Abstract
The purpose of the present study was to investigate the effectiveness of acute phase hybrid assistive limb (HAL) rehabilitation training for patients after stroke by measuring the difference in the severity of paralysis. Fifty-three acute stroke patients were enrolled in this prospective cohort study. HAL training was administered about twice per week, and the mean number of sessions was 3.9 ± 2.7. The walking training was performed on a treadmill with individually adjustable body weight support and speed and there was a 10-m walk test (10MWT) before and after each session. Assessment at baseline and at endpoint consisted of the Glasgow Coma Scale (GCS), Revised Hasegawa’s Dementia Scale (HDS-R), Brunnstrom stage (Brs), Functional Independence Measure (FIM), Barthel index (BI), and 10MWT. We measured these assessments at the first walking training session and at the end of the final training session without the HAL. To evaluate the feasibility of training with the HAL, the outcome measures of BI, FIM, and speed and number of steps of 10MWT were compared before and after training using a paired Wilcoxon’s signed-rank test in different Brs. Except for Brs IV, the Brs III or higher subgroups displayed significant amelioration in BI, and the Brs III subgroup displayed significant amelioration in FIM. The Brs V and VI subgroups displayed significant amelioration in 10-m walking speed and steps. In acute phase rehabilitation after stroke, it is thought that the HAL is more effective for patients with less lower-limb paralysis, such as Brs III or higher.
Keywords: hybrid assistive limb, acute phase rehabilitation, stroke
Introduction
Rehabilitation in the acute phase after stroke is an important treatment for improving patients’ functional outcomes.1–5) The hybrid assistive limb (HAL) suit is a wearable robot that estimates the wearer’s motion intention and enhances their motion in real time. The HAL suit enhances motion by detecting bioelectrical signals (BESs),6) recorded and amplified from hip and knee extensors and flexors via electromyography electrodes, and has been developed for the assistance of stroke patients. The HAL suit can aid rehabilitation using muscle activity and has the potential to intensify feedback. HAL-induced motion might also evoke sensory input, which has a favorable feedback effect on the central nervous system for the recovery of locomotor function.7) Although some studies have evaluated the efficacy of rehabilitation using the HAL for the recovery of chronic phase patients after stroke, studies on acute phase rehabilitation after stroke with HAL are rare.8) The purpose of the present study was to investigate the effectiveness of acute phase HAL rehabilitation training for patients after stroke by measuring the difference in the severity of paralysis.
Materials and Methods
This study was approved by the Ethics Committee of Fukuoka University Hospital. Between November 2011 and May 2014, a total of 100 patients have undergone rehabilitation training using the HAL in Fukuoka University Hospital, including 59 with stroke, 16 with brain tumor, 16 with spinal disease, and 9 with other conditions. With the exception of six convalescent phase stroke patients, 53 acute phase stroke patients were enrolled in this prospective cohort study. The training was performed by one or two physiotherapists and a doctor, who had trained to use the HAL system. In the first rehabilitation session with the HAL, the double-leg model of the HAL was used. From the second session, we used either the single-leg or the double-leg model, according to the patients’ condition; i.e., the single-leg model was mainly used for cases of hemiplegia and the double-leg model was for cases of ataxia and paraplegia.9) The physiotherapist provided instructions and feedback to the patient, and a large monitor showing the status of HAL was placed in front of the patient (Fig. 1). The patient was able to learn the moving position of their center of gravity in real time, which allowed visual feedback.
Fig. 1.
