Abstract
Delayed neurological deterioration in the absence of direct spinal cord insult following surgical decompression is a severe postoperative complication in patients with chronic severe spinal cord compression (SCC). The spinal cord ischemia-reperfusion injury (IRI) has been verified as a potential etiology of the complication. However, the exact pathophysiologic mechanisms of the decompression-related IRI remain to be defined. In this study, we developed a practical rat model of chronic severe SCC. To explore the underlying role of inflammation in decompression-related IRI, immunoreactivity of pro-inflammatory cytokines including tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β) before and after decompression were measured. In addition, expression level of TNF-α and IL-1β was examined with Western blot. Immunohistochemical staining showed negative result in gray matters in the sham group and sham-decompression group. In the severe compression group, strong positive staining of TNF-α and IL-1β were found, suggesting a dramatic infiltration of inflammatory cells in gray matters. Furthermore, the severe compression group showed a significant increase in expression level of TNF-α and IL-1β as compared with the sham group (p < 0.05). In the severe compression-decompression group, both immunostaining and Western blot showed significant increase of TNF-α and IL-1β levels in the spinal cord compared with the severe compression group (p < 0.05). The results demonstrated that surgical decompression plays a stimulative role in inflammation through increasing the expression of inflammatory cytokines in the rat model of chronic severe SCC injury. Inflammation may be one of the important pathological mechanisms of decompression-related IRI of chronic ischemia.
Keywords: rat model, chronic severe spinal cord compression, inflammation, decompression surgery, ischemia-reperfusion injury
Introduction
Chronic spinal cord compression (SCC) is commonly caused by intraspinal tumors, spondylosis, and disc herniation.1–8) Although the condition of most patients with these lesions has improved after surgery, decompression of a previously severely compressed region of the spinal cord may lead to delayed neurological deterioration during the early postoperative period.2–8) The most widely accepted pathological mechanism of the complication is spinal cord ischemia-reperfusion injury (IRI).3,6,7)
According to the pathophysiologic features, spinal cord injury (SCI) is mainly categorized into primary injury and secondary injury.9) IRI is one of the most frequent types of secondary SCI, which is defined as follows: after removing the factors that cause spinal cord ischemia and the recovery of cord blood supply, neuronal function cannot be improved and its injury is more intense than its original level, or even present in the irreversible tardive neurons death.10) Although the exact biomechanism of spinal cord IRI is not fully understood, inflammation is known to play an important role after ischemia and contributes to SCI, especially in the reperfusion period.11–13)
Tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β) are the key inflammatory mediators and play a major role in the pathological mechanisms of primary ischemic injury and secondary injury.14–16) Recent researches have also shown that TNF-α and IL-1β levels increased significantly in reperfusion period and remained at high levels after reperfusion.11–13,17,18)
In a previous study, we verified that IRI occur after decompression surgery for a rat model of chronic severe SCC. However, the exact pathophysiologic mechanisms of the decompression-related IRI, especially the role of inflammation, remain to be defined. Therefore, the aim of our study was to investigate the role of inflammation in decompression-related IRI of chronic ischemia. In this study, we continued to establish the rat model of chronic severe SCC by using water-absorbing materials, which was similar to the literature.19,20) The levels of TNF-α and IL-1β before and after decompression were measured and analyzed, to further test the hypothesis that inflammation may be one of the important pathological mechanisms of decompression-related IRI.
Methods
Sprague–Dawley (SD) male rats, 9–10 weeks of age (280–320 g), from the Experimental Animal Facilities of the hospital were used in the study. Food and water were provided ad libitum before and after the experiments. The rats were housed in a temperature-controlled environment under a light-dark cycle condition with free access to food. The study was approved by the ethnic committee of Beijing Tiantan Hospital, Capital Medical University, and performed in accordance with the policies of Chinese animal research committees and guidelines from United States National Institute of Health (NIH publication No. 96-23, revised 1996). Every effort was made to minimize the suffering and the number of animals.
I. Compression material
The compression sheet is made of a water-absorbing material. The sample is a penetrating polymer network hydrogel composed of polyvinyl alcohol and polyacrylamide, the ratio of which is 1:1. After absorbing water, it demonstrates long-lasting water retaining capability and durability. The expansion rate can gradually reach a maximum of three times its original thickness. In preliminary experiments, the material was implanted subcutaneously in the abdomen of the rats. Over the period of 12 weeks, no obvious tissue reaction was observed.
