Abstract
Context:
Maternal adiposity and overnutrition, both before and during pregnancy, plays a key role in the subsequent development of obesity and metabolic outcomes in offspring.
Objective:
We explored the hypothesis that maternal adiposity (pre-pregnancy and at 26–28 weeks' gestation) and mid-pregnancy gestational weight gain (GWG) are independently associated with offspring size and adiposity in early childhood, and determined whether these effects are ethnicity dependent.
Design:
In a prospective mother-offspring cohort study (N = 976, 56% Chinese, 26% Malay, and 18% Indian), we assessed the associations of offspring size (weight, length) and adiposity (subscapular and triceps skinfolds), measured at birth and age 6, 12, 18, and 24 mo, with maternal pre-pregnancy body mass index (ppBMI), mid-pregnancy GWG, and mid-pregnancy four-site skinfold thicknesses (triceps, biceps, subscapular, suprailiac).
Results:
ppBMI and mid-pregnancy GWG were independently associated with postnatal weight up to 2 y and skinfold thickness at birth. Weight and subscapular and triceps skinfolds at birth increased by 2.56% (95% confidence interval, 1.68–3.45%), 3.85% (2.16–5.57%), and 2.14% (0.54–3.75%), respectively for every SD increase in ppBMI. Similarly, a one-SD increase in GWG increased weight and subscapular and triceps skinfolds at birth by 2.44% (1.66–3.23%), 3.28% (1.75–4.84%), and 3.23% (1.65–4.84%), respectively. ppBMI and mid-pregnancy suprailiac skinfold independently predicted postnatal skinfold adiposity up to 2 years of age, whereas only GWG predicted postnatal length. The associations of GWG with postnatal weight and length were present only among Chinese and Indians, but not Malays (P < .05 for interaction).
Conclusions:
ppBMI and GWG are independent modifiable factors for child size and adiposity up to 2 years of age. The associations are ethnic-dependent, and underscore the importance of ethnic specific studies before generalizing the applicability of risk factors reported in other populations.
The increasing prevalence of childhood obesity (1, 2) has become a major public health issue, and emerging evidence has suggested that exposure to an adverse in utero environment, in particular maternal obesity and overnutrition, would play a key role in the subsequent development of obesity and metabolic disorders in offspring (3). Maternal obesity and overnutrition may affect offspring adiposity through permanent alterations to glucose, insulin, lipid, and amino acid metabolism (4). Maternal prepregnancy body mass index (BMI) (ppBMI) and gestational weight gain (GWG) are important determinants of the intrauterine nutritional environment, and are modifiable factors for prevention of excessive infant adiposity and obesity later in life. Skinfold thickness is also a measure of nutritional status during pregnancy (5–7). Several studies have reported positive associations of these different facets of adiposity and nutritional status during pregnancy with offspring adiposity in childhood, adolescence, and adulthood (8, 9), although these were predominantly conducted in Caucasian populations. Few studies have been conducted in Asian populations. Given that the Asian phenotype and susceptibility toward obesity and metabolic disease differ from that of Caucasians, additional study of the effect of Asian maternal adiposity on offspring adiposity is merited (10). Moreover, different Asian ethnic groups (such as Chinese, Malay, and Asian-Indian) exhibit differences in susceptibility to obesity and metabolic syndrome (11). Chinese, Malays, and Asian-Indians in Singapore exhibited significant differences in risk of obesity and type 2 diabetes mellitus; the Malay population has the highest prevalence of obesity (24.0%) whereas the Asian-Indian population has the highest prevalence of type 2 diabetes mellitus (17.2%) (12). Therefore, we embarked on this study to explore and ratify the effects of maternal ppBMI and GWG on offspring size and adiposity in a heterogeneous Asian population.
The present study seeks to prospectively characterize the relationship of ppBMI and midpregnancy GWG with child size (weight and length) and adiposity in early childhood, in a multiethnic mother-offspring Asian birth cohort. First, we evaluated whether the effects of midpregnancy GWG on child size and adiposity is independent of ppBMI and whether these associations depended on ppBMI. Second, we examined whether the effects of these two risk factors are ethnicity dependent. Finally, we hypothesized that there are facets of maternal adiposity not captured by ppBMI and midpregnancy GWG, and therefore studied the importance of maternal skinfolds on offspring's size and adiposity.
Materials and Methods
Study population
This report is part of the Growing Up in Singapore Towards Healthy Outcomes (GUSTO) study, a prospective mother-offspring birth cohort (13, 14). Pregnant women in their first trimester and of at least 18 years of age were recruited from two major public hospitals with obstetric services in Singapore, namely the KK Women's and Children's Hospital (KKH) and the National University Hospital (NUH). Eligible participants had to hold Singapore citizenship or permanent residency, intended to reside in Singapore for the next 5 years, were of Chinese, Malay or Indian ethnicity, had homogeneous parental ethnic background and had the intention of delivering at either NUH or KKH. Women who were taking chemotherapy or psychotropic drugs or had diabetes mellitus were excluded from the study. Interviewer-administered questionnaires were used to assess maternal prepregnancy weight, demographics, and maternal obstetric and medical history at enrolment. Written informed consent was obtained. The ethics boards of both KKH and NUH approved this study.
Maternal anthropometry
Maternal prepregnancy weight was self reported at enrolment. Maternal anthropometric measurements including weight, height, and skinfolds (triceps, biceps, subscapular, and suprailiac) were recorded during the 26–28-gestational-week clinic visit. Weight was recorded to the nearest 0.1 kg using a calibrated scale and taken in duplicates. Height was recorded to the nearest 0.1 cm using a stadiometer and taken in duplicates. Maternal triceps, biceps, subscapular, and suprailiac skinfolds were measured using Holtain skinfold calipers to the nearest 0.2 mm and taken in triplicates. ppBMI was calculated as self-reported prepregnancy weight (kg) divided by the squared height (m2). Midpregnancy GWG was calculated by difference between 26 and 28 weeks' gestation and prepregnancy weights.
Infant characteristics and anthropometry
Infant weight and recumbent length were measured at birth, 6, 12, 18, and 24 months of age. Infant weight was measured to the nearest gram using a calibrated scale. Recumbent infant length was measured from the top of the head to the soles of the feet using an infant mat to the nearest 0.1 cm. For reliability, all measurements were taken in duplicates. Triceps and subscapular skinfolds were measured at birth, 18, and 24 months. Triceps and subscapular skinfolds were measured in triplicates using Holtain skinfold calipers, on the right side of the body, and recorded to the nearest 0.2 mm. Child sex and gestational age (GA) were extracted from medical records.