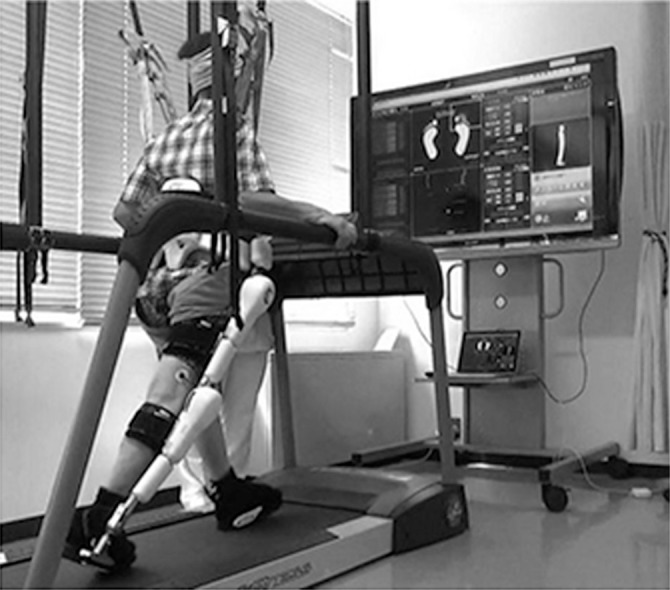
Patient performing treadmill locomotor training with the hybrid assistive limb (HAL) and body weight support system suspended.
Training started with the Cybernic Voluntary Control (CVC) mode providing complete control using BES, which was recorded by skin-surface electromyographic electrodes placed on the rectus femoris, vastus lateralis, gluteus maximus, and biceps femoris muscles. The HAL recorded the BES generated by the patient’s muscle activities and floor reaction force caused by the patient’s intended weight shifts, and the patients operated the HAL by adjusting their muscle activities. Therefore, the HAL was able to conduct training by providing motion support in response to the patient’s voluntary drive. HAL training was administered about twice per week, and the mean number of sessions was 3.9 ± 2.7. After the HAL suit was attached to the patients, knee flexion and stretch and sit-to-stand training were performed. During the training, the physiotherapist checked the BES and adjusted the motor assist level of the HAL. Next, the walking training was performed on a treadmill with individually adjustable body weight support and speed. Patients underwent a 10-m walk test (10MWT) before and after each session. The training was supervised by physiotherapists and a medical doctor. Assessment at baseline and at endpoint consisted of the Glasgow Coma Scale (GCS), Revised Hasegawa’s Dementia Scale (HDS-R), Brunnstrom stage (Brs), Functional Independence Measure (FIM), Barthel Index (BI), and the 10MWT. We measured 10MWT in patients at the start of the first walking training session and at the end of the final training session; both measurements were made without the HAL.
We divided the 53 patients into 6 groups by severity of lower-limb paralysis according to Brs (Table 1). Brs was used to assess motor recovery of the paralytic lower limb,10) and was categorized as follows: Brs I (n = 7), flaccid paralysis; Brs II (n = 5), increased muscle tone without active movement; Brs III (n = 12), increased muscle tone with active movement, mainly in rigid extension synergy; Brs IV (n = 7), increased muscle tone with alternating gross movement in extension and flexion synergies; Brs V (n = 12), normal muscle tone with some degree of selective muscle control; and Brs VI (n = 10), normal muscle tone and control.
Table 1.
Patient profiles
| Brunnstrom stage | I | II | III | IV | V | VI |
|---|---|---|---|---|---|---|
| Number of patients | 7 | 5 | 12 | 7 | 12 | 10 |
| Male : Female | 5 : 2 | 4 : 1 | 5 : 7 | 2 : 5 | 7 : 5 | 4 : 6 |
| Age | 63.7 ± 14.7 | 69.4 ± 6.2 | 60.8 ± 15.6 | 62.0 ± 21.5 | 65.1 ± 13.2 | 60.8 ± 23.0 |
| GCS | 10.4 ± 2.4 | 13.8 ± 1.6 | 13.4 ± 2.1 | 13.7 ± 1.8 | 14.3 ± 1.1 | 14.7 ± 0.9 |
| Number of training days | 13.0 ± 13.1 | 4.2 ± 2.9 | 17.0 ± 10.7 | 10.4 ± 5.2 | 11.4 ± 12.6 | 8.1 ± 7.1 |
| Number of sessions | 3.4 ± 2.2 | 2.6 ± 1.1 | 5.6 ± 3.3 | 4.7 ± 3.0 | 3.5 ± 2.5 | 2.6 ± 1.8 |
GCS: Glasgow Coma Scale.
