Abstract
New Delhi metallo-β-lactamase-1 gene (blaNDM-1) codes for New Delhi metallo-beta-lactamase-1 (NDM-1) enzyme that cleaves the amide bond of β-lactam ring, and provides resistance against major classes of β-lactam antibiotics. Dissemination of the plasmid borne blaNDM-1 through horizontal gene transfer is a potential threat to the society. In this study, a rapid non-culture method for detecting NDM-1 positive bacteria was developed by Loop Mediated Isothermal Amplification (LAMP) of blaNDM-1. Sensitivity of this method was found to be one femtogram of plasmid DNA, which translates into 2.6–25.8 copies depending on the size of the plasmid DNA. This method was applied to detect NDM-1 positive bacteria in 81 water samples that were collected from environmental and drinking water sources. NDM-1 positive bacteria were detected in three drinking water samples by LAMP but not by PCR. These three samples were collected from the water sources that were treated with chlorine for decontamination before public distribution. NDM-1 positive bacteria were not detected in lake water samples or in the samples that were collected from the water sources that were purified by reverse osmosis before public distribution. Detection of NDM-1 positive bacteria using LAMP was found to be safe, sensitive and rapid for screening large number of samples from diverse sources. This method could be developed as on-field detection kit by using fluorescent dyes to visualize the amplified blaNDM-1 gene.
Keywords: NDM-1, LAMP, blaNDM-1, Carbapenamase
Introduction
New Delhi metallo-beta-lactamase 1 (NDM-1) enzyme that confers multi-drug resistance is encoded by New Delhi metallo-beta-lactamase 1 gene (blaNDM-1) [1]. NDM-1 inactivates major classes of beta-lactam antibiotics including carbapenems by cleaving β-lactam rings. NDM-1 was reported in 11 different bacterial species including Escherichiacoli, Klebsiella sp., Shigellaboydii and Vibriocholera [2] indicating the potential of horizontal gene transfer. NDM-1 producing bacteria were reported from several countries around the world [3–7]. World Health Organization (WHO) has urged all the countries to implement infection control measures to contain the spread of these bacteria. A sensitive and rapid method for detecting NDM-1 positive bacteria would be helpful in this effort.
Currently used carbapenem hydrolysis assay for detecting NDM-1 positive bacteria requires culturing of bacteria under stringent biosafety conditions, which is not easy to perform in normal laboratories [8]. PCR and Real-time PCR based methods for detecting NDM-1 positive bacteria were reported but they need special equipment [9–11]. LAMP is a novel isothermal gene amplification method that involves auto-cycling and strand displacement DNA amplification with help of Bst DNA polymerase and four to six specially designed primers [12]. LAMP does not require any special equipment and it is highly specific as the primers bind to six distinct regions within the target DNA. The reaction takes place at isothermal temperature, and hence, does not require a thermal cycler for target DNA amplification. LAMP products could be detected visually by turbidity or by using fluorescent dyes such as ethidium bromide, calcein, and SYBR Green [13]. LAMP was successfully established as a tool for detecting pathogenic bacteria and viruses from clinical and environmental samples [14–19]. LAMP was used to detect the NDM-1 gene in sputum, urine and fecal samples [20]. In this study, a rapid method for detecting NDM-1 positive bacteria was developed from drinking and environmental water samples using LAMP based amplification of blaNDM-1. Sensitivity of LAMP in detecting NDM-1 positive bacteria was compared with that of PCR. Subsequently, LAMP was used to screen for the presence of NDM-1 positive bacteria in environmental and drinking water samples without culturing the bacteria.
