Abstract
Pravastatin is one of the most popular cholesterol-lowering drugs. Its industrial production represents a two-stage process including the microbial production of compactin and its further biocatalytic conversion to pravastatin. To increase a conversion rate, a higher compactin content in fermentation medium should be used; however, high compactin concentrations inhibit microbial growth. Therefore, the improvement of the compactin resistance of a producer still remains a relevant problem. A multi-step random UV mutagenesis of a Streptomyces xanthochromogenes strain RIA 1098 and the further selection of high-yield compactin-resistant mutants have resulted in a highly productive compactin-resistant strain S 33-1. After the fermentation medium improvement, the maximum bioconversion rate of this strain has reached 91 % at the daily compactin dose equal to 1 g/L and still remained high (83 %) even at the doubled dose (2 g/L). A 1-year study of the mutant strain stability has proved a stable inheritance of its characteristics that provides this strain to be very promising for the pravastatin-producing industry.
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-015-0537-5) contains supplementary material, which is available to authorized users.
Keywords: Pravastatin, Compactin resistance, Streptomyces xanthochromogenes, Bioconversion rate, UV mutagenesis
Introduction
Being structural inhibitors of HMG-CoA reductase, statins represent one of the basic groups of cholesterol-lowering drugs. Comparing to other hypolipidemic agents, statins have such advantages as a very high activity, low daily dosages, and relatively low toxicity. A wide use of statins to lower cholesterol and prevent coronary heart disease and its complications, the absence of serious side effects, and some additional biological effects resulted in a great success of statins in the drug market [1–3]. A high demand provides a significant interest of pharmaceutical companies to new technologies able to improve the efficiency of the statin production and, at the same time, reduce production costs.
Pravastatin is one of the first and best-studied statins on the market; since 2006, when generic pravastatin appeared on the US market, it also became one of the cheapest statins [4]. The industrial production of pravastatin represents a two-stage process, which includes the biosynthesis of compactin by Penicillium citrinum and the further microbiological hydroxylation of compactin sodium at the C6 position to obtain pravastatin. It is also possible to produce pravastatin by a chemical synthesis [5]; however, high costs and stereoisomer by-products associated with the chemical synthesis makes a bioconversion process to be more preferable.
The study of various microorganisms revealed some fungi and bacteria able to convert compactin to pravastatin. However, the possible use of fungi is significantly limited because of a fungicidal activity of compactin, whereas bacteria are more tolerant to this compound. Among bacteria, the compactin-converting activity was reported for such species as Nocardia sp., Streptomyces roseochromogenes, S. carbophilus,S. exfoliatus, S. xanthochromogenes, Actinomadura sp., Micropolyspora roseoalba, Micromonospora sp., Mortierella maculata, and Pseudonocardia carboxydivorans [6–11].
One of the main problems of the industrial pravastatin production is a sensitivity of bioconverting microorganisms to high compactin concentrations inhibiting their growth and development [12]. Thus, pravastatin production rate is limited by a necessity to maintain a low level of compactin concentration in fermentation broth that significantly increases production costs. Therefore, there is a great demand in compactin-resistant microorganisms suitable for a large-scale production of pravastatin.
In the present study we report the development of a stable compactin-resistant S. xanthochromogenes S 33-1 strain, obtained by multistage UV mutagenesis and characterized by a high compactin conversion rate.
Materials and Methods
Compactin Biosynthesis, Isolation, Purification, and Preparation
A high-yield Penicillium citrinum 21-34, obtained by a multi-stage random mutagenesis from P. citrinum SANK 18767, was used to produce compactin; the productivity of the mutant strain reached 15 g/L [13]. The strain was maintained and stored according to Ukraintseva et al. [13]. The isolation and purification of compactin from fermentation broth was performed as described by Endo et al. [14]; the purity of the final product was determined by high performance liquid chromatography (HPLC) and reached 90–95 %.
Compactin powder (lactone form) was completely dissolved in hot ethanol (55–60 °C). The resulted saturated solution of a certain molarity was cooled to 30–40 °C and mixed with the equal volume of NaOH of the same molarity. After 30-min incubation, pH was checked and, if necessary, adjusted to 6.9-7.1 by weak NaOH or HCl solutions; then sterile water was added to reduce the final ethanol concentration to 20 % or less. The content of compactin sodium in the final solution made 50–55 g/L.
