Abstract
A phenomenon of cerebral infarction following acute subdural hematoma (ASDH) in infants and young children, termed cerebral infarction following ASDH (CIASDH), has been well recognized, though both its mechanisms and risk factors have been poorly understood. The purpose of the present study was to investigate the predictors for CIASDH in a population of ASDH, and to evaluate the imaging studies to presume the mechanisms of CIASDH. We retrospectively examined consecutive children 6 years of age or younger, who were diagnosed with ASDH and were admitted to our hospital between 2000 and 2014. In 57 consecutive children with ASDH, 12 (21.1%) developed CIASDH. The multivariate analysis revealed five predictors for CIASDH: presence of seizure, consciousness disturbance at admission, absence of skull fracture, hematoma thickness ≥ 5 mm on computed tomography (CT), and midline shift ≥ 3 mm on CT (p < 0.05). In three of six patients (50%) undergoing magnetic resonance (MR) imaging/fluid-attenuated inversion recovery (FLAIR) within 5 days of admission, serpentine hyperintensities in the subarachnoid space (FLAIR vessel hyperintensities) were demonstrated. MR angiography showed neither occlusion nor stenosis of the cerebral arteries. Single photon emission CT performed at admission in one patient showed a cerebral blood flow reduction in the ASDH side. All the children with CIASDH showed unfavorable outcomes at discharge. Children showing multiple predictors at admission should be carefully observed for development of CIASDH. Evaluation of the imaging studies suggested that a blood flow disturbance in the level of peripheral arteries to microcirculation was one candidate for possible mechanisms to induce the CIASDH.
Keywords: acute subdural hematoma, big black brain, cerebral venous thrombosis, child abuse, diffuse brain swelling
Introduction
The occurrence of hypodensity lesions on computed tomography (CT) following acute subdural hematoma (ASDH) in infants and young children, termed cerebral infarction following ASDH (CIASDH) in this study, has been well recognized, which was also known as “big black brain” and “diffuse brain swelling.”1,2) In CIASDH, cerebral infarction occurs from the day of the ASDH onset to several days after the onset, which is mainly caused by child abuse or accidental fall. In many cases, subdural hematomas are small-to-moderate size, which do not show a severe mass effect. The pattern of hypodensity lesions in the cerebral hemispheres on CT of CIASDH is distinctive to spare the deep gray structures and hindbrain.1,2) Although both histopathological examination and magnetic resonance (MR) image/diffusion weighted image (DWI) studies showed that the hypodensity lesion was cerebral infarction, the pathophysiology of this cerebral infarction and its relationship to the ASDH are poorly understood.2–6) To the best of our knowledge, no previous studies have ever reported statistical predictors for CIASDH in a population of children showing ASDHs on CT, which could make us to predict and prepare the occurrence of this devastating phenomenon at the early stage of the disease.1,7)
In the present retrospective study, we first clarified predictors for CIASDH in a population of children with ASDHs by comparing between patients with and without CIASDH using multivariate analysis. Then, we evaluated imaging studies performed in the patients with CIASDH. We discussed possible mechanisms of CIASDH based on the results of the statistical study and the imaging studies.
Materials and Methods
We retrospectively assessed consecutive patients 6 years of age or younger diagnosed with ASDH, who were admitted to our hospital between January 2000 and August 2014. All patients with neurological symptoms were evaluated by neurosurgeons. Patients 7 years of age or older were excluded. Outpatients who were not admitted to our hospital were also excluded. Patients presenting with head trauma who were admitted to our neurosurgical unit routinely undergo their first non contrast CT upon arrival. Our institutional review board (Institutional Review Board for Clinical Research, Tokai University Hospital, IRB No. 13R-069) approved this retrospective study.
Clinical data were obtained through chart review. The following patient data were recorded: patient’s age and sex, cause of trauma, interval between trauma and admission, level of consciousness at admission, presence of seizure, presence of injury other than head, blood glucose level at admission, and treatment including surgery. In children younger than 24 months of age, level of consciousness at admission was evaluated by the modified coma score for infants.8) Children who were 24 months of age and older were evaluated for consciousness level using the Glasgow Coma Scale score. Scores except for the full score in the modified coma score for infants or the Glasgow Coma Scale score were regarded as scores indicating consciousness disturbance in this study. Blood glucose level > 180 mg/dL was defined as high blood glucose level. Clinical outcomes were assessed at discharge. Unfavorable outcomes were defined by the following: (1) apparent motor palsy, (2) apparent developmental disturbance, and (3) dead.