Materials and Methods
Optimization of LAMP Amplification of blaNDM-1
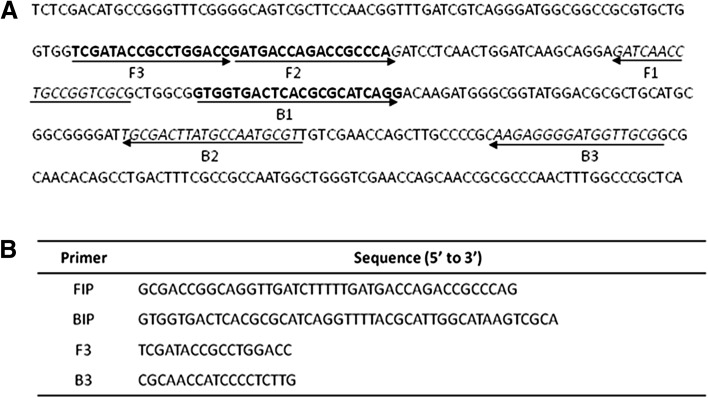
LAMP amplification of blaNDM-1 for detecting NDM-1 positive bacteria was standardized by using pNDM1 plasmid that harbours blaNDM-1. The pNDM1 plasmid DNA was isolated from NDM-1 positive E.coli. LAMP method was developed by optimizing the reaction conditions by using the plasmid DNA as template. Efficiency of LAMP was compared with that of PCR. Nucleotide sequences of different blaNDM-1 and other related metallo-beta-lactamase and beta-lactamase genes (GeneBank Accession Nos. NC_015872, AB571289, JN377410, KF031315), NC_010498, NC_011283, NC_018080, NC_012731, NC_010468, NC_021577) were retrieved from GenBank database (www.ncbi.nlm.nih.gov) and subjected to multiple sequence alignment. A region that was unique and conserved among the blaNDM-1 genes was identified as target region. LAMP primers that were targeted to this region were designed using Primer Explorer V4 (www.primerexplorer.jp). The LAMP primers consisted of two outer primers (F3 & B3) and two inner primers (FIP & BIP). FIP consists of a complementary sequence of F1 and a sense sequence of F2 and BIP consists of a complementary sequence of B2 and a sense sequence of B1. These primers are capable of recognizing six unique regions within the target DNA (Fig. 1).
Fig. 1.
LAMP primer design. a Location and sequence of LAMP targets and priming sites for NDM-1 gene (GenBank accession no.: AB571289.1). b Sequences of LAMP primers. Primer FIP consisted of the F1 complementary sequence and F2 direct sequence. Primer BIP consisted of B1 direct sequence and B2 complementary sequence
LAMP was carried out in a final volume of 25 µl containing 5 pmol F3 and B3 primers, 40 pmol FIP and BIP primers, 0.5 µl of 10 mM dNTP, 20 mM Tris–HCl, 10 mM KCl, 10 mM (NH4)2SO4, 8 mM MgSO4, 0.1 % Tween 20, 8U Bst DNA polymerase and varying quantities of pNDM1. LAMP reactions were performed isothermally at 65° C using a water bath. The amplified products were visualized on 2 % agarose gel, and stained with ethidium bromide. Ability of the additives to improve LAMP was evaluated by including different quantities of Dimethyl sulfoxide (DMSO) and Polyethylene Glycol (PEG) (4 and 8 KDa) in the reaction mixture. LAMP reaction was carried out for different durations to determine the minimum time needed to amplify blaNDM-1 to the detectable level.
Comparison of LAMP and PCR
Sensitivity of LAMP was evaluated by comparing it detection limit with that of PCR using different quantities of pNDM1 ranging between 1 ag and 40 ng per reaction. A set of PCR primers, NDM-1F (5′-CTTCCAACGGTTTGATCGTC-3′) and NDM-1R (5′-TTGGCATAAGTCGCAATCC-3′), was designed to amplify a 280 bp region of blaNDM-1. PCR was performed in a final volume of 25 µl containing 10 mM Tris–HCl (pH 8.0), 15 mM MgCl2, 40 mM KCl, 0.002 % gelatin, 10 pmol forward and reverse primers, 10 mM dNTP, 1U Taq DNA polymerase and varying quantities of pNDM1. PCR amplification was carried for 35 cycles under the following conditions: 94° C for 30 s, 55° C for 30 s and 72° C for 30 s. LAMP and PCR products were analysed on 2 and 1 % agarose gel, respectively, and stained with ethidium bromide.
Screening of Environmental and Drinking Water Samples for NDM-1 Positive Bacteria
Sample Collection
In total, 81 water samples were collected from three different water sources: Drinking water that was purified by reverse osmosis before public distribution (Group I, 17 samples), drinking water that was chlorinated before public distribution (Group II, 43 samples) and environmental water from lake (Group III, 21 samples). The samples were collected in sterile tubes and stored at −20 °C until the study.
