Abstract
In this work, 12 different yeast strains were evaluated to gauge their ability to accumulate lipids using raw glycerol as the main carbon source. Lipomyces lipofer NRRL Y-1155 stood out above the other strains, achieving 9.48 g/l biomass, 57.64 % lipid content and 5.46 g/l lipid production. The fatty acid profile was similar to vegetable oils commonly used in the synthesis of biodiesel, with the predominance of polyunsaturated acids, especially linoleic acid, reaching 68.3 % for Rhodotorula glutinis NRRL YB-252. The occurrence of palmitic acid (39.3 % for Lipomyces starkeyi NRRL Y-11557) was also notable. Thus, yeast biomass with high lipid content can be a sustainable and renewable alternative as a raw material for the biodiesel industry.
Keywords: Microbial lipids, Fatty acids, Crude glycerol, Biodiesel
Introduction
The expected decline in petroleum reserves, together with the growing concern over climate change resulting from global warming due to carbon dioxide emissions, has encouraged initiatives aimed at replacing fossil fuels [1]. In this context, biodiesel production has been encouraged because it is a renewable, biodegradable and nontoxic fuel obtained by the transesterification of an oil or fat with a monovalent alcohol, forming methyl or ethyl esters of fatty acids (biodiesel) and raw glycerol [2].
However, a major problem facing this sector is related to the increase in oilseed growing areas to meet the growing market demand, increasing competition for arable land between biofuel production and food. The cost of raw materials is also a limiting factor for the use of biodiesel on a large scale, since the use of vegetable oils, such as soybean, castor, palm, sunflower, babassu and peanut oil, is predominant [3, 4]. An alternative that has been proposed is the use of new lipid sources, which has highlighted the oleaginous yeast biomass obtained in cultivations using renewable substrates, such as agroindustrial waste, with oleaginous yeasts being considered capable of accumulating lipids at levels of more than 20 %. Therefore, studies have been proposed regarding the use of this biomass as a feedstock in the production of biodiesel [5–7].
As over 70 % of the production costs of biodiesel are due to the cost of the raw materials used, as well as the large amounts of raw glycerol generated in the process (stoichiometrically 10 %) and its low cost [8], it has become urgent to find alternative ways of converting this substrate into value-added products in order to produce biodiesel in a sustainable way with the simultaneous recycling of raw glycerol [9].
Lipids obtained from microbial sources, also called single cell oil, are produced by certain microorganisms such as yeasts, filamentous fungi, bacteria and microalgae. Generally, yeast and filamentous fungi are capable of accumulating more lipids than bacteria and microalgae, accumulating them not only as constituents of the membrane, but also in the form of triacylglycerols. The best known species of oleaginous yeast include Candida, Cryptococcus, Rhodotorula, Trichosporon and Yarrowia [10]. It has been shown that this microbial lipid source is rich in triglycerides, which can be converted into biodiesel by enzymatic or inorganic catalysis. Lipid production by oleaginous yeasts was also found to have many advantages due to its rapid growth, decreased susceptibility to changes of season, location and climate, ease of scale-up, high oil content and the similarity of their triacylglycerols with those obtained from vegetable oils and animal fats [7].
Raw glycerol from the biodiesel synthesis is 55–90 % pure. The remainder consists of unconverted triglycerides, unconverted methanol or ethanol, biodiesel, soaps and others [11]. The conversion of glycerol by the biotechnological process in commercially important products is one of the most promising alternatives for use [12, 13], and can be used with success as a carbon source by different microorganisms in the production of lipids [14].
Thus, the main goal of this work was to evaluate various yeasts capable of assimilating raw glycerol generated as a byproduct in the synthesis of biodiesel to produce biomass as a source of lipids, with potential for use as feedstock in biodiesel production.
Materials and Methods
Microorganisms
Yeasts from the National Center for Agricultural Utilization Research (Peoria, USA) were used: Rhodotorula glutinis NRRL YB-252, Lipomyces starkeyi NRRL Y-11557, Cryptococcus albidus NRRL YB-219, Lipomyces lipofer NRRL Y-11555, Cryptococcus curvatus NRRL Y-1511, Cryptococcus laurentii NRRL Y-27011, Candida cylindracea NRRL Y-17506, Yarrowia lipolytica NRRL Y-1094, Y. lipolytica NRRL Y-11853, Y. lipolytica NRRL YB-423, Candida oleophila NRRL Y-2317 and Candida lipolytica NRRL Y-1095. The lyophilized cultures were rehydrated, and transferred to Yeast Malt (YM) broth composed of (g/l): 3 malt extract, 3 yeast extract, 5 peptone and 10 glucose. They were incubated at 25 °C for 48 h and subsequently transferred to YM agar and incubated under the same conditions, after being kept stored under refrigeration (4 °C).
