Abstract
Recent studies have shown that posterior communicating artery (PComA) aneurysms are more likely to rupture. However, surgical intervention for PComA aneurysms may be associated with increased treatment-related morbidity rate. Therefore, it is meaningful to investigate the factors related to PComA aneurysm rupture. The purpose of this study was to identify morphological parameters that significantly correlate with PComA aneurysm rupture. We divided 14 pairs of mirror posterior communicating artery aneurysms (PComA-MANs) into ruptured and unruptured groups. Computed tomography angiography (CTA) imaging was evaluated with three-dimensional (3D) Slicer to generate models of the aneurysms and surrounding vasculature. Nine morphological parameters [size, height, width, neck width, aspect ratio (AR), bottleneck factor (BNF), height/width ratio (H/W), size ratio (SR), and bleb formation] were examined in the two groups for significance with respect to rupture. By contrast, statistically significant differences were found in ruptured and unruptured group for size, AR, BNF, SR, and bleb formation (P < 0.05). Parameters that had no significant differences between the two groups were height (P = 0.103), width (P = 0.078), neck width (P = 0.808), and H/W (P = 0.417). We conclude that MANs may be a useful model for the morphological analysis of intracranial aneurysm rupture. Larger size, higher AR, BNF, SR, and bleb formation may be related to rupture of PComA aneurysms. Larger sample studies minimizing the interference from patient-related factors and aneurysm type were expected for acquiring more accurate assessment of the relationship between these parameters and PComA aneurysm rupture.
Keywords: unruptured intracranial aneurysm, posterior communicating artery aneurysm, saccular aneurysm, morphology, rupture risk
Introduction
Intracranial aneurysm (IA) rupture is a main cause of spontaneous subarachnoid hemorrhage (SAH) which has a significant mortality and morbidity.1) Along with the improvement of non-invasive imaging test and elective screening, the incidental detection of unruptured intracranial aneurysms (UIAs) is increasing, from 2% to 5%.2) However, among these UIAs detected, the minority will rupture and lead to SAH in the lifetime.3)
Posterior communicating artery (PComA) is one of the most common sites for UIAs.4–6) Recent studies showed that aneurysm location could predict future rupture of unruptured IAs. And unruptured PComA aneurysms were more likely to rupture.4,7) However, as PComA gives off vital branches to supply the optic chiasm, oculomotor nerve, mammillary body, tuber cinereum, cerebral crura, ventral thalamus, and rostral portion of the caudate nucleus, clipping, or coiling PComA aneurysms may be associated with increased treatment-related morbidity rate.8,9) Accordingly, it is highly desirable to investigate factors related to the rupture of PComA aneurysms so that we can select those lesions with high rupture risk for further treatment.
With the imaging technology progressing, it is easier for doctors to acquire the morphological data of clinical cases, enabling more and more researchers to study the relationships between the risk of IA rupture and the anatomical configuration of the aneurysm along with its parent artery. Lu et al. defined “intracranial mirror aneurysms (MANs)” as bilateral saccular aneurysms at roughly the symmetric location in one patient. They can be an ideal model for morphological analysis of IA rupture as they provide an internal control for those clinical variables (such as age, sex, smoking, hypertension, and collagen genetics) and aneurysm location, which are considered related to the aneurysm rupture.10) To our knowledge, there was only one study utilizing eight pairs of PComA-MANs to investigate the correlation between the morphological parameters and the risk of PComA aneurysm rupture.11) In this research, we added a selection criteria that two symmetric aneurysms should be of the same aneurysm type, either sidewall (SW) or bifurcation (Bif), considering the possible different natural course of these two aneurysm types.12) And PComA-MANs were also used to assess the predictive ability of morphological parameters on rupture.
Materials and Methods
I. Patients
From July 2011 to May 2014, 17 consecutive patients with PComA-MANs were diagnosed by angiography in The First Affiliated Hospital of Zhejiang University. Among them, three patients with different aneurysm type of bilateral aneurysms were excluded. The remaining 14 patients were all with a ruptured aneurysm and an unruptured aneurysm in the symmetric location and the same aneurysm type. These 14 pairs of aneurysms were divided into two groups (i.e., ruptured and unruptured groups). The ruptured aneurysm was identified on the basis of the pattern of hemorrhage on conventional computed tomography (CT) scan and intraoperative findings like deposition of blood products and adhesions. In these 14 patients, 9 were female. Their ages ranged from 40 to 69, with a mean age of 59.1 years. The sidewall type occupied 6 of the 14 PComA-MANs. There were six cases with the ruptured one locating on the left side (Table 1).
