Abstract
Intraspinal angiomatous meningiomas (AMs) are rare lesions, and no case series have been reported. We retrospectively reviewed the data of 12 patients with intraspinal AMs. All patients underwent magnetic resonance imaging (MRI) of the spine. Computed tomography angiography was performed for three cases with cervical lesion. The series included six females and six males with a mean age of 49.6 years. Five tumors were located in the cervical, one in the cervicothoracic, five in the thoracic, and one in the thoracolumbar spine. The most common symptom was motor deficits and the mean duration of symptoms was 18 months. All patients were treated surgically with gross total resection (GTR) (Simpson grade I and II resection). No patients underwent embolization. After surgery immediately, the neurological function was improved in five patients, remained stable in six patients, and was deteriorated in one patient. During an average follow up of 78.6 months, 11 patients experienced an improvement in the neurological function and one patient maintained preoperative status. No tumor recurrence was observed on MRI. Compared to conventional meningiomas, AMs have no special clinical and radiological features. The accurate diagnosis depends on pathology. Timely GTR (en bloc resection) is the best treatment and embolization is not necessary for most patients. Radiotherapy is not recommended after GTR (Simpson grade I and II resection), and the risk of tumor recurrence is low.
Keywords: angiomatous meningioma, spine, surgical resection, long-term outcome
Introduction
Angiomatous meningiomas (AMs) are rare lesions, majority of which are located in the cerebral convexity.1,2) They constitute 2.1% of all meningiomas.2) Microscopically, AM can be defined as any meningioma whose vascular component exceeded 50% of the total tumor area.2,3)
AMs rarely occur in the spinal canal, and account for 1% of all intraspinal meningiomas.3) To our knowledge, no case series of intraspinal AMs have been reported. Most cases were mixed with other histopathologic subtypes and limited clinical data were provided, making interpretation of data and comparison with our series impossible.4,5) In this study, we present a surgical series of 12 patients with pathologically proven intraspinal AMs from a single center.
Patients and Methods
After the study was approved by the Institutional Review Board of Beijing Tiantan Hospital, Capital Medical University, we retrospectively reviewed the data of 12 patients with intraspinal AMs who underwent microsurgery from 2000 to 2012. All patients had performed preoperative and postoperative magnetic resonance imaging (MRI) with gadolinium-contrast enhancement. In three cases with cervical lesion, computed tomography angiography (CTA) was performed. Cranio-cervical tumors with intracranial extension and foramen magnum tumors were excluded as they represent entities other than upper cervical meningiomas.6–8)
All patients underwent laminotomy and microsurgical removal through posterior approach. No patients underwent preoperative or intraoperative embolization. Every attempt was made to resect the tumor en bloc because piecemeal resection could lead to severe bleeding. Postoperatively, no patients received radiotherapy. Specimens were sent to the Department of Pathology for histological examination. The preoperative and postoperative neurological status of the patients were classified according to the McCormick scale (Table 1).9) The patients’ follow-up status was determined during individual office visits.
Table 1.
Modified McCormick classification*
| Grade | Definition |
|---|---|
| I | Neurologically normal; gait normal; normal professional activity |
| Ib | Tired after walking several kilometers; running is impossible, or moderate sensorimotor deficit does not significantly affect the involved limb; moderate discomfort in professional activity |
| II | Presence of sensorimotor deficit affecting function of involved limb; mild to moderate gait difficulty. Severe pain or dysesthetic syndrome impairs quality of life; independent function and ambulation maintained |
| III | More severe neurological deficit; requires cane and/or brace for ambulation or maintains significant bilateral upper-extremity impairment; may or may not function independently |
| IV | Severe neurological deficit; requires wheelchair or cane and/or brace with bilateral upper-extremity impairment; usually not independent |
McCormick 1990.9)
Results
I. Clinical presentation
Among 436 patients with intraspinal meningiomas in the database of our department between 2000 and 2012, only 12 patients were definitely diagnosed with intraspinal AMs. The patients were 6 females and 6 males; with a mean age of 49.6 years (range, 28–76 years). The mean duration of symptoms was 18 months (range, 6–50 months). The symptoms included muscle weakness in 10 patients (83.3%), sensory deficits in seven patients (58.3%), pain in six patients (50%), and sphincter dysfunction in one patient (8.3%). Seven patients were at Grade II of the Modified McCormick classification and five at Grade III (Table 2).
