Abstract
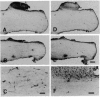
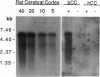
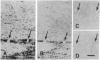
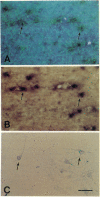
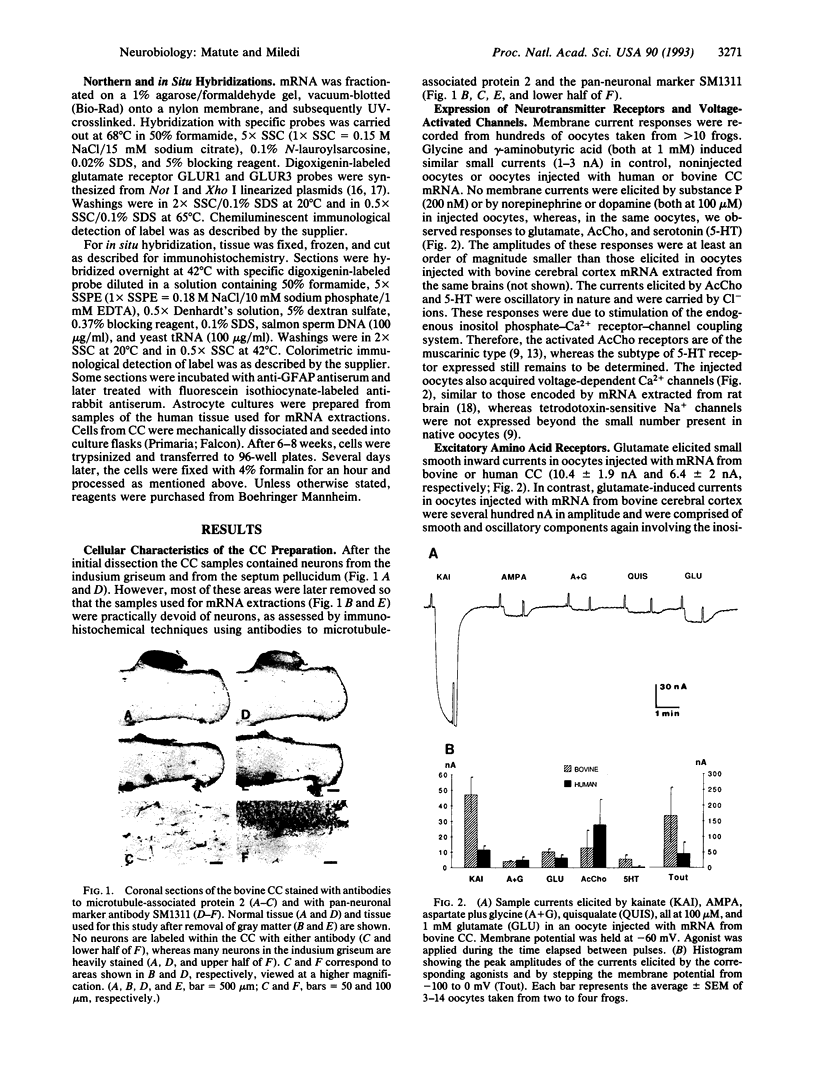
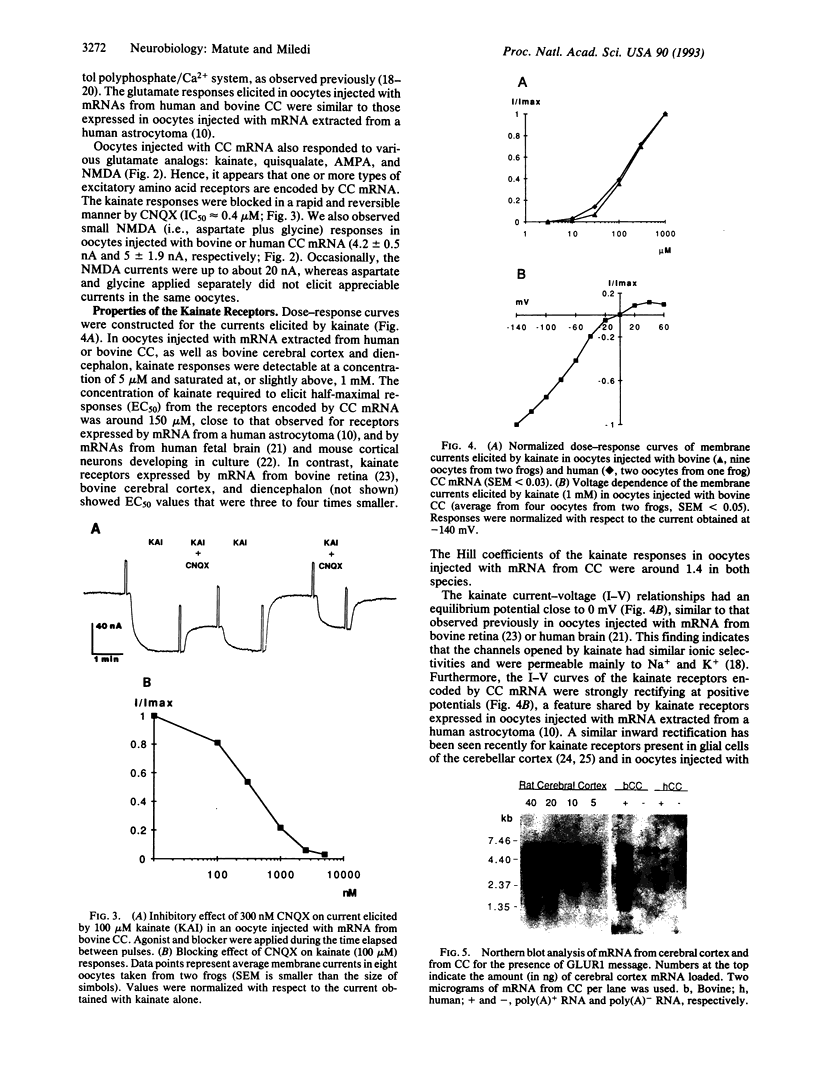
The presence of mRNAs encoding neurotransmitter receptors and voltage-gated channels in the adult human and bovine corpus callosum was investigated using Xenopus oocytes. Oocytes injected with mRNA extracted from the corpus callosum expressed functional receptors to glutamate, acetylcholine, and serotonin, and also voltage-operated Ca2+ channels, all with similar properties in the two species studied. Acetylcholine and serotonin elicited oscillatory Cl- currents due to activation of the inositol phosphate-Ca2+ receptor-channel coupling system. Glutamate and its analogs N-methyl-D-aspartate (NMDA), kainate, quisqualate, and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) induced smooth currents. The non-NMDA responses showed a strong inward rectification at positive potentials and were potently blocked by 6,7-dinitroquinoxaline-2,3-dione, as observed for the AMPA/kainate glutamate receptors GLUR1 and GLUR3. Furthermore, in situ hybridization experiments showed that GLUR1 and GLUR3 mRNAs are present in corpus callosum cells that were labeled with antiserum to glial fibrillary acid protein and that, in primary cell cultures, had the morphology of type 2 astrocytes. These results indicate that glial cells in the adult corpus callosum possess mRNA encoding functional neurotransmitter receptors and Ca2+ channels. These molecules may provide a mechanism for glial-neuronal interactions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano T., Yamakuni T., Okabe N., Kuwahara R., Ozawa F., Hishinuma F. Regulation of nerve growth factor and nerve growth factor receptor production by NMDA in C6 glioma cells. Brain Res Mol Brain Res. 1992 Jun;14(1-2):35–42. doi: 10.1016/0169-328x(92)90007-x. [DOI] [PubMed] [Google Scholar]
- Backus K. H., Kettenmann H., Schachner M. Pharmacological characterization of the glutamate receptor in cultured astrocytes. J Neurosci Res. 1989 Mar;22(3):274–282. doi: 10.1002/jnr.490220307. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Koroshetz W. J., Swartz K. J., Chun L. L., Corey D. P. Ion channel expression by white matter glia: the O-2A glial progenitor cell. Neuron. 1990 Apr;4(4):507–524. doi: 10.1016/0896-6273(90)90109-s. [DOI] [PubMed] [Google Scholar]
