Abstract
Vagus nerve stimulation (VNS) provides palliation of seizure reduction for patients with medically refractory epilepsy. VNS is indicated for symptomatic localization-related epilepsy with multiple and bilateral independent foci, symptomatic generalized epilepsy with diffuse epileptogenic abnormalities, refractory idiopathic generalized epilepsy, failed intracranial epilepsy surgery, and other several reasons of contraindications to epilepsy surgery. Programing of the parameters is a principal part in VNS. Output current and duty cycle should be adjusted to higher settings particularly when a patient does not respond to the initial setting, since the pivotal randomized trials performed in the United States demonstrated high stimulation made better responses in seizure frequency. These trials revealed that a ≥ 50% seizure reduction occurred in 36.8% of patients at 1 year, in 43.2% at 2 years, and in 42.7% at 3 years in 440 patients. Safety of VNS was also confirmed because side effects including hoarseness, throat discomfort, cough, paresthesia, and headache improved progressively during the period of 3 years. The largest retrospective study with 436 patients demonstrated the mean seizure reduction of 55.8% in nearly 5 years, and also found 75.5% at 10 years in 65 consecutive patients. The intermediate analysis report of the Japan VNS Registry showed that 60% of 164 cases got a ≥ 50% seizure reduction in 12 months. In addition to seizure reduction, VNS has positive effects in mood and improves energy level, memory difficulties, social aspects, and fear of seizures. VNS is an effective and safe option for patients who are not suitable candidates for intracranial epilepsy surgery.
Keywords: vagus nerve stimulation, medically refractory epilepsy, indications, programing, seizure reduction
Introduction
Anti-epileptic drugs (AEDs) are the fundamentals in the treatment of epilepsy. However, patients who do not attain seizure control with the first two drug regimens including combinations within 1–2 years since the beginning of treatment are unlikely to achieve remission. Then 30–40% of patients still suffer seizures after multiple trials of AEDs, and are considered to have medically refractory epilepsy.1,2) These patients should take further evaluations such as long-term video electroencephalographic (EEG) monitoring, neuroimaging studies like fluorodeoxyglucose positron emission tomography (FDG-PET), and neuropsychological tests for possible curative epilepsy surgery using craniotomy.3,4) In fact, somewhere between 10% and 50% of these are potential surgical candidates even after thorough examinations.5) This is the reason why we need palliative procedures besides epilepsy surgery. Vagus nerve stimulation (VNS) is one of the non-medical treatments and a palliative option to reduce epileptic seizures for medically refractory epilepsy. An implanted generator stimulates the vagus nerve in the left neck intermittently. VNS has proved a great boon for epilepsy patients who are not good candidates for curative surgery. More than 900 VNS Therapy systems (Cyberonics Inc., Houston, Texas, USA) in Japan and 115,000 devices for 80,000 patients worldwide have been implanted and working to palliate intractable seizures by the end of 2014.
Overview of Indications for Non-pharmacological Treatment Options
VNS is not regarded as a front-line treatment for epilepsy at the present. The Ministry of Health, Labour, and Welfare (MHLW) in Japan approved the VNS Therapy system in the Pharmaceutical Affairs Law and defined its indication as follows in January 2010. “The VNS Therapy system is an electric stimulation device to give impulses to the vagus nerve as an adjunctive treatment reducing seizure frequency for patients with medically refractory epilepsy. However, patients who are suitable candidates for epilepsy surgery should be excluded.” The terms of approval do not refer to particular indications such as epileptic syndromes or patients’ age. Then patients with generalized epilepsy or even children such as infants can be candidates for VNS based on the regulation in Japan. On the other hand, Food and Drug Administration (FDA) in the United States approved VNS in 1997, a long time ahead of Japan. However, VNS is still officially indicated for adults and adolescents over 12 years of age with partial-onset seizures that are refractory to AEDs.