Table 1.
Common conditions of 14 patients with mirror posterior communicating artery aneurysms
| Patient no. | Sex | Age | Aneurysm type | Sidedness of the ruptured one |
|---|---|---|---|---|
| 1 | F | 44 | SW* | Left |
| 2 | F | 40 | SW | Left |
| 3 | M | 69 | Bif# | Right |
| 4 | F | 59 | SW | Right |
| 5 | M | 63 | Bif | Left |
| 6 | F | 67 | Bif | Right |
| 7 | F | 67 | Bif | Right |
| 8 | M | 69 | SW | Left |
| 9 | F | 58 | Bif | Right |
| 10 | F | 61 | Bif | Right |
| 11 | F | 67 | Bif | Right |
| 12 | M | 58 | SW | Left |
| 13 | M | 58 | Bif | Right |
| 14 | F | 48 | SW | Left |
Sidewall aneurysms are those saccular aneurysms originating from only one parent vessel or from the origin of a small branch whose caliber is less than one-fifth of the parent vessel. The efferent and afferent parent vessels have similar calibers.
Bifurcation aneurysms as those saccular aneurysms located at major bifurcations in the cerebral vessel. Bif: bifurcation, F: female, M: male, SW: sidewall.
II. Reconstruction of three-dimensional (3D) models
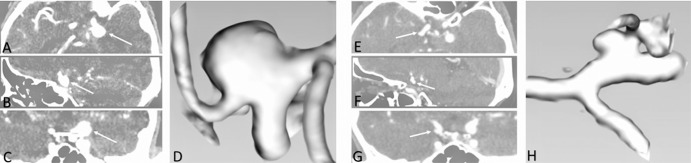
Computed tomography angiography (CTA) was routinely performed in all patients in whom conventional CT confirmed the presence of spontaneous SAH. It was performed on a GE LightSpeed VCT (General Electric Company, Fairfield, CT, USA) with a slice thickness of 0.75 mm and an increment of 0.5 mm. The CTA images were used to generate composite 3D models of the aneurysm and surrounding vasculature by 3D Slicer which is an open source, multiplatform visualization, and image analysis software developed by the Surgical Planning Laboratory at the Brigham and Women’s Hospital (Fig. 1). The 3D model of the aneurysm and parent vessels could be tumbled freely in the Slicer environment. The measure could be done in 3D space relying on fiducial-based tractography. Two observers performed the measure and calculation, and the average value was used for subsequent statistical analyses.
Fig. 1.
CTA and 3D models of a pair of PComA-MAN. A–C: Axial (A), sagittal (B), and coronal (C) CT images showing the ruptured PComA-MAN. E–G: Axial (E), sagittal (F), and coronal (G) CT images showing the unruptured PComA-MAN. D, H: Corresponding 3D reconstructed Slicer images. 3D: three-dimensional, CT: computed tomography, CTA: computed tomography angiography, PComA-MAN: mirror posterior communicating artery aneurysm.