- Barres B. A. New roles for glia. J Neurosci. 1991 Dec;11(12):3685–3694. doi: 10.1523/JNEUROSCI.11-12-03685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger T., Walz W., Schnitzer J., Kettenmann H. GABA- and glutamate-activated currents in glial cells of the mouse corpus callosum slice. J Neurosci Res. 1992 Jan;31(1):21–27. doi: 10.1002/jnr.490310104. [DOI] [PubMed] [Google Scholar]
- Bevan S., Miledi R., Grampp W. Induced transmitter release from Schwann cells and its suppression by actinomycin D. Nat New Biol. 1973 Jan 17;241(107):85–86. doi: 10.1038/newbio241085a0. [DOI] [PubMed] [Google Scholar]
- Black J. A., Waxman S. G. The perinodal astrocyte. Glia. 1988;1(3):169–183. doi: 10.1002/glia.440010302. [DOI] [PubMed] [Google Scholar]
- Boulter J., Hollmann M., O'Shea-Greenfield A., Hartley M., Deneris E., Maron C., Heinemann S. Molecular cloning and functional expression of glutamate receptor subunit genes. Science. 1990 Aug 31;249(4972):1033–1037. doi: 10.1126/science.2168579. [DOI] [PubMed] [Google Scholar]
- Burnashev N., Khodorova A., Jonas P., Helm P. J., Wisden W., Monyer H., Seeburg P. H., Sakmann B. Calcium-permeable AMPA-kainate receptors in fusiform cerebellar glial cells. Science. 1992 Jun 12;256(5063):1566–1570. doi: 10.1126/science.1317970. [DOI] [PubMed] [Google Scholar]
- Carpenter M. K., Parker I., Miledi R. Expression of GABA and glycine receptors by messenger RNAs from the developing rat cerebral cortex. Proc R Soc Lond B Biol Sci. 1988 Jul 22;234(1275):159–170. doi: 10.1098/rspb.1988.0042. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- DeFeudis F. V. Effects of electrical stimulation on the efflux of L-glutamate from peripheral nerve in vitro. Exp Neurol. 1971 Feb;30(2):291–296. doi: 10.1016/s0014-4886(71)80008-0. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Raff M. C. The oligodendrocyte-type-2 astrocyte cell lineage is specialized for myelination. 1986 Sep 25-Oct 1Nature. 323(6086):335–338. doi: 10.1038/323335a0. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Glutamate and kainate receptors induced by rat brain messenger RNA in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1984 Apr 24;221(1223):127–143. doi: 10.1098/rspb.1984.0027. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Messenger RNA from human brain induces drug- and voltage-operated channels in Xenopus oocytes. 1984 Mar 29-Apr 4Nature. 308(5958):421–424. doi: 10.1038/308421a0. [DOI] [PubMed] [Google Scholar]