I. Surgically remediable syndromes
It is necessary to understand the indications for intracranial epilepsy surgery before discussing and distinguishing indications for VNS.6) Surgically remediable syndromes are defined as7):
the pathophysiology is understood
the natural history is reasonably well known to be medically refractory or even progressive once the major first-line AEDs fail
presurgical evaluation can be accomplished noninvasively
surgery offers an excellent chance, 60% or greater, that disabling seizures will be completely eliminated
The following are the examples of these syndromes7):
mesial temporal lobe epilepsy (MTLE), that is often associated with hippocampal sclerosis
lesional focal epilepsies caused by discrete structural lesions that can be resected
catastrophic unilateral or secondary generalized epilepsies of infants and young children, related to disturbances confined to one hemisphere such as hemimegalencephaly, Sturge–Weber syndrome, Rasmussen encephalitis, cortical dysplasias, and porencephalic cysts
medically refractory epilepsy with disabling drop attacks
MTLE is one of the best indications for resective surgery since the randomized controlled trial demonstrated superior surgical results by anterior temporal lobectomy as compared to those by medical treatment (Class I evidence).8,9) If a seizure focus is obvious and resectable after evaluations including invasive monitoring with intracranial electrodes, focus resection should be prioritized before VNS. Diffuse hemispheric lesions of one side are indicated for hemispherotomy or multilobar resection.10) Patients suffering injuries with drop attacks are good candidates for corpus callosotomy (CC).11)
II. Indications for VNS
If a patient is not appropriate for cranial procedures discussed above, VNS should be considered as an alternative option. Thence, who are the suitable candidates for VNS?12–14) In a large series from one institution, the most common type of epilepsy was multifocal localization-related epilepsy (39.7%) followed by idiopathic generalized epilepsy (IGE) (17.2%) and symptomatic generalized epilepsy (16.3%). These underlying etiologies included cerebral palsy/static encephalopathy (8.0%), infection (7.6%), and neuronal migration disorders (7.6%).13)
1. Symptomatic localization-related epilepsy with multiple and bilateral independent foci
Even thorough evaluations are not able to narrow down to a single focus in this group of patients. Then if one of the foci is resected, it might be difficult to get freedom from seizure events. Main etiologies are neuronal migration disorder, cerebral palsy/static encephalopathy, traumatic brain injury, infection like encephalitis, tuberous sclerosis complex, and genetic/metabolic syndromes.13,14) These etiologies can make multiple and bilateral independent foci. VNS might also be considered for bilateral temporal lobe epilepsy when dominance of seizure onset is not easily determined.
2. Cryptogenic or symptomatic generalized epilepsy with diffuse epileptogenic abnormalities
One of the prototypes in this group is Lennox–Gastaut syndrome (LGS). These patients are possible candidates for VNS and also CC. However, it is always discussed which palliative procedure should be chosen first. Comparisons between VNS and CC have been done in many literatures.15) The meta-analysis found that CC is significantly more effective than VNS in achieving a 50% and 75% frequency reduction of atonic seizures in LGS. Then CC is especially indicated for patients with drop attacks as mentioned above. For all other seizure types such as tonic, generalized tonic-clonic, complex partial, and myoclonic seizures, VNS offers comparable rates to CC.15)
3. Refractory IGE
Most of patients with IGE are well treated only with AEDs, and lead uneventful clinical course. Some of them with intractability need VNS, however. One preliminary study demonstrated that patients with refractory IGE enjoyed both a greater reduction in seizure frequency and medication burden of AEDs by VNS as compared to those with refractory localization-related epilepsy.16)
4. Failed intracranial epilepsy surgery
If a patient chooses and undergoes a resective procedure of a seizure focus or disconnection surgery, not every patient can achieve seizure freedom. Some of them still suffer seizures postoperatively. Patients without complete resolution should take reassessment. However, reoperation would be occasionally more difficult, and would not be indicated in every case. Then VNS is indicated and works even after these invasive procedures.17)
5. Several reasons of contraindications to epilepsy surgery
After thorough discussion with the patient and the family, there might be several reasons such as memory issues, eloquent cortex such as frontal or temporal language areas overlapping ictal onset zone, etc. to hesitate going through a surgical intervention.13) VNS would be an alternative before a patient undergoes epilepsy surgery.
III. Representative cases
Case 1: The most severe case that is not suitable for intracranial epilepsy surgery
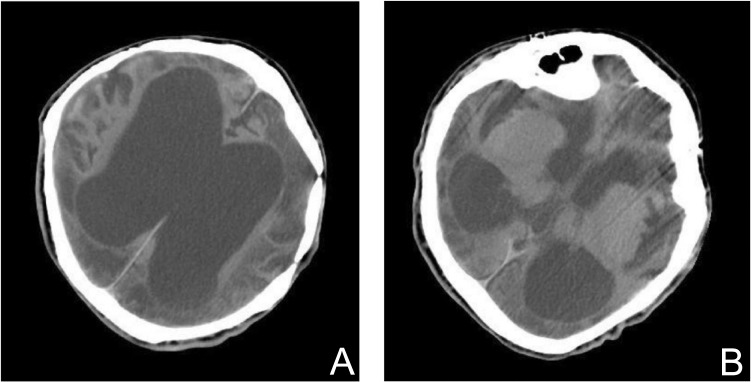
An 18-year-old male with remarkable developmental delay underwent multiple surgeries for Apert syndrome in his infancy. He sustained hypoxic brain damage postoperatively after the repair of malformed extremities. Then he developed medically refractory epilepsy with uncountable daily complex partial seizures with motionless staring and generalized tonic-clonic seizures. Almost all AEDs were tried without any results of reduction in seizure frequency. He was occasionally admitted to a local hospital because of status epilepticus and then referred to us for further evaluations and treatments. A computed tomographic scan demonstrated remarkable atrophy of the entire brain (Fig. 1A, B). Video-EEG monitoring revealed multiple bilateral spikes and sharp wave interictal discharges particularly over both the frontal through temporal regions. The seizures originated from bilateral temporal lobes independently with frequent secondary generalizations. Then conventional intracranial epilepsy surgery such as focus resection was not indicated for this patient. The VNS Therapy system was implanted and started stimulation. However, he did not show any improvements though more than a year after implantation. Its parameters were gradually adjusted to higher settings because the patient complained of discomfort around the throat. Finally he demonstrated a ≥ 50% seizure reduction almost 4 years after implantation.