The following CT findings were investigated: presence of ASDH, laterality and distribution of the ASDH, a thickness of the ASDH, a distance of midline shift, and presence of cerebral contusion. The thickness of the hematoma 5 mm and more was defined as a large hematoma. The midline shift with 3 mm and more was defined as an evident midline shift. CIASDH was defined as occurrence of low density areas on CT and/or occurrence of hyperintensity areas on MR image/T2-weighted image (WI) and/or fluid-attenuated inversion recovery (FLAIR), which was not caused by cerebral contusion. In patients with CIASDH, intervals between admission to occurrence of CIASDH and distribution of ischemic lesions were investigated. In some patients with CIASDH, MR imaging/DWI, MR imaging/FLAIR, MR angiography (time-of-flight), MR venography (time-of-flight), and single photon emission CT (SPECT) using Tc-HMPAO (Nihon Medi-Physics Co., Tokyo, Japan) were evaluated. Images were evaluated by two board-certificated neurosurgeons (HA and TS) with more than 20 years of experience in their specialties.
Statistical analysis
Univariate analyses were performed using chi-squared analysis and Fisher’s exact probability test for categorical variables and Student’s t-test or the Mann-Whitney U test for continuous variables. Numerical data are expressed as the mean ± standard deviation (SD). Each variable was analyzed using univariate analyses to identify which of the evaluated predictors were significant; the variables found to be significant at the p < 0.10 level were then included in a multivariate logistic regression analysis or a multiple linear regression analysis, which was reduced by successively removing the least significant variable from the model. All variables that were significant at the level of p < 0.10 remained in the final model. Analyses resulting in p values less than 0.05 were considered statistically significant. All statistical analyses were performed with JMP 10 (SAS Institute Inc., Cary, North Carolina, USA).
Results
Between January 2000 and August 2014, we treated 57 consecutive patients 6 years of age or less, who were diagnosed with ASDH. The mean age of these patients was 20.7 ± 23.7 months (mean ± SD), 37 patients (64.9%) were male, and 20 (35.1%) were female. Causes of head trauma were child abuse including presumed abuse in 13 patients, fall in 35, traffic accident in 7, and unknown in 2. Of the 57 patients, 52 patients (91.2%) were admitted within the day of trauma. Figure 1 shows an illustrative case of CIASDH.
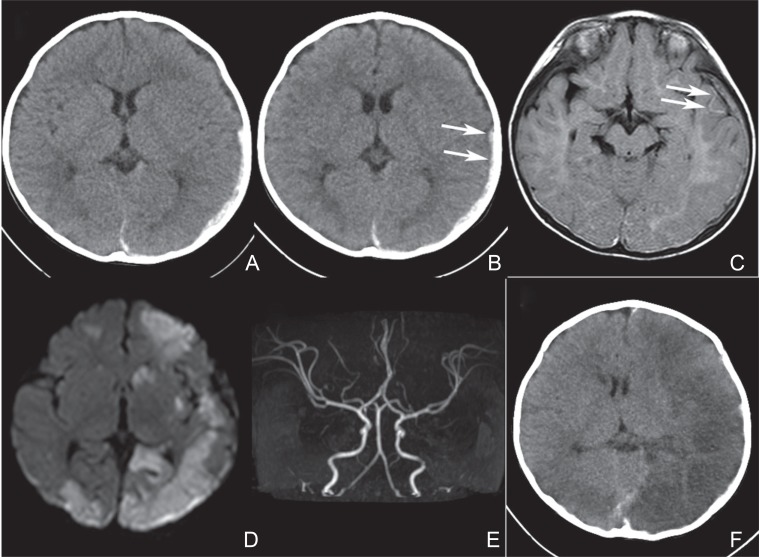
Fig. 1.
Imaging studies of an 8-month-old female, who developed cerebral infarction (CI) following acute subdural hematoma (ASDH) (Case 1). A: A computed tomography (CT) at admission showing a small left ASDH without any midline shift. B: A CT taken 1 day after admission. No major change is noticed except for a blurred margin of the ASDH (arrows). C: A magnetic resonance (MR) image/fluid attenuated inversion recovery (FLAIR) performed 2 days after admission demonstrating serpentine hyperintensity lesions in the left temporal lobe (arrows). D: An MR image/diffusion weighted image (DWI) taken 2 days after admission demonstrating several hyperintensity lesions in the left hemisphere, in the right occipital lobe, and in the right frontal lobe. Any midline shift is not noticed. E: An MR angiogram taken 2 days after admission showing neither occlusion nor stenosis of the major cerebral arteries. F: A CT performed 5 days after admission. Cerebral infarctions, which are associated with brain swelling, demonstrated mainly in the left hemisphere results in a midline shift.
