Abstract
Leptospirosis, a disease of great significance in tropical countries, presents commonly as a biphasic illness with acute febrile episode in the first phase followed by a brief afebrile period and then by the second phase of fever with or without jaundice and renal failure. However, it has varied manifestations and unusual clinical features ascribed to immunological phenomena can occur due to the additional involvement of pulmonary, cardiovascular, and neurological systems. Among the various neurological features, aseptic meningitis is the most common myeloradiculopathy, myelopathy, cerebellar dysfunction, transverse myelitis, Guillain-Barre syndrome, optic neuritis, peripheral neuropathy hare also described. Cranial neuropathy involving facial nerve is a rare, but known neurological manifestation. Sixth nerve palsy in neuroleptospirosis has so far not been reported. We hereby present the occurrence of bilateral abducent nerve palsy in a patient with leptospirosis.
Keywords: Leptospirosis, Abducent nerve palsy, Cranial nerve diseases
Introduction
Leptospirosis is a common worldwide zoonosis of great public health importance. Leptospirosis commonly presents as an acute illness occurring in a biphasic pattern of fever followed by an afebrile period and again fever with organ involvement.1 Icteric and anicteric forms can be seen in both phases. Immunological phenomenon is responsible for the features occurring in the second phase. Neurological involvement (neuroleptospirosis) is rare.2,3 Among the several manifestations of neural involvement, aseptic meningitis is the most common. Myeloradiculopathy, myelopathy, cerebellar dysfunction, transverse myelitis, Guillain-Barre syndrome, optic neuritis, peripheral neuropathyhave also been described.4,5 There are several reports of seventh cranial nerve palsy both unilateral and bilateral.6,7 The hitherto undescribed entity of bilateral abducent nerve palsy as a manifestation of neuroleptospirosis is reported herewith.
Case Report
A 22-year-old male agricultural worker presented with fever, nausea, abdominal pain, yellowish discoloration of the eyes and myalgia. He had noticed the symptoms seven days prior to admission and had been treated symptomatically at a local dispensary. Examination revealed a febrile, toxic patient. There were jaundice, conjunctival suffusion, and mild hepatomegaly. No signs of meningeal irritation were detected. He denied a history of alcohol usage. Since this was during a time when our hospital was getting numerous cases of leptospirosis, and in view of his occupation, a possible clinical diagnosis of leptospirosis was considered. Injection crystalline penicillin two million units intravenously sixth hourly was commenced while awaiting lab reports for malaria parasite and Leptospira, dengue, viral hepatitis serology.
The report revealed leukocytosis (14,600/mm3) and thrombocytopenia (59,000/mm3). ESR was 90 mm at the end of the first hour. Urea 28 mg/dL, serum creatinine 1.4 mg/dL and RBS 112 mg/dl. AST 87 IU/L and ALT 105 IU/L. Total bilirubin was 3.75 mg/dL with direct fraction 2.39/dL. Creatinine kinase was significantly elevated at 202 IU/L. Prothrombin time was 36 seconds, the control being 30 seconds. Ultrasound of the abdomen was normal. Chest X-ray was normal.
On the second night of hospitalization during routine rounds at 8.30 PM, a convergent strabismus (esotropia) was noticed (figure 1). The patient replied in the affirmative when asked about diplopia, but did not have any pain or headache. The diplopia was worse when he directed his gaze to the left or right (figure 2, diplopia chart). Onset was rapid since evaluation a few hours earlier had not shown any neurological deficit. Limitations of eye movements were confined to abduction in both eyes (figure 3). The size of the convergent squint or esotropia was larger on distant fixation than on near fixation. Bilateral abducent nerve palsy was therefore diagnosed. Other cranial nerves, fundoscopy, motor, sensory system and reflexes were normal. Plantar response was flexor bilaterally. Cranial CT scan was normal. Cerebrospinal fluid (CSF) analysis showed 16 leukocytes/mm3 - all lymphocytes, glucose of 59 mg/dL and protein level of 39 mg/dL. There was no xanthochromia.
Figure 1.

Photograph shows convergent strabismus due to weakness of abduction caused by bilateral abducens palsy.
Figure 2.

Diplopia chart depicts that diplopia was worse when gaze was directed to the left or right.
Figure 3.
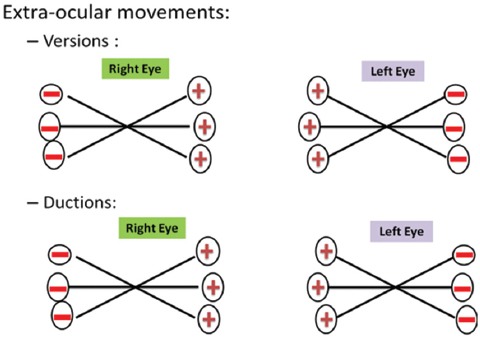
Shows extra-ocular movements; It can be seen from this chart that the limitations of eye movements were confined to abduction in both the eyes.