Table 2.
Clinical and radiological features of 12 patients with angiomatous meningioma
| Case | Age (yrs)/Sex | Duration (mos) | Level | Clinical symptoms | MRI | Preoperative diagnosis | Dural attachment | Surgery | Blood loss (ml) | McCormick grade | FU (mos) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1WI | T2WI | +GA | Dural tail sign | Pre- | Post- | Last FU | ||||||||||
| 1 | 28/M | 12 | C2–3 | Neck pain; Rt leg weakness | Iso | Hyper | Hetero | Yes | HPC | Ventral | Simpson grade II; Piecemeal resection | 400 | III | III | Ib | 152 |
| 2 | 56/F | 10 | C1–3 | Rt limbs hypaesthesia | Iso | Hyper | Homo | No | Meningioma | Nerve root | GTR; En bloc resection | 100 | II | Ib | Ib | 131 |
| 3 | 54/F | 50 | T10–11 | Bil legs numbness and weakness; sphincter dysfunction | Iso | Iso | Hetero | No | Meningioma | Ventral | Simpson grade II; Piecemeal resection | 500 | III | III | III | 120 |
| 4 | 49/M | 6 | T1–3 | Lt upper limb pain; limbs numbness and weakness | Hypo | Hyper | Homo | No | Schwannoma | Dorsal | Simpson grade I; En bloc resection | 200 | II | III | I | 108 |
| 5 | 43/F | 16 | T1 | Back pain; Rt limbs weakness | Iso | Iso | Homo | No | Schwannoma | Nerve root | GTR; En bloc resection | 100 | II | Ib | I | 99 |
| 6 | 28/F | 14 | C2–4 | Lt limbs numbness and weakness | Hypo | Hyper | Homo | No | Meningioma | Nerve root | GTR; En bloc resection | 100 | II | Ib | Ib | 86 |
| 7 | 67/F | 16 | C7–T3 | Neck pain; bil legs weakness | Mixed | Hypo | Hetero | No | Schwannoma | Lateral | Simpson grade II; En bloc resection | 300 | III | II | II | 72 |
| 8 | 76/F | 11 | T4 | Bil legs numbness and weakness | Iso | Hyper | Hetero | No | Schwannoma | Lateral | Simpson grade II; En bloc resection | 100 | III | II | Ib | 61 |
| 9 | 41/M | 18 | T11–12 | Rt leg pain and weakness | Iso | Iso | Homo | Yes | HPC | Lateral | Simpson grade II: En bloc resection | 100 | III | III | II | 53 |
| 10 | 54/M | 10 | C1–2 | Rt limbs hypaesthesia | Iso | Hyper | Hetero | Yes | HPC | Ventral | Simpson grade II; Piecemeal resection | 600 | II | II | Ib | 32 |
| 11 | 57/M | 24 | T12–L1 | Bil begs pain and weakness | Iso | Hyper | Homo | Yes | Meningioma | Lateral | Simpson grade II; Piecemeal resection | 400 | II | Ib | I | 18 |
| 12 | 42/M | 6 | C2–3 | Lt upper limb numbness and weakness | Iso | Iso | Homo | No | Meningioma | Lateral | Simpson grade II: En bloc resection | 200 | II | II | I | 11 |
Bil: bilateral, C: cervical, F: female, FU: follow-up, +GA: gadolinium administration, GTR: gross total resection, Hetero: heterogeneous, Homo: homogeneous, HPC: hemangiopericytoma, Hyper: hyperintense, Hypo: hypointense, Iso: isointense, L: lumbar, Lt: left, M: male, mos: months, MRI: magnetic resonance imaging, Pre-: preoperative, Post-: postoperative, Rt: right, T: thoracic, WI: weighted image.