Statistical analysis
Linear regression models were used to study the association of longitudinal offspring anthropometric outcomes (weight, length, subscapular and triceps skinfolds) with maternal anthropometric measures (ppBMI, GWG, and skinfolds). Child anthropometric measures were log transformed to stabilize variance. Due to the log transformation, regression coefficients were reported as percentage change in child anthropometric outcome for one SD increase in predictor variable. Due to differing variability of predictor variables, effect estimates were reported for one SD increase in predictor variable so that effect estimates of different variables were comparable. To account for repeated measures from the same child, generalized estimating equations with exchangeable working correlation structure and sandwich variance estimator were fitted using geepack package in R (15). All models adjusted for temporal effects, GA, child sex, ethnicity (except for analyses stratified by ethnicity), parity, maternal age, education, and height, chosen a priori from literature evidence. For modeling of temporal effects, first to allow for nonlinearity, distinct time points (eg, birth, 6, 12, 18, and 24 mo) were coded using binary variables (eg, five time points correspond to four binary variables, using birth as baseline). Secondly, to allow the association of offspring anthropometric measures (weight, length, subscapular and triceps skinfolds) with each variable (GA, child sex, ethnicity, parity, maternal age, education, height, ppBMI, GWG, and maternal skinfolds) to vary at each distinct time point, interaction terms between (binary variables) of time and each variable in the model were included. We report the associations of maternal adiposity variables with offspring anthropometric measures at each distinct time point.
To examine the effects of ppBMI and GWG on offspring anthropometric measures, we modeled ppBMI and GWG jointly (ie, the model includes both ppBMI and GWG), and adjusted for the aforementioned covariates. For comparison, effect estimates from modeling ppBMI and GWG separately, adjusted for covariates, are reported in Supplemental Figures 1 and 2. This analysis gave similar conclusions. To investigate whether the associations of offspring anthropometric measures with GWG depended on ppBMI, we included an interaction term between ppBMI and GWG, and adjusted for ppBMI, GWG, and covariates. To investigate whether the associations of offspring anthropometric measures with ppBMI and GWG depended on ethnicity, we included interaction terms between ethnicity and ppBMI and between ethnicity and GWG, and adjusted for ppBMI, GWG, and covariates (including ethnicity). We also report ethnicity-stratified results from modeling ppBMI and GWG jointly (ie, a regression model is fitted to each of the three ethnic groups separately). We also examined how maternal body composition (skinfolds) depends on ppBMI and GWG in each ethnic group, adjusting for parity, maternal age, education, and height. To model the effects of maternal skinfold on offspring outcomes, we tested each maternal skinfold for association with offspring anthropometric outcome separately, and adjusted for ppBMI, GWG, and covariates.
Results
ppBMI and GWG independently predicted offspring weight at birth and up to 2 years
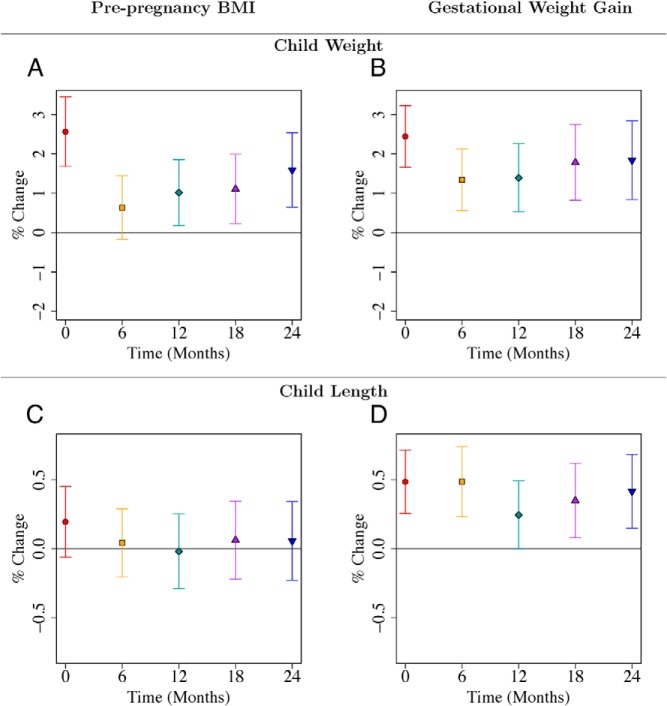
This analysis used 976 full-term neonates who had complete information in covariates and maternal anthropometry (Table 1). Five hundred fifty (56%), 249 (26%), and 177 (18%) of the mother-offspring pairs were of Chinese, Malay, and Indian ethnicity respectively. Mothers were on average 30 years old, had average maternal height of 158.2 cm, average ppBMI of 22.7 kg/m2, and average midpregnancy GWG of 8.7 kg; 62% had at least 12 years of education and 54% were multiparous. Both ppBMI and midpregnancy GWG independently predicted birth weight. The associations survived adjustment for child sex, ethnicity, GA, parity, maternal height, education, and age. The associations also survived adjustment for the other predictor, ie, ppBMI predicted offsprings' birth weight when adjusted for covariates as well as GWG (P < .001; Figure 1A; Supplemental Table 1); and GWG predicted offsprings' birth weight when adjusted for covariates and ppBMI (P < .001; Figure 1B; Supplemental Table 2). The effect of a one-SD increase in ppBMI (equivalent to 4.5 kg/m2) on birth weight was comparable to that of a one-SD increase in GWG (equivalent to 4.7 kg). Birth weight increased by 2.56% (95% confidence interval [CI], 1.68–3.45%) for every SD increase in ppBMI. A one-SD increase in GWG increased birth weight by 2.44% (95% CI, 1.66–3.23%). ppBMI and GWG continued to independently predict offspring weight up to 24 months. For both predictors, the effect sizes decreased at 6 months (and ppBMI became nonsignificant) and then increased until 24 months (Figures 1, A and B; Supplemental Tables 1 and 2).
Table 1.
