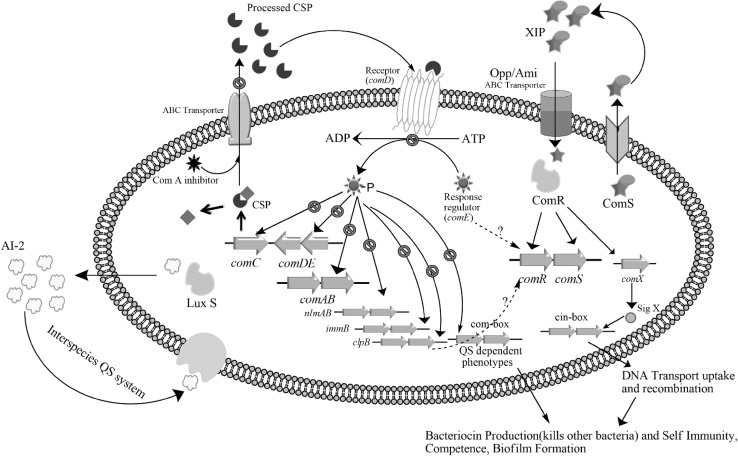
Fig. 1.
Schematic representation of quorum sensing circuits of S. mutans and blocking the communication with inhibitors.  Inhibition,
Inhibition,  Activation,
Activation,  Unknown mechanism
Unknown mechanism
ComX
ComX, an alternative sigma factor (σx) activates the late competence genes primarily involved in the processing and recombination of the foreign DNA to the chromosome of Streptococci [12]. In S. pneumoniae, the up-regulation of σx is induced in response to the activation of the competence cascade via., CSP-mediated ComCDE pathway. Upon activation with the CSP, the membrane-bound HK receptor integrates the signal to its cognate cytoplasmic response regulator (ComD) to further activate the alternate sigma factor, SigX (ComX) [60]. Also, its recognizing sites are well characterized to be a set of non-canonical consensus region (CIN box) that lie in the promoter regions (TACGAATA) of various ComX dependent genes, ssbB, dalA, ccl, celAB, cAAB, and cinA-recA [12, 61]. Similarly, in S. mutans, it is postulated that the CSP-mediated activation of σx, an alternate sigma factor up-regulates the expression of several competence genes that includes comX, comCDE (positive feedback loop) and other early com genes, comW (σx Stabilizing factor). ComX (σx) predominates to induce CSP-dependent com regulon expression and a sole key factor to resist stress responses in gram-positive bacteria, could probably be chosen as an ideal target for disarming the pathogen against its evolving trend to resist antibacterial agents.
Conclusion
Streptococcus mutans mediated dental caries is a multifactorial disease are being ignored due to its non-life threatening nature [62, 63]. An unknown fact exists as the inflammation process takes its pace slowly and gradually, it would act as a ‘silent killer’ for the patients suffering from dental caries. Therefore the untreated carious dentine can lead to the development of systemic diseases such as infective endocarditis [61–65]. Meta-analysis reports between 2003 and 2009 has shown a weak but statistically significant correlation between cardiovascular disease and dental diseases [66–68]. Based on the reports of meta-analysis, a conclusion can be drawn, that an individual with severe dental infections is at higher risk of either having or developing cardiovascular disease and rheumatoid arthritis [25, 69]. Since the association of S. mutans with dental caries was established, research was focused on recognizing its virulence properties along with their role in plaque formation and the progress of dental diseases. The review has highlighted the importance of QS pathway in S.mutans and its role in formation of biofilm along with other virulence properties. Quite a few efforts has been taken to modulate QS to reduce the production of biofilm and associated virulence factors like Hentzer and Givskov, Ravichandran et al., Arya et al., Hema et al., and Wright et al., have described the application of antagonists that act as QS inhibitors to attain the inhibition of virulence genes under QS circuit to prevent the various infections caused by Pseudomonas aeruginosa, Vibrio cholerae and Staphylococcus aureus [28, 33, 70–76]. The approach ensures its success in the fact that, anti-quorum compounds may control virulence traits of pathogenic microbes without significant effects on viability of bacterial cells [77]. Biofilm cells have been shown to be several fold more tolerant to antibiotics than planktonic cells, and this makes it hard to treat S. mutans with modern medicine [78, 79]. Thus, the importance of developing an anti-quorum compounds as alternate therapy for multi-drug resistant bacteria, has positive future perspectives with respect to medicine.
