Abstract
The traditional hypothesis of cerebrospinal fluid (CSF) hydrodynamics presumes that CSF is primarily produced in the choroid plexus (CP), then flows from the ventricles into the subarachnoid spaces, and mainly reabsorbed in the arachnoid granulations. This hypothesis is necessary to reconsider in view of recent research and clinical observations. This literature review presents numerous evidence for a new hypothesis of CSF hydrodynamics—(1) A significantly strong relationship exists between the CSF and interstitial fluid (IF), (2) CSF and IF are mainly produced and absorbed in the parenchymal capillaries of the brain and spinal cord. A considerable amount of CSF and IF are also absorbed by the lymphatic system, and (3) CSF movement is not unidirectional flow. It is only local mixing and diffusion.
Keywords: cerebrospinal fluid, choroid plexus, arachnoid granulation, lymphatic system, parenchymal capillary
Introduction
Previous literature has suggested that most of the cerebrospinal fluid (CSF) is produced in the choroid plexus (CP) and passes through the foramina of Magendie and Luschka via the cerebral aqueduct, to reach the basal cistern; from here, it moves upwards toward the subarachnoid spaces of the convexity, and then gets absorbed in the superior sagittal sinus across the arachnoid granulations (or villi). Furthermore, previous studies have also suggested that part of the CSF is circulated in the spinal subarachnoid space. However, these studies have been conducted 100 years ago, and recent research and clinical observations have brought to light the limitations of past research methods and incorrect interpretations of the results. Thus, it is necessary to reconsider the hypothesis of CSF circulation. The main purpose of the present study was to reconsider the physiology of CSF.
Production of CSF
I. Production of CSF in the CP
Literature states that CP is the main site of CSF production. The Greek physician Claudius Galenus (Galen, 129–219 AD) recognized the existence of the CP; more than 1,000 years later, Andreas Vesalius (1514–1564) at the University of Paris made an anatomical record of this region.1) Later, Thomas Willis (1621–1675) observed the gland-like structure of the CP.2) In 1914, Walter Dandy (1886–1946) conducted an experiment using a dog and demonstrated that one lateral ventricle could be enlarged by obstructing the foramen of Monro; this experiment demonstrated the intraventricular origin of CSF. Further to this experiment, Dandy blocked the foramen of Monro, while simultaneously removing the CP on the same side. This demonstrated that the lateral ventricle did not enlarge. From these experiments, conclusive evidence was obtained to show that CP is the production site of CSF.3) However, it must be noted that experiments were performed in a single dog, and it was never reproduced. Based on the results of these experiments, Dandy later attempted to perform choroid plexectomy in hydrocephalic patients; however, it was discontinued due to lack of efficacy. In 1967, Welch exposed rabbit CPs, and after vascular cannulation, the hematocrit (Ht) levels of the arterial and venous blood were compared. Based on the results, he reported that the Ht levels of venous blood were 1.16 times higher than that of arterial blood. Measurements of blood flow volume in the CP revealed that the amount of CSF produced in the CP was 0.37 μL/min.4) In 1967, Davson opined that the CP had an ideal structure for the production of CSF.5) In 2007, Brodbelt and Stoodley emphasized the large surface area of the CP and suggested that most of the CSF was produced from the CP.6) In addition, in a neuroscience textbook published in 2008, Johanson stated that more than half of the CSF is produced in the CP.7)
The CP has a villus structure and is covered with an epithelial layer. During the initial step in the production of CSF, the plasma passes across the fenestrated endothelium and is passively filtered through the basolateral membrane of CP epithelial cells. During this stage, it flows under hydrostatic pressure.8) It is then actively secreted from the apical surface of epithelial cells into the cerebral ventricles.9,10) CSF is thought to be actively produced and is not significantly affected by hydrostatic or oncotic pressure.4,10–13)
Oresković et al. used the ventriculo-cisternal perfusion method, developed by Heisey et al., to measure CSF production volume.14,15) As a result, inadequacies in this method to accurately quantify the amount of CSF produced have been pointed out.16) Contrary to the conventional hypotheses, Milhorat reported that removal of the CP in the bilateral ventricles had no significant effect on the amount and composition of CSF produced.17–21) Even after the CP was resected, the placement of a ventriculoperitoneal CSF shunt was required.22) Oresković et al. improved on Flexner and Winter’s method by implanting a cannula in the cerebral aqueduct of a cat. After blocking the cerebral aqueduct, CSF pressure in the cerebral ventricles and cisterna magna was monitored from 120 min to 190 min.23–25) If we assume that the CSF is mainly produced by the CP, then enlargement of the cerebral ventricles, elevated CSF pressure, and transmantle pressure (pressure gradient between the cerebral ventricles and subarachnoid space) should have been observed in this experiment. However, no differences in CSF pressure were observed in the lateral ventricles and cisterna magna. Cerebral ventriculography also did not reveal any enlargement of the cerebral ventricles after cerebral aqueduct blockage. In other words, elevated CSF pressure, enlargement of the cerebral ventricles, and transmantle pressure were not observed.25) The results of these studies suggest that CP is not the primary CSF production site. But for many neurosurgeons, it is hard to accept these laboratory findings. These experiments must be required verification by other groups.