Fig. 1.
Case 1: A computed tomographic scan revealed remarkable atrophy of the entire brain due to postoperative hypoxic brain damage after the repair of malformed extremities in infancy. Vagus nerve stimulation was the choice of treatment because the patient was severely disabled and electroencephalography demonstrated multiple and bilateral independent foci.
Case 2: An aged patient after stroke with poor general condition
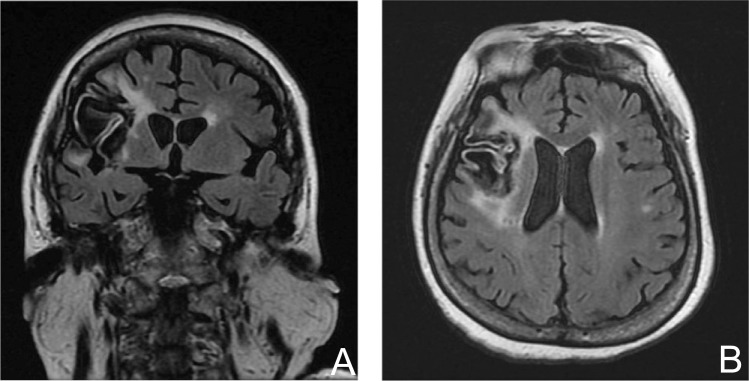
A 65-year-old female suffered from hemorrhagic infarction due to embolic stroke (Fig. 2A, B). The patient developed status epilepticus 7 months after the onset of cerebral infarction. She was hospitalized at intensive care unit (ICU) of a nearby hospital and placed on a ventilator. Medical evaluations revealed possible myelodysplastic syndrome, and she was taking warfarin to prevent recurrent embolic stroke. Her semiology was daily complex partial seizures with secondary generalizations. Video-EEG monitoring was carried out and demonstrated diffuse interictal spikes throughout the right hemisphere. Over 1 day monitoring period, four typical partial seizures were recorded during sleep. These habitual ictal events were characterized by awakening with a sudden versive turning of the eyes and head to the left, followed by a tonic extension of the left upper extremity with a subsequent generalized tonic-clonic seizure. The ictal EEG showed a diffuse spike maximally at the Fp2 electrode over the right hemisphere occurred after diffuse attenuation, followed by a high-voltage continuous spike activity. This evolved into a rhythmic delta activity (3 Hz) within 20 sec. Her seizures were resistant to a combination of 500 mg topiramate (Kyowa Hakko Kirin Co., Ltd., Tokyo), 900 mg carbamazepine (Novartis Pharma K.K., Tokyo), and 400 mg zonisamide (Sumitomo Dainippon Pharma Co., Ltd., Osaka). If we consider epilepsy surgery, placement of intracranial electrodes should be indispensable. However, her general condition could not tolerate the invasive procedure. Then she underwent implantation of the VNS Therapy system and showed excellent response in seizure frequency from daily to weekly events.
Fig. 2.
Case 2: A magnetic resonance imaging demonstrated large cerebral infarction of the frontal and temporal lobes. Intracranial epilepsy surgery was contraindicated because her general condition was not sufficient for invasive procedures. Then vagus nerve stimulation was chosen as a palliative option.
Programing
Stimulation of the vagus nerve is recommended to start 2 weeks after implantation of the VNS Therapy system. This slowness of activating stimulation might alleviate irritative sensation of the neck after complete healing of the surgical wound, particularly around the vagus nerve. The initial setting is as follows:
-
[Normal Mode]
The VNS Therapy system stimulates the vagus nerve intermittently and regularly as it is programed.- Output Current: 0.25 mA
- Signal Frequency: 30 Hz
- Pulse Width: 500 μsec
- Signal On Time: 30 sec
- Signal Off Time: 5.0 min
-
[Magnet Mode]
When an on-demand stimulation is necessary, a patient or a caregiver passes the Cyberonics magnet packed in the Patient Essentials kit over the pulse generator for at least 1 sec, a single Magnet Mode stimulation can start.- Magnet Current: 0.5 mA
- Magnet Signal Frequency: automatically follows the Normal Mode setting, usually 30 Hz at the beginning
- Magnet Pulse Width: 500 μsec
- Magnet On Time: 60 sec
There is no exact rule to follow in programing of VNS. However, adjustment to higher settings of the programing parameters is usually performed on a monthly basis,12) and is instructed in the Dosing Course Example (Table 1) of the VNS TherapyTM General Dosing Guidelines (Cyberonics Inc.) or VNS TherapyTM Technical Guide (Cyberonics Inc.).
Table 1.