Pravastatin-Producing Microorganism and Media Composition
A Streptomyces xanthochromogenes RIA 1098 from the collection of the State Research Center of Antibiotics was used as a parental strain. The strain formed compact oval colonies on agar medium; the aerial mycelium was light-beige, and no sporulation was observed. The compactin bioconversion rate of this strain did not exceed 20 % at a total daily compactin dose (DCD) equal to 1 g/L; higher doses sharply reduced the bioconversion rate to 1–2 % and inhibited culture growth.
The parental and mutant strains were grown, maintained, and stored on agar medium containing the following components (g/L): soybean flour, 10; yeast extract, 1; peptone, 10; glucose, 20; agar, 20 (pH 7.0–7.2); in the case of selective agar medium, 1 g/L of compactin was also added. Vegetation medium for seed cultures contained the following components (g/L): soybean flour, 20; yeast extract, 1; soybean peptone, 10; glucose, 3; NaCl, 5; CaCO3, 1 (pH 7.0–7.2). A selective compactin-containing fermentation medium consisted of the following components (g/L): soybean flour, 20; yeast extract, 1; soybean peptone, 5; glucose, 30; NaCl, 5; CaCO3, 3; and glycerin (2.5 mL/L) with final pH 7.0–7.2; the total DCD was 1–2 g per a liter of fermentation medium depending on the mutagenesis/selection stage.
UV Mutagenesis
After a 7-day growth at 28 °C, mycelium was washed off the agar surface with sterile water and passed through a sterile cotton filter to remove large mycelial conglomerates. Then the suspension was filtered through a glass filter (pore size 100 μm) to obtain a uniform suspension of small mycelial fragments and placed under a 50 W UV lamp (Short Wave Ultra-Violet Mineralight, USA) at a distance of 25 cm for time intervals ranging from 1 to 10 min. Because of the variability of nuclei in different fragments of mycelium, a hard irradiation destroying almost all nuclear material was used. After the UV treatment, 0.1 mL of the suspension was spread on the surface of selective agar medium and incubated for 7–10 days at 28 °C. Grown colonies were used for the further screening and selection.
Selection and Fermentation Conditions
Pieces of a 7-day mycelium of mutant strains were transferred into 100-mL flasks containing 20 mL of vegetation medium. Seed cultures were grown for 44–48 h on Infors RC-TK incubation shakers (Infors HT, Switzerland) at 28 °C and 290 rpm (2.5-cm orbit) and then transferred into 250-mL flasks containing 35 mL of selective fermentation medium. The DCD was divided into three equal portions. The first dose was added 20–22 h after the beginning of cultivation; other two doses were added after every 5–6 h. Depending on the selection stage, the total DCD was 1–2 g per a liter of fermentation medium. After the addition of the last dose, flasks were incubated on a shaker for 12–16 h at 28 °C up to the completion of the bioconversion process (the total fermentation time was 46–50 h), and then the pravastatin content in fermentation broth of survived strains was determined. Strains with the best results were used in the further mutagenesis/selection cycles.
Analysis of the Pravastatin Concentration in Fermentation Broth
Pravastatin was extracted from fermentation broth by a nine-fold volume of methanol. Pravastatin concentration and compactin bioconversion rate were determined by HPLC according to [15]. The analysis was carried out using a Gilson chromatographic system with a Reprosil ODS-A column (5 μm, 4 mm × 25 cm); the flow rate was 0.7 mL/min. The absorbance was measured at 237 nm. The mobile phase consisted of 0.1 % phosphoric acid (65 %) and acetonitrile (35 %). A standard pravastatin sodium USP RS was used as a reference sample.
Strain Stability Assessment
To exclude possible physiological adaptation and reveal any possible reversions, three successive subculturings on compactin-free agar medium followed by the inoculation on compactin-containing agar medium were performed for strains selected at the end of each mutagenesis cycle. Strains, which lose their compactin resistance or provided any revertant progeny, were excluded from the further selection.