I. Predictors for CIASDH
Of the 57 patients with ASDH, 12 patients (21.1%) had CIASDH. Table 1 shows a comparison between 12 patients with CIASDH and the remaining 45 patients. The following clinical factors were used in the comparison: age, sex, causes of trauma, intervals between trauma and admission, presence of consciousness disturbance at admission, presence of seizure, blood glucose level at admission, presence of skull fracture, side of ASDH on CT, thickness of ASDH, midline shift, distribution of ASDH, presence of cerebral contusion, and injury other than head. The multivariate analysis indicated the following five significantly related factors to CIASDH: presence of seizure (p < 0.0001), consciousness disturbance at admission (p = 0.0025), absence of skull fracture (p = 0.0078), hematoma thickness ≥ 5 mm on CT (p = 0.0171), and midline shift ≥ 3 mm on CT (p < 0.0001). All the eight patients who had both the two predictors showing p values less than 0.0001, which were presence of seizure and midline shift ≥ 3 mm on CT, developed CIASDH.
Table 1.
Comparison between children with and without cerebral infarction following acute subdural hematoma in 57 patients with acute subdural hematoma
| Variable | Children with CIASDH (n = 12) | Children without CIASDH (n = 45) | p value Univariate analysis | OR (95% CI) | p value |
|---|---|---|---|---|---|
| Multivariate analysis | |||||
| Age (months) | 14.1 ± 15.0 | 22.4 ± 25.5 | 0.1565 | ||
| Sex, male | 9 (75%) | 28 (62.2%) | 0.4099 | ||
| Fall (n = 55) | 5 (45.5%) | 31 (70.5%) | 0.1189 | ||
| Abuse (n = 55) | 6 (45.4%) | 7 (15.9%) | 0.0020 | 12,932,787 (0.59−) | 0.0925 |
| Admission within a day | 10 (83.3%) | 42 (93.3%) | 0.2766 | ||
| Consciousness disturbance at admission | 12 (100%) | 19 (43.2%) | 0.0004 | 1.46e22 (14.8–1.21e46) | 0.0025 |
| Seizure | 12 (100%) | 13 (22.9%) | < 0.0001 | 1.15e36 (405.8−) | < 0.0001 |
| Blood glucose > 180 mg/dL (n = 51) | 7 (58.3%) | 11 (28.2%) | 0.0562 | ||
| Skull fracture | 1 (8.3%) | 18 (40.0%) | 0.0387 | 3.34e−22 (−1.07e−21) | 0.0078 |
| Left hematoma | 5 (41.7%) | 22 (38.9%) | 0.5822 | ||
| Hematoma thickness ≥ 5 mm | 10 (83.3%) | 17 (37.7%) | 0.0050 | 2.56e14 (1.98e123−) | 0.0171 |
| Midline shift ≥ 3 mm | 8 (66.7%) | 3 (7.0%) | < 0.0001 | 5.0e28 (1.09e57−) | < 0.0001 |
| Hemispheric hematoma | 6 (50%) | 2 (4.4%) | < 0.0001 | ||
| Cerebral contusion | 1 (8.3%) | 5 (11.1%) | 0.7806 | ||
| Other injury | 2 (17.8%) | 3 (6.7%) | 0.2766 | ||
Underline indicates p < 0.05. CI: confidence interval, CIASDH: cerebral infarction following acute subdural hematoma, OR: odds ratio.