III. Definition of morphological parameters
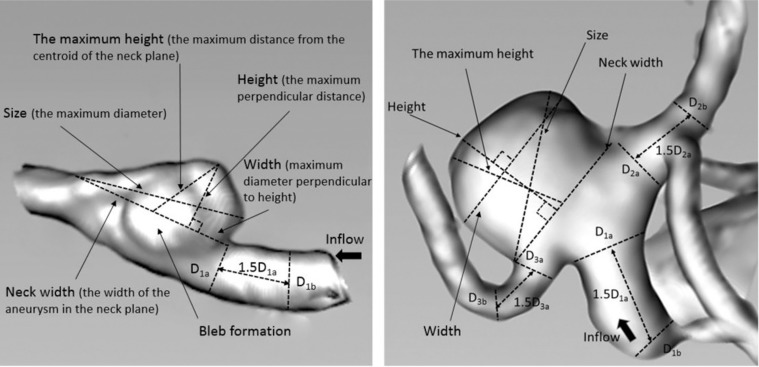
Morphological parameters analyzed and recorded for each patient included aneurysm size, aneurysm height, aneurysm width, aneurysm neck width, aspect ratio (AR), bottleneck factor (BNF), height/width ratio (H/W), size ratio (SR), and bleb formation (without bleb = 0 or with bleb = 1) (Table 2). Because of the coincidence of bilateral aneurysm type, the type of aneurysms was not included in the analysis. The IA neck plane was defined to our best ability as the location from where the aneurysmal sac pouched outward from the parent vessel. The definitions of parameters are as follows: (1) the maximum diameter of an aneurysm (size); (2) the maximum perpendicular distance of the dome from the neck plane (height); (3) the maximum (not necessarily perpendicular) distance of the dome from the centroid of the neck plane (the maximum height, Hmax); (4) the maximum diameter perpendicular to height (width); (5) width of the aneurysm in the neck plane (neck width); (6) parent artery average diameter (Dv) was the average value of vessel diameter at the proximal neck (Di) and vessel diameter at 1.5×Di upstream, i was equal to 1 or 3 according to the aneurysm type; (7) BNF = width/neck width, H/W = height/width, AR = height/neck width, SR = Hmax/Dv; and (8) bleb formation was the formation of one or more additional balloons connecting with the aneurysmal sac (Fig. 2).
Table 2.
Data of morphological parameters recorded for 14 patients with mirror posterior communicating artery aneurysms
| Patient no. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Size, mm | RIA | 9.11 | 3.82 | 2.88 | 6.58 | 10.33 | 3.18 | 8.43 | 4.73 | 4.50 | 6.67 | 6.22 | 5.78 | 5.40 | 4.00 |
| UIA | 6.14 | 3.82 | 2.36 | 4.62 | 10.20 | 2.76 | 6.34 | 4.16 | 4.92 | 4.16 | 6.86 | 3.18 | 7.32 | 2.90 | |
| Height, mm | RIA | 8.81 | 2.80 | 2.20 | 5.95 | 8.72 | 2.81 | 6.01 | 3.93 | 2.95 | 5.92 | 5.92 | 4.19 | 3.29 | 3.57 |
| UIA | 5.13 | 3.71 | 2.06 | 3.88 | 5.53 | 2.52 | 4.59 | 3.06 | 3.82 | 3.48 | 6.15 | 2.20 | 6.17 | 2.17 | |
| Width, mm | RIA | 4.50 | 3.03 | 2.88 | 4.68 | 10.31 | 2.78 | 6.18 | 4.52 | 4.50 | 6.62 | 6.15 | 5.72 | 5.36 | 3.21 |
| UIA | 3.16 | 3.48 | 1.66 | 4.60 | 10.20 | 1.42 | 6.33 | 2.56 | 4.92 | 3.20 | 3.77 | 2.60 | 6.94 | 2.89 | |
| Neck width, mm | RIA | 4.60 | 1.96 | 2.20 | 5.15 | 6.47 | 2.10 | 4.51 | 2.81 | 2.70 | 4.19 | 4.27 | 3.57 | 2.25 | 2.07 |
| UIA | 2.59 | 5.92 | 2.30 | 3.10 | 6.23 | 1.40 | 3.77 | 3.26 | 3.54 | 2.96 | 5.36 | 2.60 | 4.13 | 2.25 | |
| AR | RIA | 1.92 | 1.43 | 1.00 | 1.16 | 1.35 | 1.34 | 1.33 | 1.40 | 1.09 | 1.41 | 1.39 | 1.17 | 1.46 | 1.72 |
| UIA | 1.98 | 0.63 | 0.90 | 1.25 | 0.89 | 1.80 | 1.22 | 0.94 | 1.08 | 1.18 | 1.15 | 0.85 | 1.49 | 0.96 | |
| BNF | RIA | 0.98 | 1.55 | 1.31 | 0.91 | 1.59 | 1.32 | 1.37 | 1.61 | 1.67 | 1.58 | 1.44 | 1.60 | 2.38 | 1.55 |
| UIA | 1.22 | 0.59 | 0.72 | 1.48 | 1.64 | 1.01 | 1.68 | 0.79 | 1.39 | 1.08 | 0.70 | 1.00 | 1.68 | 1.28 | |
| H/W | RIA | 1.96 | 0.92 | 0.76 | 1.27 | 0.85 | 1.01 | 0.97 | 0.87 | 0.66 | 0.89 | 0.96 | 0.73 | 0.61 | 1.11 |
| UIA | 1.62 | 1.07 | 1.24 | 0.84 | 0.54 | 1.77 | 0.73 | 1.20 | 0.78 | 1.09 | 1.63 | 0.85 | 0.89 | 0.94 | |
| SR | RIA | 1.45 | 0.78 | 1.09 | 2.81 | 3.56 | 0.71 | 2.30 | 1.31 | 1.58 | 2.06 | 1.53 | 1.87 | 2.53 | 1.14 |
| UIA | 1.19 | 0.93 | 1.36 | 1.71 | 2.34 | 0.63 | 1.63 | 1.14 | 1.35 | 1.16 | 1.73 | 1.38 | 2.70 | 0.91 | |
| Bleb formation | RIA | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 |
| UIA | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
AR: aspect ratio, BNF: bottleneck factor, H/W: height/width ratio, RIA: ruptured intracranial aneurysm, SR: size ratio, UIA: unruptured intracranial aneurysm.