II. Rat model of chronic severe SCC
Before surgery, the rats were kept in their cages for 1 week for adaptation to the environment. The rats were anesthetized with an intraperitoneal injection of trichloroacetaldehyde (300 mg/kg, i.p. Qingdao Yulong Algae Co., Ltd., Shandong, China, No. H37022673). A longitudinal incision was made on the back, centered on the spinous processes of the T7–10. The paravertebral muscles were stripped from the spinous processes and laminae. After the yellow ligament was removed, the compression sheet (original size: 2.5 × 2.0 × 0.8 mm3; expanded size: 3.5 × 3.0 × 2.4 mm3) was inserted between the T8–9 laminae and dura. Afterwards, muscle and skin were sutured with 6-0 Vicryl (Vicryl, Ethicon, Johnson & Johnson International, Lenneke Marelaan, Belgium). Following the surgical procedure, temperatures were strictly maintained and all the rats were housed individually with free access to food. Ampicillin liquid formulation (Suzhou Two Leaves Pharmaceuticals Inc., Ltd., China, Batch No. H32021320) was injected into the back exterior muscles once per day for 3 days to prevent infection. Padding in each cage was changed every day to keep it dry. Bladders were manually emptied daily until animals regained voiding function. The rat models were elevated in the 12 weeks post-surgery and effect of the spinal cord self-repair was very limited.
III. Experimental groups
Twenty four rats were randomly allocated into four groups, six rats each: in the sham group (n = 6), rats underwent the surgical procedure but without implantation of the compression material and sacrificed the 12 weeks post-surgery. In the severe compression (SC) group (n = 6), rats underwent polymer sheet implantation, and were sacrificed the 12 weeks post-surgery. In the severe compression-decompression (SC-d) group (n = 6), rats underwent laminectomy and the removal of the expanded materials. The rats were kept alive for 24 hours after decompression, and then sacrifice took place. In the sham-decompression (sham-d) group (n = 6), rats underwent laminectomy and the postoperative protocol was the same as the SC-d group. Spinal cord samples (15 mm) were obtained from the compressed area and divided into two equal parts. Cranial parts were obtained for immunostaining; caudal parts were stored in a −20°C freezer for Western blot.
IV. Radiographic imaging
All magnetic resonance imaging (MRI) were conducted with a 3 Tesla MRI (Magnetom Trio, Siemens Medical Solutions, Erlangen, Germany). T2-weighted images (echo delay time = 92 ms; repetition time = 3,620 ms; flip angle α = 120°; slice thickness: 2 mm; field of view = 80 mm) were obtained at the 12th week following the operation to evaluate the degree of SCC. The cross-sectional area was measured using the Siemens Workstation software (NUMARIS/4).
V. Immunohistochemistry
Hydrogen streptavidin (HRP) labeled Streptavidin (S/P) kit was used for immunostaining TNF-α and IL-1β. After deparaffinization, endogenous peroxidase was quenched with 0.3% hydrogen peroxide in 60% methanol for 30 minutes. The sections were permeabilized with 0.1% Triton X-100 in phosphate-buffered saline (PBS) for 20 minutes. Nonspecific adsorption was minimized by incubating the sections in 2% normal goat serum in PBS for 20 minutes. Endogenous biotin-binding or avidin-binding sites were blocked by sequential incubation for 15 minutes with biotin and avidin, to avoid nonspecific staining. The following primary antibodies were used in experiments: rabbit-anti TNF-α (1: 200) and rabbit-anti IL-1β (1: 200). Horseradish peroxidase (HRP) conjugated goat-anti rabbit secondary antibody (Santa Cruz, California, USA) was used for 3, 3′-diaminobenzidine (DAB) staining. All primary antibodies and kits were obtained from Zhongshan Jinqiao Biotechnology (Beijing, China). The number of positive cells of high power fields (original magnification ×40) in four groups was counted.
VI. Western blot
The expression level of pro-inflammatory cytokines including TNF-α and IL-1β were measured. For Western blot, tissue proteins were extracted with RIPA Lysis buffer kit (Bio-Tek, California, USA), centrifuged at 10,000 g, and aliquoted after quantitation. Protein levels were determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and the concentration of SDS-PAGE was set to 12% and 10% for TNF-α and IL-1β, respectively. The following primary antibodies were used for Western blot: rabbit anti-TNF-α (1: 500), rabbit anti-IL-1β (1: 500), and rabbit anti-actin (1: 400, Santa Cruz, California, USA). HRP conjugated goat-anti rabbit secondary antibody (Santa Cruz, California, USA) was used for enhanced chemiluminescence. The membrane was subsequently exposed to X-ray film. Western blot results were quantified by the analysis of X-ray films using Image J software. All primary antibodies and kits were bought from Zhongshan Jinqiao Biotechnology (Beijing, China). Beta-actin was used as an internal control.