II. Imaging studies
Table 2 demonstrates the clinical characteristics and CT findings in 12 patients with CIASDH. Cerebral infarction occurred in the side of the ASDH or bilaterally in all patients. No patients showed cerebral infarction only in the opposite side to the ASDH. In all the four patients undergoing MR imaging/DWI within 5 days of admission, high intensity lesions were demonstrated on the DWIs. In three of six patients (50%) undergoing MR imaging/FLAIR within 5 days of admission, serpentine hyperintensities in the subarachnoid space, which was FLAIR vessel hyperintensity (FVH), was demonstrated (Fig. 2). MR angiograph, which was performed within 5 days in four patients, showed neither occlusion nor stenosis of the cerebral arteries (Fig. 1E). MR venography was performed within 5 days in six patients, and no apparent abnormality was demonstrated in one patient. In the other five patients, scarcity of the cortical veins in the side of the ASDH was observed on the MR venograms (Fig. 3G). In a patient (Case 8), who developed CIASDH 4 days after admission, SPECT performed on Day 0 demonstrated a decrease in cerebral blood flow in the ASDH side (Fig. 3B). This patient presented comatose at arrival. A CT at admission showed a right ASDH with a midline shift (Fig. 3A), then the patient underwent hematoma removal. Both a CT performed 6 hours after admission (Fig. 3C) and a CT on Day 2 (Fig. 3D) showed no apparent cerebral infarction. The patient developed successive seizures on Day 2 and general anesthesia was induced to control the seizures. An MR image on Day 4 demonstrated cerebral infarctions in the right frontal lobe, right basal ganglia, right occipital lobe, and the left frontal lobe (Fig. 3E, F).
Table 2.
Clinical characteristics and computed tomographic findings in 12 patients with cerebral infarction following acute subdural hematoma.
| Case no. | Age, month/Sex | Initial CT | Interval from admission to occurrence of CIASDH (days) | Hypodensity lesions on follow-up CT | |||
|---|---|---|---|---|---|---|---|
| Hematoma side | Maximum thickness of SDH (mm) | Midline shift (mm) | Side | Distribution | |||
| 1 | 8/F | Left | 3 | 1 | 2 | Bilateral | Global |
| 2 | 8/F | Bilateral | 10 | 5 | 0 | Bilateral | Global |
| 3 | 38/F | Left | 5 | 5 | 1 | Bilateral | Global |
| 4 | 46/M | Right | 12 | 8 | 0 | Right | Global |
| 5 | 6/M | Right | 5 | 3 | 0 | Bilateral | Global |
| 6 | 1/M | Bilateral | 7 | 3 | 0 | Left | Focal |
| 7 | 4/M | Left | 5 | 0 | 16 | Left | Focal |
| 8 | 15/M | Right | 10 | 9 | 4 | Bilateral | Global |
| 9 | 4/M | Left | 3 | 0 | 4 | Bilateral | Global |
| 10 | 2/M | Bilateral | 5 | 0 | 1 | Bilateral | Global |
| 11 | 9/M | Left | 13 | 8 | 2 | Bilateral | Focal (L > R) |
| 12 | 28/M | Right | 8 | 8 | 0 | Right | Focal |
CIASDH: cerebral infarction following acute subdural hematoma, CT: computed tomography, F: female, L: left, M: male, R: right, SDH: subdural hematoma.
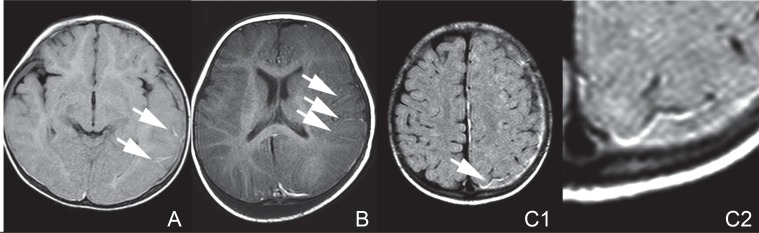
Fig. 2.
Magnetic resonance (MR) images/fluid-attenuated inversion recovery (FLAIR) performed within 3 days after admission showing FLAIR vessel hyperintensities (FVHs). Arrows indicate FVHs. A: A FLAIR image on Day 2 in Case 1. B: A FLAIR image on Day 3 in Case 2. C1: A FLAIR image on Day 2 in Case 3. C2: A magnified image of the FVH in Case 3.
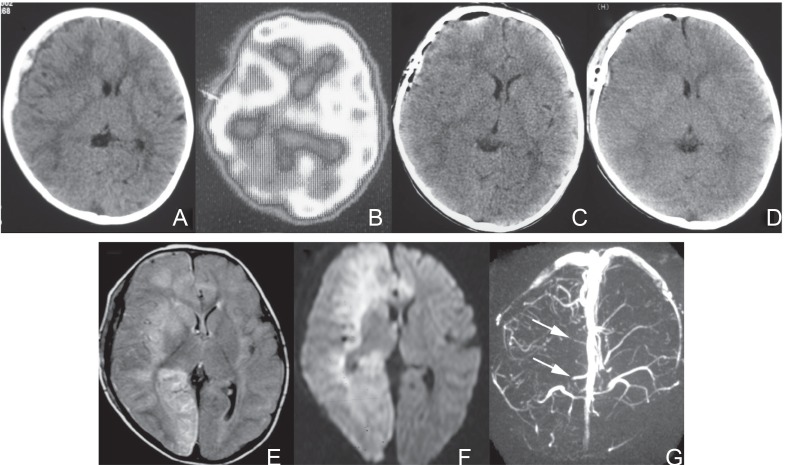
Fig. 3.
