Abstract
This article reviews the current status of surgical treatment of epilepsy and introduces the ongoing challenges. Seizure outcome of resective surgery for focal seizures associated with focal lesions is satisfactory. Particularly for mesial temporal lobe epilepsy, surgical treatment should be considered from the earlier stage of the disease. Meanwhile, surgical outcome in nonlesional extratemporal lobe epilepsy is still to be improved using various approaches. Disconnective surgeries reduce surgical complications of extensive resections while achieving equivalent or better seizure outcomes. Multiple subpial transection is still being modified expecting a better outcome by transection to the vertical cortices along the sulci- and multi-directional transection from a single entry point. Hippocampal transection is expected to preserve memory function while interrupting the abnormal epileptic synchronization. Proper selection or combination of subdural and depth electrodes and a wide-band analysis of electroencephalography may improve the accurate localization of epileptogenic region. Patients for whom curative resective surgery is not indicated because of generalized or bilateral multiple nature of their epilepsies, neuromodulation therapies are options of treatment which palliate their seizures.
Keywords: epilepsy, epilepsy surgery, neuromodulation, intracranial electroencephalography
Introduction
Twenty to forty per cent of patients with epilepsy continue to have disabling seizures in spite of adequate treatment with antiepileptic drugs (AEDs).1) In the last decade, several new-generation AEDs were approved for use in Japan after years of delay from western nations. They include gabapentin, topiramate, lamotrigine, levetiracetam, stiripentol, and rufinamide. Additionally, oxcarbazepine, lacosamide, and perampanel are under clinical trial. The rate of patients who became seizure-free by addition of novel AEDs were 2% to 6% in randomized controlled trials (RCTs) and 10% to 20% in retrospective studies.2–4) Thus, a significant number of patients remain having seizures even after addition of new-generation AEDs, making surgical treatments and neuromodulation therapies indispensable for management of epilepsy. This article reviews the current status in those two treatment modalities to drug-resistant epilepsy.
As one of the most notable progresses in the last two decades, factors predicting a favorable outcome after resective surgery for epilepsy have been clarified.5) Resective surgery became a recommended treatment for patients with those favorable factors. Surgery in the earlier stage is now recommended particularly for mesial temporal lobe epilepsy (MTLE).6,7) Meanwhile, patients in whom seizure freedom is difficult to achieve even after surgery have been characterized as well. Conventional localization and resection of epileptic focus is not sufficient to achieve favorable outcomes. In the latter part of this article, various researches and efforts being made for improvement of the outcome are reviewed, including ours.
Clinical outcome after resective surgery for focal epilepsy
Seizure outcome after resective surgery is affected by the presence or absence of magnetic resonance imaging (MRI) lesion, pathological substrates of the associated lesion, extension and location of the epileptic focus, and selection criteria of patients for surgery.8) Particularly, the presence or absence of lesion powerfully affects seizure outcome. Seizure-free rates were larger than 70% in lesional cases but it was less than 50% in nonlesional cases (Table 1).
Table 1.
Seizure outcome of epilepsy surgery5)
| Seizure-free (%) | |||
|---|---|---|---|
| Non-lesional | Lesional | ||
| Children | All | 45 | 74 |
| Temporal | 45 | 81 | |
| Extratemporal | 46 | 73 | |
| Adults | All | 36 | 72 |
| Temporal | 45 | 72 | |
| Extratemporal | 26 | 53 | |
Numbers are percentages of patients who became free of disabling seizures after surgery. Follow-up period is longer than 1 year.
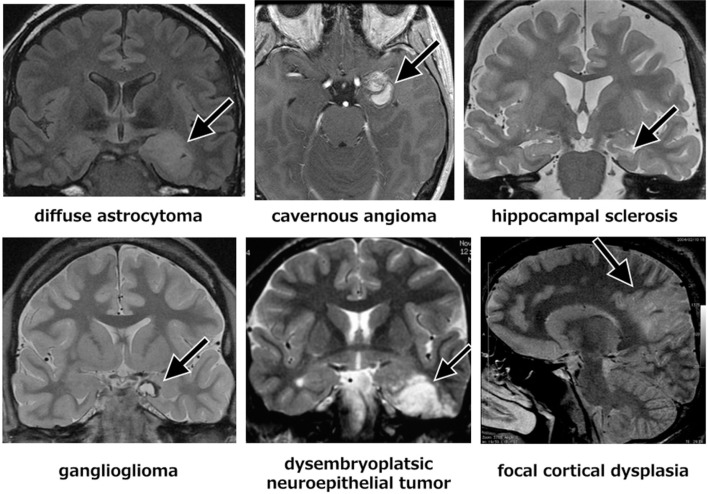
The localized lesions associated with focal epilepsy include hippocampal sclerosis, ganglioglioma, dysembryoplastic neuroepithelial tumor, diffuse astrocytoma, cavernous malformation, and focal cortical dysplasia (Fig. 1). The epileptogenic zone associated with these localized lesions is well-confined around the lesion in most cases, of which resection achieves good seizure control. Postsurgical seizure-free rates are 60–90% in hippocampal sclerosis, 85% in ganglioglioma, 70% in cavernous malformation, and 60–70% in focal cortical dysplasia.9–11)
Fig. 1.
Representative focal lesions associated with focal epilepsy demonstrated by magnetic resonance imaging.