References
- 1.Li Y-H, Tang N, Aspiras MB, Lau PC, Lee JH, Ellen RP, Cvitkovitch DG. A quorum-sensing signaling system essential for genetic competence in Streptococcus mutans is involved in biofilm formation. J Bacteriol. 2002;184:2699–2708. doi: 10.1128/JB.184.10.2699-2708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y-H, Tian X. Quorum sensing and bacterial social interactions in biofilms. Sensors. 2012;12:2519–2538. doi: 10.3390/s120302519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quirynen M, Bollen C. The influence of surface roughness and surface-free energy on supra-and subgingival plaque formation in man. J Clin Periodontol. 1995;22:1–14. doi: 10.1111/j.1600-051X.1995.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 4.Bassler BL. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr Opin Microbiol. 1999;2:582–587. doi: 10.1016/S1369-5274(99)00025-9. [DOI] [PubMed] [Google Scholar]
- 5.Kalia VC. In search of versatile organisms for quorum-sensing inhibitors: acyl homoserine lactones (AHL)-acylase and AHL-lactonase. FEMS Microbiol Lett. 2014;359:143. doi: 10.1111/1574-6968.12585. [DOI] [PubMed] [Google Scholar]
- 6.Kalia VC (ed) (2015) Microbes: the most friendly beings? In: Quorum sensing vs quorum quenching: a battle with no end in sight. Springer India, pp. 1–5. doi:10.1007/978-81-322-1982-8_1
- 7.Kalia VC, Raju SC, Purohit HJ. Genomic analysis reveals versatile organisms for quorum quenching enzymes: acyl-homoserine lactone-acylase and-lactonase. Open Microbiol J. 2011;5:1. doi: 10.2174/1874285801105010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalia VC, Rani A, Lal S, Cheema S, Raut C. Combing databases reveals potential antibiotic producers. Expert Opin Drug Discov. 2007;2:211–224. doi: 10.1517/17460441.2.2.211. [DOI] [PubMed] [Google Scholar]
- 9.Kalia VC, Kumar P (2015) The battle: quorum-sensing inhibitors versus evolution of bacterial resistance. In: Kalia VC (ed) Quorum sensing vs quorum quenching: a battle with no end in sight. Springer India, pp. 385–391. doi:10.1007/978-81-322-1982-8_31
- 10.Kleerebezem M, Quadri LE, Kuipers OP, De Vos WM. Quorum sensing by peptide pheromones and two-component signal-transduction systems in gram-positive bacteria. Mol Microbiol. 1997;24:895–904. doi: 10.1046/j.1365-2958.1997.4251782.x. [DOI] [PubMed] [Google Scholar]
- 11.Fuqua C, Parsek MR, Greenberg EP. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu Rev Genet. 2001;35:439–468. doi: 10.1146/annurev.genet.35.102401.090913. [DOI] [PubMed] [Google Scholar]
- 12.Aspiras MB, Ellen RP, Cvitkovitch DG. ComX activity of Streptococcus mutans growing in biofilms. FEMS Microbiol Lett. 2004;238:167–174. doi: 10.1016/j.femsle.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 13.Dunny GM, Leonard BA. Cell–cell communication in gram-positive bacteria. Ann Rev Microbiol. 1997;51:527–564. doi: 10.1146/annurev.micro.51.1.527. [DOI] [PubMed] [Google Scholar]
- 14.Senadheera D, Cvitkovitch DG. Quorum sensing and biofilm formation by Streptococcus mutans. Adv Exp Med Biol. 2008;631:178–188. doi: 10.1007/978-0-387-78885-2_12. [DOI] [PubMed] [Google Scholar]
- 15.Perch B, Kjems E, Ravn T. Biochemical and serological properties of Streptococcus mutans from various human and animal sources. Acta Pathol Microbiol Immunol Scand [B] 1974;82:357–370. doi: 10.1111/j.1699-0463.1974.tb02338.x. [DOI] [PubMed] [Google Scholar]