Characteristics of the Mother-Offspring GUSTO Cohort Investigated in the Analysis, Stratified by Ethnicity
| Characteristic | Time Point | All Ethnicities (n = 976) |
Chinese (n = 550) |
Indian (n = 177) |
Malay (n = 249) |
||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | Mean (sd) | N (%) | Mean (sd) | N (%) | Mean (sd) | N (%) | Mean (sd) | ||
| Offspring | |||||||||
| Sex | |||||||||
| Male | Delivery | 510 (52) | 283 (51) | 92 (52) | 135 (54) | ||||
| Female | 466 (48) | 267 (49) | 85 (48) | 114 (46) | |||||
| Parity/birth order | |||||||||
| 1 | 448 (46) | 276 (50) | 69 (39) | 103 (41) | |||||
| 2 | 335 (34) | 185 (34) | 88 (50) | 62 (25) | |||||
| >2 | 193 (20) | 89 (16) | 20 (11) | 84 (34) | |||||
| Gestational age, wk | Delivery | 976 | 38.9 (1.0) | 550 | 39.0 (1.0) | 177 | 38.9 (1.0) | 249 | 38.7 (1.0) |
| Weight, g | |||||||||
| Delivery | 944 | 3123 (390) | 533 | 3142 (386) | 168 | 3039 (376) | 243 | 3139 (404) | |
| 6 mo | 835 | 7720 (915) | 488 | 7796 (914) | 148 | 7589 (877) | 199 | 7632 (931) | |
| 12 mo | 818 | 9387 (1078) | 471 | 9384 (1021) | 150 | 9567 (1191) | 197 | 9256 (1107) | |
| 18 mo | 780 | 10 752 (1300) | 448 | 10 692 (1220) | 139 | 11 142 (1477) | 193 | 10 610 (1299) | |
| 24 mo | 789 | 11 979 (1557) | 452 | 11 925 (1494) | 140 | 12 247 (1694) | 197 | 11 913 (1585) | |
| Length, cm | |||||||||
| Delivery | 944 | 49 (2) | 533 | 49 (2) | 168 | 49 (2) | 243 | 48 (2) | |
| 6 mo | 839 | 67 (3) | 489 | 67 (3) | 149 | 67 (2) | 201 | 66 (3) | |
| 12 mo | 820 | 75 (3) | 471 | 76 (3) | 151 | 76 (3) | 198 | 74 (3) | |
| 18 mo | 674 | 82 (3) | 391 | 82 (3) | 109 | 83 (3) | 174 | 81 (3) | |
| 24 mo | 693 | 88 (4) | 391 | 88 (4) | 122 | 89 (4) | 180 | 86 (3) | |
| Subscapular skinfold, mm | |||||||||
| Delivery | 945 | 5.0 (1.2) | 535 | 5.0 (1.2) | 168 | 4.8 (1.1) | 242 | 5.0 (1.2) | |
| 18 mo | 657 | 6.4 (1.4) | 373 | 6.4 (1.2) | 113 | 6.4 (1.7) | 171 | 6.2 (1.4) | |
| 24 mo | 729 | 6.4 (1.6) | 415 | 6.4 (1.3) | 133 | 6.3 (1.7) | 181 | 6.5 (2.0) | |
| Triceps skinfold, mm | |||||||||
| Delivery | 946 | 5.4 (1.2) | 535 | 5.5 (1.2) | 168 | 5.3 (1.2) | 243 | 5.5 (1.3) | |
| 18 mo | 696 | 8.6 (1.7) | 395 | 8.5 (1.6) | 124 | 8.8 (2.1) | 177 | 8.8 (1.9) | |
| 24 mo | 706 | 8.8 (1.9) | 402 | 8.8 (1.8) | 127 | 8.8 (2.0) | 177 | 9.0 (2.1) | |
| Maternal | |||||||||
| Age, y | 30.8 (5.1) | 31.9 (4.7) | 29.9 (4.7) | 29.0 (5.5) | |||||
| Education | |||||||||
| ≥12 y | 609 (62) | 403 (73) | 127 (72) | 79 (32) | |||||
| <12 y | 367 (38) | 147 (27) | 50 (28) | 170 (68) | |||||
| ppBMI, kg/m2 | 22.7 (4.5) | 21.5 (3.3) | 24.2 (4.6) | 24.4 (5.6) | |||||
| Height, cm | 26–28 weeks' gestation | 158.2 (5.6) | 158.9 (5.5) | 157.6 (5.4) | 156.9 (5.5) | ||||
| Gestational weight gain, kg | 26–28 weeks' gestation | 8.7 (4.7) | 8.6 (3.8) | 7.9 (4.7) | 9.3 (6.1) | ||||
| Triceps skinfold, mm | 26–28 weeks' gestation | 22.4 (5.9) | 21.3 (5.5) | 23.5 (6.2) | 23.8 (6.2) | ||||
| Biceps skinfold, mm | 26–28 weeks' gestation | 11.9 (5.3) | 10.9 (4.7) | 13.0 (5.4) | 13.4 (5.8) | ||||
| Subscapular skinfold, mm | 26–28 weeks' gestation | 21.9 (6.3) | 21.0 (5.8) | 22.7 (6.5) | 23.3 (6.8) | ||||
| Suprailiac skinfold, mm | 26–28 weeks' gestation | 23.7 (5.6) | 23.4 (5.4) | 24.1 (5.6) | 24.3 (6.0) | ||||
Figure 1.
A–D, Associations of maternal prepregnancy BMI and gestational weight gain with child weight (A, B) and length (C, D) from birth to 24 mo. Point estimates and 95% CIs for associations of log-transformed child anthropometric outcome (child weight, length) with maternal prepregnancy BMI and gestational weight gain. Regression coefficients and CIs are reported as percentage change in child anthropometric outcome for one SD increase in maternal prepregnancy BMI or gestational weight gain. Each of the two rows gives results from two different linear regression models where both maternal prepregnancy BMI and gestational weight gain were included as predictors. Both models adjusted for temporal effects, child sex, ethnicity, gestational age, parity, maternal height, education, and age. Time was coded using a binary variable for each distinct time point and interaction terms of time with each variable in the model were included. Results are also reported in Supplemental Tables 1 and 2.
