Abstract
Surgery for spinal intramedullary tumors remains one of the major challenges for neurosurgeons, due to their relative infrequency, unknown natural history, and surgical difficulty. We are sure that safe and precise resection of spinal intramedullary tumors, particularly encapsulated benign tumors, can result in acceptable or satisfactory postoperative outcomes. General surgical concepts and strategies, technical consideration, and functional outcomes after surgery are discussed with illustrative cases of spinal intramedullary benign tumors such as ependymoma, cavernous malformation, and hemangioblastoma. Selection of a posterior median sulcus, posterolateral sulcus, or direct transpial approach was determined based on the preoperative imaging diagnosis and careful inspection of the spinal cord surface. Tumor-cord interface was meticulously delineated in cases of benign encapsulated tumors. Our retrospective functional analysis of 24 consecutive cases of spinal intramedullary ependymoma followed for at least 6 months postoperatively demonstrated a mean grade on the modified McCormick functional schema of 1.8 before surgery, deteriorating significantly to 2.6 early after surgery (< 1 month after surgery), and finally returning to 1.7 in the late postoperative period (> 6 months after surgery). The risk of functional deterioration after surgery should be taken into serious consideration. Functional deterioration after surgery, including neuropathic pain even long after surgery, significantly affects patient quality of life. Better balance between tumor control and functional preservation can be achieved not only by the surgical technique or expertise, but also by intraoperative neurophysiological monitoring, vascular image guidance, and postoperative supportive care. Quality of life after surgery should inarguably be given top priority.
Keywords: astrocytoma, cavernous malformation, ependymoma, hemangioblastoma, spinal intramedullary tumors
Introduction
Although surgical concepts for the treatment of spinal intramedullary tumors have been established and refined over the last century, surgery for spinal intramedullary tumors remains one of the major challenges for neurosurgeons, due to their relative infrequency, unknown natural history, and surgical difficulty.1–14) Developments in microsurgical instruments, medical equipments, and neurophysiological monitoring techniques have contributed to the safety and precision of surgery. Although a significant risk of functional deterioration after surgery exists, the probability of good functional survival needs to be pursued using careful, meticulous surgical techniques with the intent of microsurgical removal of the tumor. We remain convinced that safe and precise resection of spinal intramedullary tumors, and encapsulated benign tumors in particular, offers the promise of preserved function after surgery. In this article, we discuss the basic concepts and strategies for accomplishing safe and precise resection of spinal intramedullary tumors, with special attention to benign encapsulated tumors such as ependymoma, cavernous malformation, and hemangioblastoma.
General Remarks
I. Surgical anatomy of the spinal cord
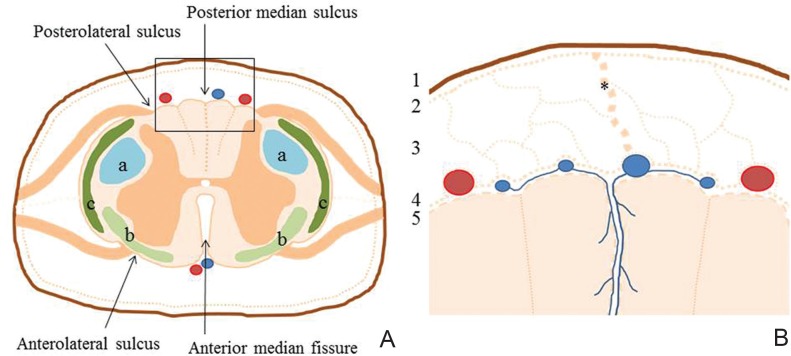
A thorough understanding of the regional neuroanatomy is essential for surgeons to accomplish successful surgery. The cross-sectional surgical anatomy of the spinal cord must be stressed. Internal divisions of the spinal cord delineate gray matter with motor and intrinsic neurons and white matter with the descending and ascending fiber system (Fig. 1A).15) A cross-section of the spinal cord shows a deep anterior median fissure and shallow posterior median sulcus, dividing the spinal cord into symmetrical right and left halves joined at the central midportion. The anterior median fissure contains a fold of pia and blood vessels. The dorsal nerve roots are attached to the spinal cord along a shallow vertical groove, the posterolateral sulcus, which lies a short distance anterior to the posterior median sulcus. The ventral nerve roots exit from the anterolateral sulcus. The spinal cord is directly covered by the thin, translucent membrane of the pia mater, which itself comprises an inner membranous layer, the intima pia, and a more superficial epipial layer (Fig. 1B).16) The intima pia, adherent to the underlying nervous tissue, follows its contours closely. The epipial layer is formed by a mesh network of collagenous fiber bundles continuous with the arachnoid trabeculae, as what we call an intermediate leptomeningeal (IL) layer.17) The blood vessels of the spinal cord lie within this epipial layer. This IL layer is closely applied to the innermost aspect of the arachnoid membrane. The IL layer reflects to form the dorsal septum and arborizes over the dorsal surface of the spinal cord, resulting in the formation of the epipial layer. The IL layer covering the inner aspect of the ventral arachnoid mater is much less substantial than that seen dorsally. The anterior spinal arteries and veins are arborized over by a dense epipial layer. The spinal cord is attached to the dura mater by a series of lateral, flattened bands of epipial tissue known as the dentate ligaments. In the region of the conus medullaris, epipial tissue forms a covering of the filum terminale. The prominence of the septa and ligaments in humans may be related to the erect posture of this species, and the fenestrated nature of the IL layer may serve to dampen the propagation of pressure waves within the spinal subarachnoid space during changes in posture.
Fig. 1.
A: Microsurgical anatomy of a cross-section of the spinal cord showing a deep anterior median fissure, shallow posterior median sulcus, and four shallow grooves of anterolateral or posterolateral sulcus. Internal divisions of the spinal cord are composed of gray matter with motor and intrinsic neurons and white matter with descending and ascending fiber systems such as the lateral corticospinal and rubrospinal tracts (a), spinothalamic tracts (b), and spinocerebellar tracts (c). B: Higher magnification of the boxed area in A shows five meningeal layers covering the spinal cord with dura mater (1), arachnoid membrane (2), and intermediate leptomeningeal layer (3) with formation of dorsal septum (*), epipial layer (4), and intima pia (5).