Images of Case 8. A: A computed tomogram (CT) at admission showing a right acute subdural hematoma with a mid-line shift. B: A single photon emission CT on Day 0 demonstrating a decrease in blood flow in the right hemisphere. C: A CT performed 6 hours after admission and immediately after hematoma removal. No apparent abnormality is demonstrated in the brain parenchyma and the mid-line shift improves. D: A CT on Day 2 showing no apparent cerebral infarction. E: A magnetic resonance (MR) image/fluid-attenuated inversion recovery (FLAIR) on Day 4 showing high intensity lesions in the right frontal lobe, right basal ganglia, right occipital lobe, and the left frontal lobe. The cerebral infarction associates with brain swelling. F: An MR image/diffusion weighted image on Day 4 demonstrated high intensity lesions in the right frontal lobe, right basal ganglia, right occipital lobe, and the left frontal lobe. G: An MR venogram on Day 4 showing scarce cortical veins in the right hemisphere (arrows).
III. Surgery and outcome
Five patients with CIASDH and five without CIASDH underwent craniotomy for hematoma removal, because of a mass effect of the hematoma. In four of the five patients with CIASDH who underwent surgery, bleeding points in the cortical veins or bridging veins were disclosed during the operation.
All the 12 patients with CIASDH showed neurological deficits at discharge. On the other hand, in 5 of the 45 patients without CIASDH, neurological deficits were observed at discharge. Unfavorable outcomes in patients with CIASDH were significantly more frequent than those in patients without CIASDH (p < 0.0001). One patient with CIASDH and two without CIASDH died during hospital stay.
Discussion
I. Predictors for CIASDH
In the present series, approximately one-fifth of the children 6 years old or younger with ASDH developed CIASDH. Many reported studies investigated CIASDH in a population of children with non-accidental head injury.9–14) On the other hand, few studies examined CIASDH in a population of children with ASDH.12,15) The real mechanisms of trauma usually provided by their parents on admission were not uncovered in the majority of child abuse. CIASDH could be caused by accidental fall as well as abusive injury.1,12) Therefore, in the present study, we investigated the occurrence of CIASDH based on the existence of ASDH, which was easily clarified using the CT at admission. A previous study reported approximately 30% of patients developing CIASDH in children with ASDH.15) One-fifth to one-third of children with ASDH have the possibility of CIASDH.
The multivariate analysis in the present study revealed five predictors for CIASDH in children with ASDH: presence of seizure, consciousness disturbance at admission, absence of skull fracture, hematoma thickness ≥ 5 mm on CT, and midline shift ≥ 3 mm on CT. All the 12 children with CIASDH presented seizure, while seizure occurred in 13 of 45 children (22.9%) without CIASDH. The reported rates of seizure in non-accidental head trauma in children ranged from 40% to 79%.16,17) Seizures have been reported to occur more frequently in children compared to adult head trauma. Seizure causes an increase in excitotoxic stress leading to the enhancement of metabolic demand. Then, seizure is one suspected mechanism for occurrence of CIASDH.2) On the contrary, seizure could be induced by the occurrence of CIASDH for itself as a manifestation of cerebra ischemia. In the present series, 10 of 27 patients (37.0%) showing ASDH with its thickness ≥ 5 mm on CT developed CIASDH. Midline shift ≥ 3 mm on CT was observed in 11 patients, and 8 of the 11 patients (72.3%) resulted in CIASDH. In two of the remaining four patients with both midline shift < 3 mm and CIASDH, ASDHs occurred within bilateral chronic subdural hematomas led to the small midline shift in spite of a mass effect of the ASDHs. A mass effect of ASDHs might relate to the development of CIASDH, though the midline shift of 3 mm was not so severe. In patients with a large ASDH showing a massive mass effect, cerebral infarctions might be caused by compression of the anterior cerebral artery against the falx and/or the posterior cerebral artery against the tentorium. All the eight patients showing both the two predictors with p values < 0.0001 in the present multivariate analysis, which were presence of seizure and midline shift ≥ 3 mm on CT, resulted in CIASDH. Combination of seizure and midline shift ≥ 3 mm associated with ASDH is possibly a warning of CIASDH.