GWG predicted offspring length at birth and up to 2 years, but not ppBMI
GWG predicted birth length after adjustment for covariates and ppBMI (P < .001; Figure 1D; Supplemental Table 2). GWG also predicted offspring length at 6, 18, and 24 months. At 24 months, child length increased by 0.41% (95% CI, 0.15–0.68%; P = .002; Supplemental Table 2) for every SD increase in GWG. In contrast, ppBMI did not predict offspring length at any time point after adjustment for covariates and GWG (Figure 1C; Supplemental Table 1).
ppBMI predicted offspring adiposity (skinfolds) at 2 years of age, but not GWG
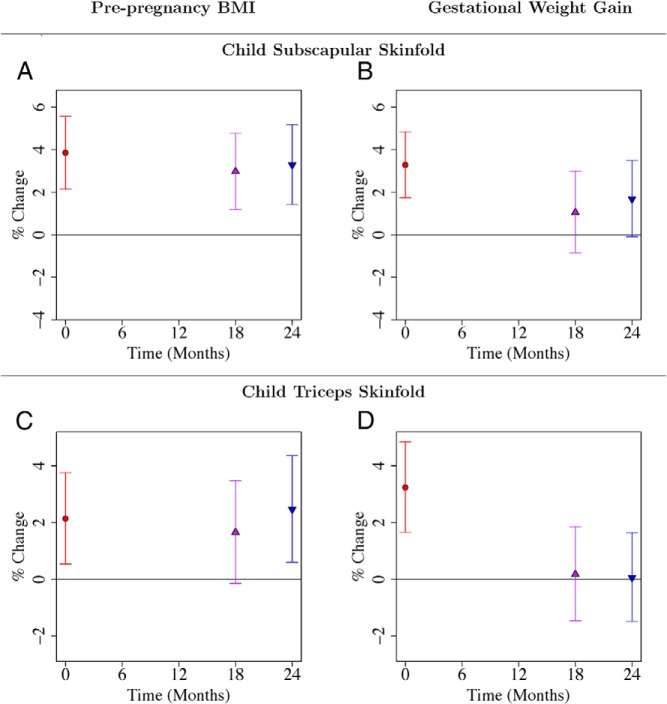
ppBMI predicted offspring adiposity at birth as measured by subscapular and triceps skinfold thickness, after adjustment for covariates and GWG (Figure 2, A and C; Supplemental Table 1); as did GWG, after adjustment for covariates and ppBMI (Figure 2, B and D; Supplemental Table 2). However, only ppBMI independently predicted infant subscapular and triceps skinfold thickness at 18 and/or 24 months of age. At 24 months, a one-SD increase in ppBMI was associated with 3.28% (95% CI, 1.43–5.17%; P < .001) and 2.46% (95% CI, 0.61–4.36%; P = .009) increases in subscapular and triceps skinfold thickness, respectively. The observed effect sizes of ppBMI on offspring triceps skinfolds were smaller compared with offspring subscapular skinfolds. This may be due to greater measurement error for triceps skinfold measurements, which are harder to measure.
Figure 2.
A–D, Associations of maternal prepregnancy BMI and gestational weight gain with child subscapular (A, B) and triceps (C, D) skinfolds from birth to 24 months. Point estimates and 95% CIs for associations of log-transformed child anthropometric outcome (child subscapular and triceps skinfolds) with maternal prepregnancy BMI and gestational weight gain. Regression coefficients and confidence intervals are reported as percentage change in child anthropometric outcome for one SD increase in maternal prepregnancy BMI or gestational weight gain. Each of the two rows gives results from two different linear regression models where both maternal prepregnancy BMI and gestational weight gain were included as predictors. Both models adjusted for temporal effects, child sex, ethnicity, gestational age, parity, maternal height, education, and age. Time was coded using a binary variable for each distinct time point and interaction terms of time with each variable in the model were included. Results are also reported in Supplemental Tables 1 and 2.
Associations of GWG with offspring anthropometry did not depend on ppBMI
To examine whether the association of GWG with offspring anthropometric outcomes depended on ppBMI, we tested for an interaction between ppBMI and GWG. The associations of GWG with all four offspring outcomes from birth to 2 years did not depend on ppBMI (P = .20 to 0.97 for interaction; Supplemental Table 3).
Effects of GWG on offspring weight and length were ethnic dependent, but not ppBMI
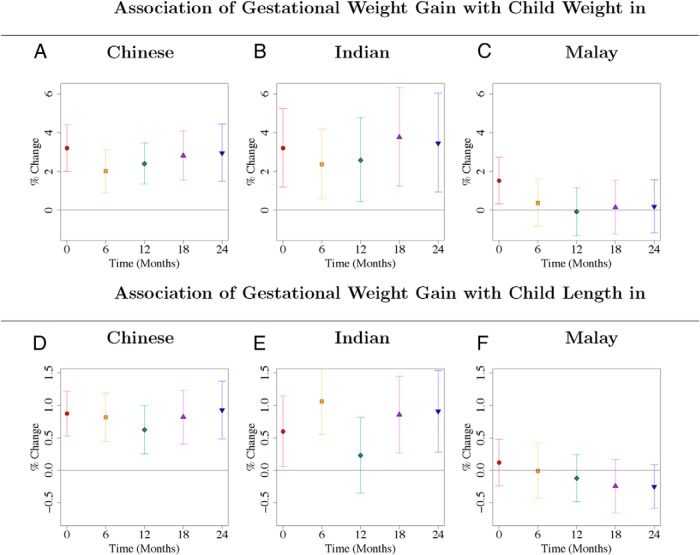
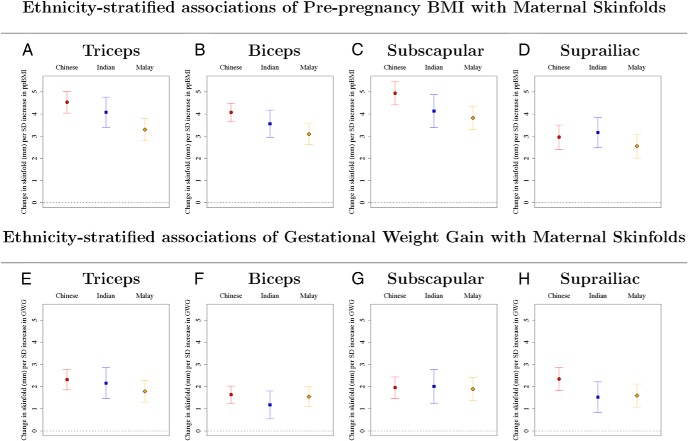
The effects of GWG on child weight and length in the Malay ethnic group were different when compared with the Chinese and Indians (P < .001 to .058 for interaction; Supplemental Tables 4 and 5). The effects for the Chinese and Indians were similar (P = .34 to .99 for interaction; Supplemental Tables 4 and 5). GWG was consistently associated with child weight (Figure 3, A and B; Supplemental Table 8) and length (Figure 3, D and E; Supplemental Table 9) from birth and to 2 years of age in the Chinese and Indian ethnic groups. In contrast, for the Malay children, GWG was only associated with neonatal weight but not subsequent infant weight (Figure 3C, Supplemental Table 8), and GWG was not associated with child length at any time point (Figure 3F, Supplemental Table 9). The associations of ppBMI (P < .005 for interaction for triceps, biceps, and subscapular) and GWG (P = .055 for suprailiac and P = .085 for triceps) with maternal skinfolds at 26–28 weeks' gestation were different for Malay women (Figure 4; Supplemental Table 10). Malay women with higher ppBMI and GWG do not tend to gain more peripheral fat (reflected by triceps skinfold), compared with Chinese and Indian women (Figure 4, A and E; Supplemental Table 10). The effects of ppBMI on child weight and length were not ethnicity dependent (P = .18 to .95 for interaction; Supplemental Tables 4 and 5). The effects of both ppBMI and GWG on child adiposity (skinfolds) were not ethnicity dependent (P = .11 to 1 for interaction; Supplemental Tables 6 and 7). In addition, we did not find consistent evidence of sex-specific effects (Supplemental Tables 11–14). Sensitivity analysis, adjusted additionally for gestational diabetes, occupational activity, alcohol, smoking, vitamin B6, B12, D, and folate levels during pregnancy (Supplemental Tables 15–18), gave similar results as those in Figures 1–3 and Supplemental Tables 1, 2, 8, and 9.