VII. Statistical analysis
Data were analyzed by the Statistical Package for the Social Sciences 16.0 software (SPSS, Chicago, Illinois, USA) and represented as mean ± standard deviation. Samples from each group were compared with independent t-test. A p value less than 0.05 was considered statistical significance.
Results
I. Neuroradiological observations
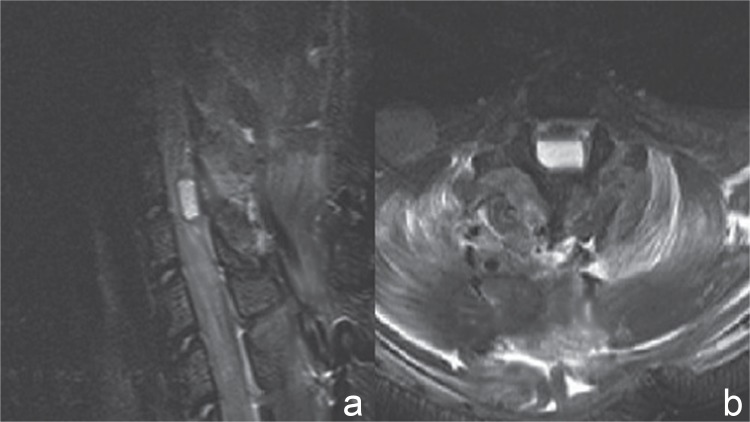
Sagittal and axial projections of the thoracic spine were obtained to ascertain the location of the compression sheet and to evaluate the degree of SCC. The expanded materials compressed the spinal cord severely and the average invasion ratio of the spinal canal was over 70%, which was measured on axial T2-weighted images (Fig. 1). The cross-sectional area in the sham group was larger than that in the compressed groups (p < 0.05) (sham: 16.20 ± 1.34 mm2; SC: 4.52 ± 1.42 mm2).
Fig. 1.
Magnetic resonance imaging after chronic severe spinal cord compression. a: The severe compression material was located dorsally within the spinal canal at the T8–9 levels on sagittal T2-weighted images. b: The sheet compressed the spinal cord severely and the invasion ratio of the spinal canal was over 70%, which was measured on axial T2-weighted images.
II. Immunohistochemical staining
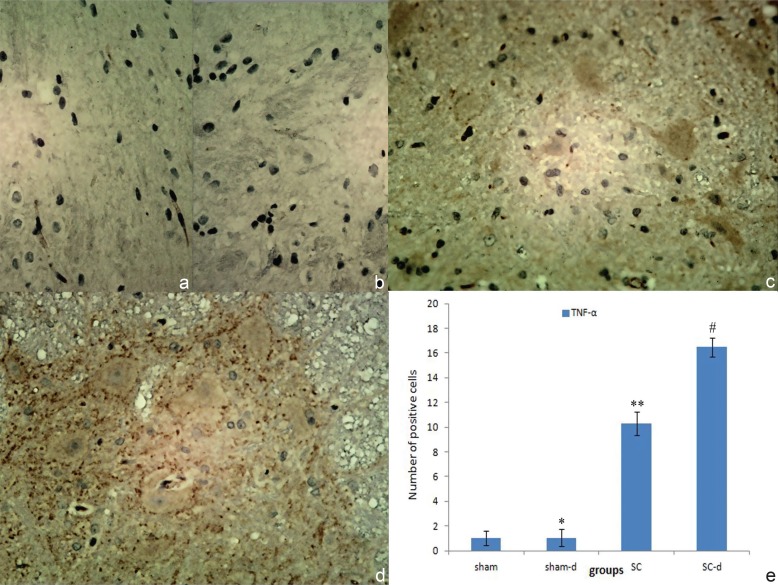
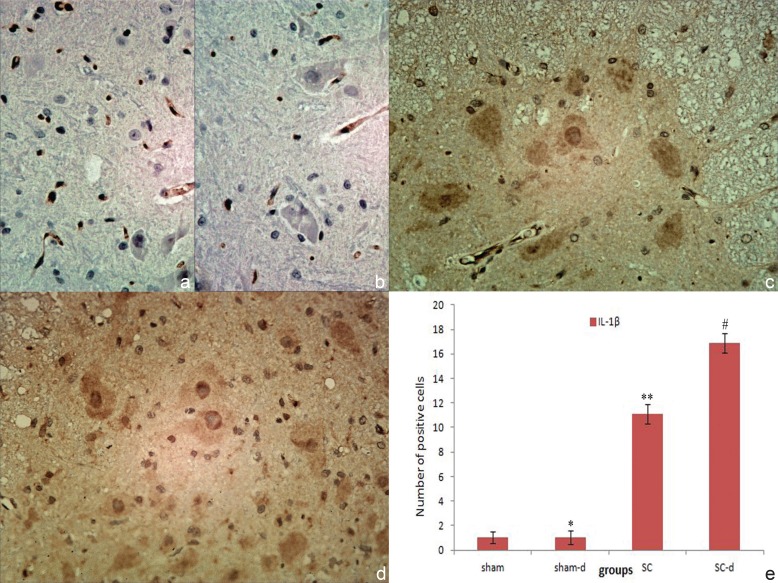
Immunohistochemical staining of TNF-α and IL-1β were displayed in Figs. 2 and 3. In the sham group and sham-d group, the negative result in the gray matters suggesting neither obvious infiltration of inflammatory cells nor obvious expression of these factors in the neurons in this area (TNF-α cells: sham 1.01 ± 0.57, sham-d 1.03 ± 0.69; IL-1β cells: sham 1.03 ± 0.48, sham-d 1.04 ± 0.55). In the SC group, strong positive staining of TNF-α and IL-1β were found, suggesting a dramatic infiltration of inflammatory cells in gray matters compared with the sham group (p < 0.05) (TNF-α cells: 10.32 ± 0.96; IL-1β cells: 11.12 ± 0.82). After surgical decompression, the degree of positive staining for TNF-α and IL-1β increased significantly and infiltration of inflammatory cells aggravated compared with the SC group (p < 0.05) (TNF-α cells: 16.47 ± 0.74; IL-1β cells: 16.88 ± 0.78).
