Abstract
N-MYC DOWNREGULATED-LIKE proteins (NDL), members of the alpha/beta hydrolase superfamily were recently rediscovered as interactors of G-protein signaling in Arabidopsis thaliana. Although the precise molecular function of NDL proteins is still elusive, in animals these proteins play protective role in hypoxia and expression is induced by hypoxia and nickel, indicating role in stress. Homology of NDL1 with animal counterpart N-MYC DOWNREGULATED GENE (NDRG) suggests similar functions in animals and plants. It is well established that stress responses leads to the microtubule depolymerization and reorganization which is crucial for stress tolerance. NDRG is a microtubule-associated protein which mediates the microtubule organization in animals by causing acetylation and increases the stability of α-tubulin. As NDL1 is highly homologous to NDRG, involvement of NDL1 in the microtubule organization during plant stress can also be expected. Discovery of interaction of NDL with protein kinesin light chain- related 1, enodomembrane family protein 70, syntaxin-23, tubulin alpha-2 chain, as a part of G protein interactome initiative encourages us to postulate microtubule stabilizing functions for NDL family in plants. Our search for NDL interactors in G protein interactome also predicts the role of NDL proteins in abiotic stress tolerance management. Based on published report in animals and predicted interacting partners for NDL in G protein interactome lead us to hypothesize involvement of NDL in the microtubule organization during abiotic stress management in plants.
Keywords: N-MYC DOWNREGULATED GENE, N-MYC DOWNREGULATED-LIKE, phospholipase D, phosphatidic acid, microtubule assembly, microtubule-associated protein, abiotic stress
Introduction
An average estimated yield loss by abiotic stress is more than 50% across the world, caused mainly by salinity, drought and temperatures (Boyer, 1982). Matter of concern is that global population is likely to reach 10 billion by 2050 (almost doubled) (Tilman et al., 2002). So the generation of stress tolerant plants is the need of the hour (Smedema et al., 2000). Salinity is the most destructive and complex stress, affects more than 45 million hectares of irrigated land worldwide, in INDIA about 8.6 million hectare area is affected by salinity (Pathak, 2000).
Right from the beginning of seed germination till crop yield, salt stress affects plant adversely via ionic imbalance leading to toxicity, nutritional disorder, hampering metabolic processes, osmotic stress leading to membrane disorganization, reduction of cell divisionand expansion, and oxidative stress (Hasegawa et al., 2000; Duan et al., 2015; Khare et al., 2015).
Although, the role of lipids in salt stress is not well understood, it has been indicated that expression of several phospholipase-D (PLD) genes is induced by salt stress (Katagiri et al., 2001; Hong et al., 2010). Hydrolysis product of PLD, phosphatidic acid (PA) is shown to bind and activate mitogen-activated protein kinase 6 (MPK6), which in turn phosphorylates salt overly sensitive 1 (SOS1) transporter in vitro (Figure 1; Yu et al., 2010). The SOS1 gene encodes a plasma membrane Na+/H+ antiporter, playing protective role in saline environment. These findings have indicated a link between lipid signaling, MAPK cascades, and salt stress tolerance in plants (Morris, 2010). Plant responses to salt stress include osmolyte biosynthesis, water flux control, and transport of ions for re-establishment of homeostasis and microtubule depolymerization and reorganization (Wang and Nick, 2001; Lü et al., 2007; Wang et al., 2007, 2010). Although all of the events are equally important for cell survival, microtubule depolymerization and reorganization are believed to be essential for plant survival under abiotic stress.
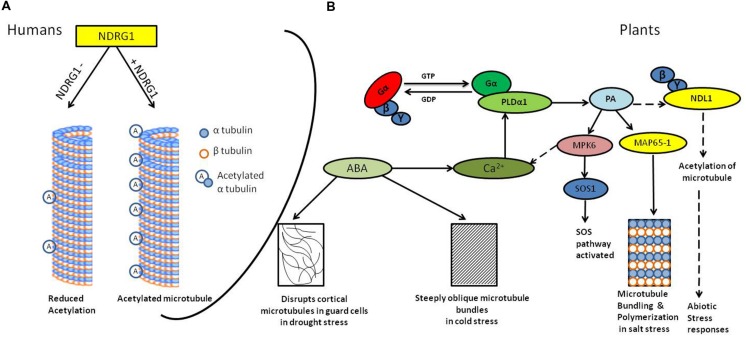
FIGURE 1.