Raw Glycerol
Raw glycerol derived from biodiesel production from degummed soybean oil via the methanolic route was used, provided by BS Bios (Passo Fundo, Brazil). The raw glycerol contained (%): 6.51 ash, 9.78 moisture, 1.22 non-glyceridic organic matter, 82.49 glycerol, pH 4.91.
Pre-selection of Oleaginous Yeasts
The pre-selection of yeasts with the greatest potential to assimilate glycerol and accumulate lipids was performed using the staining technique with Sudan Black B [15]. Colonies of each microorganism were inoculated in Petri dishes containing a growth medium capable of inducing lipid accumulation, as proposed by Evans and Ratledge [16], with the following composition (g/l): 50.0 glycerol (analytical grade), 7.0 KH2PO4, 2.0 Na2HPO4, 1.5 MgSO4·7H2O, 0.1 CaCl2, 0.008 FeCl3·6H2O, 0.0001 ZnSO4·7H2O, 0.8 yeast extract, 20 agar, pH adjusted to 5.5. The dishes (in triplicate) were incubated for 96 h at 30 °C until the colonies were approximately 2–3 mm in diameter. From the colonies of the Petri dishes, a replica of the printing was made on Whatman Grade No. 1 filter paper and with the use of Sudan Black B solution (Sigma Aldrich) it was possible to observe the typical blue color for oleaginous yeasts.
Inoculum Preparation
Two tubes of the reactivated microbial culture were scraped with 10 ml of 0.1 % peptone diluent to each tube for removal of the microorganism cells and transferred to Erlenmeyer flasks containing 180 ml of culture medium (YM broth) and incubated at 30 °C and 180 rpm (Tecnal TE-424, Brazil). The cell concentration was monitored by counting in a Neubauer chamber until there were approximately 108 cells/ml [17].
Shaken Flasks Cultivation
The yeasts selected as potential lipid producers were cultivated in a medium containing raw glycerol as the carbon source. The cultivations were performed in 500 ml Erlenmeyer flasks with an initial volume of 200 ml, resulting from the addition of the medium, yeast suspension (inoculum) and sterile distilled water. The culture medium was prepared in concentrated form to attain the following composition (g/l): 60.9 raw glycerol, 7.0 KH2PO4, 2.0 Na2HPO4, 1.5 MgSO4·7H2O, 0.1 CaCl2, 0.008 FeCl3·6H2O, 0.0001 ZnSO4·7H2O, 0.8 yeast extract, pH 5.5 [16]. The amount of raw glycerol added considered its composition in order to result an in initial glycerol concentration of 50 g/l. The flasks were inoculated with the yeast suspension previously prepared in order to achieve 107 cells/ml and maintained in a rotary shaker at 30 °C and 180 rpm.
Aliquots were taken at regular intervals, centrifuged at 1800×g for 15 min (Centribio 80-2B, Brazil) and the cells were washed with distilled water, centrifuged again and resuspended in order to determine biomass concentration. The cultivations were conducted until the stationary phase, when no significant variation was observed in the biomass concentration. At the end of the cultivation process, the biomass was recovered under the same conditions to determine the lipid content and fatty acid profile. The glycerol not consumed in the supernatant was determined. Total lipid production was calculated by multiplying biomass concentration by lipid content (dry basis) [18]. Lipid yield was calculated by the ratio of lipids produced and the consumed substrate (glycerol) [11].
Analytical Methods
Cell concentration was monitored by optical density in a spectrophotometer (Bioespectro SP 220, China) at 600 nm using a previously established standard curve for each microorganism and subsequent conversion to biomass concentration [19].
The determination of glycerol in the supernatant was performed using an enzymatic kit (LOD 0.4 mg/l, R-Biopharm, Germany), comprising the enzymes glycerol kinase, pyruvate kinase and l-lactate dehydrogenase, following the manufacturer’s recommendations. The absorbance reading was performed at 340 nm using a spectrophotometer (Bioespectro SP 220, China).
Intracellular lipids were determined using the method of Bligh and Dyer [20]. First, the dried biomass was treated with 2 M HCl for disruption of the cell wall and centrifuged at 1785×g, and the supernatant was discarded. The pellet was mixed with methanol and chloroform, following by centrifugation at 1785×g for 10 min. The procedure was repeated until total extraction of lipids. The chloroform phase was separated and the solvent was evaporated. Lipids were quantified by gravimetry.
To determine the fatty acid profile, the lipid fraction was esterified to obtain fatty acids methyl esters [21] and analyzed with a gas chromatograph (Varian Star 3400, USA) equipped with a split/splitless injector, a ZBWAX column (30 m × 0.32 mm internal diameter) and a flame ionization detector. The carrier gas was hydrogen at a flow rate of 1 ml/min, and make-up gas was nitrogen at 30 ml/min. The temperatures of the injector and detector were adjusted to 250 and 300 °C, and the injected volume was 1 μl. The fatty acids were identified by comparison with Sigma-Aldrich standards and quantified by area normalization.