To evaluate the feasibility of training with the HAL, the outcome measures of BI, FIM, and speed and number of steps of 10MWT were compared before and after training using a paired Wilcoxon’s signed-rank test in different Brs. Statistical differences of p < 0.05 were considered significant. Data analysis was performed using SPSS version 21.0.J (IBM Corp., Armonk, NewYork, USA).
Results
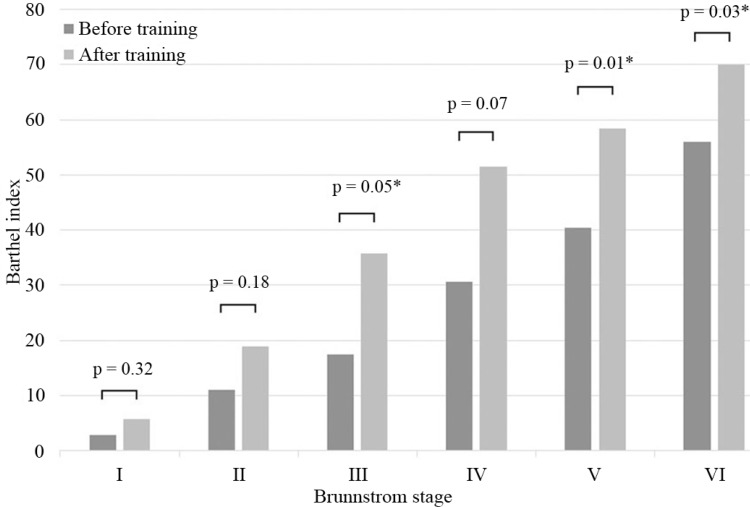
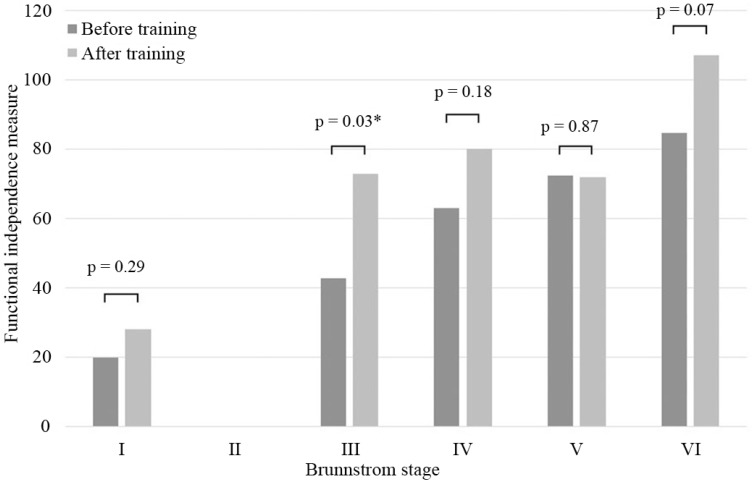
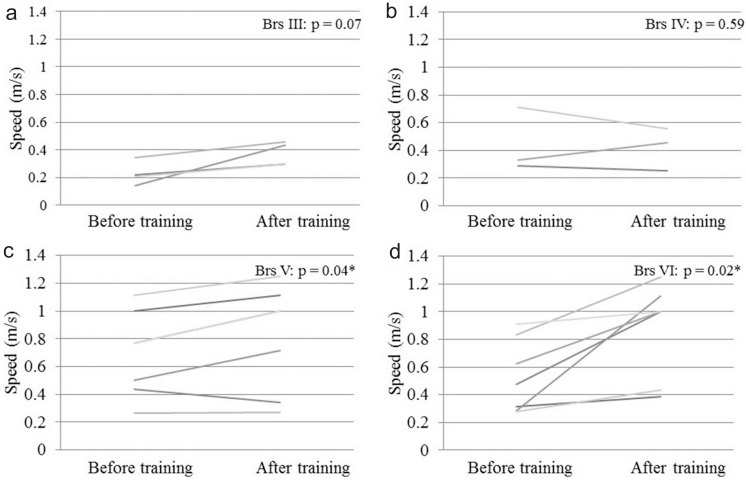
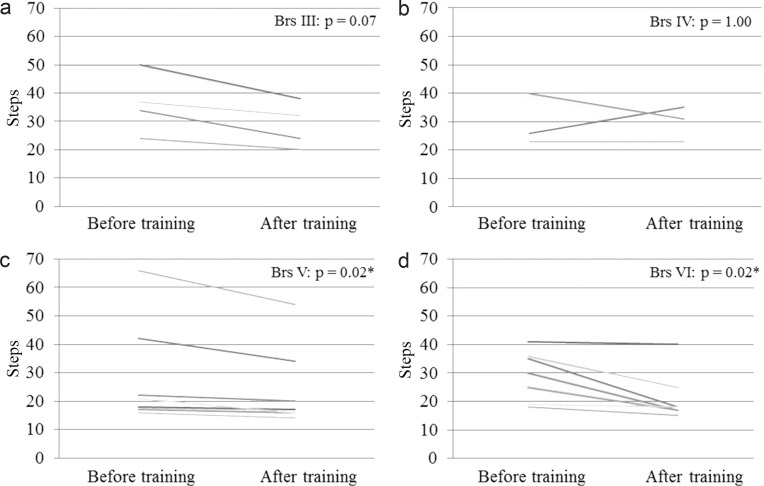
Table 1 summarizes patient information for cases in different Brs before training. Table 2 summarizes BI, FIM, and speed and steps of 10MWT before and after training. These data are shown as mean ± standard deviation (SD). Fig. 2 shows the mean improvement concerning BI in all different Brs before and after training, and statistically significant differences were seen in Brs III, V, and VI. Fig. 3 shows a change in the mean of the FIM; the score increased in Brs I, III, IV, and VI. In Brs II, the FIM was not available because we did not measure it in this group. Fig. 4 shows that all patients increased their gait speed from before to after training assessments except for two patients in Brs IV and one patient in Brs V. We