Abstract
There has been a paradigm shift in the understanding of brain function. The intrinsic architecture of neuronal connections forms a key component of the cortical organization in our brain. Many imaging studies, such as noninvasive magnetic resonance imaging (MRI) studies, have now enabled visualization of the white matter fiber tracts interconnecting the functional cortical areas in the living brain. Although such a structural connectome is essential for understanding of cortical function, the anatomical information alone is not sufficient. Practically, few techniques allow the investigation of the excitatory and inhibitory mechanisms of the cortex in vivo in humans. Several attempts have been made to track neuronal connectivity by applying direct electrical stimuli to the brain in order to stimulate subdural and/or depth electrodes and record responses from the functionally connected cortex. In vivo single-pulse electrical stimulation (SPES) and/or cortico-cortical evoked potential (CCEP) were recently introduced to track various brain networks. This article reviews the concepts, significance, methods, mechanisms, limitations, and clinical applications of CCEP in the analysis of these dynamic connections.
Keywords: cortico-cortical evoked potential, neural network, cortico-cortical, language function, motor function
Introduction
Numerous anatomical components of the human brain and their couplings with each other are critically important for functional brain activity. Recently, there has been a paradigm shift in the understanding of brain function. The earlier concept that highly localized hierarchical structures in the brain form the interstitial steps between an environmental stimulus and a response has recently given way to the new concept that brain functions are mainly intrinsic and involve acquisition and integration of external stimuli in order to interpret and respond to environmental demands.1) Therefore, the intrinsic architecture of connections is the key component of cortical organization in the brain. The present article reviews the usefulness of cortico-cortical evoked potential (CCEP) in the analysis of these dynamic links, which has improved the knowledge available in this field.
Connectivity
In general, networks are a collection of nodes and edges, with the edges representing the relationships between two nodes. In the brain, a node may correspond to a certain area of cortex or a group of neurons with synchronized actions. In order to analyze brain function accurately, the empirical data must be represented in the form of a network. The key step in this process involves defining the nodes and/or edges, namely the relevant regions or parcels. Once the nodes are defined, their mutual association can be determined by measurements of structural, functional, or effective connectivity. These pairwise relationships can be assembled in the form of a network. Structural connectivity represents the collection of white matter fiber bundles, usually visualized with neuroanatomical techniques or neuroimaging [diffusion tensor imaging (DTI)]. Functional connectivity reveals symmetric statistical relationships, extracted from the regional time course of cortical activation. This can be derived by correlating data from electroencephalography (EEG)/magnetoencephalography (MEG) and/or functional magnetic resonance imaging (fMRI). Effective connectivity provides or shows inferences of directed interregional interactions. Many techniques of time-series analysis, such as Granger causality in EEG/MEG and/or fMRI have been used to trying to visualize these connections.2)
Structural Connectivity
At a macroscopic level, the cerebral cortex could be considered as a set of hierarchically organized functional areas, each of which are formed by large groups of neurons.3) Noninvasive MRI studies have now enabled visualization of the white matter fiber tracts that interconnect these areas in the living brain. In particular, DTI sequences have a great advantage in that the random microscopic motion of water molecules is biased in the direction of connective fiber pathways. Probabilistic maps of macroscopic inter-areal tracts can be generated by combining the pattern of diffusion biases across voxels.4) In addition, another method involving a region of interest (ROI) approach has been introduced and developed. This method produces virtual representations of white matter tracts, accurate to classical postmortem descriptions, for the reconstruction of the association, projection, and commissural pathways of the living human brain.5,6) With this method, many clinico-anatomical correlative studies have broadened the view of clinical anatomy beyond the cortical surface in order to encompass dysfunctions related to connecting pathways.7,8) Although such a structural connectome is essential for a complete understanding of cortical function, information about transmission alone is not sufficient. In other words, the limitation of DTI-based tractography is that it can resolve neither the function nor the direction of anatomical links. With these results, we can find the connections that exist between two important areas. However, we neither know what kind of information travels nor whether it is one way.
Functional connectivity is usually inferred based on correlations among measurements of neuronal activity, and is defined as statistical dependencies among remote neurophysiological events. However, actual correlations can arise in a variety of ways. Two or more brain regions seem functionally connected if their dynamic correlations are statistically dependent on one another, even in a variety of ways. While it is not practical to simultaneously record from large ensembles of identified neurons in multiple cortical areas of the human brain, noninvasive neurophysiological approaches, such as EEG, MEG, or fMRI, allow delineation of human functional connectivity at a modular level with fairly high spatial resolution.9) Although this functional connectome suggests that the information flow among the ROIs provides dynamic understanding of cortical function, it does not indicate the actual dynamics of information flow.