Culturing of Water Samples and DNA Isolation
The water samples (1 ml) were inoculated in 10 ml of Luria–Bertani (LB) broth containing vancomycin (100 mg/L), kanamycin (50 mg/L) and cefotaxime (50 mg/L), and incubated at 37 °C for 16 h. Total DNA was isolated from the samples that showed bacterial growth and used as template for PCR and LAMP amplification of blaNDM-1.
Sample Preparation for Non-Culture Amplification of blaNDM-1
The water samples were filtered through 0.2 µm Whatman filter paper. These filter papers were chopped into pieces and treated with 525 µl of lysis buffer (0.4 M NaCl, 0.7 M Sucrose, 20 mM EDTA, 40 mM Tris–HCl pH9.0) and 1 mg/ml of lysozyme for 30 min at 37 °C. The samples were centrifuged at 12,000 rpm and the pellet was discarded. The DNA in the supernatant was precipitated by adding 1/10 volume of 3 M Sodium acetate (pH 5.2) and 2.5 volumes of absolute ethanol. The samples were centrifuged at 12,000 rpm for 20 min, and the pellet was dissolved in 20 µl TE buffer (10 mM Tris–HCl and 1 mM EDTA pH 8.0). This DNA was used for PCR and LAMP assay. The PCR and LAMP experiments were carried out in triplicates.
Results and Discussion
Optimization of LAMP Amplification of blaNDM-1
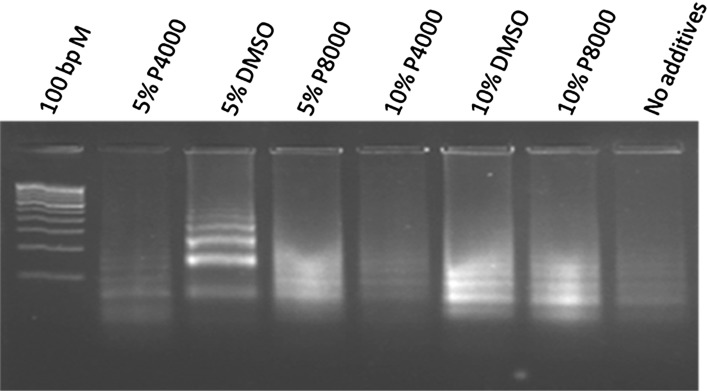
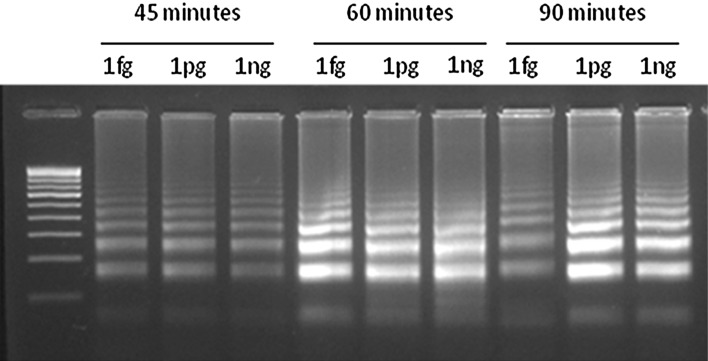
The blaNDM-1 genes from different species of bacteria showed 100 % identity among them but they showed only lower identity with the other metallo-beta-lactamase and beta-lactamase genes. Therefore, a 203 bp sequence from E.coli that is conserved and unique to NDM-1 gene was used as target region for designing LAMP primers. The genomic position and the sequence of LAMP primers are shown in Fig. 1. The FIP and BIP primers recognize both sense and anti-sense strands and creates loop like structure during the amplification. Two outer primers (F3 and B3) displace the synthesized strand with the help of Bst polymerase. LAMP amplification of blaNDM-1 failed under the standard conditions. It is reported that, amplification of DNA improves in the presence of additives like Dimethly sulfoxide (DMSO), PEG [21–23]. Therefore, the effect of DMSO, PEG 4KDa and PEG 8KDa on the efficiency of LAMP was assessed. Though amplification was improved with both additives, DMSO at a concentration of 5 % (v/v) found to be the best in amplifying the target region in blaNDM-1 (Fig. 2). Subsequently, all the LAMP reactions were performed with 5 % (v/v) DMSO. Then, LAMP was performed for different durations to find out the minimum time needed to detect the NDM-1 positive bacteria. The results showed that LAMP needed only 45 min to amplify blaNDM-1 to the detectable amount (Fig. 3), and amplification continued to higher magnitudes at 60 min and 90 min also. Subsequently, all LAMP reactions were performed for 60 min.