II. CSF production outside the CP
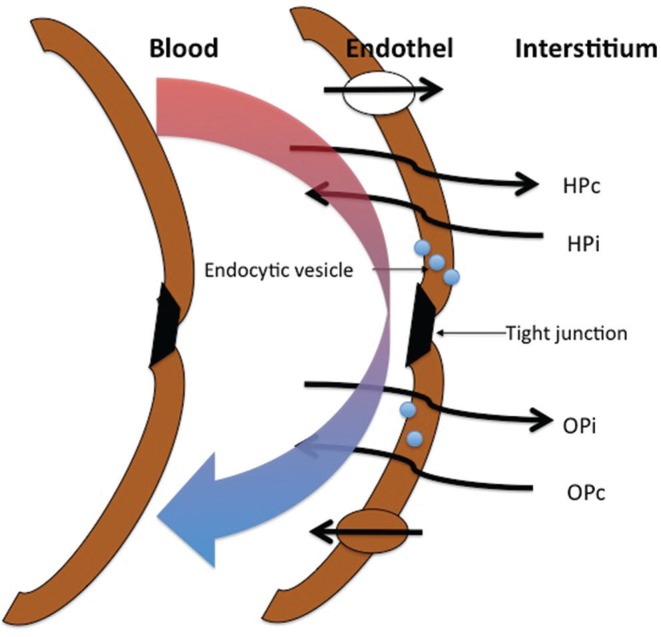
Production of CSF outside the CP has been historically debated. In 1914, Weed hypothesized that CSF is produced by the dura mater, thereby suggesting that it is produced by capillaries.26) In 1924, Hassin published a groundbreaking thesis on the relationship between CSF and extracellular fluid, where he referred to CSF as brain tissue fluid.27) However, production of CSF outside the CP has been quantitatively analyzed, since Bering and Sato published a study in 1963. They asserted that CSF is produced in the intracranial subarachnoid space.28) In 1967, Pollay et al. reported a 33% of the CSF is produced by the CP in rabbits, while Milhorat in 1971 reported a 60% is formed in rhesus monkeys, and in Bering and Sato reported a 58.8% is produced in dogs.29) Studies have demonstrated that a large amount of CSF is produced outside the CP.13,30,31) In 1971, Sato et al. also reported the production and absorption of CSF in the spinal subarachnoid space in dogs.32) The surface area of the CP (weight, 2 g) in humans is 220 cm2, and the amount of circulating blood weighed 10 times more than that in the cerebral cortex. However, it has been reported that the surface area of the capillaries in the brain is approximately 5,000 times their area in the CP. Based on this, Oresković et al. published a review article in 2010 which stated that a large amount of CSF is produced in the cerebral cortex (parenchyma).15) Bering had focused on the relationship between CSF and extracellular fluid in the brain parenchyma from early on.33) Brightman used electron microscopy to observe free-flowing ferritin and horseradish peroxidase (HRP) through the brain ependymal lining and parenchyma.34–36) Tight junctions were absent; because gap junctions exist in the ependymal cell layer, CSF and interstitial fluid can move freely. According to Bulat, Klarica, and Oresković, interstitial fluid and CSF comprise functional units; their volume is primarily regulated inside the capillaries and by the osmotic and hydrostatic pressure differences in the interstitial fluid and CSF. Furthermore, it has been proposed that the cerebral capillaries are primarily involved in the production and absorption of CSF (Fig. 1).15,22,37,38) This shows that elevation of intracranial pressure decreases CSF production, and elevation in osmotic pressure of CSF increases its production.38) This is contrary to the conventional hypothesis that CP actively secretes CSF. Large quantities of Na+-K+ antiporters exist in the capillary endothelium of the central nervous system and Na+-K+ ATPase activity is high, thereby suggesting that the capillaries control the amount of interstitial fluid and CSF produced.39)
Fig. 1.
The way CSF-ISF passes through the membrane of the capillary endothelial cells. CSF-ISF: cerebrospinal fluid-brain interstitial fluid, HPc: hydrostatic capillary pressure, HPi: hydrostatic interstitial pressure OPc: osmotic capillary pressure, Opi: osmotic interstitial pressure.
CSF Absorption
I. CSF absorption from the arachnoid villi
In the 16th and 17th centuries, Vesalius and Willis, respectively, recognized the existence of the arachnoid villi, and in 1705, Pacchioni reported the relationship between the CSF and arachnoid villi and their relationship with the superior sagittal sinus. In 1875, Key and Retzius noted that dye-colored gelatin passes through the arachnoid villi and flows from the lateral lacuna to the superior sagittal sinus; the dye was also observed in the cervical lymph nodes.40) In 1901, Cushing examined the CSF flow as part of a third circulation and hypothesized that the arachnoid villi were the drainage point connecting the CSF and blood from the sinuses.41) In 1914, Weed performed crucial experiments that showed that the arachnoid villi and granulations were important sites of CSF absorption. These are strong hypotheses, and many researchers continue to believe that CSF absorption occurs passively from the arachnoid granulations.42) Fifty years later, in 1956, Davson showed that CSF inflow into the venous blood is mainly due to a hydrostatic pressure gradient.10) In 1964, Shulman et al. elucidated and calculated the difference in CSF and venous pressure in the superior sagittal sinus.43) In 1960, Welch and Friedman stated that there was a valve between the CSF and sagittal sinus; however, this hypothesis has been rejected.44) In 1974, Tripathi published that tight junctions exist between the epithelial cells of the villi, thereby asserting that there are no morphological foramina or openings, and suggested a mechanism similar to the inflow of aqueous humor into Schlemm’s canal. Furthermore, recesses in the basal surface of epithelial cells of the villi become vacuolized, reaching the apical surface and opening into the sinus.45,46) Yamashima (1986) and Kida et al. (1988) found that the arachnoid villi in humans are comprised of four layers (a fibrous capsule, arachnoid cell layer, cap cell, and a central core); however, the villous surface is not always covered by epithelial cells. It is covered by a layer of arachnoid cells with an outer and inner zone. In some cases, it is only covered by a thin fibrous capsule. Furthermore, the authors could not prove its continuity, but stated that the CSF may be absorbed from the subarachnoid space into the lateral lacuna or sinuses via numerous extracellular cisterns, approximately 10 μm in size, on the outer and inner zones.47,48) Grzybowski et al. (2006) reported the significance of CSF absorption in arachnoid cell cultures,49) and Pollay (2010) noted that recent reviews have reaffirmed the role of the arachnoid villi.50) A large number of arachnoid villi are located in the vicinity of the middle fossa, posterior cranial fossa, and transverse sinuses as well as in the vicinity of the superior sagittal sinus.51) Furthermore, Tsutsumi et al. reported channels between the arachnoid villi and diploic veins.52) Recent research has shown that the arachnoid villi are not physiologically implicated in CSF absorption; however, when CSF pressure is elevated, they are moderately involved in CSF absorption as a secondary pathway. A reason opposing the hypothesis that the arachnoid villi is the primary site of CSF absorption is that the sinuses do not exist in rats until day 20 after birth, and the arachnoid villi do not exist prenatally in humans or sheep, but occur postpartum, increasing in number with age. Therefore, existence of a different pathway is essential for the excretion of CSF during the fetal period. The intracranial lymphatic system plays an important role in prenatal CSF excretion and seems to be an important excretion pathway for CSF in neonates.53–57)
II. CSF absorption from locations other than the arachnoid villi
In 1869, Schwalbe injected Berlin blue dye into the subarachnoid space of a dog, and was the first to observe that the lymphatic system is an important site for CSF absorption.58) Di Chiro (1964) later suggested that CSF is absorbed by various regions in the central nervous system.59) In 1993, Kida et al. reported that the arachnoid villi are only minor absorption pathways,60) and Hashimoto (2004) denied any absorption in the arachnoid villi.61) The results of the most recent studies have negated the conventional theories of CSF absorption. Bering in the 1950s and Bulat in 2011 reported that CSF is absorbed by the capillaries in the brain parenchyma.37,62)
1. CSF absorption in the ventricular walls