Effective connectivity refers explicitly to the causal influence that one neural system exerts over another, either at a synaptic or at a population level.10) There are two assumed approaches to prove effective connectivity: non-interventional and interventional. The non-interventional approaches are observational and infer causality through the analysis of simultaneous recordings of various areas in order to quantify the directionality of the functional connections with measures, such as Granger causality and dynamic causal models.11,12) In contrast, interventional approaches involve an empiric perturbation of activity in one area, which is the independent measure, and quantification of its impact or evoked response on other areas, which is the dependent measure. It is dynamic and depends on a model of interactions or coupling. The recent increase in interest in the research of stimulation-based analyses has provided new data on the investigation of the influence of directional connectivity on network topology. Interventional approaches, such as transcranial magnetic stimulation (TMS), transcranial direct current stimulation, or CCEP measure neural activity in the living human brain.
Review of the Literatures
A digital literature review was conducted using internet bibliography databases (PubMed), with the keywords “CCEP” or “cortico-cortical evoked potential.” We have found 19 papers related to TMS studies, 4 reviews13–16) and 23 original research articles of CCEP. Practically, few techniques allow the investigation of the excitatory and inhibitory mechanisms of the cortex in vivo in humans. Several attempts have been made to track neuronal connectivity by applying direct electrical stimuli to the brain in order to stimulate depth electrodes and record responses from the functionally connected cortex.17,18) In vivo single-pulse electrical stimulation (SPES) was recently introduced in humans to track various brain networks.19,20) In particular, the selective influence of premotor transcranial direct current stimulation on intracortical excitability of the ipsilateral M1 might be useful in diseases accompanied by pathological premotor activity.21) Local SPES has been studied not only in patients with mesial temporal lobe epilepsy but also in those with neocortical epilepsy. These studies have suggested that a cortical imbalance between excitation and inhibition is likely to be the pathophysiological basis for human partial epilepsy.22–24)
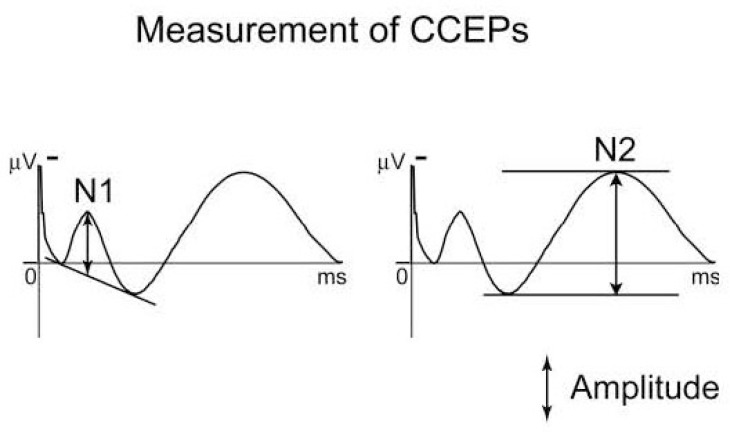
In a similar way, CCEP mapping begins with the injection of electrical current (monophasic, alternating in polarity, with 100–1,000 ms square pulse widths) between a pair of adjacent electrodes. Brief stimulation up to 15 mA is commonly tolerated without inducing afterdischarges or unwanted epileptiform discharges. This procedure is repeated 10–50 times to average the signal of the evoked response. CCEPs typically consist of an early (10–30 ms) negative surface deflection, N1, and a later (80–250 ms) slow wave, N2 (Fig. 1).25) This technique is advantageous and capable of evaluating the following: (a) cortical structures with a good spatial resolution as 1 cm in case of subdural electrodes implantation, (b) deep structures with a high spatial resolution (up to 5 mm) in case of SEEG, (c) directional dependence in signal propagation with a high temporal resolution in the order of millisecond, and (d) propagation patterns modulated by external stimuli.
Fig. 1.
Typical configuration of cortico-cortical evoked potentials (CCEPs). CCEP typically consists of an early (10–30 ms) negative surface deflection termed as N1, and a later (80–250 ms) slow wave as N2. The amplitude of N1 is measured as the height of a vertical line drawn from the negative peak of early component. The amplitude of N2 is measured as the maximum deflection through the measurement. Adapted with permission from Reference 25.