The stability of the inheritance of a compactin resistance level of the most promising strains was assessed by ten successive subculturings on compactin-free agar medium using a replica plating approach. During each subculturing, a master plate was prepared by the spreading of a dilute suspension of small mycelial fragments on the agar medium and replicated onto agar medium plates with or without compactin (1 g/L), which were further analyzed for a possible progeny disjoining.
The stability of the S 33-1 concerning its conversion rate was tested by a 1-year successive subculturing on agar medium. The strain was subcultured every month and then evaluated for its conversion activity.
Fermentation Medium Improvement
To increase the pravastatin output, fermentation medium was improved by varying of the concentration of nitrogen sources (soybean flour, yeast extract, soybean peptone) and glycerin; the fermentation was carried out as described above.
Strain Identification
The resulted mutant strain was identified by a 16S rRNA sequencing. A fragment of 16S rRNA gene was amplified by PCR using 11F (5′-GTTTGATCMTGGCTCAG-3′) and 1492R (5′-TACGGYTACCTTGTTACGACTT-3′) primers as described in [16]. Nucleotide sequences of the PCR product were determined using a Big Dye Terminator kit v.3.1 (Applied Biosystems, Inc., USA) and an ABI PRIZM 3730 DNA sequencer (Applied Biosystems, Inc., USA) as described in [17]. The reading was performed in both directions using the above primers. The obtained data were compared to the NCBI GenBank database using the BLAST and MEGA 5.05 software. Phylogenetic analysis and dendrogram construction were performed by a neighbor-joining method realized in the MEGA 5.05 software.
Results
Mutagenesis and Strain Selection
First, the survival rate of S. xanthochromogenes colonies and the frequency of morphological mutations were determined at different UV exposure time. The survival rate was determined as the ratio of colonies grown in the treated and untreated variants. The frequency of morphological mutations is an indirect measure of mutagenesis efficiency that, in turn, correlates with a possible appearance of mutant strains with improved productivity. The UV exposure time, which resulted in the maximum number of morphological mutations and, at the same time, provided a sufficient survival rate of colonies, was considered as the optimum time of treatment and was used in the further work. According to the obtained results, the optimum UV exposure time was 5–7 min; in this case, the frequency of morphological mutations was 2–3 %, and the survival rate of colonies was 0.3–0.7 %.
During this study, we observed a correlation between some morphological features of mutant strains and their compactin conversion rate. To clarify this possible correlation, we tested five revealed morphological types for their compactin conversion rate (Table 1). The obtained results showed that the mutants of the types 2–5 either were not able to convert compactin to pravastatin, or possessed a very low conversion rate, whereas those of the type 1 demonstrated an improved conversion ability. Strains of this type were selected for the further mutagenesis/selection cycles.
Table 1.
Relation between the morphological type of Streptomyces xanthochromogenes RIA 1098 mutants and their ability to convert compactin to pravastatin
| Morphological type | Aerial mycelium development | Pigmentation | Compactin conversion level (%) |
|---|---|---|---|
| 1 | Weak | Absent | >60 |
| 2 | Well-developed | Absent | <40 |
| 3 | Well-developed | Weak | <40 |
| 4 | Well-developed | Well marked | <40 |
| 5 | Weak | Well marked | <40 |
During the first cycle, the DCD in fermentation medium was chosen to be sublethal for the parental strain (1 g/L); the conversion rate of finally selected mutants varied in the range of 80–90 %. At the further cycles, DCD was sequentially increased to 1.5 and 2 g/L with the corresponding selection of survived mutants towards an increase in their conversion rate up to 80–90 %.
Finally, this series of sequential mutagenesis/selection cycles resulted in a high-yield strain S 33-1 characterized by improved compactin resistance and high conversion rate. In contrast to the parental strain, the aerial mycelium of the S 33-1 was less dense and had a grayish-white color; as colonies aged, a weak light-beige pigmentation appeared in agar around the colony. No any sporulation was observed.