A Canadian randomized controlled study published in 2001 clearly demonstrated the superiority of respective surgery over continuing medication for drug-resistant temporal lobe epilepsy (TLE).12) Seizure-free rates at a year after surgery or medical treatment were 58% and 8%, respectively. The American Academy of Neurology, American Epilepsy Society, and American Association of Neurological Surgeons published a practice parameter with recommendation that referral to an epilepsy surgery center should be strongly considered for patients who are compromised by disabling complex partial seizures based on this Class I study and many other Class IV studies.6) Early surgery is now recommended when adequate two AEDs failed to control seizure in patients with TLE.7)
Interestingly, the number of surgeries for TLE is rather decreasing recently in the United States and in the European nations.13–15) The reason for the decrease was multifactorial.14) First, the occurrence rate of MTLE decreased presumably owing to the improved and preventive care of pediatric diseases that precede MTLE such as febrile seizure. Second, many of the accumulated patients with MTLE underwent surgery in the early 1990s when MRI was introduced. Finally, the availability of newer AEDs may have led to better seizure control and a reduction in the use of surgery. We do not have exact statistics in Japan.
Surgical outcomes for non-MTLE have been accumulated as well, although the case numbers were smaller than MTLE.5,16–18) Seizure-free rates were significantly lower in extra-TLE than in TLE and in nonlesional epilepsy than in lesional epilepsy. It was only 26% in adult nonlesional extra-TLE.5) A long-term retrospective study recently reported from Mayo Clinic clearly demonstrated the difficulty to treat nonlesional extra-TLE.18) After the noninvasive presurgical evaluation, a clear hypothesis for seizure origin was possible for 47 of the 85 patients (55%), and 31 of these 47 patients (66%) proceeded to intracranial electroencephalography (EEG) monitoring. For 24 of these 31 patients undergoing long-term intracranial EEG (77%), a seizure focus was identified and surgically resected. Of these 24 patients, 9 (38%) had an excellent outcome, defined as Engel classes I–IIA, after resective epilepsy surgery. Therefore, while 38% of patients undergoing resective surgery had excellent outcomes, only 29% of patients undergoing intracranial EEG and only 11% of patient with nonlesional extratemporal lobe epilepsy had long-term excellent outcomes.
In general, the putative epileptogenic zone of nonlesional extra-TLE is diffuse and extensive. It is substantially difficult to localize and determine the area of resection even when intracranial EEG is used.16,18) While seizure control is expected to improve naturally in proportion to resection area, resection area is limited in most cases by the presence of adjacent eloquent areas.
Advances in Surgical Procedures
I. Disconnective surgery
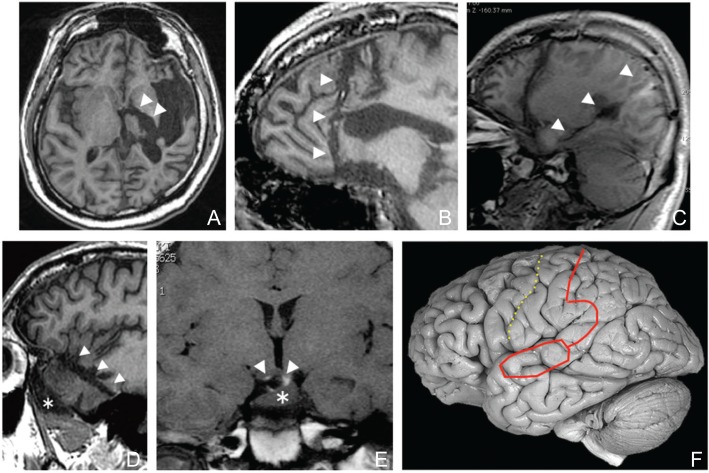
While resection of pathogenic lesion is the common basic concept of various neurosurgical procedures, disconnection of lesion is a concept specific for epilepsy surgery.19) Complete disconnection of the epileptogenic cortices from the surrounding cortices and downstream midbrain is sufficient for control of epileptic seizures even if the pathogenic lesion is left in situ. Hemispherotomy, prefrontal disconnection, and posterior disconnection were developed as replacements for hemispherectomy, frontal lobectomy, and posterior quadrantectomy, respectively. The concept is applicable as well for smaller lesions, such as those located within a lobe and hypothalamic hamartoma (Fig. 2). These disconnective procedures reduces surgical complications associated with extensive resection, such as blood loss and hydrocephalus, reduces operation time while achieving equivalent or better seizure outcomes.19–23)
Fig. 2.
Variation of disconnective surgery for epilepsy. Postsurgical MRIs demonstrating disconnection lines (arrowheads, A–E) and a conceptual figure of posterior disconnection (F). A: Hemispherotomy. B: Prefrontal disconnection. C: Posterior disconnection. This case underwent a previous prefrontal disconnection as well. D: Anterior temporal disconnection for temporal lobe epilepsy associated with a temporal tip encephalocele (asterisk). E: Disconnection of a hypothalamic hamartoma (asterisk) presenting with gelastic seizures. F: Solid line demonstrates disconnection line and dotted line demonstrates the central sulcus. MRI: magnetic resonance imaging.
Hemispherotomy was proposed as a surgical treatment for extensive epileptogenic zones in a hemisphere (Fig. 2A). The procedure reduces surgical invasiveness and occurrence of cerebrospinal fluid circulation. We recently proposed a further modification of the procedure for the purpose of less brain resection and confirmation of the complete disconnection.21) The surgical anatomy and concept of disconnection are apparently complicated and difficult to understand, but when the procedure is decomposed to each component to be disconnected i.e., the contralateral cortices and the ipsilateral mesencephalon. The commissural fibers and projection fibers are to be severed to these structures to be disconnected from epileptic brain, respectively.21) The fornix i.e., the projection fiber from the limbic system is to be severed as well. The major component of commissural fibers is the corpus callosum but the anterior commissure must be severed as well. Understanding the hemispherotomy procedure as a combination of the components to be disconnected makes it possible to tailor the procedure for each patient selecting best approach whether it is vertical or horizontal.