It has provided a unique opportunity to electrophysiologically track functional connectivity among different cortical regions,26,27) and also been specifically used to study the differences in cortical excitability between epileptogenic and normal cortical tissue.28–31) Therefore, it may become the “standard technique” against which newer neuroimaging methods of connectivity study are validated.32)
Possible Generating Mechanisms of CCEP
The generation of CCEPs consists of two phases: (a) the physiological status around the stimulation site and (b) the physiological change recorded at the projection site. If pyramidal cells play a major role in the generation of the induced current in the cortex, we should start with a discussion about stimulation-induced changes in these neurons. Current injected onto the cortical surface could affect local neurons through few assumed mechanisms. Many animal studies have suggested that CCEP generation primarily involves activation of the superficial dendritic tree of pyramidal cells in the external granular/pyramidal, internal pyramidal, and/or multiform layers. Considering the relatively late and blunt N1 potential, excitation could also occur indirectly through activation of ascending recurrent axon collaterals and excitatory interneuron.33,27) Second, local responses in the middle and deep pyramidal cells would then propagate down their axons to mono- and poly-synaptically connected regions through cortico-cortical and cortico-subcortical projections. Therefore, it is likely that responses to SPES in humans reflect both a major pyramidal cell contribution through orthodromic cortico-cortical and cortico-subcortico-cortical projections as well as a minor antidromic contribution.34) As seen from the shape and uniform orientation of the CCEPs, pyramidal cells are also dominant generators of field potentials at the recording site. The N1 of CCEPs bears great similarity to the early excitatory cortical response that results from feed-forward input, whereas the later N2 is reminiscent of the later response. It is likely that both locally-driven oscillations with sequences of excitation and inhibition, as well as recurrent relay volleys, contribute to this prolonged response to even the briefest stimulation.13)
I. Probing functional networks by CCEP: extraoperative investigation
Two different techniques are performed for intracranial monitoring of CCEP with chronically implanted electrodes. The grid and strip approach involves a craniotomy, followed by placement of two-dimensional strips or sheets of electrodes (typically, 3 mm diameter and 1 cm inter-electrode spacing), with which neural activity can be recorded from the surface of the cortex. In contrast, stereo-EEG involves multi-contact electrode leads that penetrate the brain, with which neural activity can be recorded from cerebral parenchyma. Some CCEP studies have provided new insights into the human limbic network,35) intimate connections among various regions of the limbic network in reverberating circuits,36) and the anatomical blueprint underlying the lateral parieto-frontal network.27) As for motor function, Matsumoto et al. have delineated the cortical motor network between the primary and premotor cortices.34) Subsequently, a continued series of CCEP studies related to the pre-supplementary motor area,37) supplementary motor area,38) and primary negative motor area39) have been conducted. In addition, CCEP studies have been reported not only in the hemisphere but also across the hemispheres. Both facial and non-facial motor areas send dense interhemispheric connections to the contralateral facial motor area.40) CCEP results have suggested a neural connection between the bilateral basal temporal regions, which correspond to the basal temporal language area.41) CCEP has also been used to clarify the perisylvian and extrasylvian areas that participate in the language system. In contrast to the classical Wernicke–Geschwind model, a CCEP study has revealed a bidirectional connection between Broca’s and Wernicke’s areas, probably through the arcuate fasciculus and/or the cortico-subcortico-cortical pathway.25) According to these CCEP results, we speculate that the two language areas are connected mainly through subcortical fibers from the posterior to the basal temporal language area that mediate mono- or oligo-synaptic transmissions.42,25) In addition, language reorganization might be associated with a functional shift from the termination of antero-posterior language connections to the surrounding cortices in patients with intractable partial epilepsy.13) It should be noted that language areas could be identified outside the antero-posterior language connection.43) CCEP has been applied to lobes other than the frontal/temporal lobe and even to rather deep areas, such as the insula. Stimulation of the lower-order visual cortex elicits an augmentation of gamma-activity in the higher-order visual cortex after the preceding CCEP subsides. The manner of propagation of stimulation-elicited cortical signals differed between feed-forward and feedback directions in the human occipital lobe.44) CCEP studies have suggested electrophysiological connections from the posterior cingulate gyrus to parietal, temporal, mesial occipital, and mesial frontal areas.45) The human insula is characterized by a rich and complex connectivity that varies as a function of the insular gyrus and appears to partly differ from the efferents described in nonhuman primates.46) Moreover, an analysis of the connectivity of Broca’s area with DTI tractography and CCEP was used to measure the spread of artificial currents. That this represents the electrical information flow by CCEP has been confirmed with the neural architecture that was visualized with DTI, which revealed network connectivity in the language system.47) CCEP responses are well correlated with resting state fMRI findings.48) Therefore, a multimodal group analysis co-registered to Brodmann areas enabled a multicenter, large-scale, and directional study of local and long-range cortical connectivity, which aids in the interpretation of the noninvasive functional connectome.26)