Compactin Resistance Assessment
To determine a compactin resistance range of the strain S 33-1, the effect of a total DCD on the final pravastatin output and conversion rate was studied (Table 2). While DCD remained below 2 g/L, the utilization rate of compactin sodium was almost 100 %; the further dose increase caused a sharp drop of this parameter (data not shown). The highest compactin conversion rate (91 %) was observed at the total DCD equal to 1 g/L; however, even doubled dose still provided a high bioconversion level. Thus, in addition to a high productivity, the obtained strain demonstrated an improved compactin resistance comparing to the parental S. xanthochromogenes RIA 1098, which growth almost completely inhibited at the total DCD exceeding 1 g/L.
Table 2.
Effect of a total daily dose of compactin sodium on the final pravastatin concentration and bioconversion rate of Streptomyces xanthochromogenes S 33-1
| Daily compactin dose (g/L) | Pravastatin concentrationa (g/L) | Bioconversion ratea (%) |
|---|---|---|
| 1 | 0.91 ± 0.04 | 91.0 ± 4.0 |
| 1.5 | 1.31 ± 0.03 | 87.3 ± 2.0 |
| 2 | 1.65 ± 0.05 | 83.0 ± 2.5 |
| 2.5 | 0.1 ± 0.05 | 4.0 ± 2.0 |
aData obtained for the improved fermentation medium
Fermentation Medium Improvement
To maximize the productivity of the strain S 33-1 in a lab-scale use, fermentation medium was improved at the DCD corresponding to the maximum conversion rate (1 g/L). Since actinomycetes consume carbon and nitrogen sources mainly at the growth stage (first 48 h of fermentation), the most productive strains should require a higher concentration of nutrients in the beginning of the process. Along with soybean flour, peptone and yeast extract provide the maximum increase of a biomass and accumulation of secondary metabolites. In addition, glycerin seems to be a necessary component of fermentation medium, since the use of glycerin-free medium resulted in a low conversion level (40–50 %). Therefore, we decided to optimize the concentrations of the above nutrients. Due to a relatively short duration of a shake flask fermentation, any changes in the glucose concentration were rather inexpedient, since its final level at the end of the process still remained quite sufficient (10–15 g/L) to provide a necessary consumption level.
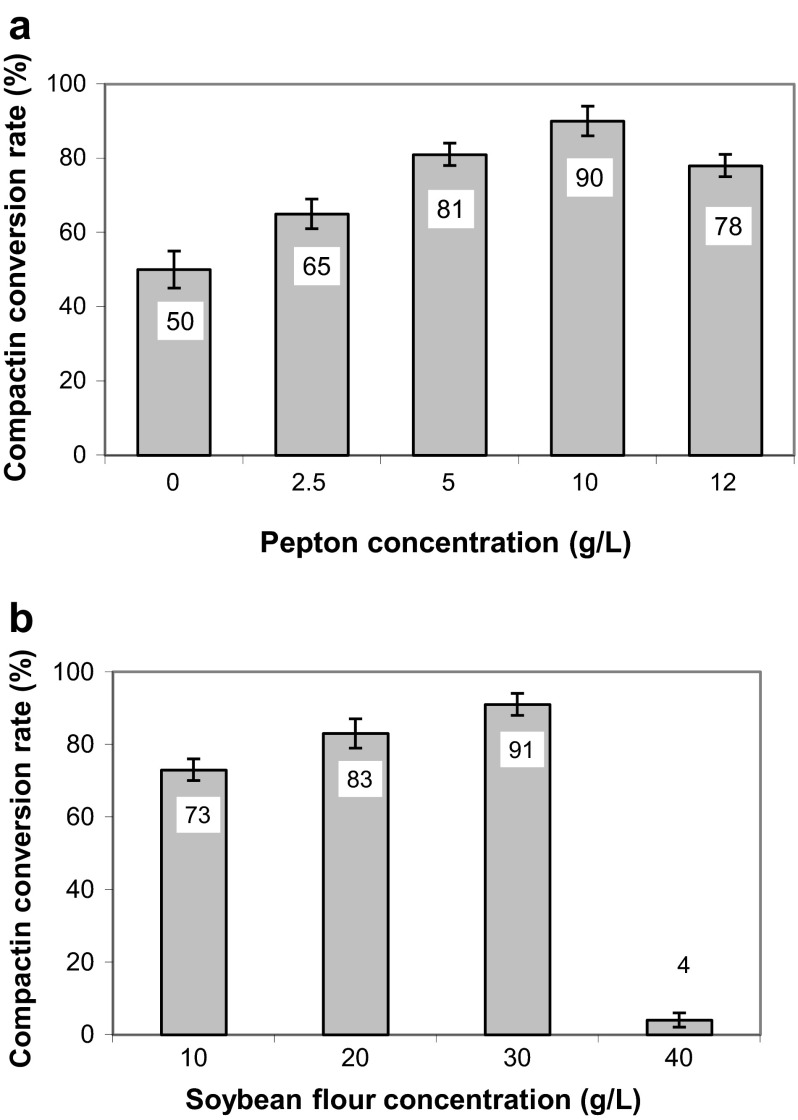
Changes in the yeast extract content in fermentation medium did not reveal any significant difference in the resulting conversion rates, so the concentration of this nutrient in improved medium was remained at the level of 1 g/L. The examination of different glycerin concentrations (1–5 mL/L) also did not reveal any significant changes in the conversion rate indicating just a small local optimum at the level of 2.5 mL/L. The results obtained for peptone and soybean flour are shown in Fig. 1a, b.