Fig. 2.
Definition of morphologic parameters. The parameters measured in three-dimensional models including size, height, maximum height (Hmax), width, neck width, and parent vessel diameter (Dv). Left: bottleneck factor (BNF) = width/neck width, height/width (H/W) ratio = height/width, aspect ratio (AR) = height/neck width, size ratio (SR) = Hmax/Dv. Right: Calculation of SR in bifurcation aneurysm. Dvi = (Dia + Dib)/2, Dia presents the vessel diameter at the neck or branching point and Dib presents the vessel diameter 1.5 Dia away from Dia (i = 1, 2, 3); Dv = (Dv1 + Dv2 + Dv3)/3; SR = Hmax/Dv.
Statistical Analysis
The means and standard deviations (SDs) of continuous variables were calculated for the ruptures and unruptured groups. Data were expressed as mean ± SD. The differences of the continuous variables were analyzed by a paired nonparametric Wilcoxon test. Categorical variables were analyzed in contingency tables using Fisher’s exact test. A probability value of less than 0.05 was considered statistically significant, and all tests were two-sided. Statistical analyses were performed using SPSS 17.0 (SPSS Inc, Chicago, IL, USA).
Results
Values for each parameter are displayed in Table 3. On average, the size of the aneurysms in ruptured group was 5.83 ± 2.24 mm, which was significantly larger than 4.98 ± 2.18 mm in the unruptured group (P = 0.042). The AR of aneurysms in ruptured group was significantly higher than that of unruptured group (1.37 ± 0.24 vs. 1.17 ± 0.37, P = 0.041), as same as BNF (1.49 ± 0.35 vs. 1.16 ± 0.38, P = 0.021). The SR was significantly different between ruptured and unruptured groups (1.77 ± 0.81 vs. 1.44 ± 0.56, P = 0.037). Bleb formation in ruptured aneurysms was more common than that in unruptured aneurysms (71.4% vs. 21.4%, P = 0.021). Parameters including height (P = 0.103), width (P = 0.078), neck width (P = 0.808), and H/W (P = 0.417) had no significant differences between the two groups.
Table 3.
Results from statistical analysis of all parameters examined in ruptured and unruptured aneurysm cases
| Parameter | Ruptured group | Unruptured group | P value |
|---|---|---|---|
| Size, mm | 5.83 ± 2.24 | 4.98 ± 2.18 | 0.042* |
| Height, mm | 4.79 ± 2.14 | 3.93 ± 1.40 | 0.103 |
| Width, mm | 5.03 ± 1.99 | 4.12 ± 2.36 | 0.078 |
| Neck width, mm | 3.48 ± 1.40 | 3.53 ± 1.44 | 0.808 |
| AR | 1.37 ± 0.24 | 1.17 ± 0.37 | 0.041* |
| BNF | 1.49 ± 0.35 | 1.16 ± 0.38 | 0.021* |
| H/W | 0.97 ± 0.33 | 1.09 ± 0.37 | 0.417 |
| SR | 1.77 ± 0.81 | 1.44 ± 0.56 | 0.037* |
| Bleb formation (%) | 10 (71.4) | 3 (21.4) | 0.021 * |
Statistical significant, AR: aspect ratio, BNF: bottleneck ratio, H/W: height/width ratio, SR: size ratio.