Statistical Analysis
The experiments were performed in triplicate. The data were analyzed by analysis of variance and the Tukey test to verify the significant differences between the microorganisms under study, at a 95 % confidence level (p ≤ 0.05), using Statistica 5.0 software (Stat Soft Inc., USA).
Results and Discussion
Pre-selection of Oleaginous Yeasts
Of the 11 yeasts analyzed by the Sudan Black B staining technique, seven showed a bluish tint and were considered potential producers of lipids and characterized according to the intensity of the color (Table 1). Duarte et al. [22] isolated yeasts from different Brazilian ecosystems using a staining technique similar to that used in this study, enabling the selection of five yeasts with potential for lipid production. Although this technique does not allow a precise response to the cellular lipid content, as it is a qualitative analysis, it does provide partial information regarding the ability of the microorganisms to accumulate lipids, selecting the most promising ones. The red yeast R. glutinis NRRL YB-252 was not analyzed by this method, since, according to Evans et al. [15], there might be a divergence in results due to a structural difference in these yeasts that hinders the entry of the pigment into the cell.
Table 1.
Qualitative analysis of yeasts using the staining technique with Sudan Black B
| Microorganism | Color intensity |
|---|---|
| C. albidus NRRL YB-219 | nc |
| C. cylindracea NRRL Y-17506 | ** |
| C. curvatus NRRL Y-1511 | *** |
| C. oleophila NRRL Y-2317 | nc |
| C. laurentii NRRL Y-27011 | nc |
| C. lipolytica NRRL Y-1095 | * |
| L. lipofer NRRL Y-11555 | ** |
| L. starkeyi NRRL Y-11557 | *** |
| Y. lipolytica NRRL Y-11853 | * |
| Y. lipolytica NRRL YB-423 | * |
| Y. lipolytica NRRL Y-1094 | nc |
Different coloration intensities of the colonies were indicated qualitatively as: * weak, ** average, *** intense, nc not colored
Shaken Flasks Cultivation
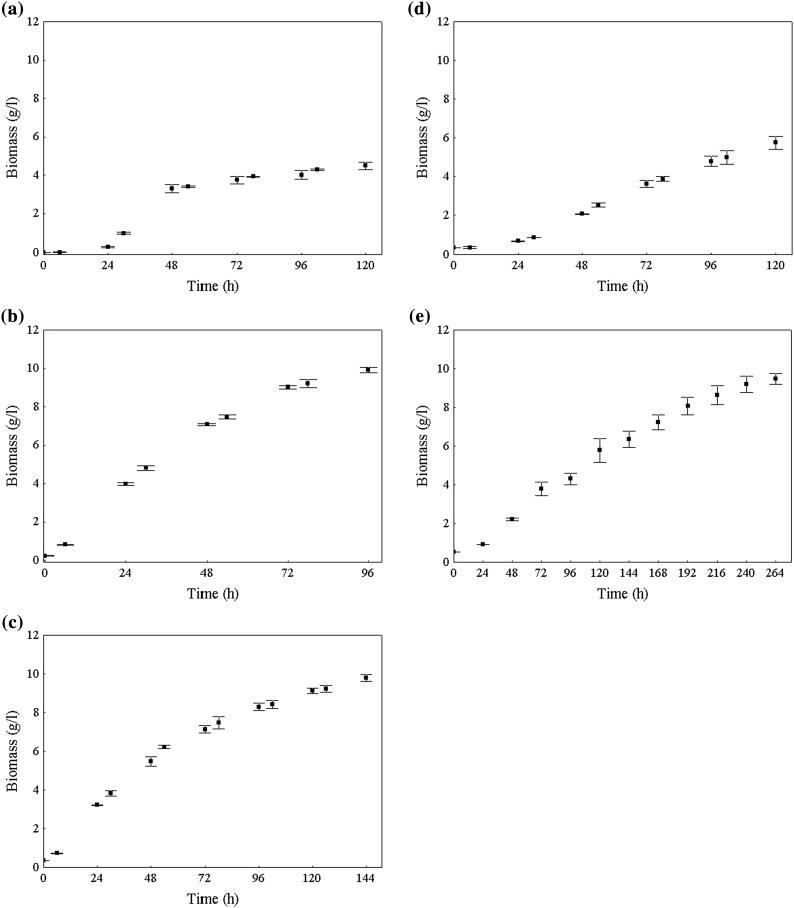
The four yeasts that showed an intense or average blue color and the red yeast were pre-selected in order to cultivate in a liquid medium containing raw glycerol as the carbon source. Figure 1 presents the growth curves for the yeasts under study. It is possible to note that the time to reach the stationary phase was different for each yeast, varying from 96 (C. cylindracea NRRL Y-17506) to 264 h (L. lipofer NRRL Y-11555).
Fig. 1.