II. Radiological findings
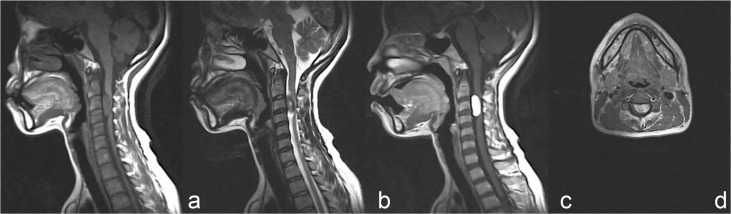
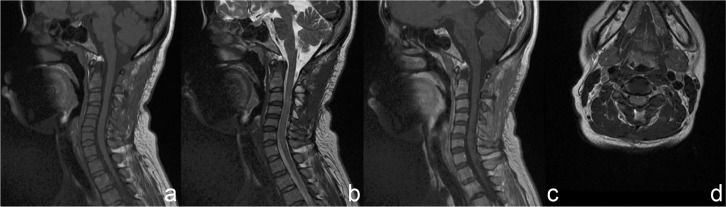
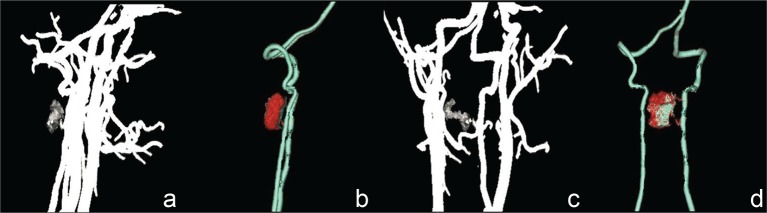
On MRI, the tumors compressed the spinal cord severely and the average invasion ratio of the spinal canal was over 70%, which was measured on axial contrast-enhanced T1-weighted images (WIs). Tumors were located in the cervical (five cases, 41.7%), cervicothoracic (one case, 8.3%), thoracic (five cases, 41.7%), and thoracolumbar (one case, 8.3%) spine. They were oval or nodular shaped, and well demarcated from the spinal cord. Based on the T1WI results, the tumor had isointensity in nine cases, hypointensity in two cases, and mixed intensity in one case. T2WI results indicated the tumors had hyperintensity with small cystic components in seven cases, isointensity in four cases, and hypointensity in one case. Contrast-enhanced T1WI revealed markedly heterogeneous enhancement in five cases and markedly homogeneous enhancement in seven cases. Dural tail sign was positive in four patients. According to the preoperative MRI, five patients were misdiagnosed as schwannomas and three were hemangiopericytomas (HPCs). Tumoral vascularization and tenuous feeding arteries of vertebral artery were noted on CTA. The detailed radiological profiles are summarized in Table 2. MRI and CTA of Case 6 are illustrated in Figs. 1–3.
Fig. 1.
Preoperative magnetic resonance imaging (MRI) showed a well-defined intradural extramedullary tumor at the C2–4 levels. The tumor was hypointense on T1-weighted image (WI) (a) and hyperintense on T2WI (b). c: Markedly homogeneous enhancement was observed on the contrast-enhanced T1WI and dural tail sign was negative. d: Axial contrast-enhanced T1WI demonstrated the tumor was located ventrally to the spinal cord, with severe cord compression.
Fig. 3.
Five years after surgery, magnetic resonance imaging showed no tumor recurrence and the spinal cord had decompressed (a: T1WI, b: T2WI, c: contrast-enhanced T1WI, d: axial contrast-enhanced T1WI, WI: weighted image).
III. Intraoperative findings
Intradural exploration exhibited well-circumscribed, flesh-red, oval, or nodular-shaped lesions with thin capsule (Fig. 4). The dural attachment was ventral in three cases, lateral in five cases, and dorsal in one case. Additionally, no dural attachment was found in three cases. The last is the tumor attached with the nerve root, so-called non-dura-based AM. Gross total resection (GTR) (Simpson grade I and II resection) was achieved in all cases. En bloc resection was performed in three cases with non-dura-based AMs, four lateral cases and one dorsal case, respectively. Piecemeal resection was achieved in three ventral cases and one lateral case. Blood loss during en bloc resection was 150 ± 55 ml (range, 100–400 ml) and that during piecemeal resection was 475 ± 83 ml (range, 400–600 ml). The blood loss during en bloc resection was significantly less (p < 0.05) than that in piecemeal resection group. The dural attachment was completely resected (Simpson grade I resection) if the tumor was located dorsally, and duraplasty was performed with artificial dura. In cases with ventral or lateral dural attachment, the dural attachment was not resected but extensively bipolar cauterized (Simpson grade II resection). The non-dura-based AM was thought to be a schwannoma during surgery, and no dural cauterization was performed.