Figure 3.
A–F, Ethnicity-stratified associations of gestational weight gain with child weight (A–C) and length (D–F) from birth to 24 months. Gestational weight gain was associated with child weight and length after birth only among Chinese and Indian children. Point estimates and 95% CIs for each ethnic group, for associations of log-transformed child anthropometric outcome (child weight, length) with gestational weight gain. Regression coefficients and CIs are reported as percentage change in child anthropometric outcome for one SD increase in gestational weight gain. Each of the six panels gives results from six different linear regression models, for each of the three ethnic groups and two child outcomes, and gestational weight gain as predictor variable. All models adjusted for temporal effects, child sex, gestational age, parity, maternal height, education, age, and ppBMI. Time was coded using a binary variable for each distinct time point and interaction terms of time with each variable in the model were included. Results are also reported in Supplemental Tables 8 and 9. Results without stratifying by ethnicity are given in Figures 1, B and D.
Figure 4.
A–H, Ethnicity-stratified associations of ppBMI (A–D) and gestational weight gain (E–H) with maternal skinfolds. Point estimates and 95% CIs for each ethnic group, for associations of maternal skinfolds (triceps, biceps, subscapular, and suprailiac), with ppBMI and gestational weight gain. Regression coefficients and CIs are reported as change in maternal skinfold (mm) for one SD increase in prepregnancy BMI or gestational weight gain. Each column gives results from three different linear regression models for each of the three ethnic groups in which both maternal prepregnancy BMI and gestational weight gain are included as predictors. All models adjusted for parity, maternal height, education and age.
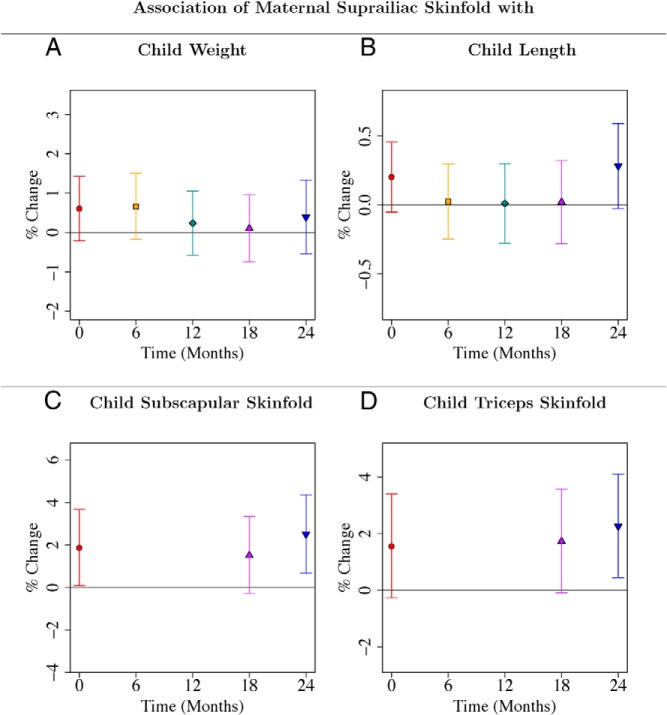
Maternal suprailiac skinfold independently predicted offspring adiposity (skinfolds) at 24 months
After adjusting for ppBMI, GWG, and other covariates, midpregnancy maternal suprailiac skinfold thickness, but not triceps, biceps, or subscapular skinfolds, predicted child subscapular skinfold (P = .007) and triceps skinfold (P = .014) at 24 months (Figures 5, C and D; Supplemental Tables 24 and 26). Offspring subscapular and triceps skinfolds at 24 months increased by 2.51% (95% CI, 0.68–4.36%) and 2.26% (95% CI, 0.44–4.10%) respectively, for every SD increase in maternal suprailiac skinfold thickness (Figure 5, C and D; Supplemental Tables 24 and 26). Maternal suprailiac skinfold was associated with offspring measures more consistently after adjustment for ppBMI and GWG, than midpregnancy triceps, biceps, or subscapular skinfolds. All four maternal skinfolds (triceps, biceps, subscapular, and suprailiac) were generally associated with offspring weight, subscapular, and triceps skinfolds at birth and 24 months, without adjusting for ppBMI and GWG (Supplemental Figures 3–6; Supplemental Tables 19, 23, 25). However, maternal suprailiac skinfold is the only maternal skinfold predictive at 24 months after the adjustment (Supplemental Figures 3–6, Supplemental Tables 20, 24, 26). Associations of maternal suprailiac skinfold thickness with offspring measures (after adjustment for ppBMI and GWG) were not ethnicity dependent (data not shown).
Figure 5.
A–D, Associations of maternal suprailiac skinfold with child weight (A), length (B), subscapular (C), and triceps (D) skinfolds from birth to 24 months. Point estimates and 95% CIs for associations of log-transformed child anthropometric outcome with maternal suprailiac skinfold. Regression coefficients and CIs are reported as percentage change in child anthropometric outcome for one SD increase in maternal suprailiac skinfold. Each of the four panels gives results from four different linear regression models with maternal suprailiac skinfold as predictor. All four models adjusted for temporal effects, child sex, ethnicity, gestational age, parity, maternal height, education, age, ppBMI, and gestational weight gain. Time was coded using a binary variable for each distinct time point and interaction terms of time with each variable in the model were included. Results are also reported in Supplemental Tables 20, 22, 24, and 26.