Fig. 2.
Immunohistochemical localizations of tumor necrosis factor-alpha (TNF-α) in spinal cord in sham group (a), sham-decompression (sham-d) group (b), severe compression (SC) group (c), and severe compression-decompression (SC-d) (d) (original magnification ×40). No obvious positive staining of TNF-α was observed in sham group and sham-d group (*p > 0.05); a substantial increase in the release of TNF-α was found in various cells in the SC group compared with the sham group (**p < 0.05); whereas TNF-α production increased significantly in the SC-d group compared with the SC group (#p < 0.05) (e).
Fig. 3.
Immunohistochemical localizations of interleukin-1β (IL-1β) in spinal cord in sham group (a), sham-decompression (sham-d) group (b), severe compression (SC) group (c), and severe compression-decompression (SC-d) (d) (original magnification ×40). No obvious positive staining of IL-1β was observed in sham group and sham-d group (*p > 0.05); a substantial increase in the release of IL-1β was found in various cells in the SC group compared with the sham group (**p < 0.05); whereas IL-1β production increased significantly in the SC-d group compared with the SC group (#p < 0.05) (e).
III. Levels of inflammatory molecules
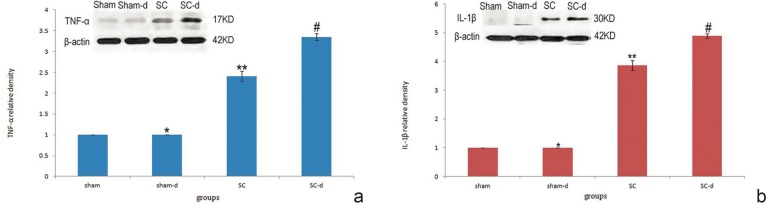
The results of Western blot for TNF-α (17 kD) and IL-1β (30 kD) are displayed in Fig. 4. The expression levels of the proteins were normalized with sham group and presented as percentages (relative density: 1). In the sham-d group, the two proteins expressed at low levels without difference from those in the sham group. The SC group showed a significant increase in expression level of TNF-α and IL-1β as compared with the sham group (p < 0.05) (TNF-α relative density: 2.412 ± 0.124; IL-1β relative density: 3.866 ± 0.172). Furthermore, SCI-induced increase of the immunoreactivity was stimulated in the SC-d group as shown by significant increase in expressions of inflammatory cytokines (p < 0.05) (TNF-α relative density: 3.352 ± 0.083; IL-1β relative density: 4.884 ± 0.079). This result indicated that surgical decompression can significantly increase the immunoreactivity in the rat model of chronic severe SCC.
Fig. 4.
Expression levels of pro-inflammatory cytokines in the four groups. No difference was observed between the sham and sham-decompression groups (*p > 0.05) (a). Severe compression resulted in higher level of tumor necrosis factor-alpha (TNF-α) in the spinal cord compared with the sham group (**p < 0.05). Meanwhile, decompression surgery substantially elevated TNF-α levels in the decompression group compared with the compression group (#p < 0.05). The levels of TNF-α were measured by Western blot analysis using actin as a standard control. No difference was observed between the sham and sham-decompression groups (*p > 0.05) (b). Severe compression resulted in higher level of interleukin-1β (IL-1β) levels compared with the sham group (**p < 0.05). Meanwhile, decompression surgery substantially elevated IL-1β levels in the decompression group compared with the compression group (#p < 0.05). The levels of IL-1β were measured by Western blot analysis using actin as a standard control.