Fig. 4.
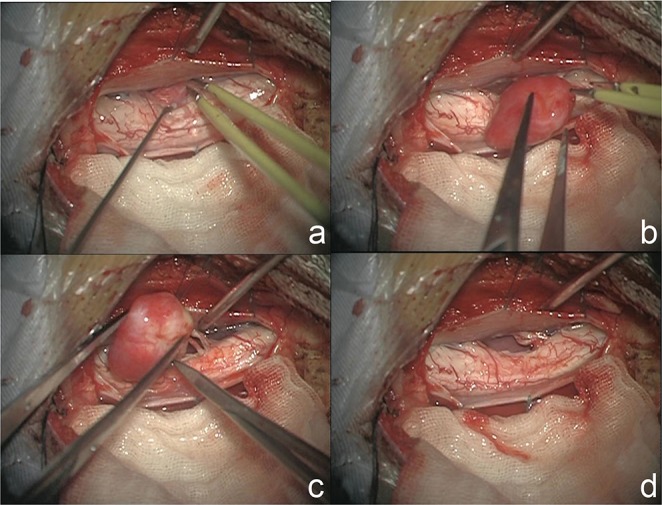
a: Intraoperative photographs showing a flesh-red tumor located ventrally to the spinal cord. b: The tumor was oval shaped, well-circumscribed, and with thin capsule. c: The nerve roots were connected with the tumor, and no dural attachment was found. d: The nerve roots were cut off, and the tumor was totally removed without dural cauterization.
IV. Pathological findings
Microscopically, the tumor consisted of dilated vascular spaces with intervening tissue showing spindle to oval cells with abundant cytoplasm and oval nuclei. The vascular component exceeded 50% of the total tumor area (Fig. 5a). No cytonuclear atypia, necrosis, or mitotic activity was observed. Immunohistochemical examination showed that the tumor cells were positive for epithelial membrane antigen (EMA) and vimentin (Fig. 5b–c). The Ki67 proliferation index was < 2% (Fig. 5d).
Fig. 5.
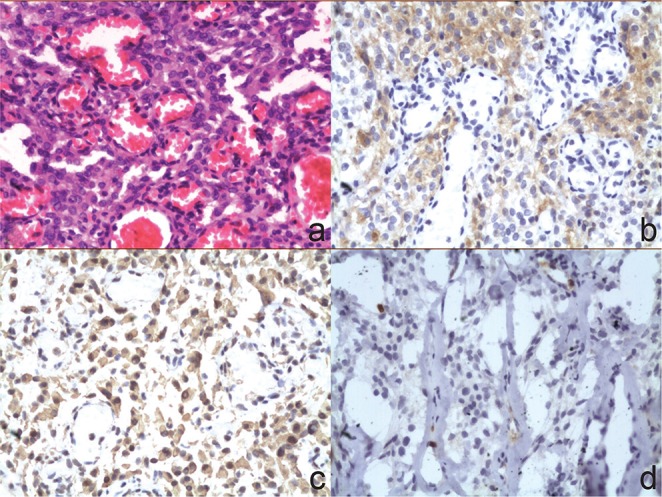
Photomicrographs illustrated that the tumor consisted of dilated vascular spaces with intervening areas showing spindle to oval cells with abundant cytoplasm and oval nuclei. There was no evidence of cytonuclear atypia, necrosis, or mitotic activity (a: H&E stain, original magnification ×200). The tumor cells were positive for epithelial membrane antigen (b: Immunohistochemical stain, original magnification ×200), vimentin (c: Immunohistochemical stain, original magnification ×200). The Ki67 proliferation index was 1.9% (d: Immunohistochemical stain, original magnification ×200). H&E: hematoxylin and eosin.