Discussion
For a neurosurgeon, the morphological parameters we used can be easily acquired from angiography images by using professional image workstations. And ascertaining the association between geometry parameters of unruptured PComA aneurysms and their rupture risks contributes to choosing the better intervention.
I. Size
Size is most frequently studied in such researches regarding the morphological parameters. It is almost recognized as larger aneurysms prone to rupture with a significantly greater frequency than smaller aneurysms. The result in our research meets the majority as well. Researchers were attempting to find the size threshold for aneurysm rupture; 7 mm was proposed as a dividing point for rupture.13,14) However, in our research, the majority aneurysms (11/14, 78.6%) in ruptured group had a smaller size than 7 mm. Jagadeesan et al. also revealed that small aneurysms (≤ 7 mm) and very small aneurysms (≤ 3 mm) occupied the majority of ruptured PComA aneurysms.15) A mean size of ruptured PComA aneurysms being 5.20 ± 1.41 mm was calculated by Xu et al., which is smaller than 7 mm.11) The results of morphological researches differing in size threshold may indicate that this threshold is likely to vary by location. It is easily understood that aneurysms arising from posterior communicating arteries with thinner walls may present with worse ability to support wall tension in the same pressure, compared to the aneurysms on larger arteries. Larger study is needed to set a reasonable threshold for the intervention of PComA aneurysm.
II. AR, BNF, and H/W ratio
AR, H/W, and BNF are initial parameters to evaluate the shape of IAs. Among the three geometric parameters, AR shows the least sensitivity to definition variation and the best association with rupture risk.16) Therefore, AR was more popular than the other two parameters in the aneurysm shape analysis. A great amount of researches have considered AR as a promising morphological parameter for IA rupture prediction.17–19) Our research has further confirmed the correlation between AR and PComA aneurysm rupture, as same as a similar research based on PComA-MANs.11) Though numerous researchers have confirmed this correlation, where the threshold values lying hasstill some discrepancy.17–20) This may be related to the different components of aneurysm locations in previous studies. Our research showed the threshold value of AR was 1.2 for PComA aneurysms. It might be more meaningful to research on the same location with a larger sample size to acquire more accurate value.
BNF and H/W have also been reported to be related to aneurysm rupture.21,22) In our research, only BNF showed statistically significant. The different results may owe to these factors: (1) both BNF and H/W are parameters merely reflecting aneurysm shape without considering the parent vessel, their performance may vary by different locations; (2) choosing of 3D or 2D angiographic data may result in different values; and (3) BNF and H/W have different prediction performance in bifurcation aneurysms and sidewall aneurysms, the different component ratio of aneurysms in a research will lead to the various results. Therefore, analyses after location and aneurysm type adjusted, as well as unification of data acquisition, are needed for the accurate risk assessment on these parameters in further studies.
III. Bleb formation
The association between aneurysm irregularity and rupture risk has been investigated for decades. Bleb formation is the most common parameter reflecting aneurysm irregularity. A great amount of clinical studies have pointed out that aneurysms with one or more blebs have larger tendency for future rupture.4,9,23) This significance reappeared in our research on PComA aneurysms. This phenomenon was well explained by hemodynamic analysis. Blebs usually form at or adjacent to impingement regions where there is high wall shear stress (WSS). Formation of blebs leads to lower WSS and higher oscillatory shear index (OSI) which may contribute to the process of rupture.23)
IV. SR
SR was first proposed by Dhar et al. for IA rupture risk assessment. It compares the size of aneurysm in a manner that accounts for the local parent artery diameter to relate aneurysm geometry to the local vessel geometry. It seemed as a promising morphological parameter according to the result of a retrospective research and a subsequent virtual experimental study by Dhar et al. and Tremmel et al.17,24) The good performance of SR continued in the following studies.25–27)
However, the SR is still a disputed parameter as some latter studies indicated that SR was not useful for bifurcation aneurysms.28,29) They argued that the aneurysm-type distribution bias existing in the prior studies could contribute to maximize SR performance. Additionally, in the sidewall subset, SR being capable of rupture prediction may owe to its components of different IA location. For example, the internal carotid artery aneurysms are more than half of the total in the unruptured group, while aneurysms on other locations whose calibers are smaller occupies the majority of the ruptured group. Strong polarity in ruptured/unruptured locations will beautify the SR performance.