observed a significant increase in gait speed in Brs V and VI. Fig. 5 showed that the number of steps was decreased in all patients except for one patient in Brs IV, and a significant difference was seen in Brs V and VI. In Brs I and II, speed and steps of 10MWT were not available; the patients had difficulty in 10-m walking in this group because of their severe paralysis.
Table 2.
Baseline and endpoint data in different Brunnstrom stages, mean ± range
| Brunnstrom stage | I | II | III | IV | V | VI | |
|---|---|---|---|---|---|---|---|
| Barthel index | Before | 2.9 ± 7.6 | 11.0 ± 16.7 | 17.5 ± 19.8 | 30.7 ± 27.9 | 40.4 ± 24.9 | 56.0 ± 22.6 |
| After | 5.7 ± 9.8 | 19.0 ± 20.1 | 35.8 ± 23.2 | 51.4 ± 33.3 | 58.3 ± 26.1 | 70.0 ± 20.8 | |
| FIM | Before | 20.0 ± 2.0 | 42.8 ± 20.0 | 63.0 ± 11.3 | 72.3 ± 21.3 | 84.6 ± 34.1 | |
| After | 28.0 ± 9.6 | N/A | 72.8 ± 19.4 | 80.0 ± 14.1 | 71.9 ± 17.6 | 107.0 ± 19.0 | |
| Speed (m/s) | Before | 0.2 ± 0.1 | 0.4 ± 0.2 | 0.7 ± 0.3 | 0.5 ± 0.3 | ||
| After | N/A | N/A | 0.4 ± 0.1 | 0.4 ± 0.2 | 0.8 ± 0.4 | 0.9 ± 0.3 | |
| Steps | Before | 36.3 ± 10.7 | 29.7 ± 9.1 | 28.9 ± 18.6 | 29.1 ± 8.8 | ||
| After | N/A | N/A | 28.5 ± 8.1 | 29.7 ± 6.1 | 24.4 ± 14.7 | 21.4 ± 8.8 |
FIM: Functional Independence Measure, N/A: not applicable.