V. Function evaluation
Meningitis occurred in one patient after surgery, and he was treated successfully by antibiotics and lumbar drainage. In the immediate postoperative period, the neurological function was improved in five patients and remained stable in six patients. One patient experienced a worsening of his symptoms, but improved later to a better status than preoperatively. During a mean follow-up of 78.6 months, no tumor recurrence was observed on MRI. Neurological status had markedly improved in 11 patients and was not improved in one patient. At the last assessment, five patients returned to Grade I and five were at Grade Ib followed by one at Grade II and one at Grade III.
Discussion
I. Epidemiology and clinical futures
Intraspinal AMs are extremely rare. We meticulously reviewed the literature and found no case series of this rare lesion. Since our institute is a tertiary referral hospital, we have more opportunities to treat intraspinal AMs. In our study, symptomatic intraspinal AMs accounted for 2.7% of all intraspinal meningiomas, thus the rarity of the tumors was obvious. Although female predominance in the conventional meningiomas has been recognized,4,8,10) Liu et al.1) and Hasselblatt et al.2) described a female/male ratio of 1.08:1 to 1.4:1 in intracranial AMs. In our series, a female/male ratio of 1:1 was noted, which was close to ratios described in the intracranial AMs but lower than that for conventional meningiomas. The mean age of 48.7 years was slightly younger than that of intraspinal conventional meningiomas.3,8,10–12) According to the literature, the most common location is the thoracic spine in more than 60% of conventional meningiomas, followed by cervical spine in fewer than 30%; lumbar location is extremely rare.3,8,13,14) In our series, 41.7% of the tumors were located in the thoracic region, 41.7% were in the cervical region, 8.3% were in the cervicothoracic region, and 8.3% were in the thoracolumbar region, which was slightly different from conventional meningiomas.
Similar to those of common intradural extramedullary tumors, the clinical symptoms of intraspinal AMs are motor or sensory deficits, local pain, and sphincter dysfunction always appears in the late stage. The clinical course was relatively slow (mean, 18 months) which may reflect the non-aggressive nature of intraspinal AMs. Despite hypervascularity, spontaneous intratumoral hemorrhage was observed only in one intracranial AM.2) Similarly, no sudden neurological deterioration caused by intratumoral hemorrhage occurred in our study. All these findings argue against a high incidence of intratumoral hemorrhage in AMs.
II. Radiological features and differentiation
MRI is doubtlessly the diagnostic tool for intraspinal tumors. On MRI, intraspinal AMs are iso- to hypointense on T1WI and iso- to hyperintense on T2WI, and contrast MRI sequences show significant enhancement. Dural tail sign was positive in 30% of our cases, which is slightly lower than 35% to 58.3% in conventional meningiomas.5,15) Intracranial AMs usually show obvious signal voids of vessels compared to conventional meningiomas,1,16,17) however, signal voids of vessels were not obvious in our series. Compared to conventional meningiomas, intraspinal AMs showed no special MRI features in our series. Three patients underwent CTA and no procedure-related complications were noted. In our experience, this noninvasive technique could delineate tumoral vascularization and feeding arteries. Therefore, we recommend CTA as an auxiliary examination following MRI.
Radiologically, intraspinal AMs are easily to be misdiagnosed with schwannomas and HPCs. The lack of dural attachment and the presence of neural foraminal extension are important clues in distinguishing schwannomas from AMs.15) Nevertheless, a reliable differentiation between intraspinal meningiomas and schwannomas is still challenging based only on MRI.8) An accurate diagnosis depends on pathological examinations. HPC is a highly vascularized, aggressive dural-based lesion that is classified as a mesenchymal nonmeningothelial tumor with uncertain malignant potential or borderline malignancy.18–20) Because of the similar microscopic features, hematoxylin and eosin staining is not sufficient to differentiate these tumors. Therefore, immunohistochemical staining is necessary. Although AMs showed diffusely positive reactivity for vimentin in our series, we agree with Ohnishi et al. that vimentin has poor specificity for meningiomas.21) EMA is the most reliable immunomarker, and exhibits positive immunoreactivity in 50–100% of meningiomas,2,22–26) whereas HPCs are immunoreactive to vimentin and CD34 but negative to EMA.20,27) In our series, all tumors exhibited abundant vascular component which exceeded 50% of the total tumor area, the lack of cytonuclear atypia, necrosis or mitotic activity, the low Ki67 proliferation index, and EMA immunoreactivity. Thus, the tumors are diagnosed with AMs (World Health Origination grade I).