Fig. 2.
Standardization of LAMP amplification of bla NDM-1. Effect of additives on LAMP assay for amplification of bla NDM-1: LAMP assay were performed using different concentration of various additives for the reactions. P4000—Polyethylene Glycol 4000 Dalton; DMSO—Dimethyl sulfoxide; P8000—Polyethylene Glycol 8000 Dalton
Fig. 3.
Effect of different reaction time and template plasmid (pNDM1) concentrations over LAMP assay for amplification of bla NDM-1. LAMP assay were performed for various reaction time and at different concentrations of pNDM1. Reaction time were varied from 45 to 90 min. fg—Femtogram/reaction, pg—Picogram/reaction, ng—Nanogram/reaction
Comparison of LAMP and PCR
Sensitivity LAMP over PCR was tested by using different quantities of pNDM1 under the standardized reaction conditions. While LAMP was able to detect blaNDM-1 up to one femtogram of plasmid DNA, PCR failed after one picogram (Fig. 4). It is well known that blaNDM-1 gene is plasmid borne, and the size of the carrier plasmid was reported to vary between 35.9 and 353.4 kb. Accordingly, the femtogram level sensitivity of LAMP translates into a detection limit of 2.62–25.8 copies. Recently it was reported that some plasmids may carry multiple copies of blaNDM-1 which should also be taken into consideration while estimating the detection limit of LAMP [24]. We observed that LAMP was 1000 time more sensitivity than PCR in detecting NDM-1 positive bacteria. Similar level of sensitivity was reported in detecting Neisseriameningitidis from the cerebrospinal fluid of humans [25], and Arcobacter species from infected chicken tissues [26]. The increased sensitivity of LAMP is attributed to the tolerance of the Bst DNA polymerase to the impurities that co-purify along with the template DNA [27, 28], and its ability to accumulate more than 109 copies of the amplicons in less than an hour [12]. With specific reference to the LAMP based detection of NDM-1, the current study reports higher sensitivity and lesser reaction time compared with the earlier report [20]. While we have used plasmid DNA as template Liu et al. [20] have used genomic DNA, which is relatively more complex. Further, we have used 5 % DMSO, which is reported to enhance primer annealing and amplification efficiency with GC rich templates [21–23].
Fig. 4.
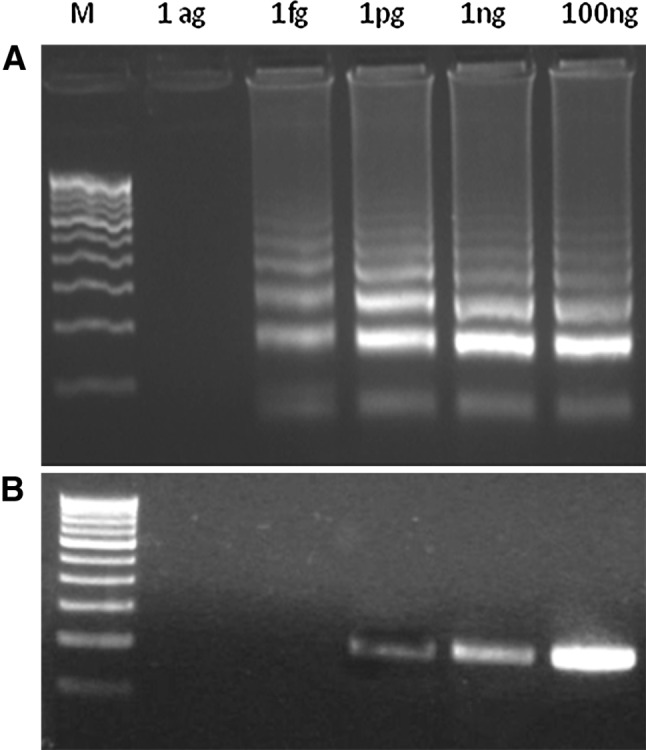
Sensitivity of the LAMP versus that of PCR in amplification of bla NDM-1. The LAMP and PCR were carried out at different quantities of pNDM1 ranging between 1 ag and 100 ng per reaction. A LAMP assay for the detection of NDM-1 gene. B PCR for the detection of NDM-1 gene. M—100 bp Marker, ag—Attogram/reaction, fg—Femtogram/reaction, pg—Picogram/reaction
Screening of Drinking and Environmental Water Samples for NDM-1 Positive Bacteria