Growth curves of the pre-selected yeasts cultivated in a medium containing raw glycerol. a C. curvatus NRRL Y-1511, b C. cylindracea NRRL Y-17506, c R. glutinis NRRL YB-252, d L. starkeyi Y-11557, e L. lipofer NRRL Y-11557
By observing the biomass concentration obtained for the different yeasts (Table 2), it appears that C. cylindracea NRRL Y-17506, R. glutinis NRRL YB-252 and L. lipofer NRRL Y-11555 did not differ from one another, reaching 9.92, 9.80 and 9.48 g/l, respectively. The best results regarding this growth parameter were obtained with these yeasts. Therefore, raw glycerol was shown to be a good carbon source for the cultivation of these yeasts, even in the presence of impurities, such as soaps, glycerides, fats, heavy metals, inorganic salts and methanol. According to Xu et al. [11], some of these impurities could act as nutritional elements that can be assimilated by microorganisms during the cultivation, increasing biomass concentration and lipid accumulation. Saenge et al. [14] reported the positive influence of inorganic salts such as sodium, calcium, potassium and magnesium in the production of lipids. In addition to these macroelements, there may also be vitamins and trace elements that diffuse into the glycerol during the reaction of the formation of biodiesel, enriching the raw glycerol [23].
Table 2.
Parameters of the pre-selected oleaginous yeasts
| Yeast | Time (h) | μamax(h−1) | Biomass (g/l) | Glycerol (g/l) | Lipid (% w/w) | Lipid production (g/l) | YaL/s (g/g) |
|---|---|---|---|---|---|---|---|
| C. curvatus | 120 | 0.14A | 4.49 ± 0.20C | 40.2 ± 0.1A | 27.90 ± 1.28D | 1.25 ± 0.09D | 0.13BC |
| C. cylindracea | 96 | 0.11B | 9.92 ± 0.13A | 39.8 ± 0.5A | 14.11 ± 0.47E | 1.40 ± 0.06D | 0.14B |
| R. glutinis | 144 | 0.09C | 9.80 ± 0.17A | 28.5 ± 0.9B | 40.85 ± 1.88C | 4.00 ± 0.12B | 0.19A |
| L. starkeyi | 120 | 0.04D | 5.74 ± 0.32B | 28.0 ± 0.5B | 50.49 ± 0.99B | 2.89 ± 0.17C | 0.13BC |
| L. lipofer | 264 | 0.03E | 9.48 ± 0.27A | 2.9 ± 0.1C | 57.64 ± 2.81A | 5.46 ± 0.34A | 0.12C |
μmax is the maximum specific growth rate; YL/S is the lipid yield
Different uppercase superscript letters in the same column indicate a statistically significant difference (p < 0.05)
aSD < 0.01
It was verified that all yeasts assimilated glycerol as a carbon source. Glycerol remaining in the medium at the end of cultivation varied from 2.9 (L. lipofer NRRL Y-11555) to 40.2 g/l (C. curvatus NRRL Y-1511) (Table 2), corresponding to a glycerol consumption of 94.2 and 19.6 %, respectively. The carbon excess positively affects lipid production, but high glycerol can inhibit cellular growth [24]. Duarte et al. [22] found glycerol consumption between 31.23 and 64.31 % when used raw glycerol (initial concentration of 40 g/l) for lipid production by wild yeasts isolated from Brazilian biodiversity. Raimondi et al. [24] found glycerol consumption between 12 and 100 % for different yeasts cultivated in a medium containing 40 g/l pure glycerol.
For the maximum specific growth rate, C. curvatus NRRL Y-1511 and C. cylindracea NRRL Y-17506 showed the highest values (0.14 and 0.11 h−1, respectively), while L. starkeyi NRRL Y-11557 and L. lipofer NRRL Y-11555 were the yeasts with the slowest (0.04 and 0.03 h−1, respectively), which resulted in longer cultivation times (120 and 264 h, respectively).
The lipid contents of the yeasts are shown in Table 2. The data show significant differences in lipid accumulation from one yeast to another, highlighting L. lipofer NRRL Y-11555 with 57.64 % and L. starkeyi NRRL Y-11557 with 50.49 %. These values are similar to that found by Duarte et al. [22] for Candida LEB-M3 (56.5 %). In addition, L. lipofer NRRL Y-11555 had the highest lipid yield, 5.49 g/l, because in addition to the highest lipid content among the yeasts in question it resulted in a high biomass concentration, despite the longer cultivation time (264 h) as shown by their slow growth (0.03 h−1).
Lipids were determined just in the stationary phase. According to Beopoulos et al. [25], the accumulation of lipids in the cell starts to rise in the growth phase (log phase), because these lipids are used in the cell membrane synthesis to support its growth. When the cell reaches its ideal size, starts to accumulate lipids as droplets within the cell. So, lipid content reaches its maximum value in the stationary phase. Once the amount of nutrients in the medium begins to decrease, lipids are rapidly degraded into free fatty acids, so removal of cells in early stationary phase is necessary to prevent lipid degradation.