Dosing course example
| Office visit 1 | Office visit 2 | Office visit 3 | Office visit 4 | Office visit 5 | Office visit 6 | Office visit 7 | Office visit 8 | |
|---|---|---|---|---|---|---|---|---|
| Output current (mA) | 0.25 | 0.5 | 0.75 | 1.0 | 1.25 | 1.5 | 1.5 | 1.5 |
| Signal frequency (Hz) | 20/30 | 20/30 | 20/30 | 20/30 | 20/30 | 20/30 | 20/30 | 20/30 |
| Pulse width (μsec) | 250/500 | 250/500 | 250/500 | 250/500 | 250/500 | 250/500 | 250/500 | 250/500 |
| Signal on time (seconds) | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Signal off time (minutes) | 5 | 5 | 5 | 5 | 5 | 5 | 3 | 1.8 |
| Magnet current (mA) | 0.5 | 0.75 | 1.0 | 1.25 | 1.5 | 1.75 | 1.75 | 1.75 |
| Magnet on time (seconds) | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 |
| Magnet pulse width (μsec) | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 |
Courtesy of Cyberonics Inc. and Nihon Kohden.
It is obvious that higher stimulation is more effective as shown in the previous studies. Two pivotal trials, the EO3 study and the EO5 study were carried out for patients with partial epilepsy.18,19) These two studies were multicenter, blind, randomized trials and compared two different types of stimulation patterns: high stimulation (30 Hz, 30 sec on, 5 min off, 500 μsec pulse width) and low stimulation (1 Hz, 30 sec on, 90–180 min off, 130 μsec pulse width). In the EO3 study, the mean seizure reduction was 6.1% at 12 weeks in the low-stimulation group and 24.5% in the high-stimulation group (p = 0.01).18) In the EO5 study correspondingly, average seizure reductions were 15% and 28% for low- and high-stimulation groups, respectively (p = 0.039).19) Both studies well demonstrated that high stimulation was more effective than low stimulation.20) Thus higher output current is necessary if a patient does not show any improvement in the early phase of VNS. Twenty percent of initial non-responders showed response after current intensity was increased.21)
Duty cycle is another important element in programing, and is calculated as (ON time + 4 sec)/(ON time + OFF time), for which ON and OFF time are measured in seconds.12,22–24) The efficacy of VNS improved significantly over the first year on the original settings of 30 sec on and 5 min off. Some patients who were initially resistant to VNS may benefit from reductions in off time to ≤ 1.1 min (increases in duty cycle).22) Duty cycles can be adjusted up to 49%. However, continuous high frequency (≥ 50 Hz) stimulation could cause nerve injury, and should be avoided (Tables 1, 2).23,25) Within the limits of safety, patients who do not respond to initial settings may respond to incremental increases in output current and duty cycle.
Table 2.
Duty cycles for various ON and OFF times
| Duty cycles (% ON time) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ON time (sec) | OFF time (min) | ||||||||
| 0.2 | 0.3 | 0.5 | 0.8 | 1.1 | 1.8 | 3 | 5 | 10 | |
| 7 | 58% | 44% | 30% | 20% | 15% | 10% | 6% | 4% | 2% |
| 14 | 69 | 56 | 41 | 29 | 23 | 15 | 9 | 6 | 3 |
| 21 | 76 | 64 | 49 | 36 | 29 | 19 | 12 | 8 | 4 |
| 30 | 81 | 71 | 57 | 44 | 35 | 25 | 16 | 10 | 5 |
| 60 | 89 | 82 | 71 | 59 | 51 | 38 | 27 | 18 | 10 |
 Not recommended.
Not recommended.
Courtesy of Cyberonics, Inc. and Nihon Kohden.
Some of the patients who are treated by higher output current or higher duty cycle may complain of throat pain and tingling sensation. Then downgrade adjustments of frequency and/or pulse width help alleviating these adverse effects.12,23)
If patients or their caregivers can sense an aura or a simple partial seizure during the initial stages of seizure events, on-demand stimulations with the Cyberonics magnet may abort, shorten, or even terminate seizures. The output current setting of the Magnet Mode should be 0.25 mA higher than the Normal Mode in many patients and increased until activation by the magnet works effectively.26)
Outcomes of VNS Therapy
I. Long-term efficacy in seizure control