II. Basic surgical strategy
There is no doubt that the surgeon should take responsibility to elaborately delineate the tumor-cord interface that may or must exist in the spinal intramedullary tumor. Although diagnostic imaging may be the first step for successful surgery, the results do not always suggest the specific pathological nature of the intramedullary tumor. The surgeon should not fully trust a diagnosis from preoperative imaging until direct inspection of the tumor can be performed. Careful intraoperative inspection and pathological diagnosis using frozen sections may guide the surgeons to continue tumor resection. The true tumor-cord interface needs to be carefully exposed, especially in possible cases of benign encapsulated tumors, with minimal damage to the spinal cord parenchyma. Identification of the tumor-cord interface represents the most critical issue. Once the intraoperative appearance of the tumor suggests a benign intramedullary tumor, the entire tumor-cord interface needs to be meticulously exposed under high magnification through the surgical microscope. However, when the surgeon cannot identify a clear tumor-cord interface or if the intraoperative appearance of the tumor suggests an anaplastic nature for the tumor, further tumor removal is not warranted. The intent of gross total resection of the tumor should be abandoned and remedial measures devised instead.
III. Intraoperative neurophysiological monitoring
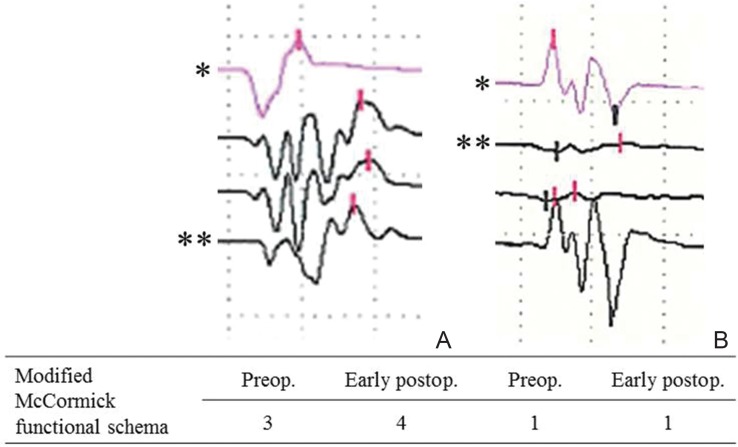
Intraoperative neurophysiological monitoring needs to be routinely set up, although surgeons of great experience may emphasize that the basic principles of a meticulous technique obviate the need for such monitoring. This technique has been used in an attempt to minimize surgery-related risks of neurological deterioration. To date, somatosensory evoked potential (SSEP) and transcranial motor evoked potential (MEP) monitoring tools have been gaining popularity. Transcranial MEP monitoring can be recorded either in the epidural space at the surgical site (spinal MEP) or from needle electrodes placed in the extremities such as tibialis anterior muscle or gastrocnemius muscle (myogenic MEP). Intraoperative neurophysiological monitoring using MEP can provide the surgeon with an almost real-time assessment of motor function, but the alarm point is still not definitive or always reliable.18) Surgeons should understand that intraoperative neurophysiological monitoring is not absolutely reliable, but respected. Previous studies have shown significant correlations between MEP waveform changes and postoperative motor deficits.19–25) Myogenic MEP waveforms recorded from muscles can be categorized into three patterns: polyphasic, biphasic, and absent. Degradation from polyphasic to biphasic waveforms may suggest an adverse reaction to descending motor pathways (Fig. 2). MEP amplitude changes are also examined to predict motor function after surgery. MEP monitoring can be used effectively to avoid excessive spinal cord manipulation and modify the surgical technique during tumor resection, particularly tumor dissection in the lateral or ventral plane, although myogenic MEP does not appear to be obtained in any patient with muscle power of 2/5 or less according to manual muscle testing. Rajshekhar et al. showed that 89% of patients with preoperative Nurick grades of 0–3 provided successful recordings, compared to only 7.1% of patients with preoperative Nurick grades of 4–5.26)
Fig. 2.
Illustrative waveforms of transcranial myogenic motor evoked potential monitoring observed during tumor resection. A: A case of spinal hemangioblastoma of the cervical spine. Gait disturbance was aggravated early after surgery despite only modest degradation of intraoperative waveforms (**) compared to baseline (*). B: A case of spinal cavernous malformation of the high cervical spine. No significant deterioration was noted after surgery, although transient degradation of intraoperative waveforms (**) compared to baseline (*) was recognized in the early stage of surgery.
Surgical Technique
I. Patient positioning
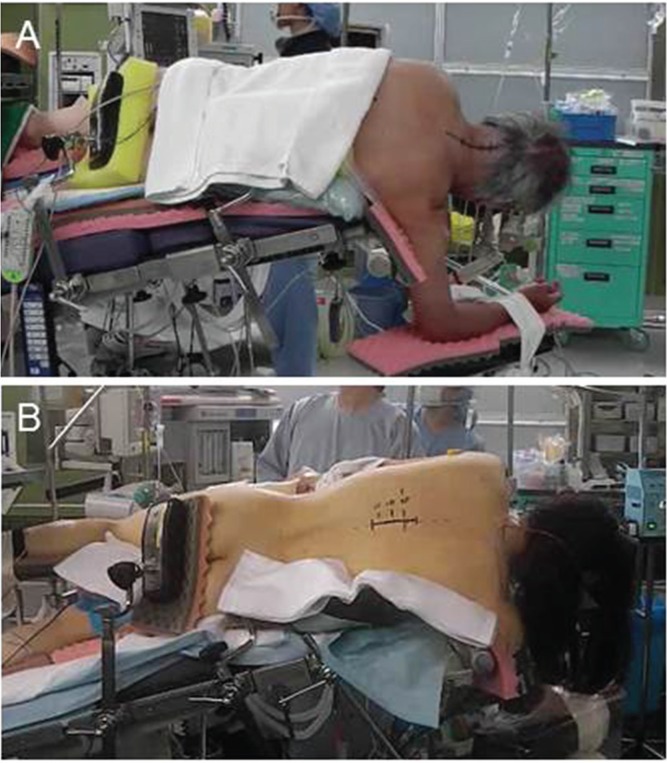
In our institute, patients with cervical intramedullary lesions were initially operated on in the semisitting position, and all patients with thoracic lesions were treated in a lateral oblique position that we call the prone oblique position or semiprone park-bench position.5,12,27,28) However, use of the semisitting position is awkward with the microscope, and air embolic stroke was encountered as an occasional complication during surgery. For the past decade, all patients with cervical and thoracic intramedullary tumors have instead been operated on using a lateral oblique position (Fig. 3).29) The side of the tumor is placed uppermost. The thorax is elevated 15° and the head is maintained in neutral flexion without rotation. All pressure points are securely padded to avoid any venous congestion or peripheral nerve injury. The lateral oblique position permits a fixed spine in good microscope focus throughout the respiratory cycle, as well as good control of epidural venous pressure. Oozing blood drains out of the operative field (thanks to gravity). We prefer not to use the prone position, because the patient’s body is lifted slightly with each respiratory movement, which may disturb fine procedures under the high magnification of the surgical microscope.