II. Imaging studies
All MR imaging/DWI performed in an acute stage showed hyperintensity areas indicating cerebral ischemia, which was similar to the previous reports.1,3,6) Vascular serpentine hyperintensities in the subarachnoid space were observed in three of six patients, in whom MR imaging/FLAIR was performed in an acute stage. FVHs are described as focal, tubular, or serpentine hyperintensities seen, often transiently, in the subarachnoid space against the relative hypointensity of cerebrospinal fluid on FLAIR sequences.18–22) This finding has been termed “FVH,” “hyperintense vessel sign,” “hyperintense vessels on FLAIR,” and the “ivy sign.”18) FVHs have been observed in the setting of acute stroke, intracranial steno-occlusive disease, and moyamoya disease.18) FVH is considered a representation of the sluggish or disordered blood flow through vessels, most often leptomeningeal collaterals distal to arterial occlusion or stenosis.21) This is the first reported study on FVH observed in patients with CIASDH. Sulcal hyperintensity, which was occasionally shown within cortical sulci near a lesion with a mass effect on MR imaging/FLAIR, indicated an increased tissue pressure. The sulcal hyperintensity was reported to be diffuse hyperintensities occupying sulci, which was different from the FVH, in which liner hyperintensities were shown.23) The sulcal hyperintensity possibly implicates venous congestion caused by an increased intracranial pressure. The observation of FVHs suggested that an arterial blood flow disturbance led to the cerebral infarction in the patients with CIASDH. MR angiography showed neither occlusion nor stenosis in the cerebral arteries, which was same as the previous reports on MR angiography and conventional cerebral angiography.2) The cerebral infarction must be caused by arterial abnormality, which could not be observed by MR angiography and cerebral angiography. Hypoperfusion in the affected side demonstrated on the SPECT at the day of admission in the present series also supported the arterial blood flow disturbance. Considering the presence of FVHs, the MR angiography findings and the hypoperfusion on the SPECT, we speculated that a blood flow disturbance occurred in the level of peripheral cerebral arteries to microcirculation.
MR venography was performed in 6 patients, and 5 patients showed absence of visualization of cortical veins in the affected side.24) Contrast medium was not used in the MR venography in the present series. Thus a slow blood flow in the cortical veins, which was caused by the arterial blood flow disturbance and/or an increased intracranial pressure, possibly resulted in no detectable blood flow signal in the cortical veins.25–27) Venous congestion, if it existed, was not an essential mechanism of CIASDH because of the following reasons. DWI has shown an extensive diminution of the apparent diffusion coefficient (ADC) in cytotoxic edema of acute arterial infarction.1,3,6) In vasogenic edema caused by venous infarction, the ADC values on DWI are increased,3,6,28,29) which has never been reported in CIASDH. A distribution of hypodensity areas was not consistent with venous territory. None of the previous reports have reported that intra-venous thromobosis plays a role in causing ASDH.19) The venous damage observed in the four operated patients, which could disturb venous blood flow, possibly caused the absence of cortical venous visualization on the MR venograms.30) A decrease in both inflow and outflow might lead a metabolic crisis under an increased intracranial pressure.
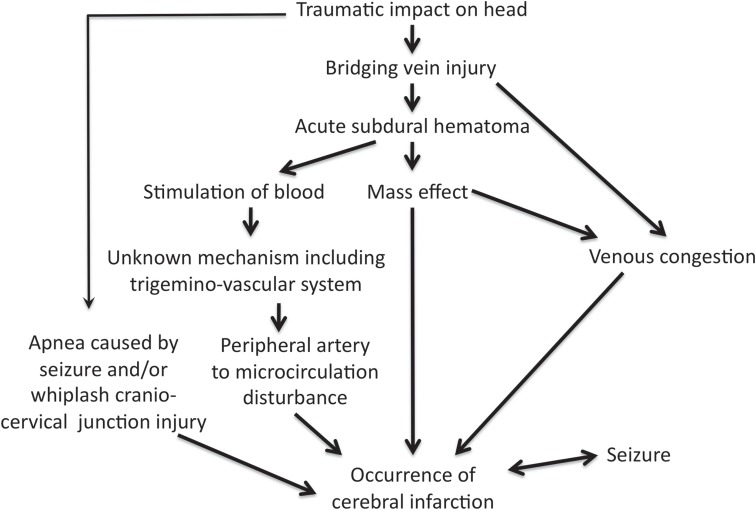
Duhaime and Durham2) and Squier et al.5) hypothesized that presence of blood in the subdural space caused the decrease in cerebral blood flow via unknown mechanisms. Squier et al. postulated that the trigeminovascular system has a role of mechanism for CIASDH in responding to trauma.5) Trigeminal nerve innervation to both the dura and the intracranial vessels mediates cerebral vascular responses to dural injury and bleeding. The trigeminal nerve sends the nerve fibers to the ipsilateral internal carotid artery, the circle of Willis, and the rostral basilar artery. The vessels supplying the deep gray nuclei and the caudal basilar artery receive minimal trigeminal innervation, which was compatible to the distribution of the cerebral infarction in CIASDH.5) Nerve density increases between 31 weeks of gestation and term, with a subsequent decrease in the first five postnatal months, remaining stable thereafter.5) These maturational changes in the trigeminal system may contribute to the differences in the response of the immature brain to traumatic injury. A subdural blood clot and its mass effect could stimulate trigeminal nociceptor, which might result in the peripheral arterial disturbance via the trigeminovascular system.5) We postulated a cascade of mechanisms for CIASDH, shown in Fig. 4.