Serum (IgM) antibodies for leptospira by ELISA were done. Titer was 50 U/ml (<15 U/ml negative, 15-20 Intermediate, >20 positive) confirming the clinical diagnosis. HIV ELISA test was negative. Antibiotic therapy was continued and supportive measures for neuropathy were instituted. However, steroids were not given.
The patient’s general condition steadily improved. He also showed complete recovery from abducent palsy over the next five days and was discharged on the eleventh day after admission.
Discussion
Leptospirosis is caused by Leptospira, sp interrogans. Around the world about 1,500-2,000 cases are reported annually. It is an important cause of acute febrile illness in China, the Indian subcontinent, Southeast Asia, Africa, South America and Central America where malaria, typhoid and dengue are also common.1
Human infection occurs by direct contact with urine or blood of an infected rodent or animal, or from water or soil contaminated by urine. The organisms can penetrate abraded skin or intact mucous membrane, and enter the circulation and rapidly disseminate to various tissues.2
The infection is commonly seen in farmers, sewage workers, veterinarians, and animal handlers. After an incubation period of 7-14 days, the disease manifests in two phases. The first phase, leptospiremic or septicemic phase, has an abrupt onset, lasts for a week and is characterized by fever, chills, headache, skin rash, myalgia and conjunctival suffusion. The second phase or the immune phase, which can last up to 30 days, is characterized by leptospiruria and the development of anti-Leptospira antibodies. The patient becomes ill again. Fever and other constitutional symptoms recur. Additional involvement of pulmonary, renal, cardiovascular, and neurological systems can occur. In this phase, unusual clinical features can occur due immunological phenomena.
The commonest central nervous system manifestation of Leptospirosis is aseptic meningitis.1,3 The rarer clinical presentations described in literature are myeloradiculopathy, myelopathy, cerebellar dysfunction,4 transverse myelitis,5 cerebrovascular accident, cerebral venous thrombosis, cerebral arteritis, subarachnoid haemorrhage, optic neuritis, mononeuritis multiplex, peripheral nerve palsy, psychosis, suicidal behaviour, encephalitis, and cerebellitis. There are many reports of facial palsy6 and one refers to bilateral facial palsy.7 Virendra et al. and Mumford et al. reported paraparesis.8,9 Transient paraparesis has been described in a recent report by Lih-shinn et al.10 Occurrence of Guillain-Barre syndrome is also known.11 Retinitis is another recently described feature of neuroleptospirosis.12 Although implicated in various neurological manifestations, leptospirosis is often overlooked by clinicians in the evaluation of febrile illnesses with neurological manifestations.13
In the case reported by Kavitha and Shastry, their patient had fever, jaundice and lower limb weakness, urinary retention and decreased sensory perceptions below T4 level. As in our case, the confirmation of the diagnosis was by positive anti-Leptospira IgM serology.5 In the report by Costa et al., facial palsy was observed following an illness consistent with leptospirosis and in their case, microscopic agglutination test (MAT)- the confirmatory test was not done, but seroconversion was observed in the macroagglutination test.6 In the report by Mumford et al., flaccid paraplegia was observed. In contrast to our patient, this case ended with fatality ascribable to renal and hepatic involvement.8
The sixth cranial nerve (abducens nerve) innervates the lateral rectus muscle in the ipsilateral eye. It abducts the ipsilateral eye. Paralyses can be due to direct damage of the sixth cranial nerve, encephalon nuclei or less frequently, diffuse axonal damage. Bilateral sixth nerve palsy is a rare clinical condition. Unlike unilateral palsy, which is often due to local causes such as trauma, increased intracranial pressure, brain stem glioblastoma etc., the bilateral form is due to systemic causes such as vasculopathy secondary to atherosclerosis and immune mediated vasculitis.
Patients with bilateral sixth nerve palsies complain about binocular horizontal diplopia being worse in the gaze direction of the paretic lateral rectus muscle. This feature was noted in the present case.
Currently, there is paucity of knowledge regarding the pathogenetic pathways involved in the above neurological manifestations. The molecular mechanisms by which spirochetes interact with cellular barriers and the chain of events involved in leptospira meningitis and other leptospirosis-related neurological phenomena remain unknown.
After invading the bloodstream, leptospira plays a role in the activation of innate immunity cells, such as macrophages, via interaction among the transmembrane toll-like receptor 2 (TLR2), CD14, and leptospiral lipopolysaccharide. The organisms migrate from the bloodstream to the CSF by crossing the blood brain barrier.14 The ensuing neurological disease is dependent on the virulence of strains and on the development of an inflammatory response. Yet, interestingly, most of the current animal models for the spirochetoses do not recreate the manifestations of the neurological spectrum. Experimental approaches to the human neurological manifestations of the spirochetoses have shown inability and difficulty in reproducing aspects of the human disease in animal models.15
Though reports of other cranial nerve palsies in leptospirosis are made in literature, abducent paralysis has not been mentioned hitherto. In our patient, typical clinical presentation of leptospirosis was noted and laboratory findings confirmed leptospirosis. The other causes of abducent palsy were excluded by medical history and by other investigations. It is also to be noted that the patient developed abducent palsy approximately on the ninth day of symptomatic disease, when the clinical manifestations attributed to leptospirosis had subsided. This is consistent with the concept that cranial palsies, including the present bilateral abducent palsy in patients with leptospirosis is mediated by immunological mechanisms.