Fig. 3.
Intraoperative photographs showing patients in the lateral oblique position. A: cervical lesion and B: thoracic lesion.
II. Tumor access and removal
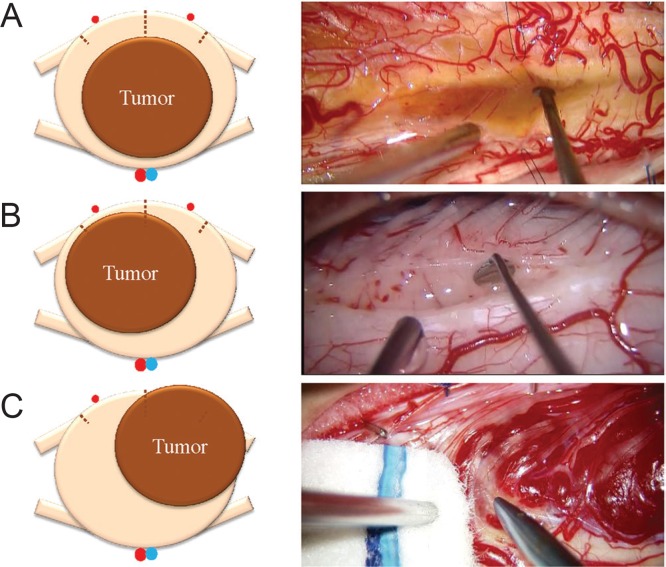
Tumor access to the intramedullary tumor needs to be carefully determined based on diagnostic imaging before surgery, as well as direct inspection of the spinal cord surface (Fig. 4). A posterior median sulcus approach is preferred for most gliomas, such as ependymoma and astrocytoma, whereas lateral myelotomy from a point at which the lesion can be recognized under the microscope may be more suitable for vascular tumors such as hemangioblastoma or cavernous malformation.1–13,27,28) The posterolateral sulcus approach represents another option, equivalent to dorsal root entry zone (DREZ) myelotomy, in selected cases of spinal intramedullary tumor with a lateral location.14,29) Careful inspection of the spinal cord surface may help surgeons to determine the surgical approach. Our basic concept for selecting the surgical approach is direct with the shortest distance to the tumor, as there are no non-eloquent neural tissues within any part of the spinal cord parenchyma.
Fig. 4.
Tumor access to the spinal intramedullary tumor. A: Posterior median sulcus approach for a centrally located tumor. Intraoperative photograph shows an illustrative case of ependymoma. B: Posterolateral sulcus approach for lateral location of the tumor. Intraoperative photograph shows an illustrative case of intramedullary metastasis. C: Direct transpial approach for subpial location of the tumor. Intraoperative photograph shows an illustrative case of hemangioblastoma.
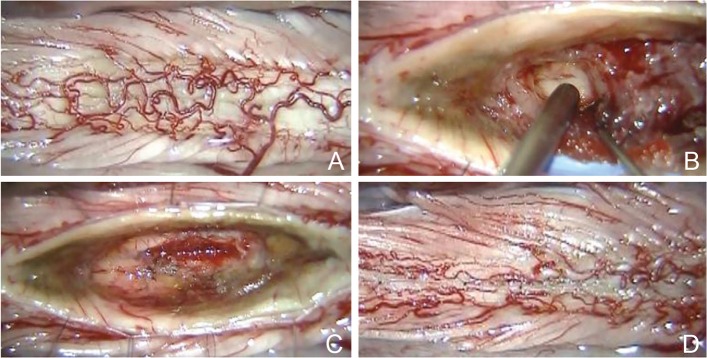
For successful removal of the tumor, atraumatic myelotomy is of great importance to clarify the tumor-cord interface (Fig. 5A, B). The tumor-cord interface needs to be exposed circumferentially with minimal damage to the spinal cord parenchyma. Pial stay sutures are helpful to secure the operative field. We believe that gliosis, edematous tissue, or hemosiderin-deposited tissue surrounding the tumor may guide the surgeon in following the tumor-cord interface (Fig. 5C). Tumor dissection should be continued carefully within the inner circle of the tumor-cord interface. Internal decompression of the tumor may be helpful in cases of relatively large tumor. Another critical point is full longitudinal exposure of the tumor. Otherwise, the surgeon may struggle with removal at the rostral or caudal pole of the tumor. We are sure that maintenance of a bloodless operative field during surgery is the key to successfully securing the tumor-cord interface circumferentially.
Fig. 5.
Basic surgical steps for successful removal of a spinal intramedullary tumor. Intraoperative photographs during resection of an ependymoma show the posterior surface of the spinal cord (A), careful exposure of the tumor-cord interface after atraumatic myelotomy using a posterior median sulcus approach (B), the tumor resection bed after total removal of the tumor (C), and restoration of the spinal cord shape by suturing the pial edges together (D).