Regarding the lipid yield, Table 2 shows that R. glutinis NRRL YB-252 had significant differences in relation to other yeasts (YL/S = 0.19 g/g), and the values obtained from the yeasts under study were similar to those reported by Xu et al. [11] for Rhodosporidium toruloides (approximately 0.14 and 0.15 g/g for pure and raw glycerol, respectively).
It is important to note that the lipid accumulation process requires the exhaustion of a nutrient, usually nitrogen, to allow the excess of carbon to be used in the lipid synthesis [26]. Angerbauer et al. [27], cultivating L. starkeyi DSM 70295 in a medium based on sewage sludge supplemented with glucose, obtained a lipid content of 68 % with a C/N ratio of 150, and a lipid content of 40 % with a C/N ratio of 60. Saenge et al. [14], investigating the influence of the C/N ratio in lipid accumulation by R. glutinis TISTR 5159, found that the highest content of lipids was achieved with a C/N ratio of 85 and glycerol concentration of 95 g/l, achieving 5.49 g/l biomass and 41.28 % lipid. In the present study, a high C/N ratio (162) was used, aiming at lipid accumulation. According to Saenge et al. [14], a low C/N ratio favors biomass production, whereas lipid accumulation is favored by a high C/N ratio. The enzyme AMP deaminase is activated by nitrogen depletion, which promotes the decrease of mitochondrial adenosine monophosphate (AMP) and increases the cellular concentration of ammonia. This decrease of AMP inhibits the enzyme isocitrate dehydrogenase, blocking the citric acid cycle and promoting the accumulation of acetyl-CoA required for the synthesis of fatty acids [10, 14].
On the other hand, cultivations strategies are important to be considered in order to provide a better performance, mainly avoiding the growth inhibition by the substrate. Candida freyschussii ATCC 18737 cultivated in shaken flasks using pure glycerol as carbon source was capable of producing 3.2 g/l lipids (11.9 g/l biomass and 26.4 % lipids) [24]. In the present work, raw glycerol was used, with lipid production varying from 1.25 (C. curvatus NRRL Y-1511) to 5.46 g/l (L. lipofer NRRL Y-11555) (Table 2). However, for C. freyschussii ATCC 18737, it was possible to increase lipid production in a laboratory-scale bioreactor cultivation (2 l of medium), reaching 4.7 g/l (batch mode) and 28 g/l (fed-batch mode), with an increase of biomass and lipid content [24]. So, it is expected that the values obtained in this work can be improved and subsequent studies will follow.
Fatty Acids
According to the literature, palmitic acid (C16:0), stearic acid (C18:0), oleic acid (C18:1), linoleic acid (C18:2) and linolenic acid (C18:3) are the major constituents of the fatty acids of microbial triacylglycerols [28]. Table 3 shows the fatty acid composition of the lipid fraction of yeasts cultivated in raw glycerol-based medium. Several authors have argued that the fatty acid composition of lipids influences the properties of biodiesel and therefore its quality; and some of the most important properties are determined by the chemical structure. Among the most significant parameters are the chain length and degree of unsaturation, capable of predicting the properties of biodiesel [29]. Data demonstrate that the predominant fatty acids are the long chain fatty acids with 16–18 carbon atoms, including palmitic acid, oleic acid, linoleic acid and linolenic acid. Stearic acid was detected only in trace amounts.
Table 3.
Fatty acid profile of the pre-selected yeasts cultivated in a medium containing raw glycerol
| Yeast | Fatty acid relative content (% w/w)a | ||||||
|---|---|---|---|---|---|---|---|
| C16:0 | C16:1 | C18:1 | C18:2 | C18:3 | C20:0 | C22:6 | |
| C. curvatus | 17.3 | – | 18.0 | 49.5 | 10.6 | 1.7 | 1.7 |
| C. cylindracea | 30.0 | 3.6 | 7.3 | 29.3 | 23.1 | 3.3 | 1.9 |
| R. glutinis | 20.4 | 1.8 | 1.1 | 68.3 | 4.0 | – | 0.8 |
| L. starkeyi | 39.3 | 4.3 | 7.8 | 42.5 | 4.2 | – | – |
| L. lipofer | 35.1 | 5.3 | 8.2 | 43.1 | 3.7 | – | 1.7 |
aSome fatty acids were detected in trace amounts and were not included in the table
Comparing the fatty acid profiles reported in previous studies for R. glutinis [30], R. mucilaginosa [31], L.starkeyi [32] and Cryptococcus sp. [33] grown on different carbon sources (dextrose, Jerusalem artichoke tubers, glucose and corncob hydrolysate, respectively), it was observed in the present study that the lipid fraction of the pre-selected and cultivated yeasts in the medium containing raw glycerol as the main carbon source was particularly rich in polyunsaturated fatty acids, especially linoleic acid, which reached 68.3 % in the yeast R. glutinis NRRL YB-252. The compositions (Table 3) differed greatly from those mentioned in the literature, in particular linoleic acid, considered as a nutritionally essential fatty acid ω6, achieving approximately 6 % for R. glutinis [30, 34], 1.2 % for L. starkeyi [32] and 7.2 % for Cryptococcus sp. [33]. According to Fakas et al. [35], the difference in fatty acid profiles may occur due to the age of the cell, the substrate that is used and the culture conditions.