II. Intraoperative investigation
In addition to these extraoperative CCEP studies, with chronically implanted intracranial electrodes, two novel attempts have been made for its clinical application to intraoperative use: one to probe epileptogenicity and the other to map the whole functional network by combining cortical and subcortical 50 Hz and 1 Hz stimulation. Alarcon and his coworkers have extensively applied SPES to probe epileptogenicity in the interictal state in the chronic extraoperative setting. By applying SPES in a longer interval of such as 5 sec or 10 sec, they found that delayed responses occurs 100 ms to 1.5 sec after the electrical stimuli and that their distribution well correspond to the topography of the seizure onset zone.22,23) Recently they applied this approach in the intraoperative setting under general anesthesia and found that delayed responses can be reliably replicated in this circumstance and its distribution generally corresponded to that under chronic implantation. Intraoperative SPES could be used as a complimentary technique to improve intraoperative electrode placement during epilepsy surgery even when no definite interictal activity is present.49)
The combination of non-invasive functional and anatomical language studies such as fMRI and DTI, and invasive CCEP mapping could provide a definite map of both functional cortical regions and the termination of white matter connections. Such a map would enable tracking of exact neuronal connections, even during operations.50) Monitoring CCEP during an awake craniotomy is feasible during the resection of brain tumors affecting language-related cerebral structures. Sequential monitoring of the arcuate fasciculus successfully prevented persistent language impairment over 20 patients in our institute (unpublished data). It can also be used to predict the language outcome after surgery and determine the optimal resection of a neoplasm.51)
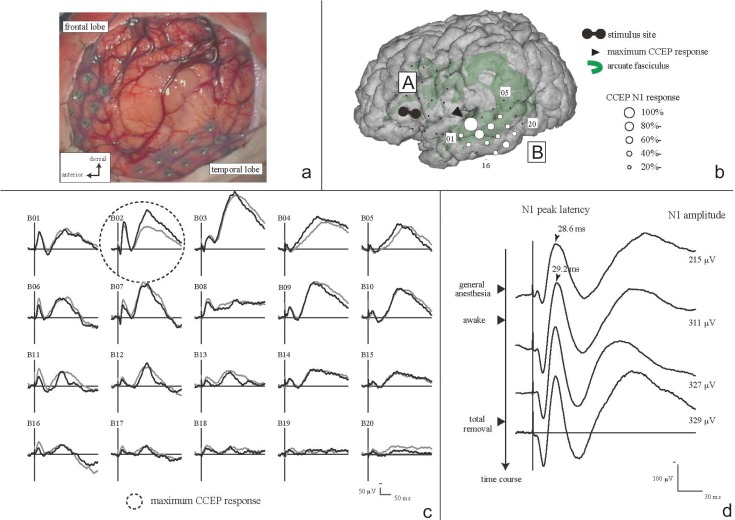
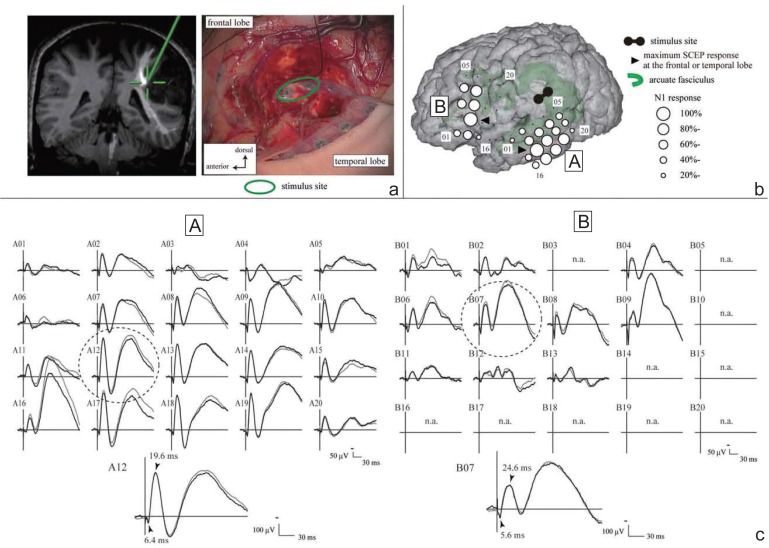
Practical procedures are illustrated as follows: The electrical stimulus, for the CCEP monitoring, consisted of constant current square wave pulses of alternating polarity with a pulse width of 0.3 ms, which were delivered at a fixed frequency of 1 Hz and intensity of 10–15 mA. Two adjacent electrodes were stimulated in a bipolar fashion to achieve more localized current flow in the cortex beneath the electrodes. A 32-channel intraoperative monitoring system (MEE 1232 Neuromaster, equipped with MS 120B electrical stimulator; Nihon-Kohden, Shinjuku-ku, Tokyo) was used in our institute for delivering electric currents, recording raw electrocorticogram (ECoG) to detect afterdischarges and/or EEG seizures, and on-line measurement of CCEP. All the data were digitized at a sampling rate of 5,000 Hz, referenced to a scalp electrode on the skin over the contralateral mastoid process. CCEP was obtained online by averaging ECoG time-locked to the stimulus onset, with a time window of 400 ms and a band-pass filter of 1–1,500 Hz. Reproducibility was confirmed by comparing at least two trials of CCEP. SPES was delivered to each candidate site of the anterior language area (AL) according to the results of preoperative fMRI and anatomical (probabilistic diffusion tensor tractography) MRI investigations. CCEP was recorded from the electrodes over the lateral temporo-parietal area covering the putative posterior language area (PL). A large CCEP response with an N1 peak around 20–40 ms in the PL was considered to represent the cortico-cortical connections between the AL and PL (Fig. 2).50) Based on the localization of the maximum CCEP response in the PL, the frontal stimulus site was selected for online sequential CCEP recordings. In other words, we determined the putative AL based on the locus of that led to peak CCEP amplitude evoked in PL (optimal CCEP connection representing the arcuate fasciculus). Throughout the surgical procedure, the integrity of the dorsal language pathway, namely, the arcuate fasciculus, can be monitored online by stimulating the putative AL and by recording CCEP from the putative PL, in a sequential fashion at 10–15 min of intervals. Of note, CCEP measurement is highly practical since (1) it is feasible even during general anesthesia, (2) it takes only a minute or less for each trial, and (3) it can probe connections even in the presence of brain edema that usually prevents accurate tract tracing by diffusion tractography. This intraoperative application has been validated by a different group.51) Intraoperative CCEP monitoring is clinically useful for evaluating the integrity of the language network (Fig. 2). In our case series, 32% decrease did not produce persistent language impairment.50) By analogy to MEP,52) further case accumulation is warranted to yield a certain cut-off value for establishing its clinical utility. Intraoperative investigation provided a unique opportunity to study the subcortical language network as well. After tumor removal, SPES has been applied to the white matter tracts beneath the floor of the removal cavity in order to trace its connections into the language-related cortices (Fig. 3). Judging from the latencies and distribution of CCEP and subcortico-cortical evoked potential (SCEP), the eloquent subcortical site was shown to connect directly with two different cortical language areas, which were already shown by CCEP to connect each other. Combined 50 Hz and 1 Hz white matter stimulation under awake craniotomy would be a promising method to probe the function and cortical targets of the large white matter bundles involved in higher brain functions such as language.