Posterior median sulcus approach:
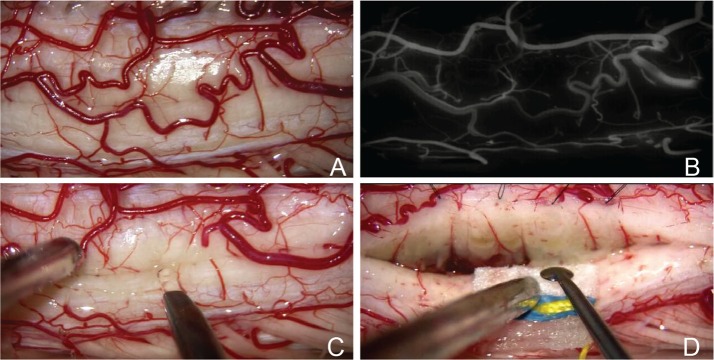
The posterior median sulcus approach, which we call posterior midline myelotomy, is preferred for most gliomas such as ependymoma or astrocytoma.1–2,4–11,13) Posterior midline myelotomy is achieved by the opening of the posterior median septum. The posterior median sulcus can be closely estimated by determining the midpoint between the posterior lateral sulci on both sides. Small veins exiting from the posterior median sulcus may also be helpful for identification. The posterior spinal veins running over the spinal cord surface need to be dissected and mobilized laterally to fully expose the posterior median sulcus, if those veins appear important for spinal venous circulation. However, preservation of the posterior venous circulation is not always necessary. Some posterior spinal veins may be sacrificed with careful consideration of the collateral venous circulation. Indocyanine green videoangiography (ICG-VA) may help the surgeon to judge the spinal venous circulation (Fig. 6A, B).30) Small crossing vessels can be coagulated at very low power levels under continuous saline irrigation. The opening in the posterior median sulcus is made using a diamond knife (Fig. 6C). The myelotomy is extended rostrally or caudally by meticulous splaying of the spinal tissue using a microdissector (Fig. 6D). Identification of a vertical array of posterior sulcal central veins on the inner surface of each posterior column ensures that the surgical orientation is well maintained. In cases showing maximal cord swelling with or without myelomalacia or syrinx formation, exact identification of the posterior median sulcus on the tumor is usually difficult. An alternative technique is to open the posterior median sulcus from the rostral or caudal edge beyond the tumor, where the posterior surface of the spinal cord appears normal. Rostral and caudal openings of the posterior median sulcus are then connected to each other to open the longitudinal plane over the extent of the tumor.
Fig. 6.
The posterior median sulcus approach. Intraoperative photographs during resection of a cavernous malformation show the posterior surface of the spinal cord (A), careful analysis of the posterior venous circulation using indocyanine green videoangiography (B), opening of the posterior median sulcus using the diamond knife (C), and identification of a vertical array of posterior sulcal central veins on the inner surface of each posterior column (D).
After the tumor is encountered, gentle dissection of the tumor-cord interface is continued in the longitudinal plane over the extent of the tumor. Pial stay sutures, typically of 8-0 monofilament nylon, are usually applied between the pia and dura mater to keep the spinal cord incision gently open, although there is an argument that pial stay sutures are disadvantageous by increasing the tension exerted on the normal spinal cord.13) Microcoagulation of the tumor surface can be used to shrink back the surface of the tumor and draw it away from the spinal cord surface, with preservation of the fine vessels and without current spread or heating of the adjacent spinal tissue. With care to protect the parenchyma of the spinal cord, the tumor capsule is removed segmentally or in one piece. Internal decompression of the tumor may help the surgeon to ensure the tumor-cord interface. Fibrous adhesions between the tumor and inner surface of the spinal cord parenchyma are carefully divided over the dorsal to lateral portion of the tumor. Surrounding hemosiderin-stained tissue or gliosis is never resected. Such histological degeneration may represent the barrier of the spinal cord parenchyma. After dissection of the lateral margins of the tumor, ventral dissection represents a final, critical step in tumor removal (Fig. 7). Ventral dissection should be directed longitudinally from either the rostral or caudal pole of the tumor. Small feeding arteries from the anterior spinal artery are carefully identified, coagulated, and divided with the preservation of normal arterial perforating arteries. Good preservation of normal perforating arteries from the anterior spinal artery can be recognized using ICG-VA after complete resection of the tumor (Fig. 8).
Fig. 7.
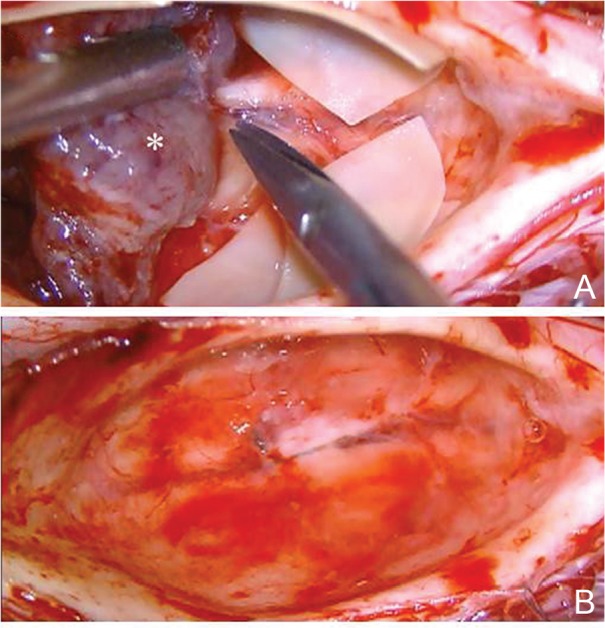
The posterior median sulcus approach. Intraoperative photographs during resection of an ependymoma (*) show the ventral dissection representing the final, critical step of tumor removal (A), and the tumor resection bed after total removal of the tumor (B). The small feeding arteries from the anterior spinal artery are carefully identified, coagulated, and divided.
Fig. 8.