- Hirono C., Ito I., Yamagishi S., Sugiyama H. Characterization of glutamate receptors induced in Xenopus oocytes after injection of rat brain mRNA. Neurosci Res. 1988 Dec;6(2):106–114. doi: 10.1016/0168-0102(88)90012-0. [DOI] [PubMed] [Google Scholar]
- Hollmann M., Hartley M., Heinemann S. Ca2+ permeability of KA-AMPA--gated glutamate receptor channels depends on subunit composition. Science. 1991 May 10;252(5007):851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Hollmann M., O'Shea-Greenfield A., Rogers S. W., Heinemann S. Cloning by functional expression of a member of the glutamate receptor family. Nature. 1989 Dec 7;342(6250):643–648. doi: 10.1038/342643a0. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambolez B., Curutchet P., Stinnakre J., Bregestovski P., Rossier J., Prado de Carvalho L. Electrophysiological and pharmacological properties of GluR1, a subunit of a glutamate receptor-channel expressed in Xenopus oocytes. Neurosci Lett. 1991 Feb 11;123(1):69–72. doi: 10.1016/0304-3940(91)90160-u. [DOI] [PubMed] [Google Scholar]
- Levi G., Patrizio M. Astrocyte heterogeneity: endogenous amino acid levels and release evoked by non-N-methyl-D-aspartate receptor agonists and by potassium-induced swelling in type-1 and type-2 astrocytes. J Neurochem. 1992 May;58(5):1943–1952. doi: 10.1111/j.1471-4159.1992.tb10073.x. [DOI] [PubMed] [Google Scholar]
- MacVicar B. A., Tse F. W. Norepinephrine and cyclic adenosine 3':5'-cyclic monophosphate enhance a nifedipine-sensitive calcium current in cultured rat astrocytes. Glia. 1988;1(6):359–365. doi: 10.1002/glia.440010602. [DOI] [PubMed] [Google Scholar]
- Matute C., Arellano R. O., Conde-Guerri B., Miledi R. mRNA coding for neurotransmitter receptors in a human astrocytoma. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3399–3403. doi: 10.1073/pnas.89.8.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982 Jul 22;215(1201):491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- Miledi R., Woodward R. M. Effects of defolliculation on membrane current responses of Xenopus oocytes. J Physiol. 1989 Sep;416:601–621. doi: 10.1113/jphysiol.1989.sp017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T., Möller T., Berger T., Schnitzer J., Kettenmann H. Calcium entry through kainate receptors and resulting potassium-channel blockade in Bergmann glial cells. Science. 1992 Jun 12;256(5063):1563–1566. doi: 10.1126/science.1317969. [DOI] [PubMed] [Google Scholar]