- 16.Ishii S, Yano T, Ebihara A, Okamoto A, Manzoku M, Hayashi H. Crystal structure of the peptidase domain of Streptococcus ComA, a bifunctional ATP-binding cassette transporter involved in the quorum-sensing pathway. J Biol Chem. 2010;285:10777–10785. doi: 10.1074/jbc.M109.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida A, Kuramitsu HK. Multiple Streptococcus mutans genes are involved in biofilm formation. Appl Environ Microbiol. 2002;68:6283–6291. doi: 10.1128/AEM.68.12.6283-6291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Princy SA, Bharath D, Krishna PV, Kaur G. Synergetic activity of a quorum sensing inhibitor and antibiotics to combat oral adhesivity of Streptococcus mutans. Biotechnol Indian J. 2014;9:175–177. [Google Scholar]
- 19.Ahn S-J, Wen ZT, Burne RA. Multilevel control of competence development and stress tolerance in Streptococcus mutans UA159. Infect Immun. 2006;74:1631–1642. doi: 10.1128/IAI.74.3.1631-1642.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chowdhary PK, Keshavan N, Nguyen HQ, Peterson JA, González JE, Haines DC. Bacillus megaterium CYP102A1 oxidation of acyl homoserine lactones and acyl homoserines. Biochemistry. 2007;46:14429–14437. doi: 10.1021/bi701945j. [DOI] [PubMed] [Google Scholar]
- 21.Bassler BL, Wright M, Showalter RE, Silverman MR. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol Microbiol. 1993;9:773–786. doi: 10.1111/j.1365-2958.1993.tb01737.x. [DOI] [PubMed] [Google Scholar]
- 22.Schauder S, Bassler BL. The languages of bacteria. Genes Dev. 2001;15:1468–1480. doi: 10.1101/gad.899601. [DOI] [PubMed] [Google Scholar]
- 23.Surette MG, Miller MB, Bassler BL. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc Natl Acad Sci. 1999;96:1639–1644. doi: 10.1073/pnas.96.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen ZT, Burne RA. LuxS-mediated signaling in Streptococcus mutans is involved in regulation of acid and oxidative stress tolerance and biofilm formation. J Bacteriol. 2004;186:2682–2691. doi: 10.1128/JB.186.9.2682-2691.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeRiso AJ, Ladowski JS, Dillon TA, Justice JW, Peterson AC. Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest. 1996;109:1556–1561. doi: 10.1378/chest.109.6.1556. [DOI] [PubMed] [Google Scholar]
- 26.Köhler B, Birkhed D, Olsson S. Acid production by human strains of Streptococcus mutans and Streptococcus sobrinus. Caries Res. 1995;29:402–406. doi: 10.1159/000262099. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan HB, Plamann L. A Myxococcus xanthus cell density-sensing system required for multicellular development. FEMS Microbiol Lett. 1996;139:89–95. doi: 10.1111/j.1574-6968.1996.tb08185.x. [DOI] [PubMed] [Google Scholar]
- 28.Ravichandiran V, Shanmugam K, Anupama K, Thomas S, Princy A. Structure-based virtual screening for plant-derived SdiA-selective ligands as potential antivirulent agents against uropathogenic Escherichia coli. Eur J Med Chem. 2012;48:200–205. doi: 10.1016/j.ejmech.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Syvitski RT, Tian X-L, Sampara K, Salman A, Lee SF, Jakeman DL, Li Y-H. Structure-activity analysis of quorum-sensing signaling peptides from Streptococcus mutans. J Bacteriol. 2007;189:1441–1450. doi: 10.1128/JB.00832-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee MS, Morrison DA. Identification of a new regulator in Streptococcus pneumoniae linking quorum sensing to competence for genetic transformation. J Bacteriol. 1999;181:5004–5016. doi: 10.1128/jb.181.16.5004-5016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar P, Patel SK, Lee J-K, Kalia VC. Extending the limits of Bacillus for novel biotechnological applications. Biotechnol Adv. 2013;31:1543–1561. doi: 10.1016/j.biotechadv.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Kalia VC, Wood TK, Kumar P. Evolution of resistance to quorum-sensing inhibitors. Microb Ecol. 2014;68:13–23. doi: 10.1007/s00248-013-0316-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eckert R, Qi F, Yarbrough DK, He J, Anderson MH, Shi W. Adding selectivity to antimicrobial peptides: rational design of a multidomain peptide against Pseudomonas spp. Antimicrob Agents Chemother. 2006;50:1480–1488. doi: 10.1128/AAC.50.4.1480-1488.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bjarnsholt T, Givskov M. Quorum sensing inhibitory drugs as next generation antimicrobials: worth the effort? Curr Infect Dis Rep. 2008;10:22–28. doi: 10.1007/s11908-008-0006-y. [DOI] [PubMed] [Google Scholar]