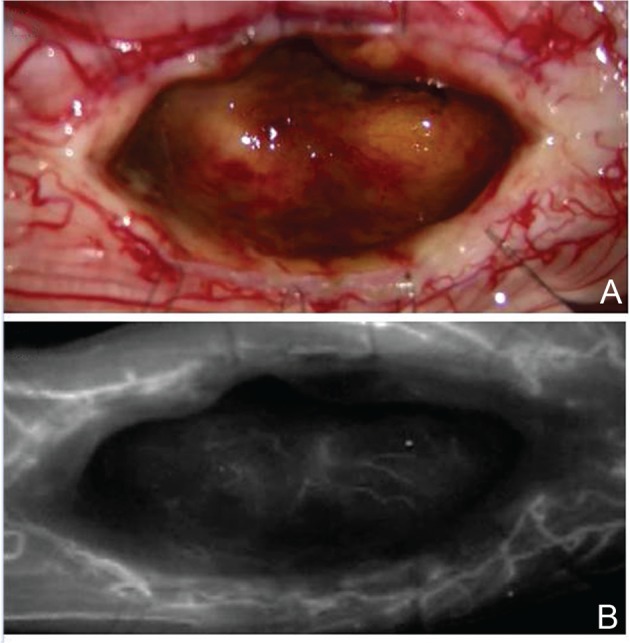
The posterior median sulcus approach. Intraoperative photographs during resection of a cavernous malformation show the tumor resection bed after total removal of the tumor (A) and good preservation of normal perforating arteries from the anterior spinal artery that can be recognized using indocyanine green videoangiography (B)
Posterolateral sulcus approach:
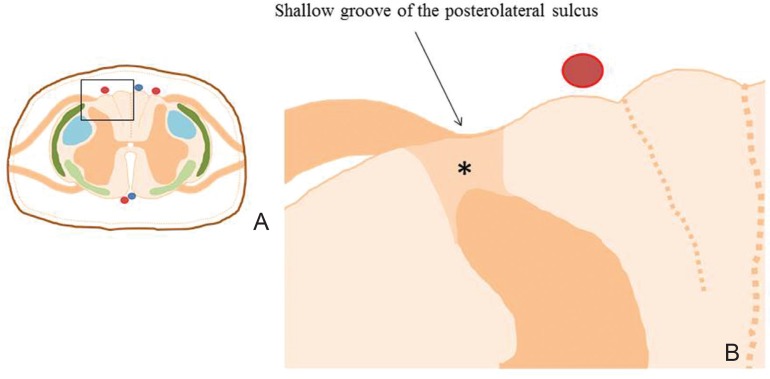
Posterolateral sulcus approach is equivalent to DREZ myelotomy (Fig. 9).14,29,31,32) A posterolateral sulcus approach may be indicated for intramedullary tumors situated laterally in the spinal cord that do not contact the posterior or lateral surfaces on magnetic resonance imaging before surgery. The basic surgical technique for the posterolateral sulcus approach is completely the same as the posterior median sulcus approach. The linear incision along the posterolateral sulcus is made just on the tumor location after careful inspection of the dorsal nerve roots and ipsilateral posterior spinal artery. ICG-VA may help the surgeon to identify the course of the posterior spinal artery (Fig. 10A, B).30) The dorsal nerve roots attach to the spinal cord along the shallow vertical groove of the posterolateral sulcus, which naturally continues to the posterolateral tract of Lissauer. An opening is made using a diamond knife. Crossing vessels are coagulated at very low power levels under continuous saline irrigation. The myelotomy is extended rostrally or caudally by meticulously splaying the spinal tissue using a microdissector. Meticulous dissection of the posterolateral tract of Lissauer reveals the cleavage plane between the dorsal and lateral columns with the microvasculature of the spinal cord. This cleavage plane leads to the substantia gelatinosa at the posteromedial aspect of the dorsal horn of the gray matter. After the tumor is encountered, gentle dissection of the tumor-cord interface is continued in the longitudinal plane over the extent of the tumor (Fig. 10C).
Fig. 9.
The posterolateral sulcus approach. Higher magnification (B) of boxed area in a cross-section of the spinal cord (A) shows the shallow vertical groove of the posterolateral sulcus where the dorsal nerve roots attach to the spinal cord. The posterolateral sulcus naturally continues to the posterolateral tract of Lissauer (*).
Fig. 10.
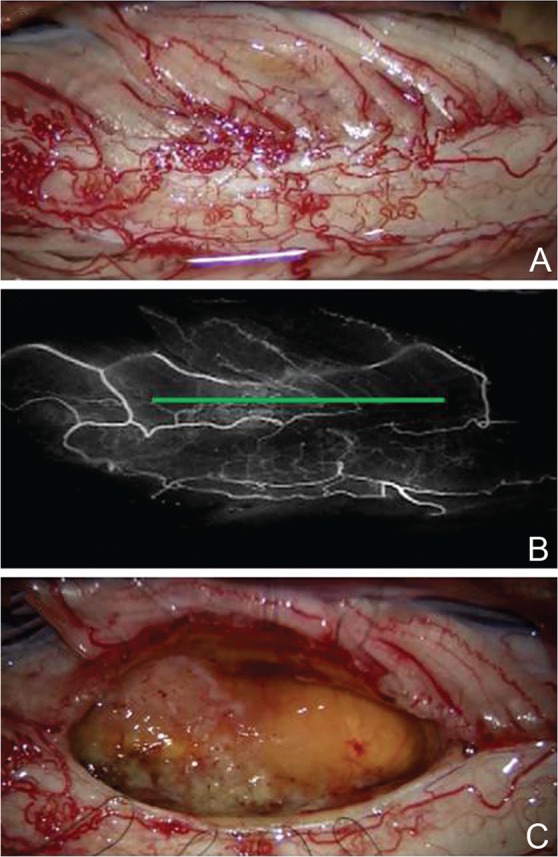
The posterolateral sulcus approach. Intraoperative photographs during resection of an ependymoma show the posterior surface of the spinal cord (A), careful analysis of the posterior arterial, and venous circulations using indocyanine green videoangiography with identification of the posterolateral sulcus (green line) (B) and the tumor resection bed after total removal of the tumor (C).
The advantages of this posterolateral sulcus approach are a reduced risk of neurological deficits related to dorsal column damage compared to the standard posterior median sulcus approach and acceptable pain relief.29) A disadvantage is the greater chance of neurological deficits related to lateral column damage compared to the standard posterior median sulcus approach, particularly in the form of damage to the corticospinal tract on the tumor side.