Prefrontal disconnection is a useful option for extensive epileptic focus of the frontal lobe (Fig. 2B). Briefly, from the disconnection line set at the dorsal prefrontal area, cutting with ultrasonic aspiration is made toward the sphenoid ridge. To avoid injury to the perforating arteries from the anterior and middle cerebral arteries, it is important to stay anterior to this plane. After reaching the sphenoid ridge, the residual posterior orbitofrontal cortex can be resected. Since the anterior perforated substance i.e., the entry points of the perforating arteries, is located just posterior to the medial and lateral striae of olfactory nerve, it is a useful technique to trace the olfactory nerve and find the olfactory trigone again to avoid injury to the perforating arteries.
Posterior disconnection is used for the extensive epileptic focus over the occipital, parietal, and temporal lobes.22,24,25) The surgical technique consists of an anterior temporal lobectomy followed by disconnection of the parietooccipital region (Fig. 2C, F). A posterior incision on the superior temporal gyrus is extended posteriorly, keeping the vein of Labbé intact, to reach the ventricle up to the trigone. The incision is then curved, crossing, but preserving, the sylvian vessels, and extending just posterior to the postcentral gyrus to the vertex. The incision is then deepened to reach the falx, transecting all the white matter from the corpus callosum to the sagittal sinus. The fornix is cut later to interrupt the hippocampal connections.
II. Non-resective surgery to minimize postsurgical functional deficit
For the treatment of epileptic foci overlapping the eloquent cortices such as the primary motor and language areas, multiple subpial transection was proposed for reducing the risk of devastating complications.26,27) The efficacy and complication varied among reports. Many centers have not adopted this approach and there is still some reservation regarding its use. The technique is still being modified to improve success rates and has changed considerably since it was initially described by Morrell in 1989.26) The outcome may improve by the use of an anesthetic agent that activates epileptiform discharges, transection to the cortices along the sulci in addition to the surface gyri, and multi-directional transection from a single entry point.27)
Postoperative memory deficit is unavoidable after the resection of the mesial temporal structures that appears normal in MRI on the language-dominant side. We developed multiple hippocampal transection, the hippocampal counterpart of cortical MST, that transects the epileptic synchronization along the longitudinal axis of the hippocampus and parahippocampal gyrus for preservation of memory function.28) It is suggested that synchronized discharges involving the complete anterior-posterior axis of the hippocampal formation underlie the spread of epileptiform discharges outside the hippocampal structures and, therefore, for the generation of epileptic seizures originating in the medial temporal lobe.29) This procedure is expected to preserve input to and output from the hippocampal formation as well as the polysynaptic circuits in the hippocampus, that are essential for memory formation and retrieval, while interrupting the abnormal synchronization associated with epilepsy. A few centers confirmed the preserved memory indices in neuropsychological evaluations and seizure outcome similar to the resection of the hippocampus.30–32)
Advances in Implantation Procedures of Intracranial Electrodes and Analysis of Intracranial EEG
There are two methods for intracranial recording of EEG, subdural electrodes, and depth electrodes. The former has been used mainly in the United States and Japan, and the latter mainly in the European countries. Each of them has advantages and drawbacks. Subdural electrodes cover the broad cortical surfaces but are difficult to be placed in the deep-seated cortices such as hippocampus, bottoms of sulci, and insular cortices. Many surgeons recently use the combination of them simultaneously.33) Stereotactic frame has been used for implantation of the depth electrodes but prevents craniotomy for implantation of subdural electrodes over broad areas of cortical surfaces. Usage of frameless stereotactic navigation system now allows the easy and accurate implantation of both types of electrodes simultaneously.34)
Owing to advances in computer analysis dealing with a large scale digital data and increased capacity of data storage, spatial density of electrodes, and frequency range of brain activity targeted for analysis have been dramatically increased. While spikes and sharp waves are considered as epileptogenic markers in conventional EEG, ictal direct current (DC) shifts and high-frequency oscillations (HFOs) are also implicated as epileptogenic markers in wide-band EEG.35–39) HFOs are usually defined as oscillatory activities higher than 80 Hz. Pathological HFOs in epilepsy primarily reflect clusters of action potentials of pyramidal cells and interneurons. Ictal DC shifts may reflect abnormal glial function.37)
While epileptic seizures are traditionally characterized as the hypersynchronous neuronal activity, examination of ictal firing patterns of single neurons revealed that neuronal spiking activity during seizure initiation and spread was highly heterogeneous, not hypersynchronous.40,41) The “epileptic ensemble or network” responsible for seizure generation are more complex and heterogeneous than previously thought. These findings were obtained for the first time using single unit recording from human cortices. Recording of single neuron activity is presently for research purpose and is being performed in highly selected centers in the United States and Germany.