Fig. 1.
Influence of peptone (a) and soybean flour (b) concentrations in fermentation broth on the compactin bioconversion rate of Streptomyces xanthochromogenes S 33-1
Thus, an improved medium for the pravastatin production by the strain S 33-1 consists of soybean flour (30 g/L), soybean peptone (10 g/L), yeast extract (1 g/L), glucose (30 g/L), glycerin (2.5 mL/L), NaCl (5 g/L), and CaCO3 (3 g/L). In this case, the maximum conversion rate of the mutant strain reached 91 %. The further scaling-up of the technology is planned.
Strain Stability Assessment
The growth of the S 33-1 on compactin-containing agar medium after three successive subculturings on compactin-free medium was not suppressed that confirmed the improved compactin resistance of this strain to be inherited trait rather than the result of physiological adaptation to high compactin concentrations.
Replica plating performed for ten successive subculturings of the S 33-1 showed no any disjoining concerning its compactin resistance; for each subculturing, the colony patterns on both compactin-containing and compactin-free replica plates were the same as that of the master plate indicating a stable inheritance of the acquired trait.
A 1-year successive subculturing of the S 33-1 did not show any decrease in the compactin conversion rate. Such stability can be probably explained by the lack of sporulation and, therefore, only vegetative reproduction of this strain.
Strain Identification
To confirm the origin of the obtained strain S 33-1, a 16S rRNA sequencing of the parental and mutant strains was performed. Almost whole sequence (1468 nucleotides) of the amplified fragment of the gene encoding 16S rRNA was determined for both strains; the resulted nucleotide sequences were identical between themselves (data not shown). The obtained sequence was compared with similar sequences from the GenBank database. The maximum similarity level (99.3 %) was revealed in the case of bacterial strains S. xanthochromogenes NRRL B-5410 (NR_043847) and S. michiganensis NBRC 12797 (NR_041071). According to the current standards [18], such similarity level is sufficient to consider the strain S 33-1 to belong to the genera Streptomyces.
To clarify the taxonomic position of the analyzed samples, a phylogenetic cladogram was constructed (Online Resource 1). The sequence of amplified fragments of the 16S rRNA gene, determined for the strain S 33-1, was clusterized with the similar sequences of S. xanthochromogenes and S. michiganensis. Thus, like the parental strain, the strain S 33-1 can be considered as S. xanthochromogenes.
Discussion
Low compactin resistance of producing strains remains a serious bottleneck still limiting the industrial production of pravastatin. There are two possible ways to solve this problem. First, one can optimize technological conditions, such as the feeding mode [12] or pre-adaptation of a producing strain to compactin [8]. However, this has a limited effect on the total productivity level, which is significantly influenced by the features of producing microorganisms. Alternatively, one can use compactin-resistant strains obtained either from natural sources, or as a result of the strain improvement.