Discussion
In this study, we characterized the joint effects of maternal prepregnancy adiposity and midpregnancy GWG on offspring size (weight and length) and adiposity in the first 2 years of life in a multiethnic Asian cohort. We demonstrated that maternal adiposity not only affected neonatal size, but also subsequent infant size in the first 2 years of life. ppBMI and GWG also captured different facets of maternal adiposity temporally and were independently associated with offspring anthropometric and adiposity measures. ppBMI was observed to be associated with offspring's sc adiposity (measured by subscapular and triceps skinfold thickness) at 2 years regardless of GWG during pregnancy. GWG was associated with child length at 2 years regardless of ppBMI, for Chinese and Indians. ppBMI represents the mother's nutrition status prior to conception, and also the heritability of adiposity (“nature”), which may influence that of her offspring. GWG represents maternal pregnancy nutritional status, which constitutes the in utero environment (“nurturing”). The genetic basis of GWG is debatable, with contrasting reports of whether such genetic influences exist (16, 17). Our report has provided evidence that maternal preconception nutrition, and possibly genetic influences, as well as maternal nutrition during gestation and in utero fetal nutrition, have independent effects on size at birth and up to 2 years of age.
We also showed that midpregnancy suprailiac skinfold, but not triceps, biceps, or subscapular skinfolds was independently predictive of offspring sc adiposity at 2 years of age after adjusting for ppBMI and GWG, and we postulate that truncal sc fat may be more heritable than the other fat layers in the body or reflect more accurately the degree of overnutrition in pregnant mothers compared with sc fat at the other skinfold sites. This association achieved statistical significance only at 2 years of age but not earlier time points, and deserves further validation in larger cohorts.
Studies have documented positive relationships between ppBMI and GWG on offspring size and adiposity conducted primarily among Caucasian subjects (18–20). There are studies however, that reported null effects of ppBMI and GWG on offspring size and adiposity (21, 22). Our findings on ppBMI are consistent with the positive associations with offspring size and adiposity in these reports. Although we observed an association of midpregnancy GWG with adiposity measured by skinfolds at birth, the association of midpregnancy GWG with adiposity at 2 years was suggestive but not statistically significant. It is also debatable whether the observed greater offspring size with greater GWG is due to increased fetal growth, in utero developmental plasticity, genetic tendency to gain weight inherited from the mothers, continued exposure postntatally to the same environment and eating habits of the mothers, or interaction of two or more of these factors (23). A study by Branum et al (24) reported no associations between GWG and offspring BMI at 4 years using 2758 family groups from the Collaborative Perinatal Project. The study was conducted within families to account for shared factors such as genetics or family diet/activity patterns, hence suggesting that the positive relation between GWG and child BMI in previous studies may be explained by shared familial characteristics.
The absence of an interaction between ppBMI and GWG on offspring size and adiposity suggests that there may not be a need to stratify GWG risk on offspring size and adiposity by different ppBMI categories. Although some studies have reported evidence of statistical interaction between ppBMI and GWG in their effects on offspring size and adiposity (25), other studies did not find evidence for interaction (26, 27). In our study however, we have considered the role of maternal adiposity (before and during pregnancy) only on offspring size and adiposity. The health implications of maternal obesity are much more extensive, given that other studies have documented associations of excessive ppBMI and GWG with increased risks of adverse pregnancy outcomes such as gestational diabetes (28, 29), hypertension and preeclampsia (30), caesarian delivery (31, 32), stillbirths (33), and maternal postpartum weight retention (34). Thus, it is possible that the GWG risk on other adverse pregnancy outcomes may depend on ppBMI. In addition, it is possible that we did not have the statistical power to detect this interaction.
Interestingly in our cohort, the effects of GWG on offspring weight and length were dependent on ethnicity. GWG did not significantly predict weight or length after birth among Malay children. In contrast, GWG predicted offspring weight and length up to 2 years of age for both Chinese and Indians. The absence of an association between GWG and offspring weight and length in Malay children was unlikely to be caused by a lack of statistical power, given that the Indian ethnic group had a smaller sample size compared with the Malay ethnic group but yet demonstrated the association. We found that Malay women had significantly different body fat composition as pregnancy progressed, whereas Malay pregnant women with higher ppBMI and GWG seemed to put on less “peripheral fat” than Chinese and Indian women. Whether this variation in maternal composition during pregnancy leads to the differing associations of GWG with offspring weight and length for the Malay ethnic group is unclear but deserves additional exploration. We did not have maternal skinfolds measured before pregnancy to fully explore this hypothesis, but the ethnicity-dependent associations of ppBMI and GWG on maternal skinfolds at 26–28 weeks' gestation is consistent with this hypothesis. The ethnicity-dependent associations of GWG with offspring weight and length underscores the importance of studying GWG in different ethnic groups to assess applicability of associations reported in other populations.
Our study has several strengths, which includes its prospective study design with a relatively high follow-up rate. In addition, the longitudinal measures allowed us to study not only neonatal but also subsequent infant size and adiposity in the first two years of life. Another strength is the inclusion of three major Asian ethnic groups in our study. These three ethnic groups compose more than 40 percent of the world's population (35). This study, however, is not without limitations. First, we have only considered midpregnancy GWG, which was calculated using only weight gain up to 26–28 weeks' gestation. If GWG increased linearly with time throughout pregnancy for each individual, then midpregnancy GWG would be (strongly) correlated with total GWG for the pregnancy, and midpregnancy GWG can be a good surrogate for total GWG. It is possible that we have not captured the totality of the effects of GWG. However, recent data have demonstrated that high rates of GWG in early and midpregnancy have stronger effects on offspring body weight and metabolic outcomes compared with GWG during late pregnancy (36–38), suggesting that much of the effect of GWG on offspring size and adiposity would be reflected during early- and midpregnancy but not during late pregnancy. Thus, looking at midpregnancy GWG can be a good surrogate for observing the total effects of GWG on child size and adiposity. Second, we have included only women with term deliveries of at least 37 weeks. GA has often been used as a surrogate for the quality of the in utero environment and maternal adiposity can affect GA. Thus, the effects of maternal adiposity on child size and adiposity might be more pronounced than reported here. Third, our findings were based on an Asian cohort, which might not be applicable or generalizable to other populations. Fourth, self-reported prepregnancy weight was used to calculate ppBMI and GWG. However, our self-reported prepregnancy weight and weight measured during first trimester were highly correlated (r = 0.97; calculated using 85% of individuals); thus, major conclusions were unlikely to have been affected by bias in self-reported weight. Lastly, confounding is a concern in any observational study. To minimize the affect of residual confounding on our findings, we have adjusted for an extensive list of covariates that were chosen a priori based on literature evidence.