Fig. 2.
Cortico-cortical evoked potential (CCEP). a: Intraoperative view of electrodes placement over frontal/temporal lobe, arranged with tumor lesion. b: Scheme of electrodes placement and CCEP distribution under general anesthesia. Electrical stimuli were delivered on electrodes A02 and A07. CCEP distributed maximum at electrode B02 over the middle to posterior part of the temporal lobe. c: CCEP waveforms in plate B, before tumor removal, under awake condition. Two trials were superimposed to check the reproducibility. CCEP distribution did not change under between general anesthesia and awake condition. d: CCEP change along with surgical procedures at the maximum CCEP response site (N1 amplitude in electrode B02). CCEP waveforms were sequentially shown from the top to the bottom along the time course. As the patient became awake, the N1 amplitude increased from 215 mV to 311 mV (145%). After tumor removal the N1 amplitude did not decline (329 mV). The patient preserved normal language function throughout the perioperative period. Adapted with permission from Reference 50.
Fig. 3.
Subcortico-cortical evoked potential (SCEP). a: Site of paired electrical stimulation on white matter. A pair of electrodes (green circle) was stimulated at the floor of the tumor removal cavity (right). The site (green cross) was located close to the arcuate fasciculus passing (white line) in the co-registered image on the neuro-navigation system (left). b: Cortical responses, evoked by the SPES to the subcortical area in the floor of removal cavity (SCEP), were identified both in the frontal (B plate) and temporal (A plate) areas. c: Maximum SCEP response was recorded on the A12 electrode in temporal lobe, and the B07 electrode in frontal lobe (dotted circle). Their onset latencies of N1 were 6.4 ms and 5.6 ms, respectively. The summation of them (12.0 ms) was very close to the onset latency of CCEP (12.8 ms) between frontal and temporal regions in this case. Adapted with permission from Reference 50.
Limitations
As addressed above, CCEP is a powerful tool for detecting functional connections in cortical networks. However, there are some limitations to its potential clinical use. First of all, the reported CCEP could not correspond to completely normal networks because most of the data have been obtained in patients with intractable epilepsy or tumor. Second, CCEP data are, moreover, limited inside the coverage area of the used electrodes with the current technique especially in the regions of interest. Due to lack of information outside the coverage area, it is unknown whether there are connections other than those obtained by recorded CCEP. Third, the mechanisms of CCEP are too complicated to be fully understood. It clarified neither the normal range of latency nor the normal range of amplitude at each time component of N1 and N2. Therefore, we need much more case accumulation to establish the promising parameter and/or cutoff line to check. Fourth, there is, on the other hand, a wide variety in stimulation parameters, including the electrode type among research groups, which make it difficult to accumulate the consistent CCEP results across different research groups. Fifth, since several studies have obtained bidirectional CCEP responses, it is not clear whether the pathway is indeed bidirectional or reflecting orthodromic and/or antidromic transmission. Suitable input-output setting of CCEP monitoring has not been established. Finally, CCEP responses could be affected by the vigilance and/or cognitive states of the patients. We should be careful about the influence of anesthetic agents, especially during intraoperative CCEP monitoring. According to our preliminary observations, CCEP amplitude or morphology may change depending on the patients’ state, but the distribution would remain the same (unpublished data).
Conclusion
Although we addressed six limitations that need to be addressed for further understanding and/or development in the future, CCEP mapping represents a potentially powerful tool that could provide details of the organization of cortical networks with high spatial and temporal resolution. The brain is composed of numerous subregions with functional specializations, which are not isolated, but largely connected with each other. Therefore, this technique is a useful method not only in basic neuroscience validating noninvasive neuroimaging techniques, but also in clinical refinement in the field of neurosurgery.