In our research, the location confounding and aneurysm-type distribution bias was eliminated. We showed SR was significantly higher in the ruptured group. The same conclusion was also obtained in another morphological research on 52 pairs of MANs including 33 pairs of PComA-MANs.30) Higher SR may predict future rupture of unruptured PComA aneurysms. It is expected that larger location and aneurysm type-specific studies for SR predictable performance should be carried out to solve the controversy in the future.
V. Limitation
First, as same as other retrospective researches about the impact of morphological parameters on aneurysm rupture, our study is based on the hypothesis that the shape or size of an aneurysm will not change after rupture. Actually, the likelihood of shape or size change during aneurysmal rupture could not be completely excluded in this study. The aneurysm morphology could have been affected by the rupture. Fortunately some studies have indicated that the size and shape of an aneurysm are not affected much by rupture31,32) Second, the data of ruptured aneurysm may be affected by vasospasm. To make vasospasm less likely to influence the results, in all patients of our research, CTA was performed within 24 hours from rupture. Then, aneurysm wall conditions which can influence its tolerance of blood flow striking have not been considered, which might cause deviations of the research. Furthermore, ours is a retrospective study based on a small sample of 14 pairs of PComA-MANs, the effectiveness and rationality of applying the results concluded on PComA-MANs to IAs on other location is controversial. What’s more, as the sample size is limited, we have not conducted multivariate logistic regression to identify the parameters that retained significance when accounting for all relevant variables. Finally, the conclusion of this study still requires verification in large prospective randomized studies.
Conclusion
We insist the importance of unifying the location and aneurysm type on morphological analysis. With an ideal model for morphological analysis on aneurysm rupture, nine parameters for correlation with rupture were examined. Larger size, higher AR, BNF, SR, and bleb formation may be related to rupture of IAs. For the relatively low prevalence of PComA-MANs, larger studies minimizing the interference from patient-related factors and aneurysm type were expected for acquiring more accurate assessment of the relationship between these parameters and PComA aneurysm rupture.
Acknowledgments
This study was supported by Foundation of Zhejiang Educational Committee (Y201120299).
References
- 1). Kissela BM, Sauerbeck L, Woo D, Khoury J, Carrozzella J, Pancioli A, Jauch E, Moomaw CJ, Shukla R, Gebel J, Fontaine R, Broderick J: Subarachnoid hemorrhage: a preventable disease with a heritable component. Stroke 33: 1321– 1326, 2002. [DOI] [PubMed] [Google Scholar]
- 2). Juvela S: Prevalence of and risk factors for intracranial aneurysms. Lancet Neurol 10: 595– 597, 2011. [DOI] [PubMed] [Google Scholar]
- 3). Greving JP, Wermer MJ, Brown RD, Morita A, Juvela S, Yonekura M, Ishibashi T, Torner JC, Nakayama T, Rinkel GJ, Algra A: Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol 13: 59– 66, 2014. [DOI] [PubMed] [Google Scholar]
- 4). UCAS Japan Investigators. Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, Hashimoto N, Nakayama T, Sakai M, Teramoto A, Tominari S, Yoshimoto T: The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med 366: 2474– 2482, 2012. [DOI] [PubMed] [Google Scholar]
- 5). Beck J, Rohde S, Berkefeld J, Seifert V, Raabe A: Size and location of ruptured and unruptured intracranial aneurysms measured by 3-dimensional rotational angiography. Surg Neurol 65: 18– 25; discussion 25–27, 2006. [DOI] [PubMed] [Google Scholar]