Discussion
Although adequate surgical decompression has been the treatment choice for chronic severe SCC with favorable surgical outcomes, delayed neurological deterioration is still an unpredictable and disastrous postoperative complication.2–8) The reported incidences of the disorder vary from 3.5% to 14.5%.2,4,5,8) In general, postoperative neurological deficit is most often due to iatrogenic spinal cord insult.2,21,22) However, the patients in previous studies could move their extremities after the surgery.2–8) Moreover, no postoperative hematomas or any compressive lesions were found.2–8) All the evidences make alternative underlying mechanisms more likely than direct spinal cord trauma. Some studies have shown that the blood flow in the compressed segment significantly reduced compared with the rostral segment, which demonstrated spinal cord ischemia caused by severe mechanical compression.23) In our previous study, blood vessels in the gray and white matter were dilated remarkablely after decompression, which suggested that blood supply of compressed segment get partially restored. The most widely accepted pathogenesis is that the sudden cord expansion and reperfusion after surgical decompression may have led to disruption in the blood brain barrier and triggered a cascade of reperfusion injury resulting in neurologic dysfunction.3,4,7,8) In our previous study, the incidence of delayed neurological deterioration was very low. Nonetheless, lipid peroxidation got worse after decompression, signifying ischemia-reperfusion did occur. Although we verified that IRI occur after decompression and may be a potential etiology of delayed neurological deterioration, the precise pathophysiologic mechanisms of the decompression-related IRI remain unclear.
Spinal cord IRI includes primary ischemic injury and delayed reperfusion injury.24,25) Recent studies have indicated that inflammatory cells play a crucial role in the pathological mechanisms of primary ischemic injury and the delayed reperfusion injury in the spinal cord.11,12,14,20) Inflammatory cells produce a number of pro-inflammatory cytokines, which recruit peripheral inflammatory cells into the spinal cord and cause delayed neuron injury.26) Thus, this study was undertaken to measure and analyze inflammatory cytokines levels before and after decompression surgery to identify the underlying role of inflammation in decompression-related IRI. The rat model of chronic SCC induced by water-absorbing materials is a well-characterized animal model, which is used to study histopathologic and neurological changes of chronic compressive SCI.19,20) This rat model reproduced the characteristic course and features of chronic severe SCC injury; reduced activity of surviving neurons, neuronal degeneration, and demyelination of axons, observed as the decrease in the capacity of locomotion.19,20,27) The mean spinal cord narrowing rate in our SC models was over 70%. We insure that the degree of compression is consistent with the criteria on the chronic severe SCC.1,27)
TNF-α and IL-1β are the key inflammatory mediators and play a major role in the inflammatory response to central nervous system injury.11,28) They share the same signaling molecules as nuclear factor (NF)-κB and have similar biological effects.11,29) Microglias are the primary sources of the two pro-inflammatory cytokines.30) TNF-α and IL-1β increase neutrophil accumulation by increasing vascular endothelial permeability and upregulating the expression of endothelial leukocyte adhesion molecules.31) They control the production of IL-6 which stimulates the growth of mature B cells and is responsible for the respiratory burst of neutrophils and release of free radicals.11,32) Additionally, they have toxic effects on oligodendrocytes and promote necrosis and apoptosis in neurons.33,34)
As the most important pro-inflammatory cytokines, there is growing amount of information implicating a possible responsibility of TNF-α and IL-1β in the pathogenesis of SCI. Elevated expression of TNF-α and IL-1β have been demonstrated in animal models of traumatic SCI.27,35,36) Yang et al. revealed that immunoreactivity of TNF-α and IL-1β increased in human neurons at both early and late phases after SCI.35) Researchers reported that levels of TNF-α and IL-1β in the spinal cord increased significantly in animal models of SCI.36) Similarly, Inukai et al. found overexpression of TNF-α in the spinal cord of twy/twy mice sustaining chronic severe compression.27)