Direct transpial approach:
The direct transpial approach is a direct myelotomy from the point at which the lesion can be recognized under the microscope, and may be suitable for hemangioblastoma, cavernous malformation, or subependymoma.3,5,11,12)
Spinal hemangioblastomas are mostly superficial, lying over and into the spinal cord, and are highly vascular tumors that require advanced surgical techniques to achieve safe and precise resection. A substantial portion of the hemagioblastoma is buried within the spinal cord and makes an appearance on the dorsal or dorsolateral pial surface (Fig. 11A). Extensive myelotomy is usually unnecessary. A critical point for safe resection of spinal hemangioblastoma is preservation of the main draining veins until the feeding arteries are well controlled. In particular, for huge hemangioblastomas, the draining vein should not be coagulated at the beginning of the procedure. Thorough inspection of the feeding arteries under microscopy is possible with the help of ICG-VA, and the effect of interrupting the feeding arteries on blood flow to the tumor can also be assessed (Fig. 11B–D).30,33) Sharp resection of the cloudy, thick pia mater encircling the tumor surface results in adequate visualization of the tumor-cord interface (Fig. 11E). After successful resection of the circumscribing pial margin, the tumor-cord interface is ready to be dissected. Cauterization of the tumor surface with a low setting for bipolar coagulation results in shrinkage of the tumor volume and facilitates tumor dissection.12) Although the majority of feeding arteries and draining veins are located at the posterior spinal pial surface, some deep feeding arteries or draining veins are encountered during lateral or ventral dissection of the tumor. Fibrous attachments or bridging vessels at the ventral dissection are carefully isolated, cauterized, and divided. Some draining veins are finally cauterized and divided after the completion of tumor dissection. The tumor is totally removed in en-bloc fashion (Fig. 11F).
Fig. 11.
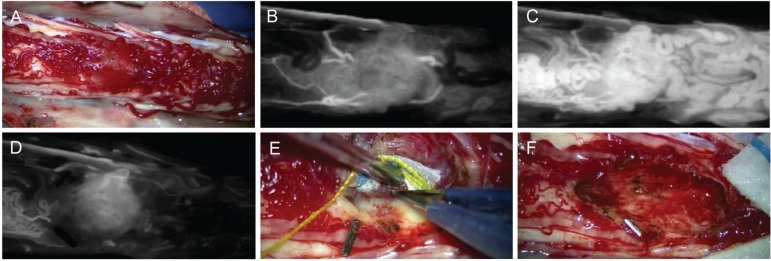
The direct transpial approach. Intraoperative photographs during resection of a hemangioblastoma show the highly vascular tumors buried within the spinal cord (A), good visualization of feeding arteries (B) and tumor stain and draining veins (C) using indocyanine green videoangiography, significantly decreased blood flow after point coagulation of feeding arteries (D), careful dissection of the tumor-cord interface (E), and the tumor resection bed after total removal of the tumor (F).
III. Closure
When removal is complete, there is usually little or no need for further hemostasis. The entire procedure is accomplished as much as possible in a field with no significant bleeding. The pial stay sutures are then removed. The shape of the spinal cord is restored by suturing the pial edges together (Fig. 5D). The arachnoid membrane is then closed with the dura mater or dural graft with fascia lata, to reduce the chance of postoperative myelopathy related to cord tethering at the level of surgery.27–29) Resected laminae are constructed in lift-up style for the cervical spine or on-lay style for the thoracic spine using a titanium mini-plate and screws (Fig. 12).34,35)
Fig. 12.
Osteoplastic laminoplasty of the cervical spine (lift-up style) (A) and thoracic spine (on-lay style) (B), after tumor resection.
Functional Outcomes
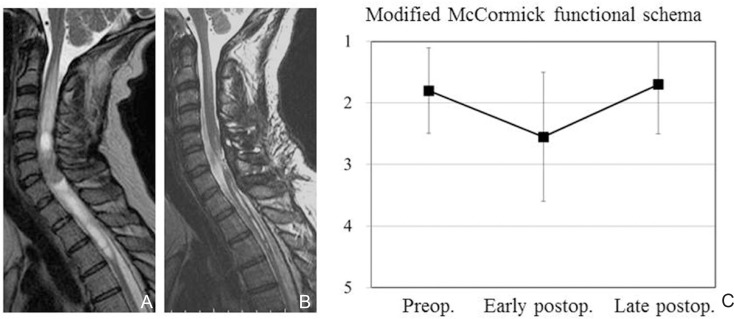
Why does neurological function deteriorate after surgery? Several arguments can be made regarding the underlying mechanisms. Surgical insult to the spinal parenchyma itself may be the primary reason, whether the effect is transient or permanent. Vascular insult via the arterial or venous circulation may be another reason. Most patients in whom the posterior median sulcus approach is used for tumor removal demonstrate sensory dysfunction early after surgery, associated with posterior column dysfunction.8,11,27) Typical manifestations are abnormalities of discriminative touch sensation and proprioception. The patient may also demonstrate gait difficulty early after surgery. Such subjective and objective impairments can recover, but may become permanent. Neuropathic pain syndrome even long after surgery is another problem, and appears common after surgery with associated syringomyelia or for lesions located in the cervical cord.36,37) Neurological recovery may be quite slow and occur over several to many months or even years. Postoperative functional recovery may well correlate with preoperative neurological status.8,27,38,39) Hoshimaru et al. suggested that the thoracic spinal cord may be susceptible to surgical manipulation, and intraoperative findings of arachnoid scarring or cord atrophy are ominous for surgical morbidity.9) Hida et al. proposed the utility of staged operations to enhance the ability of the surgeon to resect spinal intramedullary tumors.40) We retrospectively analyzed 24 consecutive cases of spinal intramedullary benign ependymoma that were treated surgically in our institute between 2003 to 2014 and followed up for at least 6 months after surgery. Microscopically gross total resection of the tumor was achieved in all cases, and no case underwent postoperative local radiation. Although longer follow-up after surgery is mandated, no case demonstrated recurrence during the postoperative follow-up period in the present analysis. Functional analysis demonstrated that a mean grade according to the modified McCormick functional schema was 1.8 ± 0.70 (mean ± standard deviation) before surgery, deteriorating significantly to 2.6 ± 1.05 early after surgery (< 1 month after surgery), and finally returning to 1.7 ± 0.80 late after surgery (> 6 months after surgery) (Fig. 13). Although the overall analysis may indicate acceptable or satisfactory recovery of neurological function or activities of daily living, the risk of functional deterioration after surgery should be taken into serious consideration. Surgical indications for asymptomatic patients with spinal intramedullary ependymoma remain unclear.41) There is no doubt that functional deterioration after surgery, including sensory impairment in the form of dysesthesia or pain even long after surgery, significantly affects the patient’s quality of life. A better balance between tumor control and functional preservation may be achieved not only by the surgical technique or expertise, but also by intraoperative neurophysiological monitoring, vascular image guidance, and postoperative supportive care including rehabilitation.
Fig. 13.