Fig. 4.
Suspected mechanisms for cerebral infarction following acute subdural hematoma in infants and young children.
Limitations
The limitations of our study include the small subject group and retrospective design. The small number of patients might underestimate risk factors other than the five factors verified as predictors in this multivariate analysis. We could not obtain any statistical conclusion on neuroimaging studies except for CT findings.
Conclusion
Approximately one-fifth of children with ASDH 6 years of age or younger developed CIASDH in the present study. Predictors for CIASDH were presence of seizure, consciousness disturbance at admission, absence of skull fracture, hematoma thickness ≥ 5 mm on CT, and midline shift ≥ 3 mm on CT. Based on the results of imaging studies, including presence of FVHs on MR images/FLAIR, no abnormal findings on MR angiograms and a hemispheric hypoperfusion on SPECT, a blood flow disturbance occurred in the level of the peripheral arteries to microcirculation was considered one candidate for possible mechanisms to induce CIASDH. Prediction and preparation for the occurrence of CIASDH at the early stage may be essential to search for future preventive measurements for CIASDH.
References
- 1). Duhaime AC, Christian CW, Rorke LB, Zimmerman RA: Nonaccidental head injury in infants—the “shaken-baby syndrome”. N Engl J Med 338: 1822– 1829, 1998. [DOI] [PubMed] [Google Scholar]
- 2). Duhaime AC, Durham S: Traumatic brain injury in infants: the phenomenon of subdural hemorrhage with hemispheric hypodensity (“Big Black Brain”). Prog Brain Res 161: 293– 302, 2007. [DOI] [PubMed] [Google Scholar]
- 3). Ichord RN, Naim M, Pollock AN, Nance ML, Margulies SS, Christian CW: Hypoxic-ischemic injury complicates inflicted and accidental traumatic brain injury in young children: the role of diffusion-weighted imaging. J Neurotrauma 24: 106– 118, 2007. [DOI] [PubMed] [Google Scholar]
- 4). Squier W: The “Shaken Baby” syndrome: pathology and mechanisms. Acta Neuropathol 122: 519– 542, 2011. [DOI] [PubMed] [Google Scholar]
- 5). Squier W, Mack J, Green A, Aziz T: The pathophysiology of brain swelling associated with subdural hemorrhage: the role of the trigeminovascular system. Childs Nerv Syst 28: 2005– 2015, 2012. [DOI] [PubMed] [Google Scholar]
- 6). Suh DY, Davis PC, Hopkins KL, Fajman NN, Mapstone TB: Nonaccidental pediatric head injury: diffusion-weighted imaging findings. Neurosurgery 49: 309– 318; discussion 318–320, 2001. [DOI] [PubMed] [Google Scholar]
- 7). Barlow KM, Thomson E, Johnson D, Minns RA: Late neurologic and cognitive sequelae of inflicted traumatic brain injury in infancy. Pediatrics 116: e174– e185, 2005. [DOI] [PubMed] [Google Scholar]
- 8). James HE, Trauner DA: The Glasgow coma scale, in James HE, Anas NG, Perkin RM. (eds): Brain Insults in Infants and Children. Orlando, Grune and Stratton, 1985, pp 179– 182 [Google Scholar]
- 9). Ghahreman A, Bhasin V, Chaseling R, Andrews B, Lang EW: Nonaccidental head injuries in children: a Sydney experience. J Neurosurg 103( 3 Suppl): 213– 218, 2005. [DOI] [PubMed] [Google Scholar]
- 10). Graupman P, Winston KR: Nonaccidental head trauma as a cause of childhood death. J Neurosurg 104( 4 Suppl): 245– 250, 2006. [DOI] [PubMed] [Google Scholar]