III. Treatment and outcomes
Since intraspinal AMs are histologically benign and usually well marginated, a good clinical outcome after GTR is anticipated. In our series, GTR rate was 100% which was higher than 82–98% in conventional meningiomas.3,10–14,28–30) Until now, there is no consensus about which procedure, Simpson grade I or II resection, is preferable for intraspinal meningiomas. For intracranial AMs, tumor recurrence can be prevented by removing the tumors and the dural attachment.1,2) Similarly, Simpson grade I resection should be the first aim for intraspinal AMs. However, if the dural attachment is located ventrally or laterally, cauterizing the dura takes priority over dural excision, preventing postoperative cerebrospinal fluid leak or neurological damage during dural repair. In our series, there was no tumor recurrence in the Simpson grade II resection group. Thus, we consider that Simpson grade II resection is adequate and effective, as reported in other series.4,10,13,29)
To reduce the blood loss during surgery, the feeding arteries were coagulated and every attempt was made to resect the tumor en bloc. But for the tumors with ventral dural attachment, en bloc resection was difficult to achieve and piecemeal resection was adopted to avoid additional neurological impairment. Although intraoperative hemorrhage in piecemeal resection group was significantly more (p < 0.05) than that in en bloc resection group, it still could be controlled by using careful microsurgical techniques and was not severe as bleeding during resection of HPCs which needed intraoperative embolization.19,31) Embolization was not performed in our series, however, if there are obvious feeding arteries exhibited on CTA or uncontrollable intraoperative bleeding, in addition to preparing sufficient compatible blood and multiple intravenous lines, embolization still could be attempted.
During a mean follow-up of 78.6 months, no tumor recurrence was observed on MRI. Although the functional outcome remained stable in five patients and deteriorated in one patient after surgery immediately, the symptoms of six patients had markedly improved at the last neurological examination. Nevertheless, the neurological function was not improved in one patient whose clinical course was more than 4 years. Long-term compression could cause permanent neurological damage to the spinal cord.10,11,13,32) Thus, surgical resection should be performed before neurological deteriorates.
Some authors advocate radiotherapy for incompletely resected intraspinal meningiomas or recurrent tumors,10,31) however, it is still controversial because of the indolent nature of the tumor, potential radiation damage, and a lack of evidence that radiotherapy reduces the risk of tumor regrowth.24,33) In our study, all patients did not receive radiotherapy and no tumor recurred. We believe that postoperative radiotherapy may be unnecessary after GTR (Simpson grade I and II resection), and the rate of tumor recurrence is very low. When a benign intraspinal meningioma recurs with clinical progression, reoperation should be considered as the primary treatment.13)
Limitations
This retrospective study was performed in a single institution and the number of patients was relatively small. Additionally, intraoperative findings in some early cases were based on the medical records without confirmation by operation videos. Despite these limitations, our results are significant, given the paucity of intraspinal AMs.
Conclusion
Compared to conventional meningiomas, intraspinal AMs have no special clinical and radiological features. The accurate diagnosis depends on pathology. Timely GTR (en bloc resection) is the best treatment and embolization is not necessary for most patients. Radiotherapy is not recommended after GTR (Simpson grade I and II resection), and the rate of tumor recurrence is very low.
Fig. 2.
a–d: Preoperative computed tomography angiography demonstrated a highly vascularized lesion fed by tenuous feeding arteries of the left vertebral artery.
Acknowledgments
The authors like to thank all the patients who trusted them and all the physicians and staff who helped them in this study.