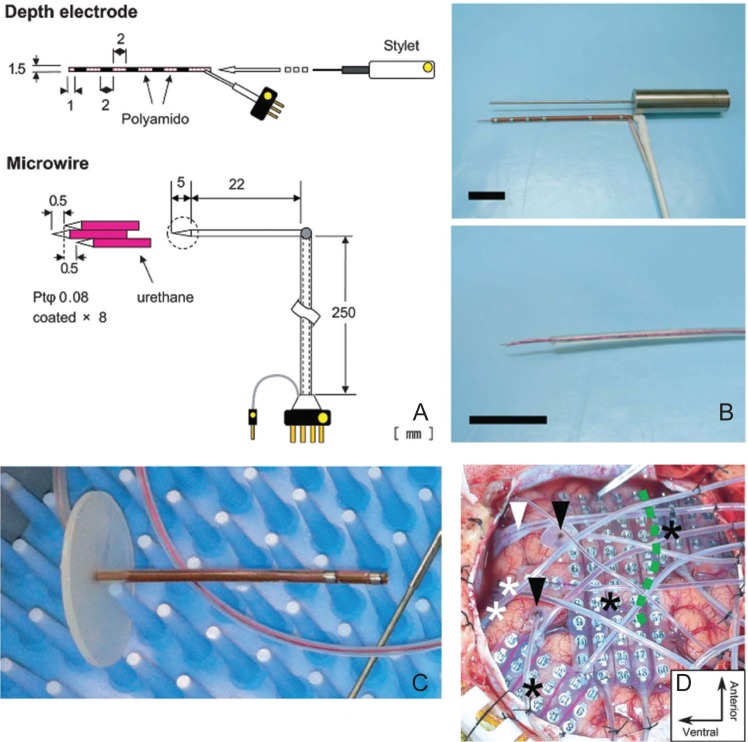
It was difficult to implant microwires and subdural electrodes during a single surgical operation because the stereotactic frame hampers flexible craniotomy. We developed newly designed electrodes that enable simultaneous recording from hippocampal neurons and broad areas of the cortical surface (Fig. 3). The depth electrode was designed so that it does not protrude into the dura and pulsates naturally with the brain. The length and tract of the depth electrode were determined preoperatively between the lateral subiculum and the lateral surface of the temporal lobe. A frameless navigation system was used to insert the depth electrode. Surface grids and ventral strips were placed before and after the insertion of the depth electrodes, respectively. Finally, a microwire bundle was inserted into the lumen of the depth electrode. As a result, depth-microwire electrodes were placed with a precision of 3.6 mm. The mean successful recording rate of single- or multiple-unit activity was 14.8%, which was maintained throughout the entire recording period.34)
Fig. 3.
Simultaneous recording of intracranial EEG and single neuron firing.34) A: Specifications of the electrode. B: Picture of the electrode. C: The cannula is equipped with EEG contacts and its length was predetermined from MRI. The microwires are inserted into the cannula. D: Intraoperative picture after insertion and placement of all electrodes. EEG: electroencephalography. MRI: magnetic resonance imaging.
Analysis of oscillatory brain activity recorded using intracranial electrodes has been introduced not only for localization of epileptogenic area but also for mapping of functional cortices. Augmentation of high gamma activity (HGA) at > 50 Hz reflects cortical activation and its distributions display strong correlations with various functions including motor, auditory, visual, language, episodic memory, working memory, and attention. We investigated and demonstrated the correlation among HGA, extraoperative electrocortical stimulation, and functional MRI as a language mapping in the clinical presurgical evaluation.42–44)
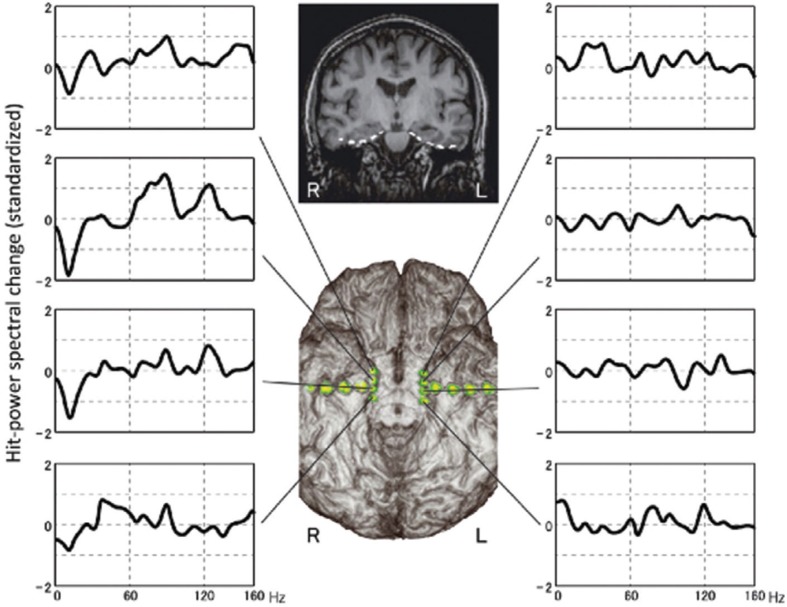
Surgery for TLEs with bilateral hippocampal sclerosis or without correspondent lesions carries a higher risk of devastating memory decline, underscoring the importance of establishing the memory-dominant side preoperatively, and adopting the most appropriate procedure. We analyzed HGA in the parahippocampal gyri and investigated the relationship between memory-related HGA and memory outcomes after hippocampal transection.45) Parahippocampal HGA could provide predictive information on whether the mesial temporal lobe can be resected without causing memory worsening. Therefore it was suggested we can select the optimal surgical strategy i.e., resection or transection, for atypical MTLE based on reliable memory lateralization from parahippocampal HGA measured using preoperative intracranial recording (Fig. 4).
Fig. 4.
A 35-year-old woman with mild atrophy in the left hippocampus underwent bilateral electrode placement on the parahippocampal gyri, because less invasive examinations failed to determine the epileptic focus. Long-term ECoG monitoring captured habitual seizures originating from the left hippocampus. Her verbal memory function was intact. Both Wada language and memory tests indicated dominant language and memory functions on the left side. Spectral analyses of the ECoG data obtained during the memory task revealed marked high gamma activity in the right parahippocampal gyrus. We performed hippocampal transection, not resection, on the left hippocampus to avoid permanent damage to her verbal memory function. Postoperative memory score, however, was normal even immediately after surgery, when transient deterioration occurs even after hippocampal transection.32) In this case, memory outcome agreed with parahippocampal high gamma activity, but not with the Wada test. According to the high gamma activity, we could have performed hippocampal resection without postoperative memory decline.45) ECoG: electrocorticography.
Neuromodulation Therapies for Epilepsy
Resective surgery for epilepsy is a curative procedure that is performed expecting to make patients seizure-free. Patients for whom curative resective surgery is not indicated because of generalized or bilateral multiple nature of their epilepsies, neuromodulation therapies are options of treatment which palliate their seizures. Currently, the single available modality in Japan is vagus nerve stimulation. However, a variety of treatments including deep brain stimulation (DBS) to various regions, closed loop reactive stimulation, and trigeminal nerve stimulation, are being developed and available in other countries.