The objective of this study was to develop a LAMP based method for the detection of NDM-1 positive bacteria in drinking and environmental water sources without culturing them. DNA was extracted directly from 81 water samples without culturing, and was used as template for LAMP and PCR. Three drinking water samples (Group II) were tested positive only by LAMP (Table 1). These samples were collected from the water sources that were treated with chlorine for decontamination before public distribution. Similarly, Walsh et al. [2] have detected NDM-1 positive bacteria in two out of fifty drinking water sources that were screened using microbiological method followed by PCR confirmation. In both PCR and LAMP, NDM-1 positive bacteria were not detected in lake water samples (Group III) or in the drinking water samples that were purified by reverse osmosis before public distribution (Group I). To reinforce the above results, all the water samples were cultured in the presence of antibiotics, and the growth was observed after the incubation period. Only those three samples that were tested positive in non-culture LAMP method, showed growth after the incubation period. Presence of NDM-1 positive bacteria in these cultures was again confirmed by LAMP. These results established that LAMP could be used as an efficient non-culture method for detecting NDM-1 bacteria.
Table 1.
Screening of environmental and drinking water samples for the detection of NDM-1 positive bacteria using LAMP and PCR
| Samples | Cultivable method | Non-culture method | |||||
|---|---|---|---|---|---|---|---|
| Samples screened | Growth+ | PCR+ | LAMP+ | Samples screened | PCR+ | LAMP+ | |
| Group I | 17 | – | – | – | 17 | – | – |
| Group II | 43 | 3 | 3 | 3 | 43 | – | 3 |
| Group II | 21 | – | – | – | 21 | – | – |
Group I—drinking water that was purified by reverse osmosis before public distribution, Group II—drinking water that were chlorinated before public distribution, Group III—environmental water from lake; Growth+—number of samples shown growth in presence of antibiotics, PCR+—number of water sample shown positive amplification by PCR, LAMP+—number of water samples shown positive amplification for bla NDM-1 by LAMP
The above results show that LAMP is a sensitive, rapid and safe method for detecting NDM-1 positive bacteria. Simplicity of this method makes it more useful for screening large number of samples. It could be further simplified in the form of on-field detection kit by substituting the UV transilluminator with fluorescent dyes for visualization.
Acknowledgments
We thank Ms. M. Sulaika, Mr. Md. Ahmar Ahmad and Mrs. Brinda Chandar for their help in this study. We also thank SRM University for their financial support for this study.
Conflict of interest
No conflict of interest declared.