Acknowledgments
This work was funded by the Grants-in-Aid for Scientific Research (KAKENHI)(B) (C), and Grant-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; Contract grant number: 26282218, 24592159, 25861273.
Department of Epilepsy, Movement Disorders and Physiology, Kyoto University Graduate School of Medicine is an endowment department, supported with grants by GlaxoSmithKline K.K., Nihon Kohden Corporation, Otsuka Pharmaceutical Co. Ltd., and UCB Japan Co. Ltd.
References
- 1). Raichle ME: A paradigm shift in functional brain imaging. J Neurosci 29: 12729– 12734, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Catani M, Thiebaut de Schotten M, Slater D, Dell’Acqua F: Connectomic approaches before the connectome. Neuroimage 80: 2– 13, 2013. [DOI] [PubMed] [Google Scholar]
- 3). Felleman DJ, Van Essen DC: Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1: 1– 47, 1991. [DOI] [PubMed] [Google Scholar]
- 4). Mukherjee P, Berman JI, Chung SW, Hess CP, Henry RG: Diffusion tensor MR imaging and fiber tractography: theoretic underpinnings. AJNR Am J Neuroradiol 29: 632– 641, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Catani M, Thiebaut de Schotten M: A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44: 1105– 1132, 2008. [DOI] [PubMed] [Google Scholar]
- 6). Mori S, Kaufmann WE, Pearlson GD, Crain BJ, Stieltjes B, Solaiyappan M, van Zijl PC: In vivo visualization of human neural pathways by magnetic resonance imaging. Ann Neurol 47: 412– 414, 2000. [PubMed] [Google Scholar]
- 7). Catani M, Dell’acqua F, Bizzi A, Forkel SJ, Williams SC, Simmons A, Murphy DG, Thiebaut de Schotten M: Beyond cortical localization in clinico-anatomical correlation. Cortex 48: 1262– 1287, 2012. [DOI] [PubMed] [Google Scholar]
- 8). Catani M, Howard RJ, Pajevic S, Jones DK: Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 17: 77– 94, 2002. [DOI] [PubMed] [Google Scholar]
- 9). Fox MD, Raichle ME: Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8: 700– 711, 2007. [DOI] [PubMed] [Google Scholar]
- 10). Friston KJ: Functional and effective connectivity: a review. Brain Connect 1: 13– 36, 2011. [DOI] [PubMed] [Google Scholar]
- 11). Brovelli A, Ding M, Ledberg A, Chen Y, Nakamura R, Bressler SL: Beta oscillations in a large-scale sensorimotor cortical network: directional influences revealed by Granger causality. Proc Natl Acad Sci USA 101: 9849– 9854, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Kiebel SJ, Garrido MI, Moran R, Chen CC, Friston KJ: Dynamic causal modeling for EEG and MEG. Hum Brain Mapp 30: 1866– 1876, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Keller CJ, Honey CJ, Mégevand P, Entz L, Ulbert I, Mehta AD: Mapping human brain networks with cortico-cortical evoked potentials. Philos Trans R Soc Lond B Biol Sci 369: pii, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Roland PE, Hilgetag CC, Deco G: Cortico-cortical communication dynamics. Front Syst Neurosci 8: 19, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Shibasaki H: Cortical activities associated with voluntary movements and involuntary movements. Clin Neurophysiol 123: 229– 243, 2012. [DOI] [PubMed] [Google Scholar]
- 16). Zappoli R: Permanent or transitory effects on neurocognitive components of the CNV complex induced by brain dysfunctions, lesions and ablations in humans. Int J Psychophysiol 48: 189– 220, 2003. [DOI] [PubMed] [Google Scholar]
- 17). Brugge JF, Volkov IO, Garell PC, Reale RA, Howard MA: Functional connections between auditory cortex on Heschl’s gyrus and on the lateral superior temporal gyrus in humans. J Neurophysiol 90: 3750– 3763, 2003. [DOI] [PubMed] [Google Scholar]
- 18). Wilson CL, Isokawa M, Babb TL, Crandall PH: Functional connections in the human temporal lobe. I. Analysis of limbic system pathways using neuronal responses evoked by electrical stimulation. Exp Brain Res 82: 279– 292, 1990. [DOI] [PubMed] [Google Scholar]
- 19). Lacruz ME, García Seoane JJ, Valentin A, Selway R, Alarcón G: Frontal and temporal functional connections of the living human brain. Eur J Neurosci 26: 1357– 1370, 2007. [DOI] [PubMed] [Google Scholar]
- 20). Oya H, Poon PW, Brugge JF, Reale RA, Kawasaki H, Volkov IO, Howard MA, 3rd: Functional connections between auditory cortical fields in humans revealed by Granger causality analysis of intra-cranial evoked potentials to sounds: comparison of two methods. Biosystems 89: 198– 207, 2007. [DOI] [PubMed] [Google Scholar]