In summary, ppBMI and GWG are modifiable independent factors for child size and adiposity in early childhood. The associations are ethnicity dependent, underscoring the importance of examining these risk factors in specific ethnic groups. Our findings also emphasize the importance for mothers to optimize both her weight before and during pregnancy to achieve optimal size and adiposity outcomes in the first 2 years of their children's lives.
Acknowledgments
We thank the contributions of the reminder of the GUSTO study group, which includes Pratibha Agarwal, Arijit Biswas, Choon Looi Bong, Birit FP Broekman, Shirong Cai, Yiong Huak Chan, Cornelia Yin Ing Chee, Helen Y. H. Chen, Yin Bun Cheung, Audrey Chia, Amutha Chinnadurai, Chai Kiat Chng, Mary Foong-Fong Chong, Shang Chee Chong, Mei Chien Chua, Chun Ming Ding, Eric Andrew Finkelstein, Doris Fok, Marielle Fortier, Anne Eng Neo Goh, Yam Thiam Daniel Goh, Joshua J. Gooley, Wee Meng Han, Mark Hanson, Christiani Jeyakumar Henry, Chin-Ying Hsu, Hazel Inskip, Jeevesh Kapur, Ivy Yee-Man Lau, Bee Wah Lee, Ngee Lek, Sok Bee Lim, Yen-Ling Low, Iliana Magiati, Lourdes Mary Daniel, Michael Meaney, Cheryl Ngo, Krishnamoorthy Naiduvaje, Wei Wei Pang, Anqi Qiu, Boon Long Quah, Victor Samuel Rajadurai, Mary Rauff, Salome A. Rebello, Jenny L. Richmond, Anne Rifkin-Graboi, Seang-Mei Saw, Lynette Pei-Chi Shek, Allan Sheppard, Borys Shuter, Leher Singh, Walter Stunkel, Lin Lin Su, Kok Hian Tan, Oon Hoe Teoh, Hugo P. S. van Bever, Rob M. van Dam, Inez Bik Yun Wong, and P. C. Wong.
Author Contributions: X.L., Y.S.C., J.D.H., and Y.S.L. conceived and designed the study. M.T.T., S.E.S., K.M.G., G.S.-H.Y., K.K., J.K.Y.C., P.D.G., Y.S.C., and F.Y. were responsible for the conception and recruitment of the GUSTO cohort. X.L. analyzed the data. X.L., I.M.A., J.D.H., and Y.S.L. interpreted the results and wrote the article. X.L., I.M.A., M.T.T., S.E.S., K.M.G., G.S.-H.Y., K.K., J.K.-Y.C., P.D.G., Y.S.C., F.Y., J.D.H., and Y.S.L. critically revised the report for intellectual and scientific content and approved the final article.
Disclosure Summary: K.M.G., P.D.G., and Y.S.C. have received reimbursement for speaking at conferences sponsored by companies selling nutritional products. They are part of an academic consortium that has received research funding from Abbott Nutrition, Nestec and Danone. The other authors declare no competing interests. X.L., I.M.A., M.T.T., S.E.S., G.S.-H.Y., K.K., J.K.-Y.C., F.Y., J.D.H., and Y.S.L. have nothing to disclose.
This study was registered in ClinicalTrials.gov as trial number NCT01174875.
This work was supported by the Translational Clinical Research (TCR) Flagship Program on Developmental Pathways to Metabolic Disease funded by the National Research Foundation and administered by the National Medical Research Council (NMRC), Singapore - NMRC/TCR/004-NUS/2008. Additional funding is provided by the Singapore Institute for Clinical Sciences, Agency for Science, Technology and Research, Singapore. K.M.G. was supported by the National Institute for Health Research (NIHR) through the NIHR Southampton Biomedical Research Centre and by the European Union's Seventh Framework Programme (FP7/2007-2013), project EarlyNutrition under Grant No. 289346.
Footnotes
- BMI
- body mass index
- CI
- confidence interval
- GA
- gestational age
- GUSTO
- Growing Up in Singapore Towards Healthy Outcomes
- GWG
- gestational weight gain
- KKH
- KK Women's and Children's Hospital
- NUH
- National University Hospital
- ppBMI
- pre-pregnancy body mass index.