- 35.Kalia VC, Kumar P (2015) Potential applications of quorum sensing inhibitors in diverse fields. In: Quorum sensing vs quorum quenching: a battle with no end in sight. Springer, pp. 359–370. doi:10.1007/978-81-322-1982-8_29
- 36.Desai K, Mashburn-Warren L, Federle MJ, Morrison DA. Development of competence for genetic transformation of Streptococcus mutans in a chemically defined medium. J Bacteriol. 2012;194:3774–3780. doi: 10.1128/JB.00337-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mashburn-Warren L, Morrison DA, Federle MJ. A novel double-tryptophan peptide pheromone controls competence in Streptococcus spp. via an Rgg regulator. Mol Microbiol. 2010;78:589–606. doi: 10.1111/j.1365-2958.2010.07361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasan S, Danishuddin M, Adil M, Singh K, Verma PK, Khan AU. Efficacy of E. officinalis on the cariogenic properties of Streptococcus mutans: a novel and alternative approach to suppress quorum-sensing mechanism. PLoS ONE. 2012;7:e40319. doi: 10.1371/journal.pone.0040319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perry JA, Cvitkovitch DG, Lévesque CM. Cell death in Streptococcus mutans biofilms: a link between CSP and extracellular DNA. FEMS Microbiol Lett. 2009;299:261–266. doi: 10.1111/j.1574-6968.2009.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qi F, Kreth J, Lévesque CM, Kay O, Mair RW, Shi W, Cvitkovitch DG, Goodman SD. Peptide pheromone induced cell death of Streptococcus mutans. FEMS Microbiol Lett. 2005;251:321–326. doi: 10.1016/j.femsle.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 41.Wenderska IB, Lukenda N, Cordova M, Magarvey N, Cvitkovitch DG, Senadheera DB. A novel function for the competence inducing peptide, XIP, as a cell death effector of Streptococcus mutans. FEMS Microbiol Lett. 2012;336:104–112. doi: 10.1111/j.1574-6968.2012.02660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshida A, Ansai T, Takehara T, Kuramitsu HK. LuxS-based signaling affects Streptococcus mutans biofilm formation. Appl Environ Microbiol. 2005;71:2372–2380. doi: 10.1128/AEM.71.5.2372-2380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rickard AH, Palmer RJ, Blehert DS, Campagna SR, Semmelhack MF, Egland PG, Bassler BL, Kolenbrander PE. Autoinducer 2: a concentration-dependent signal for mutualistic bacterial biofilm growth. Mol Microbiol. 2006;60:1446–1456. doi: 10.1111/j.1365-2958.2006.05202.x. [DOI] [PubMed] [Google Scholar]
- 44.Merritt J, Qi F, Goodman SD, Anderson MH, Shi W. Mutation of luxS affects biofilm formation in Streptococcus mutans. Infect Immun. 2003;71:1972–1979. doi: 10.1128/IAI.71.4.1972-1979.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen P, Novak J, Qi F, Caufield PW. Diacylglycerol kinase is involved in regulation of expression of the lantibiotic mutacin II of Streptococcus mutans. J Bacteriol. 1998;180:167–170. doi: 10.1128/jb.180.1.167-170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McNab R, Ford SK, El-Sabaeny A, Barbieri B, Cook GS, Lamont RJ. LuxS-based signaling in Streptococcus gordonii: autoinducer 2 controls carbohydrate metabolism and biofilm formation with Porphyromonas gingivalis. J Bacteriol. 2003;185:274–284. doi: 10.1128/JB.185.1.274-284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Ploeg JR. Regulation of bacteriocin production in Streptococcus mutans by the quorum-sensing system required for development of genetic competence. J Bacteriol. 2005;187:3980–3989. doi: 10.1128/JB.187.12.3980-3989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kreth J, Merritt J, Zhu L, Shi W, Qi F. Cell density-and ComE-dependent expression of a group of mutacin and mutacin-like genes in Streptococcus mutans. FEMS Microbiol Lett. 2006;265:11–17. doi: 10.1111/j.1574-6968.2006.00459.x. [DOI] [PubMed] [Google Scholar]