- 21). Boros K, Poreisz C, Münchau A, Paulus W, Nitsche MA: Premotor transcranial direct current stimulation (tDCS) affects primary motor excitability in humans. Eur J Neurosci 27: 1292– 1300, 2008. [DOI] [PubMed] [Google Scholar]
- 22). Valentín A, Alarcón G, García-Seoane JJ, Lacruz ME, Nayak SD, Honavar M, Selway RP, Binnie CD, Polkey CE: Single-pulse electrical stimulation identifies epileptogenic frontal cortex in the human brain. Neurology 65: 426– 435, 2005. [DOI] [PubMed] [Google Scholar]
- 23). Valentin A, Anderson M, Alarcón G, Seoane JJ, Selway R, Binnie CD, Polkey CE: Responses to single pulse electrical stimulation identify epileptogenesis in the human brain in vivo. Brain 125 (Pt 8): 1709– 1718, 2002. [DOI] [PubMed] [Google Scholar]
- 24). Wilson CL, Khan SU, Engel J, Isokawa M, Babb TL, Behnke EJ: Paired pulse suppression and facilitation in human epileptogenic hippocampal formation. Epilepsy Res 31: 211– 230, 1998. [DOI] [PubMed] [Google Scholar]
- 25). Matsumoto R, Nair DR, LaPresto E, Najm I, Bingaman W, Shibasaki H, Luders HO: Functional connectivity in the human language system: a cortico-cortical evoked potential study. Brain 127 (Pt 10): 2316– 2330, 2004. [DOI] [PubMed] [Google Scholar]
- 26). Entz L, Tóth E, Keller CJ, Bickel S, Groppe DM, Fabó D, Kozák LR, Erőss L, Ulbert I, Mehta AD: Evoked effective connectivity of the human neocortex. Hum Brain Mapp 35: 5736– 5753, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Matsumoto R, Nair DR, Ikeda A, Fumuro T, Lapresto E, Mikuni N, Bingaman W, Miyamoto S, Fukuyama H, Takahashi R, Najm I, Shibasaki H, Lüders HO: Parieto-frontal network in humans studied by cortico-cortical evoked potential. Hum Brain Mapp 33: 2856– 2872, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Enatsu R, Jin K, Elwan S, Kubota Y, Piao Z, O’Connor T, Horning K, Burgess RC, Bingaman W, Nair DR: Correlations between ictal propagation and response to electrical cortical stimulation: a cortico-cortical evoked potential study. Epilepsy Res 101: 76– 87, 2012. [DOI] [PubMed] [Google Scholar]
- 29). Enatsu R, Piao Z, O’Connor T, Horning K, Mosher J, Burgess R, Bingaman W, Nair D: Cortical excitability varies upon ictal onset patterns in neocortical epilepsy: a cortico-cortical evoked potential study. Clin Neurophysiol 123: 252– 260, 2012. [DOI] [PubMed] [Google Scholar]
- 30). Iwasaki M, Enatsu R, Matsumoto R, Novak E, Thankappen B, Piao Z, O’Connor T, Horning K, Bingaman W, Nair D: Accentuated cortico-cortical evoked potentials in neocortical epilepsy in areas of ictal onset. Epileptic Disord 12: 292– 302, 2010. [DOI] [PubMed] [Google Scholar]
- 31). Matsumoto R, Kinoshita M, Taki J, Hitomi T, Mikuni N, Shibasaki H, Fukuyama H, Hashimoto N, Ikeda A: In vivo epileptogenicity of focal cortical dysplasia: a direct cortical paired stimulation study. Epilepsia 46: 1744– 1749, 2005. [DOI] [PubMed] [Google Scholar]
- 32). David O, Job AS, De Palma L, Hoffmann D, Minotti L, Kahane P: Probabilistic functional tractography of the human cortex. Neuroimage 80: 307– 317, 2013. [DOI] [PubMed] [Google Scholar]
- 33). Matsumoto R, Nair D: Cortico-cortical evoked potentials to define eloquent cortex, in Lüders HO. (ed): Textbook of Epilepsy Surgery. Abingon, Taylor & Francis Books Ltd., 2007, pp 1049– 1059 [Google Scholar]
- 34). Matsumoto R, Nair DR, LaPresto E, Bingaman W, Shibasaki H, Lüders HO: Functional connectivity in human cortical motor system: a cortico-cortical evoked potential study. Brain 130 (Pt 1): 181– 197, 2007. [DOI] [PubMed] [Google Scholar]
- 35). Kubota Y, Enatsu R, Gonzalez-Martinez J, Bulacio J, Mosher J, Burgess RC, Nair DR: In vivo human hippocampal cingulate connectivity: a corticocortical evoked potentials (CCEPs) study. Clin Neurophysiol 124: 1547– 1556, 2013. [DOI] [PubMed] [Google Scholar]
- 36). Enatsu R, Gonzalez-Martinez J, Bulacio J, Kubota Y, Mosher J, Burgess RC, Najm I, Nair DR: Connections of the limbic network: a corticocortical evoked potentials study. Cortex 62: 20– 33, 2015. [DOI] [PubMed] [Google Scholar]