I. Vagus nerve stimulation
Vagus nerve stimulation (VNS) therapy chronically and intermittently stimulates the left cervical vagus nerve (VN), generating afferent neural impulses that stabilize the cerebral cortex and alleviate seizures. It has been an established option of palliative and adjunctive treatment for drug-resistant epilepsy that is not suitable for resective craniotomy.46) It was approved for use by Food and Drug Administration (FDA) in 1997 after two RCTs.47,48) Seizure frequency decreased down to approximately 50% after continuation of the treatment for 2 years. The rate of responder with seizure reduction rate greater than 50% was approximately 50% after 2-year treatment.49) Since seizure-free rate is approximately 5%, it is not a curative treatment but a palliative one.
In contrast to its clinical usefulness and popularity, the underlying mechanisms of action in VNS have not been fully elucidated. Elucidating those mechanisms is important since it may help develop further effective treatment. Accumulating evidence suggests that ascending vagal signals modulate abnormal cortical excitability via various pathways.50–54) However, there had been no direct evidence for an ascending conduction of neural impulses in a clinical case of VNS. Therefore, we recorded and analyzed the short-latency components of the VN evoked potential (EP) from the viewpoint of determining whether or not it is a marker for the ascending neural conduction. The early component of VN-EP, around 3 ms, was regarded as directly resulting from ascending neural conduction of A fibers of the VN, probably originating around the jugular foramen.55)
It is also unknown how VNS exerts its inhibitory effect on seizure activity while ongoing seizure is aborted by VNS without immediate change in EEG.56,57) One possibility is that VNS modulates interaction among cortical neuronal population or cortical synchrony, thus returning the epileptogenic cortex from an abnormally hyperexcitable state to a stable one. Furthermore, beneficial effects of VNS include cognitive improvements for memory and decision making, modulation of mood, and enhanced plasticity.58–60) The variable effects other than seizure control suggest that VNS plays more general roles in maintenance of homeostatic property of cortical synchrony. We hypothesized that VNS-induced modulation of cortical activity be state-dependent in order to maintain neuronal synchrony within a normal range and investigated whether and how VNS modulates local synchrony in the cortex in a state-dependent manner. We measured the local field potential with high-spatial resolution using a microelectrode array in adult rats and demonstrated that VNS increased phase-locking value (PVL) between all pairs of electrodes in a normal state, particularly in high-γ band, but decreased it in δ and low-β bands and increased it in high-γ band in an epileptic state.61) Therefore, VNS modulates synchrony in a band-specific and state-dependent manner. VNS might keep cortical synchrony within the optimal state.
II. Intracranial neurostimulation for epilepsy
Various target locations in the brain were tried for DBS for epilepsy and the results have been reported in small series.62) Those targets were the centromedian nucleus of the thalamus, the hippocampus, the subthalamic nucleus, locus ceruleus, caudate nucleus, mammillary bodies, and the cerebellum. An RCT of bilateral stimulation of the anterior thalamic nuclei in North America was completed in 2009 and palliative effect for refractory seizures was reported.63) Median declines of seizure at the end of the blinded phase were 14.5% in the control group and 40.4% in the stimulated group. Complex partial and most severe seizures were significantly reduced by stimulation. By 2 years, there was a 56% median percent reduction in seizure frequency; 54% of patients had a seizure reduction of at least 50%. There were no major adverse effects but participants in the stimulated group were more likely to report depression or memory problems as adverse events. FDA required increasing the number of patients before approval while it was approved for use in the European Union (EU).
Several epilepsy centers have used hippocampal DBS as a treatment for epilepsy, most of which have reported its efficacy. The number of patients treated by this modality is still small and the clinical results by RCTs are being waited. Interestingly, it seems from those reports that the efficacy is similar or rather better in patients without MRI lesions than with MRI lesions.64) It is expected that the treatment work in a different mechanism from hippocampal resection that modulate and inhibit the wide-spread epileptic network.
A closed-loop electrical stimulation system is expected to abolish seizures by giving electrical stimulation to seizure foci or other locations responding to the detected start of seizures, while thus far reported VNS and DBS stimulate the targets by intermittent mode irrespective of the ictal or interictal phase. The clinical trial of responsive neurostimulation (RNS) system by NeuroPace started in 2004. A signal processor was implanted in the skull, which is connected to the intracranial electrodes to detect seizure, and to the depth or subdural electrodes that automatically stimulates epileptic cortices or thalamus. Double-blinded RCT with 191 patients confirmed its efficacy and safety and it was approved for use in 2013. Mean seizure reduction and responder rate after 2-year treatment were approximately 40% and 50%, respectively.65)