The considerable presence of unsaturated fatty acids also occurs in marine microalgae. The marine microalgae Dunaliella tertiolecta, identified as a potential raw material for biodiesel production, presented 28.8 % of saturated fatty acids and 73.2 % of unsaturated fatty acids [36]. According to Menezes et al. [4], marine microalgae are extensively studied for biodiesel production, since lipid content varies from 20 to 50 % (w/w). However, microalgae need larger acreages to cultivation and long cultivation periods than other microorganisms, such as bacteria and yeasts [26].
Oleaginous yeasts can accumulate intracellular lipids for biodiesel production through cultivation on various carbohydrates and agro-industrial wastes. Glucose [6] and raw glycerol [7] were used to produce microbial oils from different oleaginous yeasts in order to obtain a sustainable raw material for the biodiesel industry. Fatty acid composition was similar to the main vegetable oils used in the biodiesel production, resulting in a biodiesel with the requisite properties.
In this work, the lipids produced by microbial cultivation with an alternative carbon source (raw glycerol generated in the synthesis of biodiesel) were particularly rich in polyunsaturated fatty acids, bearing a resemblance to the composition of vegetable oils commonly used for the synthesis of biodiesel, such as soybean and sunflower oil, mainly containing linoleic acid (53.0 and 63.1 % respectively) [37]. The occurrence of palmitic acid (39.3 % for L. starkeyi NRRL Y-11557) was also notable. Similar results were found by Gong et al. [38] using cellobiose as a substrate (38.3 % of palmitic acid for L. starkeyi AS 2.1560). Palmitic acid is a saturated fatty acid found in large quantities in palm oil (36.7 %) [37], a vegetable oil widely used in biodiesel production.
According to CONAB (National Supply Company), the 2012/2013 soybean harvest in Brazil saw a production of 81.5 million tons for an area of 27.7 million planted hectares, with a total yield of approximately 3000 kg/ha. Considering 20 % of total soybean weight, 1 ha produces about 600 kg oil per year. Assuming, for example, a microbial lipid production of 5.5 g/l obtained for cultivation of L. lipofer NRRL Y-11555 in raw glycerol, roughly 109 m3 of the culture medium would be required for the production of oil equivalent to 1 ha, in approximately 11 days. It is important to emphasize that the microbial oil has other advantages over to vegetable oil. A point in question is that its fatty acid composition can be modified depending on the source of nutrients and culture conditions used [30].
Conclusion
The results of this study show that it was possible to pre-select oleaginous yeast using a simple technique such as staining with Sudan Black B. These yeasts can grow and accumulate higher lipid content when grown in a medium containing raw glycerol as the main carbon source. The yeast had similar fatty acids composition to the main vegetable oils used in the synthesis of biodiesel, with a predominance of long chain fatty acids with 16–18 carbons, particularly polyunsaturated fatty acids such as linoleic acid. Thus, the valorization of an agroindustrial waste, such as raw glycerol, through its bioconversion to microbial oil, may be a promising alternative, with the potential to minimize waste, reduce costs, and be used to produce second generation biodiesel.
Acknowledgments
The authors would like to thank the State Research Foundation of Rio Grande do Sul (FAPERGS), the Brazilian Council for Scientific and Technological Development (CNPq) and the Brazilian Federal Agency for the Support and Evaluation of Graduate Education (CAPES) for their financial support.