- 49.Kreth J, Hung DC, Merritt J, Perry J, Zhu L, Goodman SD, Cvitkovitch DG, Shi W, Qi F. The response regulator ComE in Streptococcus mutans functions both as a transcription activator of mutacin production and repressor of CSP biosynthesis. Microbiology. 2007;153:1799–1807. doi: 10.1099/mic.0.2007/005975-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y-H, Hanna MN, Svensäter G, Ellen RP, Cvitkovitch DG. Cell density modulates acid adaptation in Streptococcus mutans: implications for survival in biofilms. J Bacteriol. 2001;183:6875–6884. doi: 10.1128/JB.183.23.6875-6884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schertzer JW, Boulette ML, Whiteley M. More than a signal: non-signaling properties of quorum sensing molecules. Trends Microbiol. 2009;17:189–195. doi: 10.1016/j.tim.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Kotake Y, Ishii S, Yano T, Katsuoka Y, Hayashi H. Substrate recognition mechanism of the peptidase domain of the quorum-sensing-signal-producing ABC transporter ComA from Streptococcus. Biochemistry. 2008;47:2531–2538. doi: 10.1021/bi702253n. [DOI] [PubMed] [Google Scholar]
- 53.Ishii S, Yano T, Okamoto A, Murakawa T, Hayashi H. Boundary of the nucleotide-binding domain of Streptococcus ComA based on functional and structural analysis. Biochemistry. 2013;52:2545–2555. doi: 10.1021/bi3017069. [DOI] [PubMed] [Google Scholar]
- 54.Orelle C, Gubellini F, Durand A, Marco S, Lévy D, Gros P, Di Pietro A, Jault J-M. Conformational change induced by ATP binding in the multidrug ATP-binding cassette transporter BmrA. Biochemistry. 2008;47:2404–2412. doi: 10.1021/bi702303s. [DOI] [PubMed] [Google Scholar]
- 55.Leonard GD, Fojo T, Bates SE. The role of ABC transporters in clinical practice. Oncologist. 2003;8:411–424. doi: 10.1634/theoncologist.8-5-411. [DOI] [PubMed] [Google Scholar]
- 56.Oancea G, O’Mara ML, Bennett WD, Tieleman DP, Abele R, Tampé R. Structural arrangement of the transmission interface in the antigen ABC transport complex TAP. Proc Natl Acad Sci. 2009;106:5551–5556. doi: 10.1073/pnas.0811260106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ward A, Reyes CL, Yu J, Roth CB, Chang G. Flexibility in the ABC transporter MsbA: alternating access with a twist. Proc Natl Acad Sci. 2007;104:19005–19010. doi: 10.1073/pnas.0709388104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hohl M, Briand C, Grütter MG, Seeger MA. Crystal structure of a heterodimeric ABC transporter in its inward-facing conformation. Nat Struct Mol Biol. 2012;19:395–402. doi: 10.1038/nsmb.2267. [DOI] [PubMed] [Google Scholar]
- 60.Pestova E, Håvarstein L, Morrison D. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol Microbiol. 1996;21:853–862. doi: 10.1046/j.1365-2958.1996.501417.x. [DOI] [PubMed] [Google Scholar]
- 61.Lunsford RD, London J. Natural genetic transformation in Streptococcus gordonii: comX imparts spontaneous competence on strain wicky. J Bacteriol. 1996;178:5831–5835. doi: 10.1128/jb.178.19.5831-5835.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hillman J, Dzuback A, Andrews S. Colonization of the human oral cavity by a Streptococcus mutans mutant producing increased bacteriocin. J Dent Res. 1987;66:1092–1094. doi: 10.1177/00220345870660060101. [DOI] [PubMed] [Google Scholar]