The screening of wild-type strains is widely used and already revealed some strains with a satisfactory conversion rate at DCD exceeding 0.5 g/L [8, 9, 11, 12, 19, 20]. However, this way is rather laborious. Recently a rational screening method using selective compactin-containing medium was designed [21]. Authors reported about 100 revealed compactin-resistant strains, but the further study showed only two of them were able to convert compactin to pravastatin with a rather low yield. Thus, though the use of selective medium is able to facilitate the screening process, the resulting efficiency is still low. Another serious disadvantage of this approach is that wild-type strains produce secondary metabolites only in amounts they need for their own competitive benefit avoiding any overproduction; therefore, such strains are often unsuitable for the direct use in industrial production. The conversion rate of the reported wild-type strains, able to survive at DCD > 1 g/L, varies within 30–64 % (Online Resource 2) that means insufficient productivity.
Genetic engineering represents the most recent approach to the strain improvement appeared with the development of molecular genetic techniques. Concerning pravastatin production, there are some reports on the development of genetically modified (GM) overproducing strains working at DCD > 0.5 g/L [22, 23], including Escherichia coli strains with 100 % conversion rate at DCD of 1.5 g/L [23]. The use of compactin-resistant Pseudonocardia autotrophica strains as hosts for the compactin-hydroxylating gene cluster from the Streptomyces sp. TM-7 strain resulted in the development of a GM strain able to almost stoichiometrically convert compactin to pravastatin at DCD of about 4 g/L in both lab and pilot producing scale [24]. Finally, the idea of a one-step pravastatin production was recently realized via the genetic reprogramming of industrial penicillin-producing Penicillium chrysogenum strain [25]. After successful removal of penicillin biosynthetic genes, a complete compactin gene cluster from P. citrinum and an evolved compactin hydroxylase CYP gene from Amycolatopsis orientalis were introduced into the host genome. The resulted strain showed high production titers (>6 g/L) at a pilot producing scale.
In spite of impressive results, the use of GM strains has some disadvantages and limitations. GM strains are often unstable in terms of the productivity and maintenance of newly acquired traits. Microorganisms possess a high genetic plasticity and have various mechanisms of horizontal gene transfer via mobile genetic elements, such as insertion sequences, transposons, plasmids, etc. This fact may influence the fate of the recombinant DNA; the possible loss of promoters or terminators within alien gene clusters is able to drastically influence on the possibility of the further industrial use of a strain. In addition, the use of GM organisms is limited by consumer acceptance issues and necessity to get a regulatory approval for their use. Moreover, the legislation of many countries directly prohibits the use of GM organisms in the production of food and drugs on their territories.
Thus, in the most cases, the efforts to improve promising strains for industrial application are now based on natural strategies for strain improvement, such as random mutagenesis. In contrast to GM strains, the use of mutant strains is not restricted by legislation or public opinion, and these strains are much more stable. Concerning the compactin production, random mutagenesis was used mainly to increase the productivity of parental strains [26, 27]. The most interesting results were reported for two mutant Saccharotrix strains, which conversion rates reached 86.7 and 72.9 % at DCD equal to 1.5 and 2.1 g/L, respectively [27]. To our knowledge, there is only one report on the use of compactin-containing (1 g/L) medium for selection of mutant strains, but the resulting strain showed sufficient conversion rate (83.3 %) only at DCD of 0.4 g/L [28]. It is also interesting that none of the reports about mutant strains mentioned the use of a multistage mutagenesis, though it represents a powerful strain improvement tool.
Summarizing all above information, one should note a rather small number of reports devoted to the purposive development of compactin-resistant strains for the pravastatin-producing industry. In spite of the significance of this issue, this area of studies seems to be underestimated and requires more attention. According to our results, multistage random mutagenesis combined with the use of selective medium provides very promising results. A comparison of the compactin resistance level and productivity of the mutant S. xanthochromogenes S 33-1 strain, developed in our laboratory, with the reported data for other natural, mutant, and GM pravastatin-producing strains (see Online Resource 2) shows it exceeds all strains from the first two groups, excepting mutant Saccharotrix strains, which characteristics are close to those of our strain. Though some of the mentioned GM strains demonstrate a higher compactin resistance or the ability to perform a single-stage pravastatin production, the existing limitations on the use of such strains hamper their application in the biopharmaceutic industry. Thus, the reported S 33-1 strain, which stability and productivity was reliably confirmed, can be considered as very promising for the industrial use.