In addition to the short-term double-blind trials, EO3 and EO5, which demonstrated its safety and efficacy as described above,18,19) there have been two prospective long-term follow-up studies.27,28) The median reduction at 12 months after completion of the initial double-blind study was 45%. Thirty-five percent of patients had a seizure reduction of more than 50% and 20% had a reduction of more than 75%.27) In the EO1–EO5 study, 440 patients were followed up for as long as 3 years. A ≥ 50% seizure reduction occurred in 36.8% of patients at 1 year, in 43.2% at 2 years, and in 42.7% at 3 years. Safety of VNS was also demonstrated, since side effects including hoarseness, throat discomfort, cough, paresthesia, and headache improved progressively during the period of 3 years.28)
The largest retrospective study with 436 patients demonstrated the mean seizure reduction of 55.8% in nearly 5 years of mean follow-up duration, and also found the mean reduction at 10 years was 75.5% in 65 consecutive patients.13,29) They concluded that long-term titration of stimulation parameters and adjustments of AEDs might help maximize the effectiveness of VNS over time.13,17)
Although VNS for children younger than 12 years is still off-label usage in the United States, many pediatric studies have been done.14,30) These studies reported comparable efficacy for children as for adult patients. When VNS was used in comprehensive approach including aggressive AED regimens and epilepsy surgery, more than 50% of children achieved at least a 50% seizure reduction. The rate of complications was also similar in patients younger than 12 years of age and those 12 years of age or older.14)
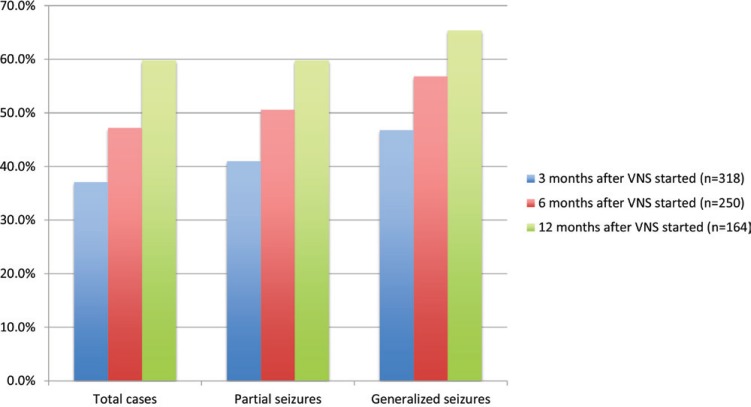
More than 10 years had passed since the approval of VNS in Europe and in the United States when MHLW approved VNS in Japan. The Japan Neurosurgical Society, the Japan Epilepsy Society, and the Epilepsy Surgery Society of Japan organized the VNS Qualifying Committee to keep quality in expanding VNS for Japanese patients, and launched an investigation and report for entire VNS cases for 3 years since January 2010 according to the request from the Pharmaceuticals and Medical Devices Agency. The intermediate analysis report of 321 cases revealed excellent results with seizure reduction of 60% in partial seizures, 68% in generalized seizures, and 58% in total cases respectively 12 months after VNS started (Table 3). The responder rates with a ≥ 50% seizure reduction were 60% in partial seizures, 65% in generalized seizures, and 60% in total cases (Fig. 3).31)
Table 3.
Seizure reduction by vagus nerve stimulation in the intermediate analysis report of the Japan VNS Registry
| Total cases | Seizure classification | |||
|---|---|---|---|---|
| Partial seizures | Generalized seizures | |||
| 3 months after VNS started | Number of cases | 318 | 199 | 233 |
| Mean (SD) | 2.81% (179.41%) | −3.19% (167.84%) | −3.95% (228.90%) | |
| Median (Min∼Max) | −20.00% (−100.0∼1,400.0%) | −20.00% (−100.0∼1,400.0%) | −37.50% (−100.0∼2,507.1%) | |
| 6 months after VNS started | Number of cases | 250 | 164 | 185 |
| Mean (SD) | −15.27% (135.41%) | −26.92% (94.64%) | 31.15% (778.38%) | |
| Median (Min∼Max) | −41.19% (−100.0∼1,400.0%) | −50.00% (−100.0∼650.0%) | −56.25% (−100.0∼10,328.6%) | |
| 12 months after VNS started | Number of cases | 164 | 107 | 127 |
| Mean (SD) | −26.84% (140.70%) | −27.16% (134.98%) | −35.75% (171.21%) | |
| Median (Min∼Max) | −58.36% (−100.0∼1,400.0%) | −60.00% (−100.0∼842.9%) | −67.75% (−100.0∼1,700.0%) | |
Modified from Kawai31) and courtesy of Nihon Koden. Max: maximum, Min: minimum, SD: standard deviation, VNS: vagus nerve stimulation.
Fig. 3.
Responder rates with a ≥ 50% seizure reduction by vagus nerve stimulation (VNS). Modified from Kawai31) and courtesy of Nihon Koden.
II. Secondary effects by VNS
A particular interest was focused on mood improvements by VNS in epilepsy patients. The study was carried out as an addition to the international multisite randomized and double-blind trial on seizure control by VNS (EO3), and demonstrated significant positive mood effects, which was independent of effects on seizure activity. Depressive symptoms were reduced in seizure responders as expected. However, positive mood changes were also shown in 75% of patients who did not respond to VNS in seizure frequency.32) The mechanisms of action in mood by VNS are not well understood as efficacy of seizure reduction in epilepsy, and then further research is necessary.