- 6). Jiang Y, Lan Q, Wang Q, Lu H, Ge F, Wang Y: Correlation between the rupture risk and 3D geometric parameters of saccular intracranial aneurysms. Cell Biochem Biophys 70: 1417– 1420, 2014. [DOI] [PubMed] [Google Scholar]
- 7). Etminan N, Beseoglu K, Barrow DL, Bederson J, Brown RD, Jr, Connolly ES, Jr, Derdeyn CP, Hänggi D, Hasan D, Juvela S, Kasuya H, Kirkpatrick PJ, Knuckey N, Koivisto T, Lanzino G, Lawton MT, LeRoux P, McDougall CG, Mee E, Mocco J, Molyneux A, Morgan MK, Mori K, Morita A, Murayama Y, Nagahiro S, Pasqualin A, Raabe A, Raymond J, Rinkel GJ, Rüfenacht D, Seifert V, Spears J, Steiger HJ, Steinmetz H, Torner JC, Vajkoczy P, Wanke I, Wong GK, Wong JH, Macdonald RL: Multidisciplinary consensus on assessment of unruptured intracranial aneurysms: proposal of an international research group. Stroke 45: 1523– 1530, 2014. [DOI] [PubMed] [Google Scholar]
- 8). Gibo H, Lenkey C, Rhoton AL: Microsurgical anatomy of the supraclinoid portion of the internal carotid artery. J Neurosurg 55: 560– 574, 1981. [DOI] [PubMed] [Google Scholar]
- 9). Matsukawa H, Fujii M, Akaike G, Uemura A, Takahashi O, Niimi Y, Shinoda M: Morphological and clinical risk factors for posterior communicating artery aneurysm rupture. J Neurosurg 120: 104– 110, 2014. [DOI] [PubMed] [Google Scholar]
- 10). Lu G, Huang L, Zhang XL, Wang SZ, Hong Y, Hu Z, Geng DY: Influence of hemodynamic factors on rupture of intracranial aneurysms: patient-specific 3D mirror aneurysms model computational fluid dynamics simulation. AJNR Am J Neuroradiol 32: 1255– 1261, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Xu J, Yu Y, Wu X, Wu Y, Jiang C, Wang S, Huang Q, Liu J: Morphological and hemodynamic analysis of mirror posterior communicating artery aneurysms. PLoS One 8: e55413, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Baharoglu MI, Lauric A, Gao BL, Malek AM: Identification of a dichotomy in morphological predictors of rupture status between sidewall- and bifurcation-type intracranial aneurysms. J Neurosurg 116: 871– 881, 2012. [DOI] [PubMed] [Google Scholar]
- 13). Wiebers DO, Whisnant JP, Huston J, Meissner I, Brown RD, Piepgras DG, Forbes GS, Thielen K, Nichols D, O’Fallon WM, Peacock J, Jaeger L, Kassell NF, Kongable-Beckman GL, Torner JC, International Study of Unruptured Intracranial Aneurysms Investigators : Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 362: 103– 110, 2003. [DOI] [PubMed] [Google Scholar]
- 14). Juvela S, Poussa K, Lehto H, Porras M: Natural history of unruptured intracranial aneurysms: a long-term follow-up study. Stroke 44: 2414– 2421, 2013. [DOI] [PubMed] [Google Scholar]
- 15). Jagadeesan BD, Delgado Almandoz JE, Kadkhodayan Y, Derdeyn CP, Cross DT, 3rd, Chicoine MR, Rich KM, Zipfel GJ, Dacey RG, Moran CJ: Size and anatomic location of ruptured intracranial aneurysms in patients with single and multiple aneurysms: a retrospective study from a single center. J Neuro Interventional Surg 6: 169– 174, 2014. [DOI] [PubMed] [Google Scholar]
- 16). Lauric A, Baharoglu MI, Malek AM: Ruptured status discrimination performance of aspect ratio, height/width, and bottleneck factor is highly dependent on aneurysm sizing methodology. Neurosurgery 71: 38– 45, 2012. [DOI] [PubMed] [Google Scholar]
- 17). Dhar S, Tremmel M, Mocco J, Kim M, Yamamoto J, Siddiqui AH, Hopkins LN, Meng H: Morphology parameters for intracranial aneurysm rupture risk assessment. Neurosurgery 63: 185– 196; discussion 196–197, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Nader-Sepahi A, Casimiro M, Sen J, Kitchen ND: Is aspect ratio a reliable predictor of intracranial aneurysm rupture? Neurosurgery 54: 1343– 1347; discussion 1347–1348, 2004. [DOI] [PubMed] [Google Scholar]