Diagrammatic representation of the signaling in abiotic stress and microtubule responses. (A) N-MYC DOWNREGULATED GENE (NDRG1) knock-down human mammary epithelial cells (hNMECs) shows decrease in tubulin acetylation as compared to cells having wild type levels of NDRG1(Kim et al., 2004). (B) Abiotic stress activates G-protein-coupled receptors (GPCRs; Yadav and Tuteja, 2011), phospholipase-Dα1 (PLDα1) interacts with activated Gα subunit (Zhao and Wang, 2004); PLD hydrolyzes membrane lipids to generate phosphatidic acid (PA). PA binds to MAP65-1, resulting into microtubule bundling and polymerization which helps in salt tolerance (Zhang et al., 2012) it also activates MPK6, which further phosphorylates SOS1 resulting into activation of SOS pathway (Yu et al., 2010). PA may interact with NDL1, interactor of Gβγ dimer and possible downstream regulator of microtubules. ABA production during cold and drought stress results into steeply oblique and disrupted microtubules, respectively, (Wang and Nick, 2001; Pollock and Pickett-Heaps, 2005). Solid lines depicts confirmed interactions, dotted line depicts hypothesized interactions
Ndrg As A Microtubule-Associated Protein (Map)
Microtubule organization is regulated by MAPs (Dixit and Cyr, 2004; Sedbrook, 2004). In animals, several MAPs have been identified and characterized. Detailed analysis of human N-MYC DOWNREGULATED GENE (NDRG) gene family showed that the family comprises of four members (NDRG1-4), each sharing 57–60% amino acid sequence similarity (Qu et al., 2002). Among these, only NDRG1 has been reported to be a MAP which participates in the spindle checkpoint in animals (Kim et al., 2004).
Microtubule dynamics is affected by an array of reversible post-translational modifications including acetylation, phosphorylation, and palmitoylation (Piperno et al., 1987; Westermann and Weber, 2003; Zhang et al., 2003). Acetylated tubulin is one of the major characteristics of stabilized microtubule structure and may contribute to regulating microtubule dynamics (Westermann and Weber, 2003; Parrotta et al., 2014). Mammalian NDRG1 knockdown cell line have decreased accumulation of acetylated -tubulin and disrupted spindle fiber formation (Figure 1; Kim et al., 2004). Moreover, growing body of evidences also show that NDRG1 recruits on recycling endosomes in the Trans Golgi Network by binding to phosphatidylinositol 4-phosphate and interacts with membrane bound Rab4aGTPase (Kachhap et al., 2007). Kachhap et al. (2007) used a prostate cancer cell line to show that NDRG1 is a novel effector for the small GTPase, Rab4a, and is important in recycling E-cadherin in proliferating cells.
Structural Similarities Between Ndrg1 And Ndl1
In plants, NDL proteins were first reported in sunflower (SF21) as stigma and transmitting tissue cell specific proteins (Kräuter-Canham et al., 1997). Thereafter, studies on SF21 proteins identified it as a small gene family with putative role as a signaling molecules in pollen-pistil interaction. Across plant species, SF21 gene has been reported in dicots (Lycopersicon esculentum, Arabidopsis thaliana) monocots (Oryza sativa) (Lazarescu et al., 2006), gymnosperms as well as in the moss, physcomitrella patens (Lazarescu et al., 2010). Arabidopsis NDL gene family has three members NDL1, NDL2, and NDL3. All family members contain NDR domain, an alpha/beta hydrolase fold, a conserved hydrophobic patch of 23 amino acids and a conserved Asp. All these mentioned features strongly suggest that NDL proteins belong to NDR protein family. NDL proteins in A. thaliana are novel effectors of G-protein signaling playing important role in root and shoot development (Mudgil et al., 2009, 2013). G-protein core complex relay signal intracellularly with the help of downstream effectors or secondary messengers.
We previously observed that Mouse NDRG1 interacts with Arabidopsis AGB1/AGG1 and AGB1/AGG2, suggesting that this interaction is evolutionarily conserved (Mudgil et al., 2009). Human NDRG1 is 93% similar to mouse NDRG1 (Mudgil et al., 2009), so we can postulate similar interaction of human NDRG1 with plant’s G protein components. Also, NDL in Arabidopsis and NDRG1 of mouse were shown to interact with the C-terminal domain of regulator of G-protein signaling (RGS1), a candidate seven-transmembrane receptor in AGB1/NDL-mediated signaling via yeast two-hybrid (Mudgil et al., 2009).
N-MYC DOWNREGULATED GENE1 functions as a MAP and acetylates microtubules in human. NDRG1 also act as novel effector for the small GTPase. In plants, protein domains search revealed that all α tubulin family subunits contain GTPase domain as the tubulin C terminal domain so NDL might also interact with α tubulin in plants.