In an experiment using rabbit hydrocephalus, Wislocki (1921) showed that CSF is absorbed by the cerebral ventricles.63) In experiments using animals with hydrocephalus, Bering and Sato (1963) and Sahar et al. (1969) reported that CSF is absorbed from the cerebral ventricular walls into the brain parenchyma.28,64) In 1976, computed tomography studies by Naidich et al. showed that in human hydrocephalus, the periventricular white matter is a notable absorption site for CSF from the cerebral ventricular walls into the brain parenchyma.65) In 1977, Drayer et al. showed that radiocontrast medium in the cerebral ventricles enter the cerebral ventricular walls.66) However, these reports that take into account patients with hydrocephalus have been criticized for tearing the ependyma. In 1980, Weller and Mitchell reported that CSF edema in the periventricular white matter is probably caused due to CSF entering the blood via the perivascular space as an alternative pathway.67)
2. Lymphatic absorption of CSF
Similar to a study conducted by Weed, Schwalbe’s historic experiment using Berlin blue dye demonstrated that the dye travels from the olfactory bulb to the olfactory nerve, nasal mucosa, and nasal and cervical lymphatic vessels.58) In an experiment performed by Mortensen and Sullivan in 1933, X-ray examination revealed that a radiocontrast medium injected into the CSF of a dog entered the cervical lymphatics, thereby demonstrating the lymphatic system is part of the CSF drainage mechanism.68) However, it was not until Bowsher (1957) that more modern methods were used for examination.69) Kozma et al. injected India ink and a radiocontrast agent, while Oehmichen and Gencic injected leukocytes into the brain parenchyma and observed that these molecules passed from the subarachnoid space through the Virchow-Robin space to the vasa vasorum of the lymphatic vessels and cervical lymph nodes via the adventitia of the extracranial vessels.70,71) Subsequently, Bradbury et al. studied in detail the absorption of CSF into the cervical lymph nodes in rabbits and cats and found that the significance of this absorption pathway is unchanged.72–74) Cserr injected a tracer into the caudate nucleus of a rat and elucidated CSF dynamics in the cerebral tissues as bulk flow. She further elucidated that a tracer injected into the caudate nucleus reaches approximately 50% of the cervical lymph nodes.75) In an experiment using cats, McComb et al. reported that a considerable amount of CSF entered the lymphatic system in cases with normal or elevated intracranial pressure, and this phenomenon may play an important role in the physiological condition of the hydrocephalus.76) Love and Leslie obtained an interesting result from a similar experiment in which artificial CSF was injected into the cisterna magna of cats in order to elucidate the absorption of CSF into the lymphatics. When artificial CSF was injected, the lymphatic drainage immediately increased, and the protein concentration of the lymph decreased as long as the injection was continued. However, even when the intracranial pressure was elevated, temporary increase in lymphatic drainage was noted.77) Alternatively, Hasuo et al. showed an increase in lymph flow volume when the intracranial pressure was elevated.78) However, the relationship between CSF absorption and the lymphatic system in humans has not been sufficiently elucidated. Arnold et al., Jackson et al., and Szentistványi et al. demonstrated that the CSF absorption pathway passes through the olfactory tract through the cribriform plate to the nasal mucosa, the retropharyngeal lymph nodes, and finally into the cervical lymph nodes.79–81) Furthermore, Foltz et al. reported CSF drainage via the olfactory nerve pathway from a pathophysiological perspective, based on the results of an experiment using dogs with hydrocephalus.82) All these findings were confirmed by detailed anatomical investigations using ultrastructural methods. Johnston et al. later showed that there is a direct link between CSF spaces and the lymphatics of the nasal mucosa in primates, including humans. Elevated CSF pressure and increased excretion were observed in the arachnoid villi and lymphatic system. When the pathway from the cribriform plate region of the ethmoid bone to the olfactory nerve was obstructed in sheep, a higher CSF pressure was necessary to obtain the same amount of excretion. Based on the results of this experiment, it was concluded that the absorption pathway functions at low pressure for the lymphatic system and at high pressure for the arachnoid villi.54) We confirmed that a high amount of lipocalin-type prostaglandin D synthase, produced by arachnoid cells and found in high concentrations in CSF, is found in the fluid in the cervical cystic lymphangioma as compared to peripheral blood, and CSF is also absorbed into the lymph in humans.
Part of the CSF absorption pathways is similar to the posterior orbital tissues as pointed out in the beginning by Field and Brierley.83) It is well known that the aqueous humor is extremely similar to CSF with regard to its production, circulation, absorption, and other dynamics. Focusing on these similarities, Tripathi performed a detailed examination of the Schlemm’s canal and examined the structure of the arachnoid villi in primates and in other mammals, including humans, using light microscopy, scanning, and transmission electron microscopy.84) The relationship among CSF, aqueous humor, and the lymphatic system has been reported by Bradbury and Cole. Gomez et al. confirmed the absorption of CSF in the periphery of the optic nerve in rabbits.85) HRP injected into the subarachnoid space under physiological pressure entered the optic nerve from the peripheral subarachnoid space of the optic nerve and finally reached the choroid. Erlich et al. performed a detailed morphological examination to ascertain the existence of CSF absorption pathways in the intraorbital region. When ferritin (molecular weight, 400,000) was perfused at physiological and high pressures, numerous channels were shown to exist in the terminal sites of the subarachnoid spaces, including the optic nerve. No barriers existed to prevent the passage of macromolecular substances in the CSF; thus, the terminal sites are open channels that are involved in the absorption of macromolecular substances, including CSF.86)
Production and Absorption of CSF in the Intraspinal Spaces
In 1971, Sato et al. reported the production and absorption of CSF in the spinal subarachnoid space.32) According to Gomez et al., the arachnoid membrane and dura mater existed in the site where the ventral and posterior roots are combined to form one root.87) Elman showed the existence of arachnoid granulations in the spinal subarachnoid space,88) and Welch and Pollay reported that a part of the arachnoid membrane showed penetration of the dura mater, similar to the cranium.89) In 2007, Greitz showed absorption by the capillaries in the spinal canal.90) Furthermore, in 2004, Edsbagge et al. also reported absorption in the spinal canal, whereas in 2007, Tubbs et al. found arachnoid villi in the spinal canal in which CSF was absorbed.91,92)
Relationship between Interstitial Fluid and CSF
In 2011, Bulat et al. classified fluid movement into three patterns: bulk flow, mixing, and diffusion.36) While bulk flow refers to the circulation or volume flow, mixing refers to the dispersion of a substance, and diffusion is a very slow process, which refers to movement in an extremely localized site. In 1924, Hassin reported that the interstitial fluid is brain tissue fluid.27) Fenstermacher and Patlak characterized the material exchange between the CSF and extracellular fluid in the brain parenchyma as diffusion.93) Cserr stated that this exchange was caused by diffusion; she also reported the existence of bulk flow, which was of great interest.94) In 1977, Cserr et al. injected HRP into the caudate nucleus of a rat and reported that the tracer moved from the intercellular space to the perivascular space and surrounding tissue of the cerebral ventricles, reaching the CSF space and entering the fenestrated capillaries. This has greatly contributed to the progress of modern physiological research on CSF.94) This is based on the hypothesis that considerable large amount of CSF is produced by the brain parenchyma.