The recently published Cochrane review on DBS and cortical stimulation for epilepsy concluded that there is a need for more, large and well-designed RCTs to validate and optimize the efficacy and safety of invasive intracranial neurostimulation treatments since only short-term RCTs are available.66)
References
- 1). Kwan P, Brodie MJ: Early identification of refractory epilepsy. N Engl J Med 342: 314– 319, 2000. [DOI] [PubMed] [Google Scholar]
- 2). Costa J, Fareleira F, Ascenção R, Borges M, Sampaio C, Vaz-Carneiro A: Clinical comparability of the new antiepileptic drugs in refractory partial epilepsy: a systematic review and meta-analysis. Epilepsia 52: 1280– 1291, 2011. [DOI] [PubMed] [Google Scholar]
- 3). Cho YJ, Heo K, Kim WJ, Jang SH, Jung YH, Ye BS, Song DB, Lee BI: Long-term efficacy and tolerability of topiramate as add-on therapy in refractory partial epilepsy: an observational study. Epilepsia 50: 1910– 1919, 2009. [DOI] [PubMed] [Google Scholar]
- 4). Labiner DM, Ettinger AB, Fakhoury TA, Chung SS, Shneker B, Tatum Iv WO, Mitchell Miller J, Vuong A, Hammer AE, Messenheimer JA: Effects of lamotrigine compared with levetiracetam on anger, hostility, and total mood in patients with partial epilepsy. Epilepsia 50: 434– 442, 2009. [DOI] [PubMed] [Google Scholar]
- 5). Téllez-Zenteno JF, Hernández Ronquillo L, Moien-Afshari F, Wiebe S: Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res 89: 310– 318, 2010. [DOI] [PubMed] [Google Scholar]
- 6). Engel J, Wiebe S, French J, Sperling M, Williamson P, Spencer D, Gumnit R, Zahn C, Westbrook E, Enos B, Quality Standards Subcommittee of the American Academy of Neurology. American Epilepsy Society. American Association of Neurological Surgeons : Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology 60: 538– 547, 2003. [DOI] [PubMed] [Google Scholar]
- 7). Engel J, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, Sperling MR, Gardiner I, Erba G, Fried I, Jacobs M, Vinters HV, Mintzer S, Kieburtz K, Early Randomized Surgical Epilepsy Trial (ERSET) Study Group : Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA 307: 922– 930, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). de Tisi J, Bell GS, Peacock JL, McEvoy AW, Harkness WF, Sander JW, Duncan JS: The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet 378: 1388– 1395, 2011. [DOI] [PubMed] [Google Scholar]
- 9). Fauser S, Schulze-Bonhage A, Honegger J, Carmona H, Huppertz HJ, Pantazis G, Rona S, Bast T, Strobl K, Steinhoff BJ, Korinthenberg R, Rating D, Volk B, Zentner J: Focal cortical dysplasias: surgical outcome in 67 patients in relation to histological subtypes and dual pathology. Brain 127: 2406– 2418, 2004. [DOI] [PubMed] [Google Scholar]
- 10). Ferroli P, Casazza M, Marras C, Mendola C, Franzini A, Broggi G: Cerebral cavernomas and seizures: a retrospective study on 163 patients who underwent pure lesionectomy. Neurol Sci 26: 390– 394, 2006. [DOI] [PubMed] [Google Scholar]
- 11). Southwell DG, Garcia PA, Berger MS, Barbaro NM, Chang EF: Long-term seizure control outcomes after resection of gangliogliomas. Neurosurgery 70: 1406– 1413; discussion 1413–1414, 2012. [DOI] [PubMed] [Google Scholar]
- 12). Wiebe S, Blume WT, Girvin JP, Eliasziw M, Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group : A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 345: 311– 318, 2001. [DOI] [PubMed] [Google Scholar]
- 13). Englot DJ, Ouyang D, Garcia PA, Barbaro NM, Chang EF: Epilepsy surgery trends in the United States, 1990–2008. Neurology 78: 1200– 1206, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Van Gompel JJ, Ottman R, Worrell GA, Marsh R, Wetjen NM, Cascino GD, Meyer FB: Use of anterior temporal lobectomy for epilepsy in a community-based population. Arch Neurol 69: 1476– 1481, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Neligan A, Haliasos N, Pettorini B, Harkness WF, Solomon JK: A survey of adult and pediatric epilepsy surgery in the United Kingdom. Epilepsia 54: e62– e65, 2013. [DOI] [PubMed] [Google Scholar]
- 16). Ansari SF, Maher CO, Tubbs RS, Terry CL, Cohen-Gadol AA: Surgery for extratemporal nonlesional epilepsy in children: a meta-analysis. Childs Nerv Syst 26: 945– 951, 2010. [DOI] [PubMed] [Google Scholar]
- 17). Ansari SF, Tubbs RS, Terry CL, Cohen-Gadol AA: Surgery for extratemporal nonlesional epilepsy in adults: an outcome meta-analysis. Acta Neurochir (Wien) 152: 1299– 1305, 2010. [DOI] [PubMed] [Google Scholar]
- 18). Noe K, Sulc V, Wong-Kisiel L, Wirrell E, Van Gompel JJ, Wetjen N, Britton J, So E, Cascino GD, Marsh WR, Meyer F, Horinek D, Giannini C, Watson R, Brinkmann BH, Stead M, Worrell GA: Long-term outcomes after nonlesional extratemporal lobe epilepsy surgery. JAMA Neurol 70: 1003– 1008, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). De Ribaupierre S, Delalande O: Hemispherotomy and other disconnective techniques. Neurosurg Focus 2008; 25: E14. [DOI] [PubMed] [Google Scholar]
- 20). Delalande O, Fohlen M: Disconnecting surgical treatment of hypothalamic hamartoma in children and adults with refractory epilepsy and proposal of a new classification. Neurol Med Chir (Tokyo) 43: 61– 68, 2003. [DOI] [PubMed] [Google Scholar]