Other studies were performed to explore changes in health-related quality of life among patients treated by VNS.33,34) Patients were evaluated with Quality of Life in Epilepsy-10 (QOLIE-10), and demonstrated significant improvements such as energy level, memory difficulties, social aspects, mental effects, and fear of seizures.34) VNS implantation and therapy is associated with a persistent and positive improvement in subjective quality of life.33)
Cost reduction is also one of the essential issues for patients with medically refractory epilepsy, since they consume a large amount of medical resources. Several studies revealed positive cost-benefit influence on health-care utilization such as outpatient visits, emergency room visits, length of hospital stay, and number of hospital admissions.35,36) Before VNS, the mean yearly epilepsy-related direct medical cost per patient was US$ 8,830 and the average number of hospital stay per year was 21 days. At 12 months after implantation, the cost decreased to US$ 4,215 (p = 0.018) and the hospital stay to 8 days (p = 0.023). Then they concluded that the cost of VNS was saved within 2 years following implantation.35)
The Near Future of Neuromodulation in Epilepsy
Neuromodulation is a promising new technology and now flourishing in many diseases of the central nervous system.37) Deep brain stimulation has been primarily used to treat movement disorders, and is now tried to apply for intractable epilepsy by stimulating the anterior nucleus of thalamus.38) The Responsive Neurostimulation (RNS) System (NeuroPace Inc., Mountain View, California, USA) is one of the recent devices approved by FDA and needs more complicated procedures as compared to those of VNS implantation. The stimulator is implanted in the skull under the scalp and connected to one or two depth and/or subdural strip leads that are implanted according to the seizure focus presumed by preoperative evaluations. This device continuously senses electrocorticographic activity and provide pulses of stimulation in response to the detection.39) Thus, thorough workup and meticulous surgical planning will be probably mandatory for implantation of this neurostimulator.
On the other hand, less invasive neuromodulators have been developed.40,41) Trigeminal nerve stimulation (TNS) is performed by using a device named MonarchTM eTNSTM System (NeuroSigma Inc., Los Angeles, California, USA), which delivers mild electrical signals stimulating branches of the trigeminal nerve on the forehead.40) Transcutaneous VNS (t-VNS) is done by NEMOS (Cerbomed GmbH, Erlangen, Deutschland), which stimulates the auricular branch of the vagus nerve.41)
Cyberonics Inc. has created a new VNS system, AspireSRTM that is already approved in Europe. The new system analyzes relative changes of heart rate, particularly ictal tachycardia, and responds to seizures automatically.
Therefore we will obtain many devices to treat medically refractory epilepsy in the very near future. Patients will also get great benefits with these devices. However, it is going to be more necessary for us to understand indications for each device and use these properly by multidisciplinary and comprehensive approach.
At present, VNS is the only device that we can use for medically refractory epilepsy in Japan. VNS is an effective treatment for patients with partial-onset seizures (Level I evidence), and effective in most seizure types indicating a broad range of activity (Level II evidence).42,43) VNS is also safe and well tolerated because side effects tend to diminish over time and VNS does not have cognitive or systemic side effects like AEDs. Therefore VNS should be offered to patients who are not suitable for intracranial epilepsy surgery.42)
References
- 1). Kwan P, Brodie MJ: Early identification of refractory epilepsy. N Eng J Med 342: 314– 319, 2000. [DOI] [PubMed] [Google Scholar]
- 2). Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, Moshé SL, Perucca E, Wiebe S, French J: Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 51: 1069– 1077, 2010. [DOI] [PubMed] [Google Scholar]
- 3). Jayakar P, Gaillard WD, Tripathi M, Libenson MH, Mathern GW, Cross JH, Task Force for Paediatric Epilepsy Surgery, Commission for Paediatrics, and the Diagnostic Commission of the International League Against Epilepsy : diagnostic test utilization in evaluation for resective epilepsy surgery in children. Epilepsia 55: 507– 518, 2014. [DOI] [PubMed] [Google Scholar]
- 4). Mathern GW, Sperling MR: Presurgical evaluation: general principles and methods, in Engel J, Jr, Pedley TA. (eds): Epilepsy: A Comprehensive Textbook, ed 2. Philadelphia, Lippincott Williams & Wilkins, 2007, pp 1771– 1778 [Google Scholar]