Microtubules Dynamics-Role In Abiotic Stress Tolerance
Microtubules are the polymers of heterodimeric protein αβ-tubulin, which provides shape to cells and maintains tracks for vesicle transport and segregation of chromosome. Microtubule organization is regulated by microtubule-associated proteins (MAPs; Dixit and Cyr, 2004; Sedbrook, 2004). A variety of MAPs have been reported in higher plants. The MAP65 family and some of kinesin family are important in bundling and polymerization of the microtubules (Smertenko et al., 2004; Van Damme et al., 2004; Mao et al., 2005; Hamada, 2007) A. thaliana genome contains nine MAP65-related genes with different functions (Hussey et al., 2002).
Calcium is a well-known second messenger which participates in the stress signaling in plants (Knight, 2000; Xiong et al., 2002; Chinnusamy et al., 2005). Cortical microtubules have been suggested to regulate the calcium levels in the cells by regulating the activity of calcium channels (Thion et al., 1996; Himschoot et al., 2015). Treatment of microtubule-destabilizing drug improved the survival and growth of A. thaliana seedlings under salt stress while treatments with microtubule-stabilizing drug caused salt stress hypersensitivity (Wang et al., 2007). Moreover, reorientation of microtubules was also observed in maize roots and tobacco BY-2 cells upon short term exposure to salt stress (Blancaflor and Hasenstein, 1995; Dhonukshe et al., 2003). In A. thaliana, long term salt stress affected the cortical microtubule organization. spr1 mutant, [SPIRAL1(SPR1), a plant-specific MT-localizing protein] has right-handed helical root growth phenotype, salt stress suppresses this phenotype (Shoji et al., 2006). Directional cell expansion (anisotropic growth) is necessary for plant morphogenesis which is achieved by well-organized interphase, cortical microtubule and SPR1 is thought to control anisotropic cell expansion through MT arrangements (Nakajima et al., 2004, 2006). Mutation in critical amino acids of tubulin gene family (mainly located at longitudinal interface of the α and β tubulins), in lateral contact region and in GTPase-activating region in α tubulin (Ishida et al., 2007) disrupts the proper organization and hence functions of microtubules (Hashimoto, 2013). Tubulin mutations affect cortical microtubule arrays in interphase resulting into altered directional growth. Mutation in TUA genes, α tubulin 6 and α tubulin 4 results into right handed helical array of cortical microtubules producing left handed helical growth phenotype, lefty 1 and lefty 2, semi dominant skewing mutants (Thitamadee et al., 2002). These results indicated that the proper organization of microtubule is one of the critical factors for growth and development.
In addition, abscisic acid (ABA), which is produced in response to salt stress, also affects the organization of cortical microtubules (Sakiyama and Shibaoka, 1990; Shibaoka, 1994). In drought stress accumulation of ABA is one of the most pronounced ways to cope up with water deficit stress. ABA leads to stomata closure thereby decrease the water loss and also enhances water uptake by root (Boudsocq and Laurière, 2005). Dehydration triggers plasmolysis of cells and it consequently destroys microtubule (Pollock and Pickett-Heaps, 2005), ABA also disrupts cortical microtubules in guard cells, but not in epidermal cells (Jiang et al., 1996). During cold stress in wheat (Chinese winter wheat) ABA produced steeply oblique microtubule bundles (Figure 1; Wang and Nick, 2001).
Phospholipase D is involved in the rearrangement of cortical microtubules (Dhonukshe et al., 2003). In A. thaliana pldα1 salt-sensitive mutant cortical microtubule showed massive depolymerization patterns (Bargmann et al., 2009; Yu et al., 2010) compared to wild type control. However, upon salt removal from the growth medium organization was recovered in wild-type plants but not in pldα1 plants indicating involvement of PLDα1 in reorganizing microtubules after depolymerization induced by salt stress (Zhang et al., 2012).
Phosphatidic acid, the end product of PLDα reaction, is a key regulator of microtubule polymerization; exogenous application of PA lead to recovery in salt-disrupted microtubule arrays in pldα1 mutant (Zhang et al., 2012). PA regulates microtubule bundling and polymerization together with MAP65-1 and their interaction is important for salt tolerance. PA could not bind or bundle microtubules and rescue microtubule disruption caused by salt in the map65-1 mutant, suggesting that MAP65-1 is necessary for PA-mediated stabilization of microtubules (Zhang et al., 2012). There are two contradictory reports regarding interaction of tubulin and PA. In the first report, a mass spectrometry based approach was used to identify the PA binding proteins which showed that TUA2 is PA binding protein (Testerink et al., 2004). However, in the second report, it was found that neither PLDα1 nor PA species bound to either α- nor β- tubulins. MAP65-1, a microtubule associated protein, was shown to bind to PA but not to other phospholipids like diacylglycerol, phosphatidylserine, phosphatidylinositol, phosphatidylethanolamine, or Phosphatidylcholines. These results indicate that PA requires other MAP to interact with microtubules (Zhang et al., 2012), further experimentation to confirm involvement/role of other MAPs is awaited.