References
- 1). Liu Z, Wang C, Wang H, Wang Y, Li JY, Liu Y: Clinical characteristics and treatment of angiomatous meningiomas: a report of 27 cases. Int J Clin Exp Pathol 6: 695– 702, 2013. [PMC free article] [PubMed] [Google Scholar]
- 2). Hasselblatt M, Nolte KW, Paulus W: Angiomatous meningioma: a clinicopathologic study of 38 cases. Am J Surg Pathol 28: 390– 393, 2004. [DOI] [PubMed] [Google Scholar]
- 3). Levy WJ, Bay J, Dohn D: Spinal cord meningioma. J Neurosurg 57: 804– 812, 1982. [DOI] [PubMed] [Google Scholar]
- 4). Boström A, Bürgel U, Reinacher P, Krings T, Rohde V, Gilsbach JM, Hans FJ: A less invasive surgical concept for the resection of spinal meningiomas. Acta Neurochir (Wien) 150: 551– 556; discussion 556, 2008. [DOI] [PubMed] [Google Scholar]
- 5). Nakamura M, Tsuji O, Fujiyoshi K, Hosogane N, Watanabe K, Tsuji T, Ishii K, Toyama Y, Chiba K, Matsumoto M: Long-term surgical outcomes of spinal meningiomas. Spine 37: E617– E623, 2012. [DOI] [PubMed] [Google Scholar]
- 6). Bassiouni H, Ntoukas V, Asgari S, Sandalcioglu EI, Stolke D, Seifert V: Foramen magnum meningiomas: clinical outcome after microsurgical resection via a posterolateral suboccipital retrocondylar approach. Neurosurgery 59: 1177– 1185; discussion 1185–1187, 2006. [DOI] [PubMed] [Google Scholar]
- 7). Samii M, Klekamp J, Carvalho G: Surgical results for meningiomas of the craniocervical junction. Neurosurgery 39: 1086– 1094; discussion 1094–1095, 1996. [DOI] [PubMed] [Google Scholar]
- 8). Setzer M, Vatter H, Marquardt G, Seifert V, Vrionis FD: Management of spinal meningiomas: surgical results and a review of the literature. Neurosurg Focus 23: E14, 2007. [DOI] [PubMed] [Google Scholar]
- 9). McCormick PC, Stein BM: Intramedullary tumors in adults. Neurosurg Clin N Am 1: 609– 630, 1990. [PubMed] [Google Scholar]
- 10). Roux FX, Nataf F, Pinaudeau M, Borne G, Devaux B, Meder JF: Intraspinal meningiomas: review of 54 cases with discussion of poor prognosis factors and modern therapeutic management. Surg Neurol 46: 458– 463; discussion 463–464, 1996. [DOI] [PubMed] [Google Scholar]
- 11). King AT, Sharr MM, Gullan RW, Bartlett JR: Spinal meningiomas: a 20-year review. Br J Neurosurg 12: 521– 526, 1998. [DOI] [PubMed] [Google Scholar]
- 12). Solero CL, Fornari M, Giombini S, Lasio G, Oliveri G, Cimino C, Pluchino F: Spinal meningiomas: review of 174 operated cases. Neurosurgery 25: 153– 160, 1989. [PubMed] [Google Scholar]
- 13). Gezen F, Kahraman S, Canakci Z, Bedük A: Review of 36 cases of spinal cord meningioma. Spine 25: 727– 731, 2000. [DOI] [PubMed] [Google Scholar]
- 14). Klekamp J, Samii M: Surgical results for spinal meningiomas. Surg Neurol 52: 552– 562, 1999. [DOI] [PubMed] [Google Scholar]
- 15). Liu WC, Choi G, Lee SH, Han H, Lee JY, Jeon YH, Park HS, Park JY, Paeng SS: Radiological findings of spinal schwannomas and meningiomas: focus on discrimination of two disease entities. Eur Radiol 19: 2707– 2715, 2009. [DOI] [PubMed] [Google Scholar]
- 16). Kim BW, Kim MS, Kim SW, Chang CH, Kim OL: Peritumoral brain edema in meningiomas: correlation of radiologic and pathologic features. J Korean Neurosurg Soc 49: 26– 30, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Whittle IR, Smith C, Navoo P, Collie D: Meningiomas. Lancet 363: 1535– 1543, 2004. [DOI] [PubMed] [Google Scholar]