Chronological change in neurological function as estimated using the modified McCormick functional schema (grade 1, neurologically normal to grade 5, severe deficit) in 24 consecutive cases of spinal intramedullary ependymoma. A, B: Illustrative case of spinal intramedullary ependymoma. Preoperative T2-weighted magnetic resonance (MR) image (A), postoperative T2-weighted MR image (B). C: Functional analysis demonstrating that mean grade was 1.8 before surgery, deteriorating significantly to 2.6 early after surgery (< 1 month after surgery), and finally returning to 1.7 late after surgery (> 6 months after surgery). Error bars indicate standard deviations.
Conclusion
The surgical goal with spinal intramedullary tumors, especially benign encapsulated tumors, is a long-term tumor control or cure with functional preservation. Better balance between tumor control and functional preservation needs to be achieved using all means available. While significant surgical risks of serious morbidity exist, the probability of satisfactory or acceptable functional survival appears much better with surgical removal of the tumor than with non-surgical treatment. Surgeons should pursue the predominance or validity of surgical treatment, but should learn humility in their own technique and experience. Quality of life after surgery should inarguably be given top priority.
References
- 1). Greenwood J: Total removal of intramedullary tumors. J Neurosurg 11: 616– 621, 1954. [DOI] [PubMed] [Google Scholar]
- 2). Guidetti B: Intramedullary tumours of the spinal cord. Acta Neurochir (Wien) 17: 7– 23, 1967. [DOI] [PubMed] [Google Scholar]
- 3). Yasargil MG, Antic J, Laciga R, de Preux J, Fideler RW, Boone SC: The microsurgical removal of intramedullary spinal hemangioblastomas. Report of twelve cases and a review of the literature. Surg Neurol ( 3): 141– 148, 1976. [PubMed] [Google Scholar]
- 4). Garrido E, Stein BM: Microsurgical removal of intramedullary spinal cord tumors. Surg Neurol 7: 215– 219, 1977. [PubMed] [Google Scholar]
- 5). Malis LI: Intramedullary spinal cord tumors. Clin Neurosurg 25: 512– 539, 1978. [DOI] [PubMed] [Google Scholar]
- 6). McCormick PC, Torres R, Post KD, Stein BM: Intramedullary ependymoma of the spinal cord. J Neurosurg 72: 523– 532, 1990. [DOI] [PubMed] [Google Scholar]
- 7). Epstein FJ, Farmer JP, Freed D: Adult intramedullary spinal cord ependymomas: the result of surgery in 38 patients. J Neurosurg 79: 204– 209, 1993. [DOI] [PubMed] [Google Scholar]
- 8). McCormick PC: Intramedullary tumors of the spinal cord , in Menezes AH, Sonntag VKH. (eds): Principles of Spinal Surgery. McGraw-Hill, 1996, pp 1355– 1370 [Google Scholar]
- 9). Hoshimaru M, Koyama T, Hashimoto N, Kikuchi H: Results of microsurgical treatment for intramedullary spinal cord ependymomas: analysis of 36 cases. Neurosurgery 44: 264– 269, 1999. [DOI] [PubMed] [Google Scholar]
- 10). Iwasaki Y, Hida K, Sawamura Y, Abe H: Spinal intramedullary ependymomas: surgical results and immunohistochemical analysis of tumour proliferation activity. Br J Neurosurg 14: 331– 336, 2000. [DOI] [PubMed] [Google Scholar]
- 11). Brotchi J: Intrinsic spinal cord tumor resection. Neurosurgery 50: 1059– 1063, 2002. [DOI] [PubMed] [Google Scholar]
- 12). Malis LI: Atraumatic bloodless removal of intramedullary hemangioblastomas of the spinal cord. J Neurosurg 97 (1 Suppl): 1– 6, 2002. [DOI] [PubMed] [Google Scholar]
- 13). Kucia EJ, Bambakidis NC, Chang SW, Spetzler RF: Surgical technique and outcomes in the treatment of spinal cord ependymomas, part 1: intramedullary ependymomas. Neurosurgery 68 (1 Suppl Operative): 57– 63; discussion 63, 2011. [DOI] [PubMed] [Google Scholar]
- 14). Mitha AP, Turner JD, Spetzler RF: Surgical approaches to intramedullary cavernous malformations of the spinal cord. Neurosurgery 68 (2 Suppl Operative): 317– 324; discussion 324, 2011. [DOI] [PubMed] [Google Scholar]
- 15). Waxman SG: Correlative Neuroanatomy, ed 23 Stamford, Connecticut, Appleton & Lange, 1996, pp 45– 68 [Google Scholar]
- 16). Parent A: Meninges and cerebrospinal fluid, in Carpenter’s Human Neuroanatomy, ed 9 Media, Pennsylvania, Williams & Wilkins, 1996, pp 3– 24 [Google Scholar]
- 17). Nicholas DS, Weller RO: The fine anatomy of the human spinal meninges. A light and scanning electron microscopy study. J Neurosurg 69: 276– 282, 1988. [DOI] [PubMed] [Google Scholar]
- 18). Kobayashi S, Matsuyama Y, Shinomiya K, Kawabata S, Ando M, Kanchiku T, Saito T, Takahashi M, Ito Z, Muramoto A, Fujiwara Y, Kida K, Yamada K, Wada K, Yamamoto N, Satomi K, Tani T: A new alarm point of transcranial electrical stimulation motor evoked potentials for intraoperative spinal cord monitoring: a prospective multicenter study from the Spinal Cord Monitoring Working Group of the Japanese Society for Spine Surgery and Related Research. J Neurosurg Spine 20: 102– 107, 2014. [DOI] [PubMed] [Google Scholar]
- 19). Morota N, Deletis V, Constantini S, Kofler M, Cohen H, Epstein FJ: The role of motor evoked potentials during surgery for intramedullary spinal cord tumors. Neurosurgery 41: 1327– 1336, 1997. [DOI] [PubMed] [Google Scholar]
- 20). Kothbauer KF, Deletis V, Epstein FJ: Motor-evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical and neurophysiological data in a series of 100 consecutive procedures. Neurosurg Focus 4: e1, 1998. [DOI] [PubMed] [Google Scholar]