Our analysis of existing information on NDL1 interactome shows interaction with Annexin 1 (ANNAT1) which has role in drought stress (Konopka-Postupolska et al., 2009), sodium and lithium-tolerant 1 (SLT1) which is involved in salt stress (Matsumoto et al., 2001) whereas lesion stimulating disease 1(LSD1) regulates cell death trigged by cold stress (Huang et al., 2010), O-Acetylserine (THIOL) Lyase (OAS-TL) Isoform A1 (OASA1) shows increased cadmium tolerance (Domínguez-Solís et al., 2001) and Arabidopsis Ribosomal Protein S27 (ARS27A) is involved in genotoxic stress (Revenkova et al., 1999). Also, comparative analysis shows overlap of NDRG1 and NDL1 interactors involved in similar pathways (Table 1).
Table 1.
N-MYC DOWNREGULATED GENE (NDRG1) and N-MYC DOWNREGULATED-LIKE (NDL1) shared interactors which are involved in common pathways/processes.
| NDRG1 a | NDL1 b | Reference | |
|---|---|---|---|
| Cyclin-dependent kinases | Cyclin-dependent kinase 15 | Cyclin-dependent kinase – G1 Cyclin-dependent kinase regulatory subunit 2 | a (Huttlin et al., 2015) |
| b (Klopffleisch et al., 2011) | |||
| Calcium-dependent phospholipid binding proteins | Annexin A5 | Annexin 1 | a (Havugimana et al., 2012) |
| b (Klopffleisch et al., 2011) | |||
| Heat shock protein | HSPA4 HSPA5 HSP90AA1 | BOBBER 1 | a (Tu et al., 2007; Ambrosini et al., 2009) |
| b (Klopffleisch et al., 2011) | |||
| Eukaryotic translation initiation factor | Eukaryotic translation initiation factor 2 | Eukaryotic initiation factor 4A-III | a (Tu et al., 2007; Kristensen et al., 2012) |
| Eukaryotic translation initiation factor 3 | DEAD-box ATP-dependent RNA helicase 2 | b (Klopffleisch et al., 2011) | |
| Eukaryotic translation initiation factor 4H | |||
| DEAD (Asp-Glu-Ala-Asp) box helicase 1 | |||
| DEAD (Asp-Glu-Ala-Asp) box helicase 5 | |||
| DEAD (Asp-Glu-Ala-Asp) box polypeptide 39B | |||
| Protein phosphatases | Protein phosphatase 2, regulatory subunit B, alpha | protein phosphatase 2A subunit A2 | a (Tu et al., 2007) |
| b (Klopffleisch et al., 2011) | |||
| Components of cytoskeleton machinery | ACTG1, Actin, gamma 1 kinesin family member 5B | TUA2, Tubulin alpha-2 chain KINESIN LIGHT CHAIN-RELATED 1 | a (Tu et al., 2007) |
| b (Klopffleisch et al., 2011) | |||
| Glutathione reductases | Glutathione reductase HEL-75 | HOT5, S-nitrosoglutathione reductase | a (Kristensen et al., 2012) |
| b (Klopffleisch et al., 2011) | |||
| Fatty acid pathway | Fatty acid synthase (FASN) | KCS9 (3-KETOACYL-COA SYNTHASE 9); acyltransferase/catalytic/transferase, transferring acyl groups other than amino-acyl groups | a (Tu et al., 2007; Kristensen et al., 2012) |
| Acyl-CoA synthetase long-chain family member 3 (ACSL-3) | b (Klopffleisch et al., 2011) | ||
| Acyl-CoA thioesterase 7 (ACOT7) | Lipoxygenase (LOX2) | ||
| Salinity response | ATPase, Na+/K+ transporting, alpha 1 polypeptide | SLT1 (sodium- and lithium-tolerant 1) | a (Tu et al., 2007) |
| b (Klopffleisch et al., 2011) | |||
Our proposed hypothesis that NDL might be playing role in stress mediated processes by regulating microtubule organization (Figure 1) can be easily tested by checking NDL1 effect on microtubules bundling and polymerization in vitro using purified NDL1 and tubulin proteins. Already available ndl loss of function mutants can be used for checking and comparing status of acetylated tubulin in the absence and presence of NDL. Effects of various stress responses on tubulin pattern in relation to NDL levels can be further studied by analyzing GFP-tagged α tubulin (35S: GFP-TUA2) patterns in NDL up and downregulated backgrounds.