- 18). Harris DJ, Fornasier VL, Livingston KE: Hemangiopericytoma of the spinal canal. Report of three cases. J Neurosurg 49: 914– 920, 1978. [DOI] [PubMed] [Google Scholar]
- 19). Lee CH, Kim KJ, Jahng TA, Kim HJ: Spinal Hemangiopericytoma Which Needed Intraoperative Embolization due to Unexpected Bleeding. J Korean Neurosurg Soc 54: 253– 256, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Nakashima H, Imagama S, Sakai Y, Nakamura H, Katayama Y, Ito Z, Wakao N, Matsuyama Y, Ishiguro N: Dumbbell-type hemangiopericytoma in the cervical spine: a case report and review. J Orthop Sci 18: 849– 855, 2013. [DOI] [PubMed] [Google Scholar]
- 21). Ohnishi Y, Iwatsuki K, Morii E, Kobayashi M, Hori Y, Moriwaki T, Ishihara M, Yoshimura K, Umegaki M, Yoshimine T: Histopathological study of spinal meningioma originating from the arachnoid villi. Brain Tumor Pathol 28: 77– 81, 2011. [DOI] [PubMed] [Google Scholar]
- 22). Takeuchi H, Llena JF, Hirano A: Epithelial differentiation in intraspinal meningiomas. Brain Tumor Pathol 14: 113– 117, 1997. [DOI] [PubMed] [Google Scholar]
- 23). Schnitt SJ, Vogel H: Meningiomas. Diagnostic value of immunoperoxidase staining for epithelial membrane antigen. Am J Surg Pathol 10: 640– 649, 1986. [DOI] [PubMed] [Google Scholar]
- 24). Ng HK, Tse CC, Lo ST: Meningiomas and arachnoid cells: an immunohistochemical study of epithelial markers. Pathology 19: 253– 257, 1987. [DOI] [PubMed] [Google Scholar]
- 25). Perry A, Gutmann DH, Reifenberger G: Molecular pathogenesis of meningiomas. J Neurooncol 70: 183– 202, 2004. [DOI] [PubMed] [Google Scholar]
- 26). Rao S, Rajkumar A, Kuruvilla S: Angiomatous meningioma: a diagnostic dilemma. Indian J Pathol Microbiol 51: 53– 55, 2008. [DOI] [PubMed] [Google Scholar]
- 27). D’Amore ES, Manivel JC, Sung JH: Soft-tissue and meningeal hemangiopericytomas: an immunohistochemical and ultrastructural study. Hum Pathol 21: 414– 423, 1990. [DOI] [PubMed] [Google Scholar]
- 28). Gottfried ON, Gluf W, Quinones-Hinojosa A, Kan P, Schmidt MH: Spinal meningiomas: surgical management and outcome. Neurosurg Focus 14: e2, 2003. [DOI] [PubMed] [Google Scholar]
- 29). Sandalcioglu IE, Hunold A, Müller O, Bassiouni H, Stolke D, Asgari S: Spinal meningiomas: critical review of 131 surgically treated patients. Eur Spine J 17: 1035– 1041, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Schaller B: Spinal meningioma: relationship between histological subtypes and surgical outcome? J Neurooncol 75: 157– 161, 2005. [DOI] [PubMed] [Google Scholar]
- 31). Fitzpatrick D, Mahajan J, Lewkowitz M, Black K, Setton A, Woldenberg R: Intradural hemangiopericytoma of the lumbar spine: a rare entity. AJNR Am J Neuroradiol 30: 152– 154, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Cohen-Gadol AA, Zikel OM, Koch CA, Scheithauer BW, Krauss WE: Spinal meningiomas in patients younger than 50 years of age: a 21-year experience. J Neurosurg 98: 258– 263, 2003. [DOI] [PubMed] [Google Scholar]
- 33). Mirimanoff RO, Dosoretz DE, Linggood RM, Ojemann RG, Martuza RL: Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg 62: 18– 24, 1985. [DOI] [PubMed] [Google Scholar]