Fig. 2.
The average of BI in each Brs between before and after training. Significant amelioration was seen in Brs III, V, and VI. BI: Barthel index, Brs: Brunnstrom stage. *p < 0.05; Wilcoxon’s signed-rank test.
Fig. 3.
The average of the FIM in each Brs between before and after training. The scores were significantly ameliorated in Brs I, II, III, and VI. FIM was not measured in Brs II. Brs: Brunnstrom stage, FIM: Functional Independence Measure.
Fig. 4.
Change of gait speed in 10-m walking test for patients after HAL training. a: Brs III, b: Brs IV, c: Brs V, d: Brs VI. All patients increased gait speed from before to after training assessments except for two patients in Brs IV and one patient in Brs V. We observed a significant increase in gait speed in Brs III and IV. Brs: Brunnstrom stage, HAL: hybrid assistive limb. *p < 0.05; Wilcoxon’s signed-rank test.
Fig. 5.
Change of number of steps in 10-m walking test for patients after HAL training. a: Brs III, b: Brs IV, c: Brs V, d: Brs VI. The number of steps was decreased in all patients except for one patient in Brs IV, and significantly decreased in Brs III and IV. Brs: Brunnstrom stage, HAL: hybrid assistive limb. *p < 0.05; Wilcoxon’s signed-rank test.
Discussion
Some past reports have demonstrated electromechanical devices for gait rehabilitation, such as the Lokomat,11,12) Gait Trainer,13) and Gait Master.14) These devices were developed to help patients in the swing phase, and control patient movements using a personal computer to assist them in moving their legs in a physiological gait pattern on a moving treadmill. Patients cannot influence the motion by themselves because these systems use a fixed gait pattern. The HAL for the lower limbs is a robotic suit that assists voluntary control of knee and hip joint motion by the detection of BES on the skin surface. It generates power-assisted torque by amplifying the patients’ joint torque, which is estimated by the BES recorded on their skin. The HAL’s characteristic feature is that it is a wearable robot that estimates the wearer’s motion intention and enhances his or her motion in real time. The HAL suit can potentially intensify feedback when aiding rehabilitation using muscle activity. HAL-induced motion might also evoke sensory input, which has a favorable feedback effect on the central nervous system toward recovery of locomotor function.7)
Many recent studies have reported the efficacy of rehabilitation using the HAL.8,15–17) It is difficult to directly compare these studies and our study because of differences in disease, severity and duration of the disorder, robotic features, methods of investigation, and outcome measures. Some studies have demonstrated that HAL rehabilitation is feasible and safe in the acute phase after stroke.8,15) Studies of chronic phase rehabilitation with the HAL have been performed a number of times; however, frequent training with the HAL for patients in the acute phase is not easy because an early transfer to a convalescence hospital is needed. An improvement was also seen in BI, FIM, and speed and number of steps of 10MWT in our study, despite the small amount of training with the HAL (average of about four sessions), which implies the usefulness of the HAL in acute phase rehabilitation. To investigate our findings on the feasibility of training using the HAL, we explored the effectiveness of the HAL in each of the subgroups based on Brs. Except for Brs IV, all Brs III or higher subgroups displayed significant amelioration in BI, and the Brs III subgroup displayed significant amelioration in FIM. The Brs V and VI subgroups displayed significant amelioration in the speed and steps of 10MWT. These results suggest that training with the HAL was effective for patients with lower-limb paralysis of Brs III or higher.