- 21). Kawai K, Morino M, Iwasaki M: Modification of vertical hemispherotomy for refractory epilepsy. Brain Dev 36: 124– 129, 2014. [DOI] [PubMed] [Google Scholar]
- 22). Sugano H, Nakanishi H, Nakajima M, Higo T, Iimura Y, Tanaka K, Hosozawa M, Niijima S, Arai H: Posterior quadrant disconnection surgery for Sturge-Weber syndrome. Epilepsia 55: 683– 689, 2014. [DOI] [PubMed] [Google Scholar]
- 23). Mohamed AR, Freeman JL, Maixner W, Bailey CA, Wrennall JA, Harvey AS: Temporoparietooccipital disconnection in children with intractable epilepsy. J Neurosurg Pediatr 7: 660– 670, 2011. [DOI] [PubMed] [Google Scholar]
- 24). Daniel RT, Meagher-Villemure K, Farmer JP, Andermann F, Villemure JG: Posterior quadrantic epilepsy surgery: technical variants, surgical anatomy, and case series. Epilepsia 48: 1429– 1437, 2007. [DOI] [PubMed] [Google Scholar]
- 25). Dorfer C, Czech T, Mühlebner-Fahrngruber A, Mert A, Gröppel G, Novak K, Dressler A, Reiter-Fink E, Traub-Weidinger T, Feucht M: Disconnective surgery in posterior quadrantic epilepsy: experience in a consecutive series of 10 patients. Neurosurg Focus 34: E10, 2013. [DOI] [PubMed] [Google Scholar]
- 26). Morrell F, Whisler WW, Bleck TP. Multiple subpial transection: a new approach to the surgical treatment of focal epilepsy. J Neurosurg 70: 231– 239, 1989. [DOI] [PubMed] [Google Scholar]
- 27). Ntsambi-Eba G, Vaz G, Docquier MA, van Rijckevorsel K, Raftopoulos C: Patients with refractory epilepsy treated using a modified multiple subpial transection technique. Neurosurgery 72: 890– 897; discussion 897–898, 2013. [DOI] [PubMed] [Google Scholar]
- 28). Shimizu H, Kawai K, Sunaga S, Sugano H, Yamada T: Hippocampal transection for treatment of left temporal lobe epilepsy with preservation of verbal memory. J Clin Neurosci 13: 322– 328, 2006. [DOI] [PubMed] [Google Scholar]
- 29). Umeoka SC, Lüders HO, Turnbull JP, Koubeissi MZ, Maciunas RJ: Requirement of longitudinal synchrony of epileptiform discharges in the hippocampus for seizure generation: a pilot study. J Neurosurg 116: 513– 524, 2012. [DOI] [PubMed] [Google Scholar]
- 30). Patil AA, Andrews R: Long term follow-up after multiple hippocampal transection (MHT). Seizure 22: 731– 734, 2013. [DOI] [PubMed] [Google Scholar]
- 31). Uda T, Morino M, Ito H, Minami N, Hosono A, Nagai T, Matsumoto T: Transsylvian hippocampal transection for mesial temporal lobe epilepsy: surgical indications, procedure, and postoperative seizure and memory outcomes. J Neurosurg 119: 1098– 1104, 2013. [DOI] [PubMed] [Google Scholar]
- 32). Kawai K, Kamada K, Ohta T, Momose T, Aoki S, Kawashima A, Saito N: Multiple hippocampal transection: seizure outcome and postoperative neuropsychometry. Epilepsia 47 (Suppl 4): 12– 13, 2006. [Google Scholar]
- 33). Desai A, Bekelis K, Darcey TM, Roberts DW: Surgical techniques for investigating the role of the insula in epilepsy: a review. Neurosurg Focus 32: E6, 2012. [DOI] [PubMed] [Google Scholar]
- 34). Matsuo T, Kawai K, Uno T, Kunii N, Miyakawa N, Usami K, Kawasaki K, Hasegawa I, Saito N: Simultaneous recording of single-neuron activities and broad-area intracranial electroencephalography: electrode design and implantation procedure. Neurosurgery 73 (2 Suppl Operative): ons146– ons154, 2013. [DOI] [PubMed] [Google Scholar]
- 35). Haegelen C, Perucca P, Châtillon CE, Andrade-Valença L, Zelmann R, Jacobs J, Collins DL, Dubeau F, Olivier A, Gotman J: High-frequency oscillations, extent of surgical resection, and surgical outcome in drug-resistant focal epilepsy. Epilepsia 54: 848– 857, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Jacobs J, Levan P, Châtillon CE, Olivier A, Dubeau F, Gotman J: High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain 132: 1022– 1037, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Kanazawa K, Matsumoto R, Imamura H, Matsuhashi M, Kikuchi T, Kunieda T, Mikuni N, Miyamoto S, Takahashi R, Ikeda A: Intracranially recorded ictal direct current shifts may precede high frequency oscillations in human epilepsy. Clin Neurophysiol 126: 47– 59, 2015. [DOI] [PubMed] [Google Scholar]
- 38). Worrell GA, Gardner AB, Stead SM, Hu S, Goerss S, Cascino GJ, Meyer FB, Marsh R, Litt B: High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain 131: 928– 937, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Zijlmans M, Jiruska P, Zelmann R, Leijten FS, Jefferys JG, Gotman J: High-frequency oscillations as a new biomarker in epilepsy. Ann Neurol 71: 169– 178, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Bower MR, Stead M, Meyer FB, Marsh WR, Worrell GA: Spatiotemporal neuronal correlates of seizure generation in focal epilepsy. Epilepsia 53: 807– 816, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Truccolo W, Donoghue JA, Hochberg LR, Eskandar EN, Madsen JR, Anderson WS, Brown EN, Halgren E, Cash SS: Single-neuron dynamics in human focal epilepsy. Nat Neurosci 14: 635– 641, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Kunii N, Kamada K, Ota T, Greenblatt RE, Kawai K, Saito N: The dynamics of language-related high-gamma activity assessed on a spatially-normalized brain. Clin Neurophysiol 124: 91– 100, 2013. [DOI] [PubMed] [Google Scholar]
- 43). Kunii N, Kamada K, Ota T, Kawai K, Saito N: A detailed analysis of functional magnetic resonance imaging in the frontal language area: a comparative study with extraoperative electrocortical stimulation. Neurosurgery 69: 590– 596; discussion 596–597, 2011. [DOI] [PubMed] [Google Scholar]