- 37). Swann NC, Cai W, Conner CR, Pieters TA, Claffey MP, George JS, Aron AR, Tandon N: Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: electrophysiological responses and functional and structural connectivity. Neuroimage 59: 2860– 2870, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Kikuchi T, Matsumoto R, Mikuni N, Yokoyama Y, Matsumoto A, Ikeda A, Fukuyama H, Miyamoto S, Hashimoto N: Asymmetric bilateral effect of the supplementary motor area proper in the human motor system. Clin Neurophysiol 123: 324– 334, 2012. [DOI] [PubMed] [Google Scholar]
- 39). Enatsu R, Matsumoto R, Piao Z, O’Connor T, Horning K, Burgess RC, Bulacio J, Bingaman W, Nair DR: Cortical negative motor network in comparison with sensorimotor network: a cortico-cortical evoked potential study. Cortex 49: 2080– 2096, 2013. [DOI] [PubMed] [Google Scholar]
- 40). Terada K, Umeoka S, Usui N, Baba K, Usui K, Fujitani S, Matsuda K, Tottori T, Nakamura F, Inoue Y: Uneven interhemispheric connections between left and right primary sensori-motor areas. Hum Brain Mapp 33: 14– 26, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Umeoka S, Terada K, Baba K, Usui K, Matsuda K, Tottori T, Usui N, Nakamura F, Inoue Y, Fujiwara T, Mihara T: Neural connection between bilateral basal temporal regions: cortico-cortical evoked potential analysis in patients with temporal lobe epilepsy. Neurosurgery 64: 847– 855; discussion 855, 2009. [DOI] [PubMed] [Google Scholar]
- 42). Araki K, Terada K, Usui K, Usui N, Araki Y, Baba K, Matsuda K, Tottori T, Inoue Y: Bidirectional neural connectivity between basal temporal and posterior language areas in humans. Clin Neurophysiol 19: pii, S1388– S2457, 2014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43). Enatsu R, Kubota Y, Kakisaka Y, Bulacio J, Piao Z, O’Connor T, Horning K, Mosher J, Burgess RC, Bingaman W, Nair DR: Reorganization of posterior language area in temporal lobe epilepsy: a cortico-cortical evoked potential study. Epilepsy Res 103: 73– 82, 2013. [DOI] [PubMed] [Google Scholar]
- 44). Matsuzaki N, Juhász C, Asano E: Cortico-cortical evoked potentials and stimulation-elicited gamma activity preferentially propagate from lower- to higher-order visual areas. Clin Neurophysiol 124: 1290– 1296, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45). Enatsu R, Bulacio J, Nair DR, Bingaman W, Najm I, Gonzalez-Martinez J: Posterior cingulate epilepsy: clinical and neurophysiological analysis. J Neurol Neurosurg Psychiatr 85: 44– 50, 2014. [DOI] [PubMed] [Google Scholar]
- 46). Almashaikhi T, Rheims S, Jung J, Ostrowsky-Coste K, Montavont A, De Bellescize J, Arzimanoglou A, Keo Kosal P, Guénot M, Bertrand O, Ryvlin P: Functional connectivity of insular efferences. Hum Brain Mapp 35: 5279– 5294, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47). Conner CR, Ellmore TM, DiSano MA, Pieters TA, Potter AW, Tandon N: Anatomic and electro-physiologic connectivity of the language system: a combined DTI-CCEP study. Comput Biol Med 41: 1100– 1109, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48). Keller CJ, Bickel S, Entz L, Ulbert I, Milham MP, Kelly C, Mehta AD: Intrinsic functional architecture predicts electrically evoked responses in the human brain. Proc Natl Acad Sci USA 108: 10308– 10313, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49). Kokkinos V, Alarcón G, Selway RP, Valentín A: Role of single pulse electrical stimulation (SPES) to guide electrode implantation under general anaesthesia in presurgical assessment of epilepsy. Seizure 22: 198– 204, 2013. [DOI] [PubMed] [Google Scholar]
- 50). Yamao Y, Matsumoto R, Kunieda T, Arakawa Y, Kobayashi K, Usami K, Shibata S, Kikuchi T, Sawamoto N, Mikuni N, Ikeda A, Fukuyama H, Miyamoto S: Intraoperative dorsal language network mapping by using single-pulse electrical stimulation. Hum Brain Mapp 35: 4345– 4361, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51). Saito T, Tamura M, Muragaki Y, Maruyama T, Kubota Y, Fukuchi S, Nitta M, Chernov M, Okamoto S, Sugiyama K, Kurisu K, Sakai KL, Okada Y, Iseki H: Intraoperative cortico-cortical evoked potentials for the evaluation of language function during brain tumor resection: initial experience with 13 cases. J Neurosurg 121: 827– 838, 2014. [DOI] [PubMed] [Google Scholar]
- 52). Macdonald DB: Intraoperative motor evoked potential monitoring: overview and update. J Clin Monit Comput 20: 347– 377, 2006. [DOI] [PubMed] [Google Scholar]