Conflict of Interest Statement
The Guest Associate Editor Girdhar Kumar Pandey declares that, despite being affiliated with the same institute as the authors Nisha Khatri and Yashwanti Mudgil, the review process was handled objectively. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the UGC Major and DU-DST Purse grant to YM.
References
- Ambrosini G., Seelman S. L., Schwartz G. K. (2009). Differentiation-related gene-1 decreases Bim stability by proteasome-mediated degradation. Cancer Res. 69 6115–6121. 10.1158/0008-5472.CAN-08-3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann B. O. R., Laxalt A. M., Riet B., Van Schooten B., Merquiol E., Testerink C., et al. (2009). Multiple PLDs required for high salinity and water deficit tolerance in plants. Plant Cell Physiol. 50 78–89. 10.1093/pcp/pcn173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancaflor E. B., Hasenstein K. H. (1995). Time course and auxin sensitivity of cortical microtubule reorientation in maize roots. Protoplasma 185 72–82. 10.1007/BF01272755 [DOI] [PubMed] [Google Scholar]
- Boudsocq M., Laurière C. (2005). Osmotic signaling in plants. Multiple pathways mediated by emerging kinase families. Plant Physiol. 138 1185–1194. 10.1104/pp.105.061275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S. (1982). Plant productivity and environment. Science 218 443–448. 10.1126/science.218.4571.443 [DOI] [PubMed] [Google Scholar]
- Chinnusamy V., Jagendorf A., Zhu J.-K. (2005). Understanding and improving salt tolerance in plants. Crop Sci. 45:437 10.2135/cropsci2005.0437 [DOI] [Google Scholar]
- Dhonukshe P., Laxalt A. M., Goedhart J., Gadella T. W. J., Munnik T. (2003). Phospholipase d activation correlates with microtubule reorganization in living plant cells. Plant Cell 15 2666–2679. 10.1105/tpc.014977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R., Cyr R. (2004). The cortical microtubule array: from dynamics to organization. Plant Cell 16 2546–2552. 10.1105/tpc.104.161030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-Solís J. R., Gutiérrez-Alcalá G., Romero L. C., Gotor C. (2001). The Cytosolic O-Acetylserine(thiol)lyase gene is regulated by heavy metals and can function in cadmium tolerance. J. Biol. Chem. 276 9297–9302. 10.1074/jbc.M009574200 [DOI] [PubMed] [Google Scholar]
- Duan L., Sebastian J., Dinneny J. R. (2015). “Salt-stress regulation of root system growth and architecture in arabidopsis seedlings,” in Plant Cell Expansion, ed Estevez J. M. (Berlin: Springer; ), 105–122. [DOI] [PubMed] [Google Scholar]
- Hamada T. (2007). Microtubule-associated proteins in higher plants. J. Plant Res. 120 79–98. 10.1007/s10265-006-0057-9 [DOI] [PubMed] [Google Scholar]
- Hasegawa P. M., Bressan R. A., Zhu J.-K., Bohnert H. J. (2000). Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51 463–499. 10.1146/annurev.arplant.51.1.463 [DOI] [PubMed] [Google Scholar]
- Hashimoto T. (2013). Dissecting the cellular functions of plant microtubules using mutant tubulins. Cytoskeleton 70 191–200. 10.1002/cm.21099 [DOI] [PubMed] [Google Scholar]
- Havugimana P. C., Hart G. T., Nepusz T., Yang H., Turinsky A. L., Li Z., et al. (2012). A census of human soluble protein complexes. Cell 150 1068–1081. 10.1016/j.cell.2012.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himschoot E., Beeckman T., Friml J., Vanneste S. (2015). Calcium is an organizer of cell polarity in plants. Biochim. Biophys. Acta Mol. Cell Res. 1853 2168–2172. 10.1016/j.bbamcr.2015.02.017 [DOI] [PubMed] [Google Scholar]
- Hong Y., Zhang W., Wang X. (2010). Phospholipase D and phosphatidic acid signalling in plant response to drought and salinity. Plant Cell Environ. 33 627–635. 10.1111/j.1365-3040.2009.02087.x [DOI] [PubMed] [Google Scholar]
- Huang X., Li Y., Zhang X., Zuo J., Yang S. (2010). The Arabidopsis LSD1 gene plays an important role in the regulation of low temperature-dependent cell death. New Phytol. 187 301–312. 10.1111/j.1469-8137.2010.03275.x [DOI] [PubMed] [Google Scholar]