- 44). Kunii N, Kamada K, Ota T, Kawai K, Saito N: Characteristic profiles of high gamma activity and blood oxygenation level-dependent responses in various language areas. Neuroimage 65: 242– 249, 2013. [DOI] [PubMed] [Google Scholar]
- 45). Kunii N, Kawai K, Kamada K, Ota T, Saito N: The significance of parahippocampal high gamma activity for memory preservation in surgical treatment of atypical temporal lobe epilepsy. Epilepsia 55: 1594– 1601, 2014. [DOI] [PubMed] [Google Scholar]
- 46). Fisher RS, Handforth A: Reassessment: vagus nerve stimulation for epilepsy: a report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 53: 666– 669, 1999. [DOI] [PubMed] [Google Scholar]
- 47). Handforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, Tecoma ES, Henry TR, Collins SD, Vaughn BV, Gilmartin RC, Labar DR, Morris GL, 3rd, Salinsky MC, Osorio I, Ristanovic RK, Labiner DM, Jones JC, Murphy JV, Ney GC, Wheless JW: Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology 51: 48– 55, 1998. [DOI] [PubMed] [Google Scholar]
- 48). The Vagus Nerve Stimulation Study Group: A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. The Vagus Nerve Stimulation Study Group. Neurology 45: 224– 230, 1995. [DOI] [PubMed] [Google Scholar]
- 49). Elliott RE, Morsi A, Kalhorn SP, Marcus J, Sellin J, Kang M, Silverberg A, Rivera E, Geller E, Carlson C, Devinsky O, Doyle WK: Vagus nerve stimulation in 436 consecutive patients with treatment-resistant epilepsy: long-term outcomes and predictors of response. Epilepsy Behav 20: 57– 63, 2011. [DOI] [PubMed] [Google Scholar]
- 50). Groves DA, Bowman EM, Brown VJ: Recordings from the rat locus coeruleus during acute vagal nerve stimulation in the anaesthetised rat. Neurosci Lett 379: 174– 179, 2005. [DOI] [PubMed] [Google Scholar]
- 51). Manta S, Dong J, Debonnel G, Blier P: Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. J Psychiatry Neurosci 34: 272– 280, 2009. [PMC free article] [PubMed] [Google Scholar]
- 52). Zagon A, Kemeny AA: Slow hyperpolarization in cortical neurons: a possible mechanism behind vagus nerve simulation therapy for refractory epilepsy? Epilepsia 41: 1382– 1389, 2000. [DOI] [PubMed] [Google Scholar]
- 53). Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Meglio M, Colicchio G, Barba C, Papacci F, Tonali PA: Effects of vagus nerve stimulation on cortical excitability in epileptic patients. Neurology 62: 2310– 2312, 2004. [DOI] [PubMed] [Google Scholar]
- 54). Henry TR, Votaw JR, Pennell PB, Epstein CM, Bakay RA, Faber TL, Grafton ST, Hoffman JM: Acute blood flow changes and efficacy of vagus nerve stimulation in partial epilepsy. Neurology 52: 1166– 1173, 1999. [DOI] [PubMed] [Google Scholar]
- 55). Usami K, Kawai K, Sonoo M, Saito N: Scalp-recorded evoked potentials as a marker for afferent nerve impulse in clinical vagus nerve stimulation. Brain Stimul 6: 615– 623, 2013. [DOI] [PubMed] [Google Scholar]
- 56). Salinsky MC, Burchiel KJ: Vagus nerve stimulation has no effect on awake EEG rhythms in humans. Epilepsia 34: 299– 304, 1993. [DOI] [PubMed] [Google Scholar]
- 57). Zabara J: Inhibition of experimental seizures in canines by repetitive vagal stimulation. Epilepsia 33: 1005– 1012, 1992. [DOI] [PubMed] [Google Scholar]
- 58). Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA: Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci 2: 94– 98, 1999. [DOI] [PubMed] [Google Scholar]
- 59). Kilgard MP: Harnessing plasticity to understand learning and treat disease. Trends Neurosci 35: 715– 722, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60). Elger G, Hoppe C, Falkai P, Rush AJ, Elger CE: Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res 42: 203– 210, 2000. [DOI] [PubMed] [Google Scholar]
- 61). Usami K, Kano R, Kawai K, Noda T, Shiramatsu TI, Saito N, Takahashi H: Modulation of cortical synchrony by vagus nerve stimulation in adult rats. Conf Proc IEEE Eng Med Biol Soc 2013: 5348– 5351, 2013. [DOI] [PubMed] [Google Scholar]
- 62). Wu C, Sharan AD: Neurostimulation for the treatment of epilepsy: a review of current surgical interventions. Neuromodulation 16: 10– 24; discussion 24, 2013. [DOI] [PubMed] [Google Scholar]
- 63). Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, Oommen K, Osorio I, Nazzaro J, Labar D, Kaplitt M, Sperling M, Sandok E, Neal J, Handforth A, Stern J, DeSalles A, Chung S, Shetter A, Bergen D, Bakay R, Henderson J, French J, Baltuch G, Rosenfeld W, Youkilis A, Marks W, Garcia P, Barbaro N, Fountain N, Bazil C, Goodman R, McKhann G, Babu Krishnamurthy K, Papavassiliou S, Epstein C, Pollard J, Tonder L, Grebin J, Coffey R, Graves N, SANTE Study Group : Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 51: 899– 908, 2010. [DOI] [PubMed] [Google Scholar]
- 64). Cukiert A, Cukiert CM, Burattini JA, Lima AM: Seizure outcome after hippocampal deep brain stimulation in a prospective cohort of patients with refractory temporal lobe epilepsy. Seizure 23: 6– 9, 2013. [DOI] [PubMed] [Google Scholar]
- 65). Morrell MJ, RNS System in Epilepsy Study Group : Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 77: 1295– 1304, 2011. [DOI] [PubMed] [Google Scholar]
- 66). Sprengers M, Vonck K, Carrette E, Marson AG, Boon P: Deep brain and cortical stimulation for epilepsy. Cochrane Database Syst Rev 6: CD008497, 2014. [DOI] [PubMed] [Google Scholar]