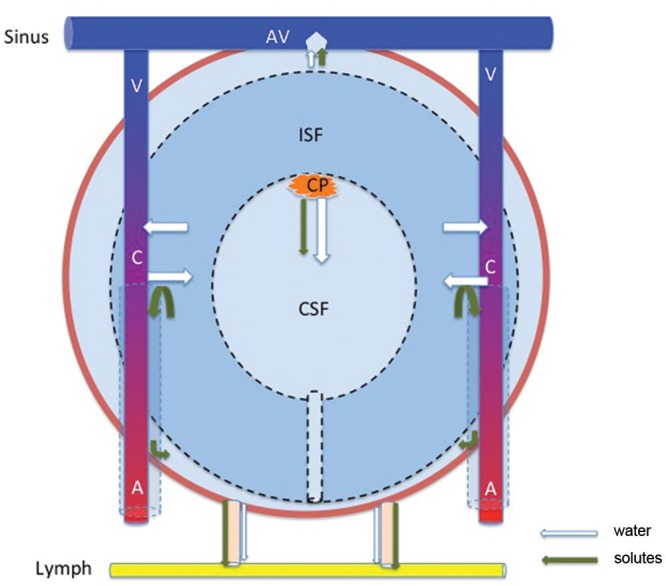
Raimondi (1994) and Hashimoto (2004) reported that CSF and all other intracranial extracellular fluid should be considered as a single entity.61,95) In 2004, Agre et al. reported the significant role of aquaporins (AQPs) in water movement.96) Williams et al. (2008), the leading authority on normal pressure hydrocephalus, reported that CSF is the same as extracellular fluid.97) In 2010, Oresković et al. asserted that CSF is produced and absorbed by all surfaces of the central nervous system that come into contact with CSF.15) In 2011, Tsutsumi and Ito showed by magnetic resonance imaging (MRI) that the brain surface and cerebral ventricles are connected by Virchow-Robin space.98) In other words, CSF and interstitial fluid both continuously form extracellular fluid. In humans, the total amount of extracellular fluid is 450 mL (CSF, 150 mL; interstitial fluid, 300 mL) (Fig. 2).
Fig. 2.
Diagram of the CSF-ISF inflow and outflow system. A: artery, AV: arachnoid villi, C: capillary, CP: choroid plexus, CSF-ISF: cerebrospinal fluid-brain interstitial fluid, V: vein.
Existence of CSF Circulation
According to the conventional theory, newly produced CSF is pushed out and flows in a single direction. Key and Retzius40) and Weed42) hypothesized the so-called vis-a-tergo. According to this theory, the pulsation of the CP propels CSF flow. In 1933 Hassin casted doubt on this flow of CSF.27) In 1964, Di Chiro was able to visualize CSF flow to a certain degree through cisternography, which caused confusion.99) In 1974, Cserr et al. showed that interstitial fluid flows through intercellular space; this observation significantly modified the understanding of CSF flow.100,101) In 2008, Yamada et al. showed that MRI (Time-Spatial Inversion Pulse method) revealed no movement of CSF in the convexity region.102) Oresković et al.15) and Klarica et al.25) inserted cannulae into the cerebral aqueduct of cats and observed CSF flow for more than 3 h. While pulsation of CSF was observed, collection of CSF via the cannulae could not be achieved. These outcomes questioned the conventional theory of CSF circulation. When artificial CSF was simultaneously injected (13 Lμ/min) into the lateral ventricles, significant transmantle pressure was observed. However, after injection of artificial CSF, the CSF pressure returned to the physiological levels and the transmantle pressure disappeared. The results of this experiment suggest that CSF is absorbed in the cerebral ventricles.
Bulat, Oresković, and Klarica et al. performed an experiment by slowly injecting 3H-water (tritium water) into the lateral ventricles of a cat.62,103) CSF did not flow from the cerebral ventricles to the cisterns, and rapid absorption was observed in the periventricular capillaries. Fenstermacher et al. showed that 3H-water passes across brain ependymal into caudate nucleus only a few millimeters being rapidly eliminated into the brain capillaries (half-life, 1.5 min).104) An experiment in which acute blockage of the cerebral aqueduct was performed demonstrated that the CSF pressure in the lateral ventricles did not change; this supports the hypothesis that CSF is rapidly absorbed into periventricular capillaries. In contrast to this, completely different dynamics are observed when large molecular weight substances are injected into the subarachnoid space. The results showed that when 3H-inulin (molecular weight, 5,500) was injected into the subarachnoid space, it was excreted extremely slowly from the circulatory system. Furthermore, it diffused in various directions in the subarachnoid space. Renkin and Crone observed the slow diffusion of 3H-inulin from the cisterna magna to the basal and lumbar cisterns.105,106)
This type of macromolecule has been used in past physiological studies of CSF generating misunderstandings about the circulation and absorption of CSF. In past experiments, in order to observe CSF circulation, macromolecules were injected into the cerebral ventricles, incorrectly suggesting that CSF moved from the lateral ventricles to the third and fourth ventricles and flowed into the subarachnoid space from the cisterna magna.
In recent years, Iliff et al. injected tracers into the cerebral ventricles and subarachnoid space in mice and showed that the time taken to penetrate the brain parenchyma differed with the molecular weight of the substance injected, and the tracer was transported through the spaces between the cerebral capillaries and foot processes of the astrocytes “glymphatic system” (gliovascular clearance system).107) The CSF movement appears across all blood vessels in and outside the brain.
Conclusion
CSF is not produced by the CP alone, and the arachnoid granulations (or villi) are not the primary absorption sites of CSF. A considerable amount of CSF is also absorbed by the lymphatic system.