In this study, patients with severe hemiparesis, such as those in Brs I and II, did not show significant amelioration in all the BI, FIM, and 10MWT, possibly because the HAL could not act effectively on them. According to Maeshima et al.,18) walking while wearing the HAL suit requires adaptation to a new gait pattern and coordination of movements, and severely hemiplegic patients may experience difficulty in rapidly adapting to a new gait pattern. One reason is that it is difficult for severely hemiplegic patients to perform activities using their muscles because the HAL suit uses only weak BES to provide power assistance, resulting in decreased walking speed. To address this problem, before we started gait training with the HAL for severely hemiplegic patients in particular, we performed sit-to-stand training and balance training in a standing position. This training was done because it would aid the patients in producing BES.19) We made the best of training with HAL although there were no significant improvements of BI, FIM, or 10MWT in our study for patients with severe hemiplegia.
Kubota et al.7) showed that the improvement in gait speed with HAL training was mainly brought about by improvement in cadence, and HAL training improved stride frequency more than stride length; this finding is in agreement with that of a previous robotic training study.20) In our study, the Brs V and VI subgroups showed a significantly increased 10MWT speed and a decreased number of steps. It was thought that patients with mild paralysis could produce relatively strong BES and that the HAL acted on them more effectively, and the improved walking rate and decreased number of steps in 10MWT were a result of the walk balance improving via training with the HAL.
The mean number of sessions in this study was 3.9 ± 2.7; the SD was high. One reason is that 10 of the 53 patients could undergo rehabilitation with the HAL only once, mainly because it did not accommodate their height or abdominal circumference, and because acute phase patients did not find it easy to receive instruction and to comprehend the use of the HAL because of their reduced higher-order brain functions.
Study Limitations
This study had some limitations. This study was not a randomized controlled trial and could not compare the efficacy of HAL training with conventional rehabilitation. Because only a small number of patients were included in each Brs subgroup, the statistical power was low. We could not exclude pathological bias because the term “stroke” was used for both brain ischemia and brain hemorrhage. Further studies are needed to assess the HAL’s effectiveness.
Conclusion
In acute phase rehabilitation after stroke, the HAL was found to be more effective for patients with less lower-limb paralysis, such as Brs III or more. When using the HAL for severely hemiplegic patients, it might be useful to record stronger BES to perform sit-to-stand training and balance training in a standing position thoroughly before gait training. Further studies are needed to analyze the effectiveness of the evaluation and the methods for the effective use of the HAL.
Acknowledgments
This study was partly supported by the Clinical Research Foundation, Japan.
References
- 1). Bernhardt J, Dewey H, Thrift A, Collier J, Donnan G: A very early rehabilitation trial for stroke (AVERT): phase II safety and feasibility. Stroke 39: 390– 396, 2008. [DOI] [PubMed] [Google Scholar]
- 2). Cumming TB, Thrift AG, Collier JM, Churilov L, Dewey HM, Donnan GA, Bernhardt J: Very early mobilization after stroke fast-tracks return to walking: further results from the phase II AVERT randomized controlled trial. Stroke 42: 153– 158, 2011. [DOI] [PubMed] [Google Scholar]
- 3). Di Lauro A, Pellegrino L, Savastano G, Ferraro C, Fusco M, Balzarano F, Franco MM, Biancardi LG, Grasso A: A randomized trial on the efficacy of intensive rehabilitation in the acute phase of ischemic stroke. J Neurol 250: 1206– 1208, 2003. [DOI] [PubMed] [Google Scholar]