- 21). Skinner SA, Nagib M, Bergman TA, Maxwell RE, Msangi G: The initial use of free-running electromyography to detect early motor tract injury during resection of intramedullary spinal cord lesions. Neurosurgery 56 (2 Suppl): 299– 314; discussion 299–314, 2005. [DOI] [PubMed] [Google Scholar]
- 22). Quiñones-Hinojosa A, Lyon R, Zada G, Lamborn KR, Gupta N, Parsa AT, McDermott MW, Weinstein PR: Changes in transcranial motor evoked potentials during intramedullary spinal cord tumor resection correlate with postoperative motor function. Neurosurgery 56: 982– 993; discussion 982–993, 2005. [PubMed] [Google Scholar]
- 23). Kothbauer KF: Intraoperative neurophysiologic monitoring for intramedullary spinal-cord tumor surgery. Neurophysiol Clin 37: 407– 414, 2007. [DOI] [PubMed] [Google Scholar]
- 24). Hyun SJ, Rhim SC: Combined motor and somatosensory evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical and neurophysiological data in 17 consecutive procedures. Br J Neurosurg 23: 393– 400, 2009. [DOI] [PubMed] [Google Scholar]
- 25). Cheng JS, Ivan ME, Stapleton CJ, Quinones-Hinojosa A, Gupta N, Auguste KI: Intraoperative changes in transcranial motor evoked potentials and somatosensory evoked potentials predicting outcome in children with intramedullary spinal cord tumors. J Neurosurg Pediatr 13: 591– 599, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Rajshekhar V, Velayutham P, Joseph M, Babu KS: Factors predicting the feasibility of monitoring lower-limb muscle motor evoked potentials in patients undergoing excision of spinal cord tumors. J Neurosurg Spine 14: 748– 753, 2011. [DOI] [PubMed] [Google Scholar]
- 27). Ohata K, Takami T, Gotou T, El-Bahy K, Morino M, Maeda M, Inoue Y, Hakuba A: Surgical outcome of intramedullary spinal cord ependymoma. Acta Neurochir (Wien) 141: 341– 346; discussion 346–347, 1999. [DOI] [PubMed] [Google Scholar]
- 28). Goto T, Ohata K, Takami T, Nishikawa M, Nishio A, Morino M, Tsuyuguchi N, Hara M: Prevention of postoperative posterior tethering of spinal cord after resection of ependymoma. J Neurosurg 99 (2 Suppl): 181– 187, 2003. [DOI] [PubMed] [Google Scholar]
- 29). Takami T, Yamagata T, Ohata K: Posterolateral sulcus approach for spinal intramedullary tumor of lateral location: technical note. Neurol Med Chir (Tokyo) 53: 920– 927, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Takami T, Yamagata T, Naito K, Arima H, Ohata K: Intraoperative assessment of spinal vascular flow in the surgery of spinal intramedullary tumors using indocyanine green videoangiography. Surg Neurol Int 4: 135, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Sindou M, Mifsud JJ, Boisson D, Goutelle A: Selective posterior rhizotomy in the dorsal root entry zone for treatment of hyperspasticity and pain in the hemiplegic upper limb. Neurosurgery 18: 587– 595, 1986. [DOI] [PubMed] [Google Scholar]
- 32). Nashold BS, Ostdahl RH: Dorsal root entry zone lesions for pain relief. J Neurosurg 51: 59– 69, 1979. [DOI] [PubMed] [Google Scholar]
- 33). Hwang SW, Malek AM, Schapiro R, Wu JK: Intraoperative use of indocyanine green fluorescence videography for resection of a spinal cord hemangioblastoma. Neurosurgery 67 (3 Suppl Operative): ons300– ons303; discussion ons303, 2010. [DOI] [PubMed] [Google Scholar]
- 34). Goto T, Ohata K, Takami T, Nishikawa M, Tsuyuguchi N, Morino M, Matusaka Y, Nishio A, Inoue Y, Hara M: Hydroxyapatite laminar spacers and titanium miniplates in cervical laminoplasty. J Neurosurg 97 (3 Suppl): 323– 329, 2002. [DOI] [PubMed] [Google Scholar]
- 35). Takami T, Ohata K, Goto T, Nishikawa M, Nishio A, Tsuyuguchi N, Hara M: Lift-up laminoplasty for myelopathy caused by ossification of the posterior longitudinal ligament of the cervical spine. Neurol India 52: 59– 63, 2004. [PubMed] [Google Scholar]
- 36). Klekamp J: Treatment of intramedullary tumors: analysis of surgical morbidity and long-term results. J Neurosurg Spine 19: 12– 26, 2013. [DOI] [PubMed] [Google Scholar]
- 37). Nakamura M, Tsuji O, Iwanami A, Tsuji T, Ishii K, Toyama Y, Chiba K, Matsumoto M: Central neuropathic pain after surgical resection in patients with spinal intramedullary tumor. J Orthop Sci 17: 352– 357, 2012. [DOI] [PubMed] [Google Scholar]
- 38). Nakamura M, Ishii K, Watanabe K, Tsuji T, Takaishi H, Matsumoto M, Toyama Y, Chiba K: Surgical treatment of intramedullary spinal cord tumors: prognosis and complications. Spinal Cord 46: 282– 286, 2008. [DOI] [PubMed] [Google Scholar]
- 39). Matsuyama Y, Sakai Y, Katayama Y, Imagama S, Ito Z, Wakao N, Sato K, Kamiya M, Yukawa Y, Kanemura T, Yanase M, Ishiguro N: Surgical results of intramedullary spinal cord tumor with spinal cord monitoring to guide extent of resection. J Neurosurg Spine 10: 404– 413, 2009. [DOI] [PubMed] [Google Scholar]
- 40). Hida K, Iwasaki Y, Seki T, Yano S: Two-stage operation for resection of spinal cord astrocytomas: technical case report of three cases. Neurosurgery 58 (4 Suppl 2): ONS– E373; discussion ONS-E373, 2006. [DOI] [PubMed] [Google Scholar]
- 41). Aghakhani N, David P, Parker F, Lacroix C, Benoudiba F, Tadie M: Intramedullary spinal ependymomas: analysis of a consecutive series of 82 adult cases with particular attention to patients with no preoperative neurological deficit. Neurosurgery 62: 1279– 1285; discussion 1285–1286, 2008. [DOI] [PubMed] [Google Scholar]