References
- 1.Yong D, Toleman M, Giske CG, Cho HS, Sundman K, Lee K, Walsh TR. Characterization of a new metallo-beta-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiellapneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53:5046–5054. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh TR, Weeks J, Livermore DM, Toleman MA. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis. 2011;11:355–362. doi: 10.1016/S1473-3099(11)70059-7. [DOI] [PubMed] [Google Scholar]
- 3.Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, Chaudhary U, Doumith M, Giske CG, Irfan S, Krishnan P, Kumar AV, Maharajan S, Mustaq S, Noorie T, Paterson DL, Pearson A, Perry C, Pike R, Rao B, Ray U, Sarma JB, Sharma M, Sheridan E, Thirunarayan MA, Turton J, Upadhyay S, Warner M, Welfar W, Livermore DM, Woodford N. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poirel L, Lagrutta E, Taylor P, Pham J, Nordmann P. Emergence of metallo-β-lactamase NDM-1-producing multidrug-resistant Escherichiacoli in Australia. Antimicrob Agents Chemother. 2010;54:4914–4916. doi: 10.1128/AAC.00878-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Struelens MJ, Monnet DL, Magiorakos AP, Santos O’Connor F, Giesecke J, the European NDM-1 Survey Participants (2010) New Delhi metallo-beta-lactamase 1–producing Enterobacteriaceae: emergence and response in Europe. Euro Surveill 15(46). http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19716 [DOI] [PubMed]
- 6.Ho PL, Lo WU, Yeung MK, Lin CH, Chow KH, Ang I, Tong AHY, Bao TYJ, Lok S, Lo JYC. Complete sequencing of pNDM-HK encoding NDM-1 carbapenemase from a multidrug-resistant Escherichiacoli strain isolated in Hong Kong. PLoS One. 2011;6:17989. doi: 10.1371/journal.pone.0017989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulvey MR, Grant JM, Plewes K, Roscoe D, Boyd DA. New Delhi metallo-β-lactamase in Klebsiellapneumoniae and Escherichiacoli, Canada. Emerging Infect Dis. 2011;17:103–106. doi: 10.3201/eid1701.101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nordmann P, Gniadkowski M, Giske CG, Poirel L, Woodford N, Miriagou V. Identification and screening of carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect. 2012;18:432–438. doi: 10.1111/j.1469-0691.2012.03815.x. [DOI] [PubMed] [Google Scholar]
- 9.Naas T, Ergani A, Carrër A, Nordmann P. Real-time PCR for detection of NDM-1 carbapenemase genes from spiked stool samples. Antimicrob Agents Chemother. 2011;55:4038–4043. doi: 10.1128/AAC.01734-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ong DCT, Koh TH, Syahidah N, Krishnan P, Tan TY. Rapid detection of the blaNDM-1 gene by real-time PCR. J Antimicrob Chemother. 2011;66:1647–1649. doi: 10.1093/jac/dkr184. [DOI] [PubMed] [Google Scholar]
- 11.Bonnin RA, Naas T, Poirel L, Nordmann P. Phenotypic, biochemical, and molecular techniques for detection of metallo-β-lactamase NDM in Acinetobacterbaumannii. J Clin Microbiol. 2012;50:1419–1421. doi: 10.1128/JCM.06276-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goto M, Honda E, Ogura A, Nomoto A, Hanaki KI. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. Biotechniques. 2009;46:167–172. doi: 10.2144/000113072. [DOI] [PubMed] [Google Scholar]
- 14.Minami M, Ohta M, Ohkura T, Ando T, Torii K, Hasegawa T, Goto H. Use of a combination of brushing technique and the loop-mediated isothermal amplification method as a novel, rapid, and safe system for detection of Helicobacterpylori. J Clin Microbiol. 2006;44:4032–4037. doi: 10.1128/JCM.00898-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bista BR, Ishwad C, Wadowsky RM, Manna P, Randhawa PS, Gupta G, Adikari M, Tyagi R, Gasper G, Vats A. Development of a loop-mediated isothermal amplification assay for rapid detection of BK virus. J Clin Microbiol. 2007;45:1581–1587. doi: 10.1128/JCM.01024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill J, Beriwal S, Chandra I, Paul VK, Kapil A, Singh T, Wadowsky RM, Singh V, Goyal A, Jahnukainen T, Johnson JR, Tarr P, Vats A. Loop-mediated isothermal amplification assay for rapid detection of common strains of Escherichiacoli. J Clin Microbiol. 2008;46:2800–2804. doi: 10.1128/JCM.00152-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang CH, Lai GH, Lee MS, Lin WH, Lien YY, Hsueh SC, Kao JY, Chang WT, Lu TC, Lin WN, Chen HJ, Lee MS. Development and evaluation of Loop Mediated Isothermal Amplification assay for rapid detection of chicken anemia virus. J Appl Microbiol. 2009;108:917–924. doi: 10.1111/j.1365-2672.2009.04481.x. [DOI] [PubMed] [Google Scholar]