References
- 1. Lobstein T, Baur L, Uauy R. Obesity in children and young people: A crisis in public health. Obes Rev. 2004;5(Suppl 1):4–85. [DOI] [PubMed] [Google Scholar]
- 2. Pwint MK, Lee YS, Wong TY, Saw SM. Prevalence of overweight and obesity in Chinese preschoolers in Singapore. Ann Acad Med Singapore. 2013;42:66–72 . [PubMed] [Google Scholar]
- 3. Gluckman PD, Hanson M, Zimmet P, Forrester T. Losing the war against obesity: The need for a developmental perspective. Sci Transl Med. 2011;3:93cm19. [DOI] [PubMed] [Google Scholar]
- 4. Lawlor DA, Relton C, Sattar N, Nelson SM. Maternal adiposity—A determinant of perinatal and offspring outcomes? Nat Rev Endocrinol. 2012;8:679–688. [DOI] [PubMed] [Google Scholar]
- 5. Clark PM, Atton C, Law CM, Shiell A, Godfrey K, Barker DJ. Weight gain in pregnancy, triceps skinfold thickness, and blood pressure in offspring. Obstet Gynecol. 1998;91:103–107. [DOI] [PubMed] [Google Scholar]
- 6. Viegas OA, Cole TJ, Wharton BA. Impaired fat deposition in pregnancy: An indicator for nutritional intervention. Am J Clin Nutr. 1987;45:23–28. [DOI] [PubMed] [Google Scholar]
- 7. Villar J, Cogswell M, Kestler E, Castillo P, Menendez R, Repke JT. Effect of fat and fat-free mass deposition during pregnancy on birth weight. Am J Obstet Gynecol. 1992;167:1344–1352. [DOI] [PubMed] [Google Scholar]
- 8. Viswanathan M, Siega-Riz AM, Moos MK, et al. Outcomes of maternal weight gain. Evid Rep Technol Assess. 2008;1–223. [PMC free article] [PubMed] [Google Scholar]
- 9. Lau EY, Liu J, Archer E, McDonald SM, Liu J. Maternal weight gain in pregnancy and risk of obesity among offspring: A systematic review. J Obes. 2014;2014:524939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kurpad AV, Varadharajan KS, Aeberli I. The thin-fat phenotype and global metabolic disease risk. Curr Opin Clin Nutr Metab Care. 2011;14:542–547. [DOI] [PubMed] [Google Scholar]
- 11. Gao H, Salim A, Lee J, Tai ES, van Dam RM. Can body fat distribution, adiponectin levels and inflammation explain differences in insulin resistance between ethnic Chinese, Malays and Asian Indians? Int J Obes (Lond). 2012;36:1086–1093. [DOI] [PubMed] [Google Scholar]
- 12. Ministry of Health Singapore. National Health Survey, 2010. [Google Scholar]
- 13. Soh SE, Tint MT, Gluckman PD, et al. Cohort profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int J Epidemiol. 2014;43:1401–1409. [DOI] [PubMed] [Google Scholar]
- 14. Soh SE, Chong YS, Kwek K, et al. Insights from the Growing Up in Singapore Towards Healthy Outcomes (GUSTO) cohort study. Ann Nutr Metab. 2014;64:218–225. [DOI] [PubMed] [Google Scholar]
- 15. Halekoh U, Højsgaard S, Yan J. The R package geepack for generalized estimating equations. J Stat Softw. 2006;15:1–11. [Google Scholar]
- 16. Lawlor DA, Fraser A, Macdonald-Wallis C, et al. Maternal and offspring adiposity-related genetic variants and gestational weight gain. Am J Clin Nutr. 2011;94:149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stuebe AM, Lyon H, Herring AH, et al. Obesity and diabetes genetic variants associated with gestational weight gain. Am J Obstet Gynecol. 2010;203:283.e1–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196:322.e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hinkle SN, Sharma AJ, Swan DW, Schieve LA, Ramakrishnan U, Stein AD. Excess gestational weight gain is associated with child adiposity among mothers with normal and overweight prepregnancy weight status. J Nutr. 2012;142:1851–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patro B, Liber A, Zalewski B, Poston L, Szajewska H, Koletzko B. Maternal and paternal body mass index and offspring obesity: A systematic review. Ann Nutr Metab. 2013;63:32–41. [DOI] [PubMed] [Google Scholar]
- 21. Ehrenthal DB, Maiden K, Rao A, et al. Independent relation of maternal prenatal factors to early childhood obesity in the offspring. Obstet Gynecol. 2013;121:115–121. [DOI] [PubMed] [Google Scholar]
- 22. Lawlor DA, Timpson NJ, Harbord RM, et al. Exploring the developmental overnutrition hypothesis using parental-offspring associations and FTO as an instrumental variable. PLoS Med. 2008;5:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deierlein AL, Siega-Riz AM, Herring AH, Adair LS, Daniels JL. Gestational weight gain and predicted changes in offspring anthropometrics between early infancy and 3 years. Pediatr Obes. 2012;7:134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Branum AM, Parker JD, Keim SA, Schempf AH. Prepregnancy body mass index and gestational weight gain in relation to child body mass index among siblings. Am J Epidemiol. 2011;174:1159–1165. [DOI] [PubMed] [Google Scholar]
- 25. Weight gain during pregnancy: Re-examining the guidelines. Institute of Medicine. Washington, DC: The National Academic Press, 2009. [Google Scholar]
- 26. Ensenauer R, Chmitorz A, Riedel C, et al. Effects of suboptimal or excessive gestational weight gain on childhood overweight and abdominal adiposity: Results from a retrospective cohort study. Int J Obes (Lond). 2013;37:505–512. [DOI] [PubMed] [Google Scholar]
- 27. Starling AP, Brinton JT, Glueck DH, et al. Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start study. Am J Clin Nutr. 2015;101:302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hedderson MM, Williams MA, Holt VL, Weiss NS, Ferrara A. Body mass index and weight gain prior to pregnancy and risk of gestational diabetes mellitus. Am J Obstet Gynecol. 2008;198:409.e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus. Obstet Gynecol. 2010;115:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saftlas A, Wang W, Risch H, Woolson R, Hsu C, Bracken M. Prepregnancy body mass index and gestational weight gain as risk factors for preeclampsia and transient hypertension. Ann Epidemiol. 2000;10:475. [DOI] [PubMed] [Google Scholar]
- 31. Barau G, Robillard PY, Hulsey TC, et al. Linear association between maternal pre-pregnancy body mass index and risk of caesarean section in term deliveries. BJOG. 2006;113:1173–1177. [DOI] [PubMed] [Google Scholar]
- 32. Sherrard A, Platt RW, Vallerand D, Usher RH, Zhang X, Kramer MS. Maternal anthropometric risk factors for caesarean delivery before or after onset of labour. BJOG. 2007;114:1088–1096. [DOI] [PubMed] [Google Scholar]
- 33. Yao R, Ananth CV, Park BY, Pereira L, Plante LA. Obesity and the risk of stillbirth: A population-based cohort study. Am J Obstet Gynecol. 2014;210:457.e1–e9. [DOI] [PubMed] [Google Scholar]
- 34. Haugen M, Brantsæter AL, Winkvist A, et al. Associations of pre-pregnancy body mass index and gestational weight gain with pregnancy outcome and postpartum weight retention: A prospective observational cohort study. BMC Pregnancy Childbirth. 2014;14:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. United Nations Department of Economic and Social Affairs PD. World population prospects: The 2012 revision, Highlights and Advance Tables. 2012. [Google Scholar]
- 36. Andersen CS, Gamborg M, Sørensen TI, Nohr EA. Weight gain in different periods of pregnancy and offspring's body mass index at 7 years of age. Int J Pediatr Obes. 2011;6:e179–e186. [DOI] [PubMed] [Google Scholar]
- 37. Gaillard R, Steegers EA, Franco OH, Hofman A, Jaddoe VW. Maternal weight gain in different periods of pregnancy and childhood cardio-metabolic outcomes. The Generation R Study. Int J Obes (Lond). 2015;39(4):677–685. [DOI] [PubMed] [Google Scholar]
- 38. Ay L, Kruithof CJ, Bakker R, et al. Maternal anthropometrics are associated with fetal size in different periods of pregnancy and at birth. The Generation R Study. BJOG. 2009;116:953–963. [DOI] [PubMed] [Google Scholar]