References
- 1.Yang F, Hanna MA, Sun R (2012) Value-added uses for crude glycerol—a byproduct of biodiesel production. Biotechnol Biofuels 5:13. doi:10.1186/1754-6834-5-13 [DOI] [PMC free article] [PubMed]
- 2.Beatriz A, Araújo YJK, Lima DP. Glicerol: um breve histórico e aplicação em sínteses estereosseletivas. Quim Nova. 2011;34:306–319. doi: 10.1590/S0100-40422011000200025. [DOI] [Google Scholar]
- 3.Poli JS, Lützhoft HH, Karakashev DB, Valente P, Angelidaki I. An environmentally-friendly fluorescent method for quantification of lipid contents in yeast. Bioresour Technol. 2014;151:388–391. doi: 10.1016/j.biortech.2013.09.128. [DOI] [PubMed] [Google Scholar]
- 4.Menezes RS, Leles MIG, Soares AT, Brandão PI, Franco M, Antoniosi Filho NR. Avaliação da potencialidade de microalgas dulcícolas como fonte de matéria-prima graxa para a produção de biodiesel. Quim Nova. 2013;36:10–15. doi: 10.1590/S0100-40422013000100003. [DOI] [Google Scholar]
- 5.Ryu B, Kim J, Kim K, Choi Y, Han J, Yang J. High-cell-density cultivation of oleaginous yeast Cryptococcus curvatus for biodiesel production using organic waste from the brewery industry. Bioresour Technol. 2013;135:357–364. doi: 10.1016/j.biortech.2012.09.054. [DOI] [PubMed] [Google Scholar]
- 6.Liu B, Zhao Z. Biodiesel production by direct methanolysis of oleaginous microbial biomass. J Chem Technol Biotechnol. 2007;82:775–780. doi: 10.1002/jctb.1744. [DOI] [Google Scholar]
- 7.Thiru M, Sankh S, Rangaswamy V. Process for biodiesel production from Cryptococcus curvatus. Bioresour Technol. 2011;102:10436–10440. doi: 10.1016/j.biortech.2011.08.102. [DOI] [PubMed] [Google Scholar]
- 8.Leoneti AB, Aragão-Leoneti V, Oliveira SVWB. Glycerol as a by-product of biodiesel production in Brazil: alternatives for the use of unrefined glycerol. Renew Energy. 2012;45:138–145. doi: 10.1016/j.renene.2012.02.032. [DOI] [Google Scholar]
- 9.Makri A, Fakas S, Aggelis G. Metabolic activities of biotechnological interest in Yarrowia lipolytica grown on glycerol in repeated batch cultures. Bioresour Technol. 2010;101:2351–2358. doi: 10.1016/j.biortech.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 10.Beopoulos A, Nicaud JM, Gaillardin C. An overview of lipid metabolism in yeasts and its impact on biotechnological processes. Appl Microbiol Biotechnol. 2011;90:1193–1206. doi: 10.1007/s00253-011-3212-8. [DOI] [PubMed] [Google Scholar]
- 11.Xu J, Zhao X, Wang W, Du W, Liu D. Microbial conversion of biodiesel byproduct glycerol to triacylglycerols by oleaginous yeast Rhodosporidium toruloides and the individual effect of some impurities on lipid production. Biochem Eng J. 2012;65:30–36. doi: 10.1016/j.bej.2012.04.003. [DOI] [Google Scholar]
- 12.Papanikolaou S, Fakas S, Fick M, Chevalot I, Galiotou-Panayotou M, Komaitis M, Marc I, Aggelis G. Biotechnological valorisation of raw glycerol discharged after bio-diesel (fatty acid methyl esters) manufacturing process: production of 1,3-propanediol, citric acid and single cell oil. Biomass Bioenerg. 2008;32:60–71. doi: 10.1016/j.biombioe.2007.06.007. [DOI] [Google Scholar]
- 13.Kumar P, Mehariya S, Ray S, Mishra A, Kalia VC. Biodiesel industry waste: a potential source of bioenergy and biopolymers. Indian J Microbiol. 2015;55:1–7. doi: 10.1007/s12088-014-0509-1. [DOI] [Google Scholar]
- 14.Saenge C, Cheirsilp B, Suksaroge TT, Bourtoom T. Potential use of oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process Biochem. 2011;46:210–218. doi: 10.1016/j.procbio.2010.08.009. [DOI] [Google Scholar]
- 15.Evans CT, Ratledge C, Gilbert SC. A rapid screening method for lipid-accumulating yeast using a replica-printing technique. J Microbiol Methods. 1985;4:203–210. doi: 10.1016/0167-7012(85)90038-7. [DOI] [Google Scholar]
- 16.Evans CT, Ratledge C. Biochemical activities during lipid accumulation in Candida curvata. Lipids. 1983;18:630–635. doi: 10.1007/BF02534674. [DOI] [PubMed] [Google Scholar]
- 17.Santos EO, Michelon M, Furlong EB, Burkert JFM, Kalil SJ, Burkert CAV. Evaluation of the composition of culture medium for yeast biomass production using raw glycerol from biodiesel synthesis. Braz J Microbiol. 2012;43:432–440. doi: 10.1590/S1517-83822012000200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai CC, Tao J, Xie F, Dai YJ, Zhao M. Biodiesel generation from oleaginous yeast Rhodotorula glutinis with xylose assimilating capacity. Afr J Biotechnol. 2007;6:2130–2134. [Google Scholar]
- 19.Zhang Y, Rittmann BE, Wang J, Sheng Y, Yu J, Shi H, Qian Y. High-carbohydrate wastewater treatment by IAL-CHS with immobilized Candida tropicalis. Process Biochem. 2005;40:857–863. doi: 10.1016/j.procbio.2004.02.010. [DOI] [Google Scholar]