- Hussey P. J., Hawkins T. J., Igarashi H., Kaloriti D., Smertenko A. (2002). The plant cytoskeleton: recent advances in the study of the plant microtubule-associated proteins MAP-65, MAP-190 and the Xenopus MAP215-like protein, MOR1. Plant Mol. Biol. 50 915–924. 10.1023/A:1021236307508 [DOI] [PubMed] [Google Scholar]
- Huttlin E. L., Ting L., Bruckner R. J., Gebreab F., Gygi M. P., Szpyt J., et al. (2015). The BioPlex network: a systematic exploration of the human interactome. Cell 162 425–440. 10.1016/j.cell.2015.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T., Kaneko Y., Iwano M., Hashimoto T. (2007). Helical microtubule arrays in a collection of twisting tubulin mutants of Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 104 8544–8549. 10.1073/pnas.0701224104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C. J., Nakajima N., Kondo N. (1996). Disruption of microtubules by abscisic acid in guard cells of Vicia faba L. Plant Cell Physiol. 37 697–701. 10.1093/oxfordjournals.pcp.a029001 [DOI] [Google Scholar]
- Kachhap S. K., Faith D., Qian D. Z., Shabbeer S., Galloway N. L., Pili R., et al. (2007). The N-Myc down regulated gene1 (NDRG1) is a Rab4a effector involved in vesicular recycling of E-cadherin. PLoS ONE 2:844 10.1371/journal.pone.0000844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri T., Takahashi S., Shinozaki K. (2001). Involvement of a novel Arabidopsis phospholipase D, AtPLDδ, in dehydration-inducible accumulation of phosphatidic acid in stress signalling. Plant J. 26 595–605. 10.1046/j.1365-313X.2001.01060.x [DOI] [PubMed] [Google Scholar]
- Khare T., Kumar V., Kishor P. B. (2015). Na+ and Cl- ions show additive effects under NaCl stress on induction of oxidative stress and the responsive antioxidative defense in rice. Protoplasma 252 1149–1165. 10.1007/s00709-014-0749-2 [DOI] [PubMed] [Google Scholar]
- Kim K. T., Ongusaha P. P., Hong Y. K., Kurdisiani S. K., Nakamura M., Lu K. P., et al. (2004). Function of Drg1/Rit42 in p53-dependent mitotic spindle checkpoint. J. Biol. Chem. 279 38597–38602. 10.1074/jbc.M400781200 [DOI] [PubMed] [Google Scholar]
- Klopffleisch K., Phan N., Augustin K., Bayne R. S., Booker K. S., Botella J. R., et al. (2011). Arabidopsis G-protein interactome reveals connections to cell wall carbohydrates and morphogenesis. Mol. Syst. Biol. 7:532 10.1038/msb.2011.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H. (2000). Calcium signaling during abiotic stress in plants. Int. Rev. Cytol. 195 269–324. 10.1016/S0074-7696(08)62707-2 [DOI] [PubMed] [Google Scholar]
- Konopka-Postupolska D., Clark G., Goch G., Debski J., Floras K., Cantero A., et al. (2009). The role of annexin 1 in drought stress in Arabidopsis. Plant Physiol. 150 1394–1410. 10.1104/pp.109.135228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräuter-Canham R., Bronner R., Evrard J. L., Hahne G., Friedt W., Steinmetz A. (1997). A transmitting tissue- and pollen-expressed protein from sunflower with sequence similarity to the human RTP protein. Plant Sci. 129 191–202. 10.1016/S0168-9452(97)00182-9 [DOI] [Google Scholar]
- Kristensen A. R., Gsponer J., Foster L. J. (2012). A high-throughput approach for measuring temporal changes in the interactome. Nat. Methods 9 907–909. 10.1038/nmeth.2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarescu E., Friedt W., Horn R., Steinmetz A. (2006). Expression analysis of the sunflower SF21 gene family reveals multiple alternative and organ-specific splicing of transcripts. Gene 374 77–86. 10.1016/j.gene.2006.01.025 [DOI] [PubMed] [Google Scholar]
- Lazarescu E., Friedt W., Steinmetz A. (2010). Organ-specific alternatively spliced transcript isoforms of the sunflower SF21C gene. Plant Cell Rep. 29 673–683. 10.1007/s00299-010-0853-5 [DOI] [PubMed] [Google Scholar]
- Lü B., Gong Z., Wang J., Zhang J., Liang J. (2007). Microtubule dynamics in relation to osmotic stress-induced ABA accumulation in Zea mays roots. J. Exp. Bot. 58 2565–2572. 10.1093/jxb/erm107 [DOI] [PubMed] [Google Scholar]