- 5). Engel J: Why is there still doubt to cut it out? Epilepsy Curr 13: 198– 204, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Duchowny MS, Harvey AS, Sperling MR, Williamson PD: Indications and criteria for surgical intervention, in Engel J, Jr, Pedley TA. (eds): Epilepsy: A Comprehensive Textbook, ed 2. Philadelphia, Lippincott Williams & Wilkins, 2007, pp 1751– 1759. [Google Scholar]
- 7). Engel J, Jr, Cascino GD, Shields WD: Surgically remediable syndromes, in Engel J, Jr, Pedley TA. (eds): Epilepsy: A Comprehensive Textbook, ed 2. Philadelphia, Lippincott Williams & Wilkins, 2007, pp 1761– 1769 [Google Scholar]
- 8). Wiebe S, Blume WT, Girvin JP, Eliasziw M, Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group : A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Eng J Med 345: 311– 318, 2001. [DOI] [PubMed] [Google Scholar]
- 9). Téllez-Zenteno JF, Dhar R, Wiebe S: Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain 128: 1188– 1198, 2005. [DOI] [PubMed] [Google Scholar]
- 10). Bulteau C, Otsuki T, Delalande O: Epilepsy surgery for hemispheric syndromes in infants: hemimegalencepahly and hemispheric cortical dysplasia. Brain Dev 35: 742– 747, 2013. [DOI] [PubMed] [Google Scholar]
- 11). Douglass LM, Salpekar J: Surgical options for patients with Lennox-Gastaut syndrome. Epilepsia 55 (Suppl 4): 21– 28, 2014. [DOI] [PubMed] [Google Scholar]
- 12). Yamamoto T: [Vagus nerve stimulation in the treatment of intractable epilepsy—knacks in programming and results of long-term follow-up]. Neurological Medicine 80: 223– 230, 2014. (Japanese) [Google Scholar]
- 13). Elliott RE, Morsi A, Kalhorn SP, Marcus J, Sellin J, Kang M, Silverberg A, Rivera E, Geller E, Carlson C, Devinsky O, Doyle WK: Vagus nerve stimulation in 436 consecutive patients with treatment-resistant epilepsy: long-term outcomes and predictors of response. Epilepsy Behav 20: 57– 63, 2011. [DOI] [PubMed] [Google Scholar]
- 14). Elliott RE, Rodgers SD, Bassani L, Morsi A, Geller E, Carlson C, Devinsky O, Doyle WK: Vagus nerve stimulation for children with treatment-resistant epilepsy: a consecutive series of 141 cases. J Neurosurg Pediatr 7: 491– 500, 2011. [DOI] [PubMed] [Google Scholar]
- 15). Lancman G, Virk M, Shao H, Mazumdar M, Greenfield JP, Weinstein S, Schwartz TH: Vagus nerve stimulation vs. corpus callosotomy in the treatment of Lennox-Gastaut syndrome: a meta-analysis. Seizure 22: 3– 8, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Ng M, Devinsky O: Vagus nerve stimulation for refractory idiopathic generalised epilepsy. Seizure 13: 176– 178, 2004. [DOI] [PubMed] [Google Scholar]
- 17). Amar AP, Apuzzo ML, Liu CY: Vagus nerve stimulation therapy after failed cranial surgery for intractable epilepsy: results from the vagus nerve stimulation therapy patient outcome registry. Neurosurgery 55: 1086– 1093, 2004. [DOI] [PubMed] [Google Scholar]
- 18). A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. The Vagus Nerve Stimulation Study Group. Neurology 45: 224– 230, 1995. [DOI] [PubMed] [Google Scholar]
- 19). Handforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, Tecoma ES, Henry TR, Collins SD, Vaughn BV, Gilmartin RC, Labar DR, Morris GL, Salinsky MC, Osorio I, Ristanovic RK, Labiner DM, Jones JC, Murphy JV, Ney GC, Wheless JW: Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology 51: 48– 55, 1998. [DOI] [PubMed] [Google Scholar]
- 20). Schachter SC, Boon P: Vagus nerve stimulation, in Engel J, Jr, Pedley TA. (eds): Epilepsy: A Comprehensive Textbook, ed 2. Philadelphia, Lippincott Williams & Wilkins, 2007, pp 1395– 1399 [Google Scholar]
- 21). Bunch S, DeGiorgio CM, Krahl S, Britton J, Green P, Lancman M, Murphy J, Olejniczak P, Shih J, Heck CN: Vagus nerve stimulation for epilepsy: is output current correlated with acute response? Acta Neurol Scand 116: 217– 220, 2007. [DOI] [PubMed] [Google Scholar]
- 22). DeGiorgio CM, Thompson J, Lewis P, Arrambide S, Naritoku D, Handforth A, Labar D, Mullin P, Heck C, VNS U.S. Study Group : Vagus nerve stimulation: analysis of device parameters in 154 patients during the long-term XE5 study. Epilepsia 42: 1017– 1020, 2001. [DOI] [PubMed] [Google Scholar]
- 23). Heck C, Helmers SL, DeGiorgio CM: Vagus nerve stimulation therapy, epilepsy, and device parameters: scientific basis and recommendations for use. Neurology 59 (6 Suppl 4): S31– S37, 2002. [DOI] [PubMed] [Google Scholar]
- 24). DeGiorgio C, Heck C, Bunch S, Britton J, Green P, Lancman M, Murphy J, Olejniczak P, Shih J, Arrambide S, Soss J: Vagus nerve stimulation for epilepsy: randomized comparison of three stimulation paradigms. Neurology 65: 317– 319, 2005. [DOI] [PubMed] [Google Scholar]
- 25). Agnew WF, McCreery DB: Considerations for safety with chronically implanted nerve electrodes. Epilepsia 31 (Suppl 2): S27– S32, 1990. [DOI] [PubMed] [Google Scholar]