Recently, researches have indicated that TNF-α and IL-1β levels increased significantly in reperfusion period and remained at high levels after reperfusion in animals suffered from spinal cord IRI. For example, Huang et al. have found significant increase of TNF-α level in serum and spinal cord 24 hours after IRI.12) Fan et al. reported that spinal cord sections from the 48 hours reperfusion group exhibited a strong positive staining for TNF-α and IL-1β, mainly localized in the gray matter.37) Hasturk et al. showed that TNF-α and IL-1β levels were significantly higher in 24 hours IRI group than those in the sham group.11) Furthermore, there were no significant changes for TNF-α and IL-1β levels between the 24 hours and 48 hours after IRI.11) There are some other reports supporting that increase in inflammatory cytokines release is correlated to spinal cord IRI. Lu et al. reported that administration of U0126 (a specific inhibitor of MAPK/ERK kinases 1/2) significantly increased neuronal survival by reducing microglia accumulation and IL-1β expression.13) Similarly, interleukine-18 binding protein plays anti-inflammatory roles in spinal cord IRI by reducing TNF-α expression.25) Moreover, tetramethylpyrazine might also exert neuroprotective effects on spinal cord IRI by reducing the expressions of TNF-α and IL-1β through inhibition of NF-κB activity.9,37) Hydrogen gas is a new popular therapeutic agent for IRI. In a rabbit model of spinal cord IRI, the beneficial effects of hydrogen gas treatment on IRI were associated with reduce of TNF-α in serum and spinal cord.12) The above data confirm that inflammation induced by TNF-α and IL-1β, is an important pathological mechanism of injury caused by blood reperfusion of ischemic spinal cord.12,35–37)
In the present study, both immunostaining and Western blot showed low level of TNF-α and IL-1β in the spinal cord in the sham-d group without difference from those in the sham group. It suggests that decompression surgery has no direct spinal cord insult. Additionally, we found significant increase of TNF-α and IL-1β after chronic severe SCC. Briefly, in the SC group, the expression of the two pro-inflammatory cytokines in the spinal cord reached significantly higher levels as compared with the sham group. The results indicate that infiltration of neutrophil and increase of pro-inflammatory cytokines are involved in chronic severe compressive SCI. These observations complied with the data revealed by compressive SCI studies in the literature.37) Furthermore, these findings were more prominent in the SC-d group. We observed that the degree of positive staining for TNF-α and IL-1β increased significantly and infiltration of inflammatory cells aggravated in the SC-d group compared with the SC group. Western blot also showed significant increase of TNF-α and IL-1β in the spinal cord 24 hours after surgical decompression. These results suggest that surgical decompression play a stimulative role in inflammatory cascade in chronic severe SCC model. Our study supported the hypothesis that inflammation may be one of the important pathological mechanisms of decompression-related IRI of chronic ischemia. Such knowledge will facilitate the development of anti-inflammation approaches for prevention and/or treatment of this severe complication.
Conclusion
Decompression surgery plays a stimulative role in inflammatory processes by increasing the expression levels of inflammatory cytokines in the chronic severe compressive SCC model. Inflammation may be one of the important pathological mechanisms of decompression-related IRI. Such knowledge will facilitate the development of anti-inflammation approaches for prevention and/or treatment of this severe complication.
Acknowledgments
The authors like to thank all the physicians and staff who helped them in this study.
Footnotes
Ethical Standards
This study was approved by Institutional Review Board of Beijing Tiantan Hospital, Capital Medical University.
References
- 1). Xu P, Gong WM, Li Y, Zhang T, Zhang K, Yin DZ, Jia TH: Destructive pathological changes in the rat spinal cord due to chronic mechanical compression. Laboratory investigation. J Neurosurg Spine 8: 279– 285, 2008 [DOI] [PubMed] [Google Scholar]