- 20.Bligh EG, Dyer JW. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 21.Metcalfe LD, Schmitz AA, Pelka JR. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal Chem. 1966;38:514–515. doi: 10.1021/ac60235a044. [DOI] [Google Scholar]
- 22.Duarte SH, Andrade CCP, Ghiselli G, Maugeri F. Exploration of Brazilian biodiversity and selection of a new oleaginous yeast strain cultivated in raw glycerol. Bioresour Technol. 2013;138:377–381. doi: 10.1016/j.biortech.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Çelik E, Ozbay N, Oktar N, Çalik P. Use of biodiesel byproduct crude glycerol as the carbon source for fermentation processes by recombinant Pichia pastoris. Ind Eng Chem Res. 2008;47:2985–2990. doi: 10.1021/ie071613o. [DOI] [Google Scholar]
- 24.Raimondi S, Rossi M, Leonardi A, Bianchi MM, Rinaldi T, Amaretti A. Getting lipids from glycerol: new perspectives on biotechnological exploitation of Candida freyschussii. Microb Cell Fact. 2014;13:1–11. doi: 10.1186/1475-2859-13-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beopoulos A, Mrozova Z, Thevenieau F, Le Dall MT, Hapala I, Papanikolaou S, Chardot T, Nicaud JM. Control of lipid accumulation in the yeast Yarrowia lipolytica. Appl Environ Microbiol. 2008;74:7779–7789. doi: 10.1128/AEM.01412-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng X, Yang J, Xu X, Zhang L, Nie Q, Xian M. Biodiesel production from oleaginous microorganisms. Renew Energy. 2009;34:1–5. doi: 10.1016/j.renene.2008.04.014. [DOI] [Google Scholar]
- 27.Angerbauer C, Siebenhofer M, Mittelbach M, Guebitz GM. Conversion of sewage sludge into lipids by Lipomyces starkeyi for biodiesel production. Bioresour Technol. 2008;99:3051–3056. doi: 10.1016/j.biortech.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 28.Davoli P, Mierau V, Weber RSW. Carotenoids and fatty acids in red yeasts Sporobolomyces roseus and Rhodotorula glutinis. Prikl Biokhim Mikrobiol. 2004;40:460–465. [PubMed] [Google Scholar]
- 29.Pinzi S, Garcia IL, Lopez-Gimenez FJ, Luque de Castro MD, Dorado G, Dorado MP. The ideal vegetable oil-based biodiesel composition: a review of social, economical and technical implications. Energy Fuels. 2009;23:2325–2341. doi: 10.1021/ef801098a. [DOI] [Google Scholar]
- 30.Easterling ER, French WT, Hernandez R, Licha M. The effect of glycerol as a sole and secondary substrate on the growth and fatty acid composition of Rhodotorula glutinis. Bioresour Technol. 2009;100:356–361. doi: 10.1016/j.biortech.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 31.Zhao C-H, Zhang T, Li M, Chi Z-M. Single cell oil production from hydrolysates of inulin and extract of tubers of Jerusalem artichoke by Rhodotorula mucilaginosa TJY15a. Process Biochem. 2010;45:1121–1126. doi: 10.1016/j.procbio.2010.04.002. [DOI] [Google Scholar]
- 32.Lin J, Shen H, Tan H, Zhao X, Wu S, Hu C, Zhao ZK. Lipid production by Lipomyces starkeyi cells in glucose solution without auxiliary nutrients. J Biotechnol. 2011;152:184–188. doi: 10.1016/j.jbiotec.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Chang Y-H, Chang K-S, Hsu C-L, Chuang L-T, Chen C-Y, Huang F-Y, Jang H-D. A comparative study on batch and fed-batch cultures of oleaginous yeast Cryptococcus sp. in glucose-based media and corncob hydrolysate for microbial oil production. Fuel. 2013;105:711–717. doi: 10.1016/j.fuel.2012.10.033. [DOI] [Google Scholar]
- 34.Lian J, Garcia-Perez M, Chen S. Fermentation of levoglucosan with oleaginous yeasts for lipid production. Bioresour Technol. 2013;133:183–189. doi: 10.1016/j.biortech.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 35.Fakas S, Papanikolaou S, Bastos A, Galiotoupanayotou M, Mallouchos A, Aggelis G. Evaluating renewable carbon sources as substrates for single cell oil production by Cunninghamella echinulata and Mortierella isabellina. Biomass Bioenerg. 2009;33:573–580. doi: 10.1016/j.biombioe.2008.09.006. [DOI] [Google Scholar]
- 36.Tang H, Abunasser N, Garcia MED, Chen M, Simon NKY, Salley SO. Potential of microalgae oil from Dunaliella tertiolecta as a feedstock for biodiesel. Appl Energy. 2011;88:3324–3330. doi: 10.1016/j.apenergy.2010.09.013. [DOI] [Google Scholar]
- 37.Ramos MJ, Fernández CM, Casas A, Rodríguez L, Pérez A. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol. 2009;100:261–268. doi: 10.1016/j.biortech.2008.06.039. [DOI] [PubMed] [Google Scholar]
- 38.Gong ZW, Wang Q, Shen HW, Hu CM, Jin GJ, Zhao ZBK. Co-fermentation of cellobiose and xylose by Lipomyces starkeyi for lipid production. Bioresour Technol. 2012;117:20–24. doi: 10.1016/j.biortech.2012.04.063. [DOI] [PubMed] [Google Scholar]