- Mao T., Jin L., Li H., Liu B., Yuan M. (2005). Two microtubule-associated proteins of the Arabidopsis MAP65 family function differently on microtubules. Plant Physiol. 138 654–662. 10.1104/pp.104.052456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T. K., Pardo J. M., Takeda S., Bressan R. A., Hasegawa P. M. (2001). Tobacco and Arabidiopsis SLT1 mediate salt tolerance of yeast. Plant Mol. Biol. 45 489–500. 10.1023/A:1010659207604 [DOI] [PubMed] [Google Scholar]
- Morris P. C. (2010). Integrating lipid signalling, mitogen-activated protein kinase cascades and salt tolerance. New Phytol. 188 640–643. 10.1111/j.1469-8137.2010.03507.x [DOI] [PubMed] [Google Scholar]
- Mudgil Y., Uhrig J. F., Zhou J., Temple B., Jiang K., Jones A. M. (2009). Arabidopsis N-MYC DOWNREGULATED-LIKE1, a positive regulator of auxin transport in a G protein-mediated pathway. Plant Cell 21 3591–3609. 10.1105/tpc.109.065557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudgil Y., Ghawana S., Jones A. M. (2013). N-MYC down-regulated-like proteins regulate meristem initiation by modulating auxin transport and MAX2 expression. PLoS ONE 8:e77863 10.1371/journal.pone.0077863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K., Furutani I., Tachimoto H., Matsubara H., Hashimoto T. (2004). SPIRAL1 encodes a plant-specific microtubule-localized protein required for directional control of rapidly expanding Arabidopsis cells. Plant Cell 16 1178–1190. 10.1105/tpc.017830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K., Kawamura T., Hashimoto T. (2006). Role of the SPIRAL1 gene family in anisotropic growth of Arabidopsis thaliana. Plant Cell Physiol. 47 513–522. 10.1093/pcp/pcj020 [DOI] [PubMed] [Google Scholar]
- Parrotta L., Cresti M., Cai G. (2014). Accumulation and post-translational modifications of plant tubulins. Plant Biol. 16 521–527. 10.1111/plb.12104 [DOI] [PubMed] [Google Scholar]
- Pathak P. S. (2000). Agro forestry: a tool for arresting land degradation. Indian Farm. 49 15–19. [Google Scholar]
- Piperno G., LeDizet M., Chang X. J. (1987). Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J. Cell Biol. 104 289–302. 10.1083/jcb.104.2.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock F. M., Pickett-Heaps J. D. (2005). Spatial determinants in morphogenesis: recovery from plasmolysis in the diatom Ditylum. Cell Motil. Cytoskeleton 60 71–82. 10.1002/cm.20044 [DOI] [PubMed] [Google Scholar]
- Qu X., Zhai Y., Wei H., Zhang C., Xing G., Yu Y., et al. (2002). Characterization and expression of three novel differentiation-related genes belong to the human NDRG gene family. Mol. Cell. Biochem. 229 35–44. 10.1023/A:1017934810825 [DOI] [PubMed] [Google Scholar]
- Revenkova E., Masson J., Koncz C., Afsar K., Jakovleva L., Paszkowski J. (1999). Involvement of Arabidopsis thaliana ribosomal protein S27 in mRNA degradation triggered by genotoxic stress. EMBO J. 18 490–499. 10.1093/emboj/18.2.490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakiyama M., Shibaoka H. (1990). Effects of abscisic acid on the orientation and cold stability of cortical microtubules in epicotyl cells of the dwarf pea. Protoplasma 157 165–171. 10.1007/BF01322649 [DOI] [Google Scholar]
- Sedbrook J. C. (2004). MAPs in plant cells: delineating microtubule growth dynamics and organization. Curr. Opin. Plant Biol. 7 632–640. 10.1016/j.pbi.2004.09.017 [DOI] [PubMed] [Google Scholar]
- Shibaoka H. (1994). Plant hormone-induced changes in the orientation of cortical microtubules: alterations in the cross-linking between microtubules and the plasma membrane. Annu. Rev. Plant Biol. 45 527–544. 10.1146/annurev.pp.45.060194.002523 [DOI] [Google Scholar]
- Shoji T., Suzuki K., Abe T., Kaneko Y., Shi H., Zhu J. K., et al. (2006). Salt stress affects cortical microtubule organization and helical growth in Arabidopsis. Plant Cell Physiol. 47 1158–1168. 10.1093/pcp/pcj090 [DOI] [PubMed] [Google Scholar]
- Smedema L. K., Abdel-Dayem S., Ochs W. J. (2000). Drainage and agricultural development. Irrig. Drain. Syst. 14 223–235. 10.1023/A:1026570823692 [DOI] [Google Scholar]