CSF is produced and absorbed by various sites in the central nervous system. In previous studies, Weed and Di Chiro hypothesized that CSF is mainly produced and absorbed in the parenchymal capillaries of the brain and spinal cord. Recently, the same has been proposed by Greitz, Oresković and Klarica, and Bulat et al. hypothesized CSF production via arterial hydrostatic pressure differences and absorption through venous osmotic pressure differences in the brain parenchymal capillaries. This hypothesis must be verified by new modern methods.
CSF movement is not unidirectional flow. It is only local mixing and diffusion.
AQP 4 is one of the most common membrane transport proteins in the central nervous system and is often observed at the end feet membrane of astrocytes and the basolateral membrane of ependymal cells. There are no tight junctions between the cells of the pia mater and ependyma; therefore, water and other substances pass freely between the subarachnoid space and brain parenchyma. The Virchow-Robin space appears along the perforating vessels and deep within the brain parenchyma; a significantly strong relationship exists between the CSF and interstitial fluid. This relationship is important for the maintenance of a stable brain environment.
Acknowledgments
The authors wish to express their thanks to O. Sato (Tokai University, Kanagawa) and all members of the Study Group of cerebrospinal fluid (CSF); M. Mase (Nagoya City University, Nagoya), S. Yamada (Toshiba Rinkan Hospital, Kanagawa), T. Inagaki (The University of Utah, Utah), K. Nishiyama (University of Niigata, Niigata), Y. Hayashi (Kanazawa University, Kanazawa), and S. Kida (Fukui Prefectural Hospital, Fukui) for suggestions of the CSF physiology.
This work was partly supported by Grant-in-Aids for Scientific Research (KAKENHI 23592106, 23592142); a Research Grant from the Ministry of Health, Labor, and Welfare of Japan (2012-Nanchi); and the Juntendo University Research Institute for Disease and Old Age.
References
- 1). Galen C: De anatomics administrationibus. Book IX: On the Brain, Vol 177, Reprint. New York, Oxford University Press; [Google Scholar]
- 2). Willis T: Cerebri Anatome: cui accessit nervorum description et usus. London, Tho. Roycroft, Impensis Jo. Martyn, Ja. Allestry, 1664. [Google Scholar]
- 3). Dandy WE, Blackfan KD: Internal hydrocephalus: an experimental, clinical and pathological study. Am J Dis Child 8: 406– 482, 1914. [Google Scholar]
- 4). Welch K: The secretion of cerebrospinal fluid by lamina epithelialis. Monogr Surg Sci 4: 155– 192, 1967. [PubMed] [Google Scholar]
- 5). Davson H: Physiology of the Cerebrospinal Fluid. London, JA Churchill, 1967. [Google Scholar]
- 6). Brodbelt A, Stoodley M: CSF pathways: a review. Br J Neurosurg 21: 510– 520, 2007. [DOI] [PubMed] [Google Scholar]
- 7). Johanson C: Choroid plexus-CSF circulatory dynamics: impact on brain growth, metabolism and repair, in Totowa CP. (ed): Neuroscience in Medicine. New Jersey, The Humana Press, 2008, pp 173–200 [Google Scholar]
- 8). Pollay M, Stevens A, Roberts PA: Alteration in choroid plexus blood flow and cerebrospinal fluid formation by increased ventricular pressure, in Wood JH. (ed): Neurobiology of Cerebrospinal Fluid, Vol. 2 New York, Plenum Press, 1983, pp 687–695 [Google Scholar]
- 9). Brown PD, Davies SL, Speake T, Millar ID: Molecular mechanisms of cerebrospinal fluid production. Neuroscience 129: 957– 970, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Davson H, Welch K, Segal MB: Physiology and Pathophysiology of the Cerebrospinal Fluid. Edinburgh, Churchill Livingstone, 1987. [Google Scholar]
- 11). Johanson CE, Duncan JA, Klinge PM, Brinker T, Stopa EG, Silverberg GD: Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res 5: 10, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). O’Connell JE: Cerebrospinal fluid mechanics. Proc R Soc Med 63: 507– 518, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Pollay M, Curl F: Secretion of cerebrospinal fluid by the ventricular ependyma of the rabbit. Am J Physiol 213: 1031– 1038, 1967. [DOI] [PubMed] [Google Scholar]
- 14). Heisey SR, Held D, Pappenheimer JR: Bulk flow and diffusion in the cerebrospinal fluid system of the goat. Am J Physiol 203: 775– 781, 1962. [DOI] [PubMed] [Google Scholar]
- 15). Oresković D, Klarica M: The formation of cerebrospinal fluid: nearly a hundred years of interpretations and misinterpretations. Brain Res Rev 64: 241– 262, 2010. [DOI] [PubMed] [Google Scholar]
- 16). Maraković J, Oresković D, Jurjević I, Rados M, Chudy D, Klarica M: Potential error in ventriculocisternal perfusion method for determination of cerebrospinal fluid formation rate in cats. Coll Antropol 35 (Suppl 1): 73– 77, 2011. [PubMed] [Google Scholar]
- 17). Hammock MK, Milhorat TH: Recent studies on the formation of cerebrospinal fluid. Dev Med Child Neurol Suppl 27–34, 1973. [DOI] [PubMed] [Google Scholar]
- 18). Milhorat TH: Choroid plexus and cerebrospinal fluid production. Science 166: 1514– 1516, 1969. [DOI] [PubMed] [Google Scholar]
- 19). Milhorat TH: The third circulation revisited. J Neurosurg 42: 628– 645, 1975. [DOI] [PubMed] [Google Scholar]
- 20). Milhorat TH: Structure and function of the choroid plexus and other sites of cerebrospinal fluid formation. Int Rev Cytol 47: 225– 288, 1976. [DOI] [PubMed] [Google Scholar]
- 21). Milhorat TH, Hammock MK, Chien T, Davis DA: Normal rate of cerebrospinal fluid formation five years after bilateral choroid plexectomy. Case report. J Neurosurg 44: 735– 739, 1976. [DOI] [PubMed] [Google Scholar]