Conclusions
A multi-step random mutagenesis of S. xanthochromogenes RIA 1098 combined with the further screening of mutants on compactin-containing medium resulted in the obtaining of S. xanthochromogenes S 33-1 possessing an improved compactin resistance and a high compactin conversion rate. After the improvement of fermentation medium, the maximum conversion rate of S. xanthochromogenes S 33-1 reached 91 % at the DCD equal to 1 g/L; a high conversion rate (up to 83 %) was maintained even at the doubled DCD (2 g/L) that is twice higher than the sublethal DCD for the parental strain and, as far as we know, exceeds the same characteristics of many other reported pravastatin-producing microorganisms. The compactin resistance level and conversion rate of the obtained strain remained stable for 1 year under regular subculturing that proves a stable inheritance of the improved characteristics.
Electronic supplementary material
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Dulak J, Józkowicz A. Anti-angiogenic and anti-inflammatory effects of statins: relevance to anti-cancer therapy. Curr Cancer Drug Targets. 2005;5:579–594. doi: 10.2174/156800905774932824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kataria P, Kaur J, Parvez E, Maurya RP. Statins: the paradigm shift in periodontal regeneration. SRM J Res Dent Sci. 2014;5:26–30. doi: 10.4103/0976-433X.129069. [DOI] [Google Scholar]
- 3.dos Santos LF, de Carvalho JC, Rubel R, Soccol CR. Microbial statins. In: Brar SK, Dhillon GS, Soccol CR, editors. Biotransformation of waste biomass into high value biochemicals. New York: Springer; 2013. pp. 313–335. [Google Scholar]
- 4.del Sol AI, Nanayakkara PWB. Pravastatin: An evidence-based statin? Expert Opin Drug Metab Toxicol. 2008;4:821–825. doi: 10.1517/17425255.4.6.821. [DOI] [PubMed] [Google Scholar]
- 5.Al-Badr AA, Mostafa GAE. Pravastatin sodium. In: Brittain HG, editor. Profiles of drug substances, excipients, and related methodology. San Diego: Elsevier; 2014. pp. 433–513. [DOI] [PubMed] [Google Scholar]
- 6.Salat J, Mozes J, Bidlo M, Horvath G, Boros S, Barta I, Birincsik L, Albrecht K, Ambrus G, Konya A, Andor A, Jekkel A, Lang I, Ilkoy E, Szabo I (2005) Hydroxylation of compactin to pravastatin by Micromonospora. US Patent 6,905,851
- 7.Salat J, Mozes NSJ, Bidlo NIG, Horvath G, Boros S, Barta I, Somogyi G, Birincsik L, Albrecht K, Ambrus G, Konya A, Andor A, Jekkel A, Lang I, Ilkoy E, Szabo I (2007) Microbial process for preparing pravastatin. Canadian Patent 2361701
- 8.Lin C-L, Tang Y-L, Lin S-M. Efficient bioconversion of compactin to pravastatin by the quinoline-degrading microorganism Pseudonocardia carboxydivorans isolated from petroleum-contaminated soil. Bioresour Technol. 2011;102:10187–10193. doi: 10.1016/j.biortech.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 9.Mei M, Ji X, Gao X, Chen Y, Li Y, Yao Y, Zhuo Z, Xu J (2009) Microorganism and the process for preparation of pravastatin sodium. US Patent 7,582,464
- 10.Peng YL, Demain AL. Bioconversion of compactin to pravastatin by Actinomadura sp. ATCC 55678. J Mol Catal B Enzym. 2000;10:151–156. doi: 10.1016/S1381-1177(00)00123-5. [DOI] [Google Scholar]
- 11.Zhang L, Zhang J, Yang W, Bai G. Classification of Streptomyces strain Z314 and purification of its product pravastatin. Wei Sheng Wu Xue Bao. 2008;48:33–37. [PubMed] [Google Scholar]
- 12.Park JW, Lee JK, Kwon TJ, Yi DH, Kim YJ, Moon SH, Suh HH, Kang SM, Park YI. Bioconversion of compactin into pravastatin by Streptomyces sp. Biotechnol Lett. 2003;25:1827–1831. doi: 10.1023/A:1026281914301. [DOI] [PubMed] [Google Scholar]