- Smertenko A. P., Chang H.-Y., Wagner V., Kaloriti D., Fenyk S., Sonobe S., et al. (2004). The Arabidopsis microtubule-associated protein AtMAP65-1: molecular analysis of its microtubule bundling activity. Plant Cell 16 2035–2047. 10.1105/tpc.104.023937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testerink C., Dekker H. L., Lim Z. Y., Johns M. K., Holmes A. B., De Koster C. G., et al. (2004). Isolation and identification of phosphatidic acid targets from plants. Plant J. 39 527–536. 10.1111/j.1365-313X.2004.02152.x [DOI] [PubMed] [Google Scholar]
- Thion L., Mazars C., Thuleau P., Graziana A., Rossignol M., Moreau M., et al. (1996). Activation of plasma membrane voltage-dependent calcium-permeable channels by disruption of microtubules in carrot cells. FEBS Lett. 393 13–18. 10.1016/0014-5793(96)00844-7 [DOI] [PubMed] [Google Scholar]
- Thitamadee S., Tuchihara K., Hashimoto T. (2002). Microtubule basis for left-handed helical growth in Arabidopsis. Nature 417 193–196. 10.1038/417193a [DOI] [PubMed] [Google Scholar]
- Tilman D., Cassman K. G., Matson P. A., Naylor R., Polasky S. (2002). Agricultural sustainability and intensive production practices. Nature 418 671–677. 10.1038/nature01014 [DOI] [PubMed] [Google Scholar]
- Tu L. C., Yan X., Hood L., Lin B. (2007). Proteomics analysis of the interactome of N-myc downstream regulated gene 1 and its interactions with the androgen response program in prostate cancer cells. Mol. Cell. Proteomics 6 575–588. 10.1074/mcp.M600249-MCP200 [DOI] [PubMed] [Google Scholar]
- Van Damme D., Van Poucke K., Boutant E., Ritzenthaler C., Inzé D., Geelen D. (2004). In vivo dynamics and differential microtubule-binding activities of MAP65 proteins. Plant Physiol. 136 3956–3967. 10.1104/pp.104.051623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Li J., Yuan M. (2007). Salt tolerance requires cortical microtubule reorganization in Arabidopsis. Plant Cell Physiol. 48 1534–1547. 10.1093/pcp/pcm123 [DOI] [PubMed] [Google Scholar]
- Wang C., Zhang L., Yuan M., Ge Y., Liu Y., Fan J., et al. (2010). The microfilament cytoskeleton plays a vital role in salt and osmotic stress tolerance in Arabidopsis. Plant Biol. 12 70–78. 10.1111/j.1438-8677.2009.00201.x [DOI] [PubMed] [Google Scholar]
- Wang Q. Y., Nick P. (2001). Cold acclimation can induce microtubular cold stability in a manner distinct from abscisic acid. Plant Cell Physiol. 42 999–1005. 10.1093/pcp/pce135 [DOI] [PubMed] [Google Scholar]
- Westermann S., Weber K. (2003). Post-translational modifications regulate microtubule function. Nat. Rev. Mol. Cell Biol. 4 938–947. 10.1038/nrm1260 [DOI] [PubMed] [Google Scholar]
- Xiong L., Schumaker K. S., Zhu J. (2002). Cell signaling during cold, drought, and salt stress. Plant Cell 14(Suppl.), S165–S184. 10.1105/tpc.000596.S166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav D. K., Tuteja N. (2011). Rice G-protein coupled receptor (GPCR): in silico analysis and transcription regulation under abiotic stress. Plant Signal. Behav. 6 1079–1086. 10.4161/psb.6.8.15771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Nie J., Cao C., Jin Y., Yan M., Wang F., et al. (2010). Phosphatidic acid mediates salt stress response by regulation of MPK6 in Arabidopsis thaliana. New Phytol. 188 762–773. 10.1111/j.1469-8137.2010.03422.x [DOI] [PubMed] [Google Scholar]
- Zhang Q., Lin F., Mao T., Nie J., Yan M., Yuan M., et al. (2012). Phosphatidic acid regulates microtubule organization by interacting with MAP65-1 in response to salt stress in Arabidopsis. Plant Cell 24 4555–4576. 10.1105/tpc.112.104182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Li N., Caron C., Matthias G., Hess D., Khochbin S., et al. (2003). HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J. 22 1168–1179. 10.1093/emboj/cdg115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Wang X. (2004). Arabidopsis phospholipase Dα1 interacts with the heterotrimeric G-protein α-subunit through a motif analogous to the DRY motif in G-protein-coupled receptors. J. Biol. Chem. 279 1794–1800. 10.1074/jbc.M309529200 [DOI] [PubMed] [Google Scholar]