- 22). Tamburrini G, Caldarelli M, Di Rocco F, Massimi L, D’Angelo L, Fasano T, Di Rocco C: The role of endoscopic choroid plexus coagulation in the surgical management of bilateral choroid plexuses hyperplasia. Childs Nerv Syst 22: 605– 608, 2006. [DOI] [PubMed] [Google Scholar]
- 23). Flexner LB: The water of the cerebrospinal fluid. Variations of its rate of flow with variation of ventricular pressure. Am J Physiol 106: 170– 174, 1933. [Google Scholar]
- 24). Flexner LB, Winters H: The rate of formation of cerebrospinal fluid in etherized cats. Am J Physiol 101: 697– 710, 1932. [Google Scholar]
- 25). Klarica M, Oresković D, Bozić B, Vukić M, Butković V, Bulat M: New experimental model of acute aqueductal blockage in cats: effects on cerebrospinal fluid pressure and the size of brain ventricles. Neuroscience 158: 1397– 1405, 2009. [DOI] [PubMed] [Google Scholar]
- 26). Weed LH: Studies on Cerebro-Spinal Fluid. No. IV: The dual source of Cerebro-Spinal Fluid. J Med Res 31: 93–118. 11, 1914. [PMC free article] [PubMed] [Google Scholar]
- 27). Hassin GB: Notes on the nature and origin of the cerebrospinal fluid. J Nerv Ment Dis 59: 113– 121, 1924. [Google Scholar]
- 28). Bering EA, Sato O: Hydrocephalus: changes in formation and absorption of cerebrospinal fluid within the cerebral ventricles. J Neurosurg 20: 1050– 1063, 1963. [DOI] [PubMed] [Google Scholar]
- 29). Milhorat TH, Hammock MK, Fenstermacher JD, Levin VA: Cerebrospinal fluid production by the choroid plexus and brain. Science 173: 330– 332, 1971. [DOI] [PubMed] [Google Scholar]
- 30). Pollay M, Curl F: Secretion of cerebrospinal fluid by the ventricular ependyma of the rabbit. Am J Physiol 213: 1031– 1038, 1967. [DOI] [PubMed] [Google Scholar]
- 31). Sato O, Bering EA, Yagi M, Tsugane R, Hara M, Amano Y, Asai T: Bulk flow in the cerebrospinal fluid system of the dog. Acta Neurol Scand 51: 1– 11, 1975. [DOI] [PubMed] [Google Scholar]
- 32). Sato O, Asai T, Amano Y, Hara M, Tsugane R, Yagi M: Formation of cerebrospinal fluid in spinal subarachnoid space. Nature 233: 129– 130, 1971. [DOI] [PubMed] [Google Scholar]
- 33). Bering EA: Water exchange of central nervous system and cerebrospinal fluid. J Neurosurg 9: 275– 287, 1952. [DOI] [PubMed] [Google Scholar]
- 34). Brightman MW: The distribution within the brain of ferritin injected into cerebrospinal fluid compartments. 1. Ependymal distribution. J Cell Biol 99–123, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Brightman MW: The distribution within the brain of ferritin injected into cerebrospinal fluid compartments. II. Parenchymal distribution. Am J Anat 117: 193– 219, 1965. [DOI] [PubMed] [Google Scholar]
- 36). Brightman MW: The intracerebral movement of proteins injected into blood and cerebrospinal fluid of mice. Prog Brain Res 29: 19– 40, 1968. [DOI] [PubMed] [Google Scholar]
- 37). Bulat M, Klarica M: Recent insights into a new hydrodynamics of the cerebrospinal fluid. Brain Res Rev 65: 99– 112, 2011. [DOI] [PubMed] [Google Scholar]
- 38). Maraković J, Oresković D, Rados M, Vukić M, Jurjević I, Chudy D, Klarica M: Effect of osmolarity on CSF volume during ventriculo-aqueductal and ventriculo-cisternal perfusions in cats. Neurosci Lett 484: 93– 97, 2010. [DOI] [PubMed] [Google Scholar]
- 39). Kalaria RN, Premkumar DR, Lin CW, Kroon SN, Bae JY, Sayre LM, LaManna JC: Identification and expression of the Na+/H+ exchanger in mammalian cerebrovascular and choroidal tissues: characterization by amiloride-sensitive [3H]MIA binding and RT-PCR analysis. Brain Res Mol Brain Res 58: 178– 187, 1998. [DOI] [PubMed] [Google Scholar]
- 40). Key A, Retzius G: Studien in der Anatomie des Nervensystems und des Bindegewebes. Stockholm, Samson & Wallin, 1875. [Google Scholar]
- 41). Cushing H: Studies on the cerebrospinal fluid: I. Introduction. J Med Res 31: 1– 19, 1914. [PMC free article] [PubMed] [Google Scholar]
- 42). Weed LH: Studies on Cerebro-Spinal Fluid. No. III: The pathways of escape from the Subarachnoid Spaces with particular reference to the Arachnoid Villi. J Med Res 31: 51– 91, 1914. [PMC free article] [PubMed] [Google Scholar]
- 43). Shulman K, Yarnell P, Ransohoff J: Dural sinus pressure. in normal and hydrocephalic dogs. Arch Neurol 10: 575– 580, 1964. [DOI] [PubMed] [Google Scholar]
- 44). Welch K, Friedman V: The relation between the structure of arachnoid villi and their functions. Surg Forum 10: 767– 769, 1960. [Google Scholar]
- 45). Tripathi BJ, Tripathi RC: Vacuolar transcellular channels as a drainage pathway for cerebrospinal fluid. J Physiol (Lond) 239: 195– 206, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46). Tripathi R: Tracing the bulk outflow route of cerebrospinal fluid by transmission and scanning electron microscopy. Brain Res 80: 503– 506, 1974. [DOI] [PubMed] [Google Scholar]
- 47). Kida S, Yamashima T, Kubota T, Ito H, Yamamoto S: A light and electron microscopic and immunohistochemical study of human arachnoid villi. J Neurosurg 69: 429– 435, 1988. [DOI] [PubMed] [Google Scholar]
- 48). Yamashima T: Ultrastructural study of the final cerebrospinal fluid pathway in human arachnoid villi. Brain Res 384: 68– 76, 1986. [DOI] [PubMed] [Google Scholar]
- 49). Grzybowski DM, Holman DW, Katz SE, Lubow M: In vitro model of cerebrospinal fluid outflow through human arachnoid granulations. Invest Ophthalmol Vis Sci 47: 3664– 3672, 2006. [DOI] [PubMed] [Google Scholar]
- 50). Pollay M: The function and structure of the cerebrospinal fluid outflow system. Cerebrospinal Fluid Res 7: 9, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51). Chen F, Deng XF, Liu B, Zou LN, Wang DB, Han H: Arachnoid granulations of middle cranial fossa: a population study between cadaveric dissection and in vivo computed tomography examination. Surg Radiol Anat 33: 215– 221, 2011. [DOI] [PubMed] [Google Scholar]