- 18.Kawai Y, Miyashita N, Kishi F, Tabuchi M, Oda K, Yamaguchi T, Ouchi K. Development and evaluation of a loop-mediated isothermal amplification method for the rapid detection of Chlamydophilapneumoniae. Eur J Clin Microbiol Infect Dis. 2009;28:801–805. doi: 10.1007/s10096-009-0710-z. [DOI] [PubMed] [Google Scholar]
- 19.Kurosaki Y, Sakuma T, Fukuma A, Fujinami Y, Kawamoto K, Kamo N, Maikino S, Yasuda J. A simple and sensitive method for detection of Bacillus anthracis by loop-mediated isothermal amplification. J Appl Microbiol. 2009;107:1947–1956. doi: 10.1111/j.1365-2672.2009.04379.x. [DOI] [PubMed] [Google Scholar]
- 20.Liu W, Zou D, Li Y, Wang X, He X, Wei X, Shao C, Li X, Shang W, Yu K, Liu D, Li Y, Guo J, Yin Z, Yuan J. Sensitive and rapid detection of the new Delhi metallo-beta-lactamase gene by loop-mediated isothermal amplification. J Clin Microbiol. 2012;50:1580–1585. doi: 10.1128/JCM.06647-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitade Y, Ootsuka S, Iitsuka O, Saga N. Effect of DMSO on PCR of Porphyrayezoensis (Rhodophyta) gene. J Appl Phycol. 2003;15:555–557. doi: 10.1023/B:JAPH.0000004460.36849.13. [DOI] [Google Scholar]
- 22.Kang J, Lee MS, Gorenstein DG. The enhancement of PCR amplification of a random sequence DNA library by DMSO and betaine: application to in vitro combinatorial selection of aptamers. J Biochem Biophys Methods. 2005;64:147–151. doi: 10.1016/j.jbbm.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Arbeli Z, Fuentes CL. Improved purification and PCR amplification of DNA from environmental samples. FEMS Microbiol Lett. 2007;272:269–275. doi: 10.1111/j.1574-6968.2007.00764.x. [DOI] [PubMed] [Google Scholar]
- 24.Huang T, Chen T, Chen Y, Lauderdale T, Liao T, Lee T, Chen C, Liu Y, Lin A, Chang Y, Wu K, Kirby R, Lai J, Tan M, Siu L, Chang C, Fung C, Tsai S. Copy number change of the NDM-1 sequence in a multidrug-resistant Klebsiellapneumoniae clinical isolate. PLoS One. 2013;8:62774. doi: 10.1371/journal.pone.0062774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee D, Kim EJ, Kilgore PE, Kim SA, Takahashi H, Ohnishi M, Anh DD, Dong BQ, Kim JS, Tomon J, Miyamoto S, Notomi T, Kim DW, Seki M. Clinical evaluation of a loop-mediated isothermal amplification (LAMP) assay for rapid detection of Neisseriameningitidis in cerebrospinal fluid. PLoS One. 2015;10:e0122922. doi: 10.1371/journal.pone.0122922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Seo D, Lee MH, Choi C. Comparison of conventional PCR, multiplex PCR, and loop- mediated isothermal amplification assays for rapid detection of Arcobacter species. J Clin Microbiol. 2014;52:557–563. doi: 10.1128/JCM.02883-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohtsuka K, Yanagawa K, Takatori K, Hara-Kudo Y. Detection of Salmonella enterica in naturally contaminated liquid eggs by loop-mediated isothermal amplifi cation, and characterization of Salmonella isolates. Appl Environ Microbiol. 2005;71:6730–6735. doi: 10.1128/AEM.71.11.6730-6735.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poon LL, Wong BW, Ma EH, Chan KH, Chow LM, Abeyewickreme W, Tangpukdee N, Yuen KY, Guan Y, Looareesuwan S, Peiris JS. Sensitive and inexpensive molecular test for falciparum malaria: detecting Plasmodiumfalciparum DNA directly from heat-treated blood by loop-mediated isothermal amplification. Clin Chem. 2006;52:303–306. doi: 10.1373/clinchem.2005.057901. [DOI] [PubMed] [Google Scholar]