- Nakanishi N., Shneider N. A., Axel R. A family of glutamate receptor genes: evidence for the formation of heteromultimeric receptors with distinct channel properties. Neuron. 1990 Nov;5(5):569–581. doi: 10.1016/0896-6273(90)90212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Sumikawa K., Miledi R. Messenger RNA from bovine retina induces kainate and glycine receptors in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1985 Jul 22;225(1238):99–106. doi: 10.1098/rspb.1985.0052. [DOI] [PubMed] [Google Scholar]
- Ragsdale D. S., Miledi R. Expressional potency of mRNAs encoding receptors and voltage-activated channels in the postmortem rat brain. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1854–1858. doi: 10.1073/pnas.88.5.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siepl C., Bodmer S., Hofer E., Wrann M., Frei K., Fontana A. Glioblastoma-cell-derived T-cell suppressor factor (G-TsF). Sequence analysis and biologic mechanism of G-TsF. Ann N Y Acad Sci. 1988;540:437–439. doi: 10.1111/j.1749-6632.1988.tb27126.x. [DOI] [PubMed] [Google Scholar]
- Sumikawa K., Parker I., Miledi R. Partial purification and functional expression of brain mRNAs coding for neurotransmitter receptors and voltage-operated channels. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7994–7998. doi: 10.1073/pnas.81.24.7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse F. W., Fraser D. D., Duffy S., MacVicar B. A. Voltage-activated K+ currents in acutely isolated hippocampal astrocytes. J Neurosci. 1992 May;12(5):1781–1788. doi: 10.1523/JNEUROSCI.12-05-01781.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usowicz M. M., Gallo V., Cull-Candy S. G. Multiple conductance channels in type-2 cerebellar astrocytes activated by excitatory amino acids. Nature. 1989 Jun 1;339(6223):380–383. doi: 10.1038/339380a0. [DOI] [PubMed] [Google Scholar]
- Weinreich D., Hammerschlag R. Nerve impulse-enhanced release of amino acids from non-synaptic regions of peripheral and central nerve trunks of bullfrog. Brain Res. 1975 Jan 24;84(1):137–142. doi: 10.1016/0006-8993(75)90807-0. [DOI] [PubMed] [Google Scholar]
- Wyllie D. J., Mathie A., Symonds C. J., Cull-Candy S. G. Activation of glutamate receptors and glutamate uptake in identified macroglial cells in rat cerebellar cultures. J Physiol. 1991 Jan;432:235–258. doi: 10.1113/jphysiol.1991.sp018383. [DOI] [PMC free article] [PubMed] [Google Scholar]