- 26). Tatum IV WO, 4th, Helmers SL: Vagus nerve stimulation and magnet use: optimizing benefits. Epilepsy Behav 15: 299– 302, 2009. [DOI] [PubMed] [Google Scholar]
- 27). DeGiorgio CM, Schachter SC, Handforth A, Salinsky M, Thompson J, Uthman B, Reed R, Collins S, Tecoma E, Morris GL, Vaughn B, Naritoku DK, Henry T, Labar D, Gilmartin R, Labiner D, Osorio I, Ristanovic R, Jones J, Murphy J, Ney G, Wheless J, Lewis P, Heck C: Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia 41: 1195– 1200, 2000. [DOI] [PubMed] [Google Scholar]
- 28). Morris GL, Mueller WM: Long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. The Vagus Nerve Stimulation Study Group E01-E05. Neurology 53: 1731– 1735, 1999. [DOI] [PubMed] [Google Scholar]
- 29). Elliott RE, Morsi A, Tanweer O, Grobelny B, Geller E, Carlson C, Devinsky O, Doyle WK: Efficacy of vagus nerve stimulation over time: review of 65 consecutive patients with treatment-resistant epilepsy treated with VNS > 10 years. Epilepsy Behav 20: 478– 483, 2011. [DOI] [PubMed] [Google Scholar]
- 30). Valencia I, Holder DL, Helmers SL, Madsen JR, Riviello JJ: Vagus nerve stimulation in pediatric epilepsy: a review. Pediatr Neurol 25: 368– 376, 2001. [DOI] [PubMed] [Google Scholar]
- 31). Kawai K: [Vagus nerve stimulation therapy for medically refractory epilepsy—the intermediate analysis report of the Japan VNS Registry]. VNS Advancement for Epilepsy, Vol. 1 Tokyo, Nihon Koden, 2014, pp 1– 4 (Japanese) [Google Scholar]
- 32). Elger G, Hoppe C, Falkai P, Rush AJ, Elger CE: Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res 42: 203– 210, 2000. [DOI] [PubMed] [Google Scholar]
- 33). Ergene E, Behr PK, Shih JJ: Quality-of-Life assessment in patients treated with vagus nerve stimulation. Epilepsy Behav 2: 284– 287, 2001. [DOI] [PubMed] [Google Scholar]
- 34). Cramer JA: Exploration of changes in health-related quality of life after 3 months of vagus nerve stimulation. Epilepsy Behav 2: 460– 465, 2001. [DOI] [PubMed] [Google Scholar]
- 35). Boon P, Vonck K, Vandekerckhove T, D’have M, Nieuwenhuis L, Michielsen G, Vanbelleghem H, Goethals I, Caemaert J, Calliauw L, De Reuck J: Vagus nerve stimulation for medically refractory epilepsy; efficacy and cost-benefit analysis. Acta Neurochir (Wien) 141: 447– 452; discussion 453, 1999. [DOI] [PubMed] [Google Scholar]
- 36). Bernstein AL, Barkan H, Hess T: Vagus nerve stimulation therapy for pharmacoresistant epilepsy: Effects on health care utilization. Epilepsy Behav 10: 134– 137, 2007. [DOI] [PubMed] [Google Scholar]
- 37). Theodore WH, Fisher RS: Brain stimulation for epilepsy. Lancet Neurol 3: 111– 118, 2004. [DOI] [PubMed] [Google Scholar]
- 38). Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, Oommen K, Osorio I, Nazzaro J, Labar D, Kaplitt M, Sperling M, Sandok E, Neal J, Handforth A, Stern J, DeSalles A, Chung S, Shetter A, Bergen D, Bakay R, Henderson J, French J, Baltuch G, Rosenfeld W, Youkilis A, Marks W, Garcia P, Barbaro N, Fountain N, Bazil C, Goodman R, McKhann G, Krishnamurthy KB, Papavassiliou S, Epstein C, Pollard J, Tonder L, Grebin J, Coffey R, Graves N, SANTE Study Group : Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 51: 899– 908, 2010. [DOI] [PubMed] [Google Scholar]
- 39). Heck CN, King-Stephens D, Massey AD, Nair DR, Jobst BC, Barkley GL, Salanova V, Cole AJ, Smith MC, Gwinn RP, Skidmore C, Van Ness PC, Bergey GK, Park YD, Miller I, Geller E, Rutecki PA, Zimmerman R, Spencer DC, Goldman A, Edwards JC, Leiphart JW, Wharen RE, Fessler J, Fountain NB, Worrell GA, Gross RE, Eisenschenk S, Duckrow RB, Hirsch LJ, Bazil C, O’Donovan CA, Sun FT, Courtney TA, Seale CG, Morrell MJ: Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia 55: 432– 441, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). DeGiorgio CM, Soss J, Cook IA, Markovic D, Gornbein J, Murray D, Oviedo S, Gordon S, Corralle-Leyva G, Kealey CP, Heck CN: Randomized controlled trial of trigeminal nerve stimulation for drug-resistant epilepsy. Neurology 80: 786– 791, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Stefan H, Kreiselmeyer G, Kerling F, Kurzbuch K, Rauch C, Heers M, Kasper BS, Hammen T, Rzonsa M, Pauli E, Ellrich J, Graf W, Hopfengärtner R: Transcutaneous vagus nerve stimulation (t-VNS) in pharmacoresistant epilepsies: a proof of concept trial. Epilepsia 53: e115– e118, 2012. [DOI] [PubMed] [Google Scholar]
- 42). Ben-Menachem E: Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol 1: 477– 482, 2002. [DOI] [PubMed] [Google Scholar]
- 43). Fisher RS, Krauss GL, Ramsay E, Laxer K, Gates J: Assessment of vagus nerve stimulation for epilepsy: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 49: 293– 297, 1997. [DOI] [PubMed] [Google Scholar]