- 63.Opal SM. Communal living by bacteria and the pathogenesis of urinary tract infections. PLoS Med. 2007;4:349. doi: 10.1371/journal.pmed.0040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cecil RL, Angevine DM. Clinical and experimental observations on focal infection, with an analysis of 200 cases of rheumatoid arthritis. Ann Intern Med. 1938;12:577–584. doi: 10.7326/0003-4819-12-5-577. [DOI] [Google Scholar]
- 65.Higashi Y, Goto C, Jitsuiki D, Umemura T, Nishioka K, Hidaka T, Takemoto H, Nakamura S, Soga J, Chayama K. Periodontal infection is associated with endothelial dysfunction in healthy subjects and hypertensive patients. Hypertension. 2008;51:446–453. doi: 10.1161/HYPERTENSIONAHA.107.101535. [DOI] [PubMed] [Google Scholar]
- 66.Otomo-Corgel J, Pucher JJ, Rethman MP, Reynolds MA. State of the science: chronic periodontitis and systemic health. J Evid Based Dent Pract. 2012;12:20–28. doi: 10.1016/S1532-3382(12)70006-4. [DOI] [PubMed] [Google Scholar]
- 67.Janket S-J, Baird AE, Chuang S-K, Jones JA. Meta-analysis of periodontal disease and risk of coronary heart disease and stroke. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:559–569. doi: 10.1067/moe.2003.107. [DOI] [PubMed] [Google Scholar]
- 68.Kaur G, Princy SA. Reciprocal regulation of the periodontitis and diabetes. As Pac J Mol Biol Biotech. 2014;22:172–179. [Google Scholar]
- 69.Persson GR. Rheumatoid arthritis and periodontitis inflammatory and infectious connections. Review of the literature. J Oral Microbiol. 2012 doi: 10.3402/jom.v4i0.11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hentzer M, Givskov M. Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J Clin Invest. 2003;112:1300. doi: 10.1172/JCI20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arya R, Princy SA. An insight into pleiotropic regulators Agr and Sar: molecular probes paving the new way for antivirulent therapy. Future Microbiol. 2013;8:1339–1353. doi: 10.2217/fmb.13.92. [DOI] [PubMed] [Google Scholar]
- 72.Arya R, Princy SA. Computational approach to design small molecule inhibitors and identify SarA as a potential therapeutic candidate. Med Chem Res. 2013;22:1856–1865. doi: 10.1007/s00044-012-0185-9. [DOI] [Google Scholar]
- 73.Ravichandiran V, Shanmugam K, Princy SA. Screening of SdiA inhibitors from Melia dubia seeds extracts towards the hold back of uropathogenic E. coli quorum sensing-regulated factors. Med Chem. 2013;9:819–827. doi: 10.2174/1573406411309060006. [DOI] [PubMed] [Google Scholar]
- 74.Wright JS, Jin R, Novick RP. Transient interference with staphylococcal quorum sensing blocks abscess formation. Proc Natl Acad Sci U S A. 2005;102:1691–1696. doi: 10.1073/pnas.0407661102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hema M, Balasubramanian S, Princy SA. Meddling Vibrio cholerae murmurs: a neoteric advancement in cholera research. Indian J Microbiol. 2015;55:121–130. doi: 10.1007/s12088-015-0520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arya R, Ravikumar R, Santhosh RS, Princy S. SarA based novel therapeutic candidate against Staphylococcus aureus associated with vascular graft infections. Front Microbiol. 2015;6:416. doi: 10.3389/fmicb.2015.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kalia VC, Kumar P, Pandian SK, Sharma P (2015) Biofouling control by quorum quenching. In: Kim S-K (ed) Hb25_Springer Handbook of Marine Biotechnology. Springer Berlin Heidelberg, pp. 431–440. doi:10.1007/978-3-642-53971-8_15
- 78.Stewart PS, William Costerton J. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–138. doi: 10.1016/S0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 79.Turovskiy Y, Kashtanov D, Paskhover B, Chikindas ML. Quorum sensing: fact, fiction, and everything in between. Adv Appl Microbiol. 2007;62:191. doi: 10.1016/S0065-2164(07)62007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]