- 52). Tsutsumi S, Ogino I, Miyajima M, Nakamura M, Yasumoto Y, Arai H, Ito M: Cranial arachnoid protrusions and contiguous diploic veins in CSF drainage. AJNR Am J Neuroradiol 35: 1735– 1739, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53). Gomez DG, Ehrmann JE, Gordon Potts D, Pavese AM, Gilanian A: The arachnoid granulations of the newborn human: An ultrastructural study. Int J Dev Neurosci 1: 139– 147, 1983. [DOI] [PubMed] [Google Scholar]
- 54). Johnston M, Zakharov A, Papaiconomou C, Salmasi G, Armstrong D: Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res 1: 2, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55). Koh L, Zakharov A, Johnston M: Integration of the subarachnoid space and lymphatics: is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal Fluid Res 2: 6, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56). Oi S, Di Rocco C: Proposal of “evolution theory in cerebrospinal fluid dynamics” and minor pathway hydrocephalus in developing immature brain. Childs Nerv Syst 22: 662– 669, 2006. [DOI] [PubMed] [Google Scholar]
- 57). Osaka K, Handa H, Matsumoto S, Yasuda M: Development of the cerebrospinal fluid pathway in the normal and abnormal human embryos. Childs Brain 6: 26– 38, 1980. [DOI] [PubMed] [Google Scholar]
- 58). Schwalbe G: Der arachnoidalraum ein lympharaum und sein zusammenhang mir den perichoroidalraum. Zentralbl Med Wiss 7: 465, 1869. [Google Scholar]
- 59). Dichiro G: Movement of the cerebrospinal fluid in human beings. Nature 204: 290– 291, 1964. [DOI] [PubMed] [Google Scholar]
- 60). Kida S, Pantazis A, Weller RO: CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol 19: 480– 488, 1993. [DOI] [PubMed] [Google Scholar]
- 61). Hashimoto PH: The cerebrospinal fluid as a tissue fluid of the nervous system. The role of CSF circulation and its clinical significance. Nervous System in Children 29: 217– 223, 2004. [Google Scholar]
- 62). Bulat M, Lupret V, Oresković D, Klarica M: Transventricular and transpial absorption of cerebrospinal fluid into cerebral microvessels. Coll Antropol 32 (Suppl 1): 43– 50, 2008. [PubMed] [Google Scholar]
- 63). Wislocki GB, Putnam TJ: Absorption from the ventricles in experimentally produced internal hydrocephalus. Am J Anat 29: 313– 320, 1921. [Google Scholar]
- 64). Sahar A, Hochwald GM, Ransohoff J: Alternate pathway for cerebrospinal fluid absorption in animals with experimental obstructive hydrocephalus. Exp Neurol 25: 200– 206, 1969. [DOI] [PubMed] [Google Scholar]
- 65). Naidich TP, Epstein F, Lin JP, Kricheff II, Hochwald GM: Evaluation of pediatric hydrocephalus by computed tomography. Radiology 119: 337– 345, 1976. [DOI] [PubMed] [Google Scholar]
- 66). Drayer BP, Rosenbaum AE, Higman HB: Cerebrospinal fluid imaging using serial metrizamide CT cisternography. Neuroradiology 13: 7– 17, 1977. [DOI] [PubMed] [Google Scholar]
- 67). Weller RO, Mitchell J: Cerebrospinal fluid edema and its sequelae in hydrocephalus. Adv Neurol 28: 111– 123, 1980. [PubMed] [Google Scholar]
- 68). Mortensen OA, Sullivan WE: The cerebrospinal fluid and the cervical lymph nodes. Anat Rec 56: 359– 363, 1933. [Google Scholar]
- 69). Bowsher D: Pathways of absorption of protein from the cerebrospinal fluid: an autoradiographic study in the cat. Anat Rec 128: 23– 39, 1957. [DOI] [PubMed] [Google Scholar]
- 70). Kozma M, Zoltãn OT, Csillik B: Die anatomischen Grundlagen des prälymphatischen Systems im Gehirn. Acta Anat (Basel) 81: 409– 420, 1972. [PubMed] [Google Scholar]
- 71). Oehmichen W, Genćić M: Experimental studies on kinetics and functions of monuclear phagozytes of the central nervous system. Acta Neurolpathol Suppl Suppl 6: 285– 290, 1975. [DOI] [PubMed] [Google Scholar]
- 72). Bradbury MW: Proportion of cerebrospinal fluid draining into jugular lymphatic trunks of the cat [Proceeding]. J Physiol 276: 67–68, 1978. [PubMed] [Google Scholar]
- 73). Bradbury MW, Cole DF: The role of the lymphatic system in drainage of cerebrospinal fluid and aqueous humour. J Physiol (Lond) 299: 353– 365, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74). Bradbury MW, Cserr HF, Westrop RJ: Drainage of cerebral interstitial fluid into deep cervical lymph of the rabbit. Am J Physiol 240: F329– F336, 1981. [DOI] [PubMed] [Google Scholar]
- 75). Cserr HF: Convection of brain interstitial fluid and its drainage into deep cervical lymph. Abstract 097, The International Symposium on Intracranial Pressure 1982, p 228 [Google Scholar]
- 76). McComb JG, Hyman S, Weiss MH: Lymphatic drainage of cerebrospinal fluid in the cat, in Shapiro K, Marmarou A, Portony H. (eds): Hydrocephalus. New York, Raven Press, 1984, pp 83–98 [Google Scholar]
- 77). Love JA, Leslie RA: The effects of raised ICP on lymph flow in the cervical lymphatic trunks in cats. J Neurosurg 60: 577– 581, 1984. [DOI] [PubMed] [Google Scholar]
- 78). Hasuo M, Asano Y, Teraoka M, Ikeyama A, Kageyama N: Cerebrospinal fluid absorption into the lymphatic system in increased intracranial pressure, in Ishii S, Nagai H, Brock M. (eds): Intracranial Pressure V. Berlin, Hidelberg, Springer-Verlag, 1983, pp 611–617 [Google Scholar]
- 79). Arnold W, Ritter R, Wagner WH: Quantitative studies on the drainage of the cerebrospinal fluid into the lymphatic system. Acta Otolaryngol 76: 156– 161, 1973. [DOI] [PubMed] [Google Scholar]
- 80). Jackson RT, Tigges J, Arnold W: Subarachnoid space of the CNS, nasal mucosa, and lymphatic system. Arch Otolaryngol 105: 180– 184, 1979. [DOI] [PubMed] [Google Scholar]