- 4). Drummond AE, Pearson B, Lincoln NB, Berman P: Ten year follow-up of a randomised controlled trial of care in a stroke rehabilitation unit. BMJ 331: 491– 492, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Langhorne P, Stott D, Knight A, Bernhardt J, Barer D, Watkins C: Very early rehabilitation or intensive telemetry after stroke: a pilot randomised trial. Cerebrovasc Dis 29: 352– 360, 2010. [DOI] [PubMed] [Google Scholar]
- 6). Kawamoto H, Taal S, Niniss H, Hayashi T, Kamibayashi K, Eguchi K, Sankai Y: Voluntary motion support control of Robot Suit HAL triggered by bioelectrical signal for hemiplegia. Conf Proc IEEE Eng Med Biol Soc 462– 466, 2010. [DOI] [PubMed] [Google Scholar]
- 7). Kubota S, Nakata Y, Eguchi K, Kawamoto H, Kamibayashi K, Sakane M, Sankai Y, Ochiai N: Feasibility of rehabilitation training with a newly developed wearable robot for patients with limited mobility. Arch Phys Med Rehabil 94: 1080– 1087, 2013. [DOI] [PubMed] [Google Scholar]
- 8). Ueba T, Hamada O, Ogata T, Inoue T, Shiota E, Sankai Y: Feasibility and safety of acute phase rehabilitation after stroke using the hybrid assistive limb robot suit. Neurol Med Chir (Tokyo) 53: 287– 290, 2013. [DOI] [PubMed] [Google Scholar]
- 9). Hamada O, Samura K, Ogawa S, Fukuda H, Saita K, Goto K, Ogata T, Shiota E, Inoue T: [A study of neurorehabilitation with appropriate use of the single-leg model and double-leg model of the hybrid assistive limb]. Currently Practical Neurosurgery 24: 898– 903, 2014. (Japanese) [Google Scholar]
- 10). Brunnstrom S: Motor testing procedures in hemiplegia: based on sequential recovery stages. Phys Ther 46: 357– 375, 1966. [DOI] [PubMed] [Google Scholar]
- 11). Colombo G, Wirz M, Dietz V: Driven gait orthosis for improvement of locomotor training in paraplegic patients. Spinal Cord 39: 252– 255, 2001. [DOI] [PubMed] [Google Scholar]
- 12). Jezernik S, Colombo G, Keller T, Frueh H, Morari M: Robotic orthosis lokomat: a rehabilitation and research tool. Neuromodulation 6: 108– 115, 2003. [DOI] [PubMed] [Google Scholar]
- 13). Hesse S, Uhlenbrock D: A mechanized gait trainer for restoration of gait. J Rehabil Res Dev 37: 701– 708, 2000. [PubMed] [Google Scholar]
- 14). Tanaka N, Saitou H, Takao T, Iizuka N, Okuno J, Yano H, Tamaoka A, Yanagi H: Effects of gait rehabilitation with a footpad-type locomotion interface in patients with chronic post-stroke hemiparesis: a pilot study. Clin Rehabil 26: 686– 695, 2012. [DOI] [PubMed] [Google Scholar]
- 15). Nilsson A, Vreede KS, Häglund V, Kawamoto H, Sankai Y, Borg J: Gait training early after stroke with a new exoskeleton—the hybrid assistive limb: a study of safety and feasibility. J Neuroeng Rehabil 11: 92, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Sakakima H, Ijiri K, Matsuda F, Tominaga H, Biwa T, Yone K, Sankai Y: A newly developed robot suit hybrid assistive limb facilitated walking rehabilitation after spinal surgery for thoracic ossification of the posterior longitudinal ligament: a case report. Case Rep Orthop 2013: 621405, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Kawamoto H, Kamibayashi K, Nakata Y, Yamawaki K, Ariyasu R, Sankai Y, Sakane M, Eguchi K, Ochiai N: Pilot study of locomotion improvement using hybrid assistive limb in chronic stroke patients. BMC Neurol 13: 141, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Maeshima S, Osawa A, Nishio D, Hirano Y, Takeda K, Kigawa H, Sankai Y: Efficacy of a hybrid assistive limb in post-stroke hemiplegic patients: a preliminary report. BMC Neurol 11: 116, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Hamada O, Ueba T, Ogata T, Nonaka M, Fukuda H, Shiota E, Inoue T: [Detection of bioelectric potential by a hybrid assistive limb during acute stage rehabilitation]. Jpn J Neurosurg 22: 792– 797, 2013. (Japanese) [Google Scholar]
- 20). Nooijen CF, Ter Hoeve N, Field-Fote EC: Gait quality is improved by locomotor training in individuals with SCI regardless of training approach. J Neuroeng Rehabil 6: 36, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]