- 13.Ukraintseva SN, Voinova TM, Dzhavakhiya VG. Penicillium citrinum strain improvement for compactin production by induced-mutagenesis and optimization of obtained mutant cultivation conditions. In: Zaikov GE, editor. Biotechnology and medicine. New York: Nova Science Publisher Inc.; 2004. pp. 71–78. [Google Scholar]
- 14.Endo A, Kuroda M, Tsujita Y. ML-236A, ML-236B and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinum. J Antibiot (Tokyo) 1976;29:1346–1348. doi: 10.7164/antibiotics.29.1346. [DOI] [PubMed] [Google Scholar]
- 15.Dzhavakhiya VV, Voinova TM. Optimization of fermentation conditions for high lovastatin producing mutant 45–50 of fungus Aspergillus terreus. In: Zaikov GE, editor. Biotechnology and industry. New York: Nova Science Publisher Inc.; 2004. pp. 81–87. [Google Scholar]
- 16.Lane DJ. 16S/23S sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. New York: Wiley; 1991. pp. 115–175. [Google Scholar]
- 17.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;84:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stackebrandt E, Ebers J. Taxonomic parameters revisited: tarnished gold standards. Microbiol Today. 2006;33:152–155. [Google Scholar]
- 19.Lee C-K, Kim D-Y, Suh J-W, Chang J-H (2004) Streptomyces sp. CJPV975652 capable of converting compactin to pravastatin and method for producing pravastatin using the same. International Patent Application WO2004111205
- 20.Lee J-K, Park J-W, Seo D-J, Lee S-C, Kim J-Y (2001) Microorganism Streptomyces exfoliatus YJ-118 and a method for producing pravastatin sodium by using the strain. US Patent 6,306,629
- 21.Chen C-H, Hu H-Y, Cho Y-C, Hsu W-H. Screening of compactin-resistant microorganisms capable of converting compactin to pravastatin. Curr Microbiol. 2006;53:108–112. doi: 10.1007/s00284-005-0276-7. [DOI] [PubMed] [Google Scholar]
- 22.Szabo L, Tchelet R (2010) Process for constructing strain having compactin hydroxylation ability. US Patent 7,700,337
- 23.Fujii T, Fujii Y, Machida K, Ochiai A, Ito M. Efficient biotransformations using Escherichia coli with tolC acrAB mutations expressing cytochrome P450 genes. Biosci Biotechnol Biochem. 2009;73:805–810. doi: 10.1271/bbb.80627. [DOI] [PubMed] [Google Scholar]
- 24.Fujii Y, Norihisa K, Fujii T, Aritoku Y, Kagawa Y, Sallam KI, Johdo O, Arisawa A, Tamura T. Construction of a novel expression vector in Pseudonocardia autotrophica and its application to efficient biotransformation of compactin to pravastatin, a specific HMG-CoA reductase inhibitor. Biochem Biophys Res Commun. 2011;404:511–516. doi: 10.1016/j.bbrc.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 25.McLean KJ, Hans M, Meijrink B, van Scheppingen WB, Vollebregt A, Tee KL, van der Laan J-M, Leys D, Munro AW, van den Berg MA. Single-step fermentative production of the cholesterol-lowering drug pravastatin via reprogramming of Penicillium chrysogenum. Proc Natl Acad Sci. 2015;112:2847–2852. doi: 10.1073/pnas.1419028112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kostova I, Ivanova N, Losev V, Dimitrova A, Vasileva R, Todorova D (2004) Method for production of pravastatin by fermentation. European Patent 1452602
- 27.Lee F-Y, Lee M-L, Hong AC, Chiu S-C (2007) Strains of Saccharotrix, process for producing pravastatin using the strains and isolation process of (HMG-CoA) reductase. US Patent 7,202,062
- 28.Choi N-H, Tak K-T, Lee K-W, Kim N-H, Jun J-C, Kong Y-L, Lee K-M (2007) Process for preparing pravastatin sodium. US Patent 7,223,590
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.