- 81). Szentistványi I, Patlak CS, Ellis RA, Cserr HF: Drainage of interstitial fluid from different regions of rat brain. Am J Physiol 246: F835– F844, 1984. [DOI] [PubMed] [Google Scholar]
- 82). Foltz E, Blanks J, Morton ME: Experimental transcerebral fistula. Perineural olfactory CSF flow in the normal, hydrocephalic, and postoperative hydrocephalic dog shown by radionuclide ventriculography. J Neurosurg 61: 355– 364, 1984. [DOI] [PubMed] [Google Scholar]
- 83). Field EJ, Brierley JB: The retro-orbital tissues as a site of outflow of cerebrospinal fluid. Proc R Soc Med 42: 447– 450, 1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84). Tripathi RC: The functional morphology of the outflow systems of ocular and cerebrospinal fluids. Exp Eye Res 25 Suppl: 65– 116, 1977. [DOI] [PubMed] [Google Scholar]
- 85). Gomez DG, Manzo RP, Fenstermacher JD, Potts DG: Cerebrospinal fluid absorption in the rabbit. Optic pathways. Graefes Arch Clin Exp Ophthalmol 226: 1– 7, 1988. [DOI] [PubMed] [Google Scholar]
- 86). Erlich SS, McComb JG, Hyman S, Weiss MH: Ultrastructure of the orbital pathway for cerebrospinal fluid drainage in rabbits. J Neurosurg 70: 926– 931, 1989. [DOI] [PubMed] [Google Scholar]
- 87). Gomez DG, Chambers AA, Di Benedetto AT, Potts DG: The spinal cerebrospinal fluid absorptive pathways. Neuroradiology 8: 61– 66, 1974. [DOI] [PubMed] [Google Scholar]
- 88). Elman R: Spinal arachnoid granulations with especial reference to the cerebrospinal fluid. Johns Hopkins Hosp Bull 34: 99– 104, 1923. [Google Scholar]
- 89). Welch K, Pollay M: The spinal arachnoid villi of the monkeys Cercopithecus aethiops sabaeus and Macaca irus. Anat Rec 145: 43– 48, 1963. [DOI] [PubMed] [Google Scholar]
- 90). Greitz D: Paradigm shift in hydrocephalus research in legacy of Dandy’s pioneering work: rationale for third ventriculostomy in communicating hydrocephalus. Childs Nerv Syst 23: 487– 489, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91). Edsbagge M, Tisell M, Jacobsson L, Wikkelso C: Spinal CSF absorption in healthy individuals. Am J Physiol Regul Integr Comp Physiol 287: R1450– R1455, 2004. [DOI] [PubMed] [Google Scholar]
- 92). Tubbs RS, Hansasuta A, Stetler W, Kelly DR, Blevins D, Humphrey R, Chua GD, Shoja MM, Loukas M, Oakes WJ: Human spinal arachnoid villi revisited: immunohistological study and review of the literature. J Neurosurg Spine 7: 328– 331, 2007. [DOI] [PubMed] [Google Scholar]
- 93). Fenstermacher JD, Patlak CS: The exchange of material between cerebrospinal fluid and brain, in Cserr HF, Fenstermacher JD, Fencl V. (eds): Fluid Environment of the Brain. New York, Academic Press Inc, 1975, pp 201–214 [Google Scholar]
- 94). Cserr HF, Cooper DN, Milhorat TH: Flow of cerebral interstitial fluid as indicated by the removal of extracellular markers from rat caudate nucleus. Exp Eye Res 25 Suppl: 461– 473, 1977. [DOI] [PubMed] [Google Scholar]
- 95). Raimondi AJ: A unifying theory for the definition and classification of hydrocephalus. Childs Nerv Syst 10: 2– 12, 1994. [DOI] [PubMed] [Google Scholar]
- 96). Agre P, Nielsen S, Ottersen OP: Towards a molecular understanding of water homeostasis in the brain. Neuroscience 129: 849– 850, 2004. [DOI] [PubMed] [Google Scholar]
- 97). Williams MA, McAllister JP, Walker ML, Kranz DA, Bergsneider M, Del Bigio MR, Fleming L, Frim DM, Gwinn K, Kestle JR, Luciano MG, Madsen JR, Oster-Granite ML, Spinella G: Priorities for hydrocephalus research: report from a National Institutes of Health-sponsored workshop. J Neurosurg 107 (5 Suppl): 345– 357, 2007. [DOI] [PubMed] [Google Scholar]
- 98). Tsutsumi S, Ito M, Yasumoto Y, Tabuchi T, Ogino I: The Virchow-Robin spaces: delineation by magnetic resonance imaging with considerations on anatomofunctional implications. Childs Nerv Syst 27: 2057– 2066, 2011. [DOI] [PubMed] [Google Scholar]
- 99). Di Chiro G: Observations on the circulation of the cerebrospinal fluid. Acta Radiol Diagn (Stockh) 5: 988– 1002, 1966. [DOI] [PubMed] [Google Scholar]
- 100). Cserr HF, Ostrach LH: Bulk flow of interstitial fluid after intracranial injection of blue dextran 2000. Exp Neurol 45: 50– 60, 1974. [DOI] [PubMed] [Google Scholar]
- 101). Cserr HF: Bulk flow of cerebral extracellular fluid as a possible mechanism of CSF-Brain exchange, in Cserr HF, Fenstermacher JD, Fencl V. (eds): Fluid Environment of the Brain. New York, Academic Press Inc, 1975, pp 215–224 [Google Scholar]
- 102). Yamada S, Miyazaki M, Kanazawa H, Higashi M, Morohoshi Y, Bluml S, McComb JG: Visualization of cerebrospinal fluid movement with spin labeling at MR imaging: preliminary results in normal and pathophysiologic conditions. Radiology 249: 644– 652, 2008. [DOI] [PubMed] [Google Scholar]
- 103). Bulat M: Dynamics and statics of the cerebrospinal fluid: the classic and new hypothesis, in Avezaat CJJ. (ed): Intracranial Pressure VIII. Berlin, Springer-Verlag, 1993, pp 726–730 [Google Scholar]
- 104). Fenstermacher J, Kaye T: Drug “diffusion” within the brain. Ann N Y Acad Sci 531: 29– 39, 1988. [DOI] [PubMed] [Google Scholar]
- 105). Crone C: The permeability of capillaries in various organs as determined by use of the ‘indicator diffusion’ method. Acta Physiol Scand 58: 292– 305, 1963. [DOI] [PubMed] [Google Scholar]
- 106). Renkin EM, Cron C: Microcirculation and capillary exchange, in Greger R, Windhorst U. (eds): Comprehensive Human Physiology. Berlin, Springer, 1996, pp 1965–1979 [Google Scholar]
- 107). Iliff JJ, Wang M., Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M: A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 4: 147ra111, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]