- 2). Hasegawa K, Homma T, Chiba Y: Upper extremity palsy following cervical decompression surgery results from a transient spinal cord lesion. Spine (Phila Pa 1976) 32: E197– E202, 2007. [DOI] [PubMed] [Google Scholar]
- 3). Chin KR, Seale J, Cumming V: “White cord syndrome” of acute tetraplegia after anterior cervical decompression and fusion for chronic spinal cord compression: a case report. Case Rep Orthop 2013: 697918, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Sakaura H, Hosono N, Mukai Y, Ishii T, Yoshikawa H: C5 palsy after decompression surgery for cervical myelopathy: review of the literature. Spine (Phila Pa 1976) 28: 2447– 2451, 2003. [DOI] [PubMed] [Google Scholar]
- 5). Nassr A, Eck JC, Ponnappan RK, Zanoun RR, Donaldson WF, Kang JD: The incidence of C5 palsy after multilevel cervical decompression procedures: a review of 750 consecutive cases. Spine (Phila Pa 1976) 37: 174– 178, 2012. [DOI] [PubMed] [Google Scholar]
- 6). Taher F, Lebl DR, Cammisa FP, Pinter DW, Sun DY, Girardi FP: Transient neurological deficit following midthoracic decompression for severe stenosis: a series of three cases. Eur Spine J 22: 2057– 2061, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Lee KS, Shim JJ, Doh JW, Yoon SM, Bae HG, Yun IG: Transient paraparesis after laminectomy in a patient with multi-level ossification of the spinal ligament. J Korean Med Sci 19: 624– 626, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Young WF, Baron E: Acute neurologic deterioration after surgical treatment for thoracic spinal stenosis. J Clin Neurosci 8: 129– 132, 2001 [DOI] [PubMed] [Google Scholar]
- 9). Shin JW, Moon JY, Seong JW, Song SH, Cheong YJ, Kang C, Sohn NW: Effects of tetramethylpyrazine on microglia activation in spinal cord compression injury of mice. Am J Chin Med 41: 1361– 1376, 2013 [DOI] [PubMed] [Google Scholar]
- 10). Kieffer E, Chiche L, Cormier E, Guegan H: Recurrent spinal cord ischemia after endovascular stent grafting for chronic traumatic aneurysm of the aortic isthmus. J Vasc Surg 45: 831– 833, 2007 [DOI] [PubMed] [Google Scholar]
- 11). Hasturk A, Atalay B, Calisaneller T, Ozdemir O, Oruckaptan H, Altinors N: Analysis of serum pro-inflammatory cytokine levels after rat spinal cord ischemia/reperfusion injury and correlation with tissue damage. Turk Neurosurg 19: 353– 359, 2009 [PubMed] [Google Scholar]
- 12). Huang Y, Xie K, Li J, Xu N, Gong G, Wang G, Yu Y, Dong H, Xiong L: Beneficial effects of hydrogen gas against spinal cord ischemia-reperfusion injury in rabbits. Brain Res 1378: 125– 136, 2011 [DOI] [PubMed] [Google Scholar]
- 13). Lu K, Cho CL, Liang CL, Chen SD, Liliang PC, Wang SY, Chen HJ: Inhibition of the MEK/ERK pathway reduces microglial activation and interleukin-1-beta expression in spinal cord ischemia/reperfusion injury in rats. J Thorac Cardiovasc Surg 133: 934– 941, 2007 [DOI] [PubMed] [Google Scholar]
- 14). Reece TB, Okonkwo DO, Ellman PI, Warren PS, Smith RL, Hawkins AS, Linden J, Kron IL, Tribble CG, Kern JA: The evolution of ischemic spinal cord injury in function, cytoarchitecture, and inflammation and the effects of adenosine A2A receptor activation. J Thorac Cardiovasc Surg 128: 925– 932, 2004 [DOI] [PubMed] [Google Scholar]
- 15). Nakata T, Kawachi K, Nagashima M, Yasugi T, Izutani H, Ryugo M, Okamura T, Shikata F, Imagawa H, Yano H, Takahashi H, Tanaka J: Transient ischemia-induced paresis and complete paraplegia displayed distinct reactions of microglia and macrophages. Brain Res 1420: 114– 124, 2011 [DOI] [PubMed] [Google Scholar]
- 16). Seekamp A, Jochum M, Ziegler M, van Griensven M, Martin M, Regel G: Cytokines and adhesion molecules in elective and accidental trauma-related ischemia/reperfusion. J Trauma 44: 874– 882, 1998 [DOI] [PubMed] [Google Scholar]
- 17). Matsumoto S, Matsumoto M, Yamashita A, Ohtake K, Ishida K, Morimoto Y, Sakabe T: The temporal profile of the reaction of microglia, astrocytes, and macrophages in the delayed onset paraplegia after transient spinal cord ischemia in rabbits. Anesth Analg 96: 1777– 1784, table of contents, 2003 [DOI] [PubMed] [Google Scholar]
- 18). Ning N, Dang X, Bai C, Zhang C, Wang K: Panax notoginsenoside produces neuroprotective effects in rat model of acute spinal cord ischemia-reperfusion injury. J Ethnopharmacol 139: 504– 512, 2012 [DOI] [PubMed] [Google Scholar]
- 19). Kim P, Haisa T, Kawamoto T, Kirino T, Wakai S: Delayed myelopathy induced by chronic compression in the rat spinal cord. Ann Neurol 55: 503– 511, 2004 [DOI] [PubMed] [Google Scholar]