- 19). Ujiie H, Tachibana H, Hiramatsu O, Hazel AL, Matsumoto T, Ogasawara Y, Nakajima H, Hori T, Takakura K, Kajiya F: Effects of size and shape (aspect ratio) on the hemodynamics of saccular aneurysms: a possible index for surgical treatment of intracranial aneurysms. Neurosurgery 45: 119– 129; discussion 129–130, 1999. [DOI] [PubMed] [Google Scholar]
- 20). Amenta PS, Yadla S, Campbell PG, Maltenfort MG, Dey S, Ghosh S, Ali MS, Jallo JI, Tjoumakaris SI, Gonzalez LF, Dumont AS, Rosenwasser RH, Jabbour PM: Analysis of nonmodifiable risk factors for intracranial aneurysm rupture in a large, retrospective cohort. Neurosurgery 70: 693– 699; discussion 699–701, 2012. [DOI] [PubMed] [Google Scholar]
- 21). Elsharkawy A, Lehečka M, Niemelä M, Kivelev J, Billon-Grand R, Lehto H, Kivisaari R, Hernesniemi J: Anatomic risk factors for middle cerebral artery aneurysm rupture: computed tomography angiography study of 1009 consecutive patients. Neurosurgery 73: 825– 837; discussion 836–837, 2013. [DOI] [PubMed] [Google Scholar]
- 22). Hoh BL, Sistrom CL, Firment CS, Fautheree GL, Velat GJ, Whiting JH, Reavey-Cantwell JF, Lewis SB: Bottleneck factor and height-width ratio: association with ruptured aneurysms in patients with multiple cerebral aneurysms. Neurosurgery 61: 716– 722; discussion 722–723, 2007. [DOI] [PubMed] [Google Scholar]
- 23). Cebral JR, Sheridan M, Putman CM: Hemodynamics and bleb formation in intracranial aneurysms. AJNR Am J Neuroradiol 31: 304– 310, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Tremmel M, Dhar S, Levy EI, Mocco J, Meng H: Influence of intracranial aneurysm-to-parent vessel size ratio on hemodynamics and implication for rupture: results from a virtual experimental study. Neurosurgery 64: 622– 630; discussion 630–631, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Rahman M, Smietana J, Hauck E, Hoh B, Hopkins N, Siddiqui A, Levy EI, Meng H, Mocco J: Size ratio correlates with intracranial aneurysm rupture status: a prospective study. Stroke 41: 916– 920, 2010. [DOI] [PubMed] [Google Scholar]
- 26). Xiang J, Natarajan SK, Tremmel M, Ma D, Mocco J, Hopkins LN, Siddiqui AH, Levy EI, Meng H: Hemodynamic-morphologic discriminants for intracranial aneurysm rupture. Stroke 42: 144– 152, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Ma D, Tremmel M, Paluch RA, Levy EI, Meng H, Mocco J: Size ratio for clinical assessment of intracranial aneurysm rupture risk. Neurol Res 32: 482– 486, 2010. [DOI] [PubMed] [Google Scholar]
- 28). Lin N, Ho A, Gross BA, Pieper S, Frerichs KU, Day AL, Du R: Differences in simple morphological variables in ruptured and unruptured middle cerebral artery aneurysms. J Neurosurg 117: 913– 919, 2012. [DOI] [PubMed] [Google Scholar]
- 29). Lauric A, Baharoglu MI, Gao BL, Malek AM: Incremental contribution of size ratio as a discriminant for rupture status in cerebral aneurysms: comparison with size, height, and vessel diameter. Neurosurgery 70: 944– 951; discussion 951–952, 2012. [DOI] [PubMed] [Google Scholar]
- 30). Li M, Jiang Z, Yu H, Hong T: Size ratio: a morphological factor predictive of the rupture of cerebral aneurysm? Can J Neurol Sci 40: 366– 371, 2013. [DOI] [PubMed] [Google Scholar]
- 31). Backes D, Vergouwen MD, Velthuis BK, van der Schaaf IC, Bor AS, Algra A, Rinkel GJ: Difference in aneurysm characteristics between ruptured and unruptured aneurysms in patients with multiple intracranial aneurysms. Stroke 45: 1299– 1303, 2014. [DOI] [PubMed] [Google Scholar]
- 32). Ujiie H, Tamano Y, Sasaki K, Hori T: Is the aspect ratio a reliable index for predicting the rupture of a saccular aneurysm? Neurosurgery 48: 495– 502; discussion 502–503, 2001. [DOI] [PubMed] [Google Scholar]