The novelty of this case is that whereas numerous case reports exist of other cranial nerve palsies occurring in leptospirosis, the present case is probably the first one that exemplifies abducent nerve palsy developing as a result of antecedent Leptospira infection.
Conclusion
The present case report has highlighted the hitherto unreported manifestation of bilateral abducent nerve palsy. Neuroleptospirosis should be considered in the differential diagnosis of all neuroinfections especially in endemic areas. Practicing physicians in developing countries, where advanced diagnostic facilities are not available, should be aware of the different patterns of clinical presentation and the various rarer manifestations of leptospirosis to be able to make a clinical diagnosis even when confronted with the unusual associations of leptospirosis.
Acknowledgement
We sincerely thank Dr (Prof) Mahesh B.S. Head of the Department of Ophthalmology, JSS Hospital, Mysore and Dr (Prof) Harsha Sundaramurthy, Neurophysician, Head of the Department of Neurology, JSS Hospital, Mysore for the help rendered.
Conflict of Interest: None declared.
References
- 1.Bal AM. Unusual clinical manifestations of leptospirosis. J Postgrad Med. 2005;51:179–83. [PubMed] [Google Scholar]
- 2.Sethi S, Sharma N, Kakkar N, Taneja J, Chatterjee SS, Banga SS, et al. Increasing trends of leptospirosis in northern India: a clinico-epidemiological study. PLoS Negl Trop Dis. 2010;4:e579. doi: 10.1371/journal.pntd.0000579. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panicker JN, Mammachan R, Jayakumar RV. Primary neuroleptospirosis. Postgrad Med J. 2001;77:589–90. doi: 10.1136/pmj.77.911.589. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathew T, Satishchandra P, Mahadevan A, Nagarathna S, Yasha TC, Chandramukhi A, et al. Neuroleptospirosis - revisited: experience from a tertiary care neurological centre from south India. Indian J Med Res. 2006;124:155–62. [PubMed] [Google Scholar]
- 5.Kavitha S, Shastry BA. Leptospirosis with transverse myelitis. J Assoc Physicians India. 2005;53:159–60. [PubMed] [Google Scholar]
- 6.Costa E, Sacramento E, Lopes AA, Bina JC. Facial nerve palsy associated with leptospirosis. Rev Soc Bras Med Trop. 2001;34:219–20. doi: 10.1590/S0037-86822001000200011. [DOI] [PubMed] [Google Scholar]
- 7.Maldonado F, Portier H, Kisterman JP. Bilateral facial palsy in a case of leptospirosis. Scand J Infect Dis. 2004;36:386–8. doi: 10.1080/00365540410020299. [DOI] [PubMed] [Google Scholar]
- 8.Mumford C, Dudley N, Terry H. Leptospirosis presenting as a flaccid paraplegia. Postgrad Med J. 1990;66:218–20. doi: 10.1136/pgmj.66.773.218. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patil VC, Patil HV, Sakaria A, Tryambake S. An unusual case of Weil’s syndrome with paraparesis. Indian J Crit Care Med. 2011;15:130–3. doi: 10.4103/0972-5229.83014. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang LS, Wang CC, Huang SH, Chao H, Lin SH, Chang JH, et al. Leptospirosis with transient paraparesis and thrombocytopenia: a case report. J Microbiol Immunol Infect. 2012;45:75–8. doi: 10.1016/j.jmii.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Bal AM, Bharadwaj RS, Gita N, Joshi SA, Thakare JP. Guillain-Barre syndrome in a pediatric patient following infection due to Leptospira. Jpn J Infect Dis. 2003;56:29–31. [PubMed] [Google Scholar]
- 12.Basumatary LJ, Das S, Das M, Goswami M, Kayal AK. Leptospirosis presenting as neuroretinitis. J Neurosci Rural Pract. 2012;3:224–6. doi: 10.4103/0976-3147.98264. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Souza AL. Neuroleptospirosis: unexplored & overlooked. Indian J Med Res. 2006;124:125–8. [PubMed] [Google Scholar]
- 14.Romero EC, Billerbeck AE, Lando VS, Camargo ED, Souza CC, Yasuda PH. Detection of Leptospira DNA in patients with aseptic meningitis by PCR. J Clin Microbiol. 1998;36:1453–5. doi: 10.1128/jcm.36.5.1453-1455.1998. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Monco JC, Benach JL. A disconnect between the neurospirochetoses in humans and rodent models of disease. PLoS Pathog. 2013;9:e1003288. doi: 10.1371/journal.ppat.1003288. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]