- 20). Wang J, Rong W, Hu X, Liu X, Jiang L, Ma Y, Dang G, Liu Z, Wei F: Hyaluronan tetrasaccharide in the cerebrospinal fluid is associated with self-repair of rats after chronic spinal cord compression. Neuroscience 210: 467– 480, 2012 [DOI] [PubMed] [Google Scholar]
- 21). Kou J, Fischgrund J, Biddinger A, Herkowitz H: Risk factors for spinal epidural hematoma after spinal surgery. Spine (Phila Pa 1976) 27: 1670– 1673, 2002. [DOI] [PubMed] [Google Scholar]
- 22). Cramer DE, Maher PC, Pettigrew DB, Kuntz C: Major neurologic deficit immediately after adult spinal surgery: incidence and etiology over 10 years at a single training institution. J Spinal Disord Tech 22: 565– 570, 2009 [DOI] [PubMed] [Google Scholar]
- 23). Kurokawa R, Murata H, Ogino M, Ueki K, Kim P: Altered blood flow distribution in the rat spinal cord under chronic compression. Spine (Phila Pa 1976) 36: 1006– 1009, 2011. [DOI] [PubMed] [Google Scholar]
- 24). Zhu P, Li JX, Fujino M, Zhuang J, Li XK: Development and treatments of inflammatory cells and cytokines in spinal cord ischemia-reperfusion injury. Mediators Inflamm 2013: 701970, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Karavelioğlu E, Gönül Y, Kokulu S, Hazman Ö, Bozkurt F, Koçak A, Eser O: Anti-inflammatory and antiapoptotic effect of interleukine-18 binding protein on the spinal cord ischemia-reperfusion injury. Inflammation 37: 917– 923, 2014 [DOI] [PubMed] [Google Scholar]
- 26). Ilhan A, Yilmaz HR, Armutcu F, Gurel A, Akyol O: The protective effect of nebivolol on ischemia/reperfusion injury in rabbit spinal cord. Prog Neuropsychopharmacol Biol Psychiatry 28: 1153– 1160, 2004 [DOI] [PubMed] [Google Scholar]
- 27). Inukai T, Uchida K, Nakajima H, Yayama T, Kobayashi S, Mwaka ES, Guerrero AR, Baba H: Tumor necrosis factor-alpha and its receptors contribute to apoptosis of oligodendrocytes in the spinal cord of spinal hyperostotic mouse (twy/twy) sustaining chronic mechanical compression. Spine (Phila Pa 1976) 34: 2848– 2857, 2009. [DOI] [PubMed] [Google Scholar]
- 28). Hayashi M, Ueyama T, Nemoto K, Tamaki T, Senba E: Sequential mRNA expression for immediate early genes, cytokines, and neurotrophins in spinal cord injury. J Neurotrauma 17: 203– 218, 2000 [DOI] [PubMed] [Google Scholar]
- 29). Nguyen MD, Julien JP, Rivest S: Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci 3: 216– 227, 2002. [DOI] [PubMed] [Google Scholar]
- 30). Byrnes KR, Garay J, Di Giovanni S, De Biase A, Knoblach SM, Hoffman EP, Movsesyan V, Faden AI: Expression of two temporally distinct microglia-related gene clusters after spinal cord injury. Glia 53: 420– 433, 2006 [DOI] [PubMed] [Google Scholar]
- 31). Baki ED, Aldemir M, Kokulu S, Koca HB, Ela Y, Sıvacı RG, Öztürk NK, Emmiler M, Adalı F, Uzel H: Comparison of the effects of desflurane and propofol anesthesia on the inflammatory response and s100β protein during coronary artery bypass grafting. Inflammation 36: 1327– 1333, 2013 [DOI] [PubMed] [Google Scholar]
- 32). Hassan W, Ding L, Gao RY, Liu J, Shang J: Interleukin-6 signal transduction and its role in hepatic lipid metabolic disorders. Cytokine 66: 133– 142, 2014 [DOI] [PubMed] [Google Scholar]
- 33). Ismailoğlu Ö, Oral B, Sütcü R, Kara Y, Tomruk O, Demir N: Neuroprotective effects of raloxifene on experimental spinal cord injury in rats. Am J Med Sci 345: 39– 44, 2013 [DOI] [PubMed] [Google Scholar]
- 34). Hermann GE, Rogers RC, Bresnahan JC, Beattie MS: Tumor necrosis factor-alpha induces cFOS and strongly potentiates glutamate-mediated cell death in the rat spinal cord. Neurobiol Dis 8: 590– 599, 2001 [DOI] [PubMed] [Google Scholar]
- 35). Yang L, Blumbergs PC, Jones NR, Manavis J, Sarvestani GT, Ghabriel MN: Early expression and cellular localization of proinflammatory cytokines interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in human traumatic spinal cord injury. Spine (Phila Pa 1976) 29: 966– 971, 2004. [DOI] [PubMed] [Google Scholar]
- 36). Pineau I, Lacroix S: Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol 500: 267– 285, 2007 [DOI] [PubMed] [Google Scholar]
- 37). Fan L, Wang K, Shi Z, Die J, Wang C, Dang X: Tetramethylpyrazine protects spinal cord and reduces inflammation in a rat model of spinal cord ischemia-reperfusion injury. J Vasc Surg 54: 192– 200, 2011 [DOI] [PubMed] [Google Scholar]