- 11). Hymel KP, Makoroff KL, Laskey AL, Conaway MR, Blackman JA: Mechanisms, clinical presentations, injuries, and outcomes from inflicted versus noninflicted head trauma during infancy: results of a prospective, multicentered, comparative study. Pediatrics 119: 922– 929, 2007. [DOI] [PubMed] [Google Scholar]
- 12). Nishimoto H, Kurihara J: [Re-estimation of acute subdural hematoma in children caused by trivial household head trauma]. Nervous System in Children 31: 215– 223, 2006. (Japanese) [Google Scholar]
- 13). Nishimoto H, Kurihara J: [The present situation and problems of non-accidental head injury in children: with special reference to infantile acute subdural hematoma]. Jpn J Neurosurg (Tokyo) 13: 822– 829, 2004. (Japanese) [Google Scholar]
- 14). Park YS, Kotani Y, Sugimoto T, Motoyama Y, Nakasa H, Kogeichi Y, Okuchi K: [Clinical comparative analysis of abusive injuries and accidental injury in infantile subdural hematoma]. Nervous System in Children 38: 354– 363, 2013. (Japanese) [Google Scholar]
- 15). Fujimotoi K, Shimomura T, Okumura Y, Sakaki T: [CT shows ipsilateral cerebral hemispheric low density area after acute subdural hematoma in intants]. Nervous System in Children 24: 504– 508, 1999. (Japanese) [Google Scholar]
- 16). Barlow KM, Spowart JJ, Minns RA: Early posttraumatic seizures in non-accidental head injury: relation to outcome. Dev Med Child Neurol 42: 591– 594, 2000. [DOI] [PubMed] [Google Scholar]
- 17). Goldstein JL, Leonhardt D, Kmytyuk N, Kim F, Wang D, Wainwright MS: Abnormal neuroimaging is associated with early in-hospital seizures in pediatric abusive head trauma. Neurocrit Care 15: 63– 69, 2011. [DOI] [PubMed] [Google Scholar]
- 18). Azizyan A, Sanossian N, Mogensen MA, Liebeskind DS: Fluid-attenuated inversion recovery vascular hyperintensities: an important imaging marker for cerebrovascular disease. AJNR Am J Neuroradiol 32: 1771– 1775, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). McLean LA, Frasier LD, Hedlund GL: Does intracranial venous thrombosis cause subdural hemorrhage in the pediatric population? AJNR Am J Neuroradiol 33: 1281– 1284, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Sanossian N, Ances BM, Shah SH, Kim D, Saver JL, Liebeskind DS: FLAIR vascular hyperintensity may predict stroke after TIA. Clin Neurol Neurosurg 109: 617– 619, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Sanossian N, Saver JL, Alger JR, Kim D, Duckwiler GR, Jahan R, Vinuela F, Ovbiagele B, Liebeskind DS: Angiography reveals that fluid-attenuated inversion recovery vascular hyperintensities are due to slow flow, not thrombus. AJNR Am J Neuroradiol 30: 564– 568, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Toyoda K, Ida M, Fukuda K: Fluid-attenuated inversion recovery intraarterial signal: an early sign of hyperacute cerebral ischemia. AJNR Am J Neuroradiol 22: 1021– 1029, 2001. [PMC free article] [PubMed] [Google Scholar]
- 23). Taoka T, Yuh WT, White ML, Quets JP, Maley JE, Ueda T: Sulcal hyperintensity on fluid-attenuated inversion recovery MR images in patients without apparent cerebrospinal fluid abnormality. AJR Am J Roentgenol 176: 519– 524, 2001. [DOI] [PubMed] [Google Scholar]
- 24). Matsushige T, Nakaoka M, Kiya K, Takeda T, Kurisu K: Cerebral sinovenous thrombosis after closed head injury. J Trauma 66: 1599– 1604, 2009. [DOI] [PubMed] [Google Scholar]
- 25). Ayanzen RH, Bird CR, Keller PJ, McCully FJ, Theobald MR, Heiserman JE: Cerebral MR venography: normal anatomy and potential diagnostic pitfalls. AJNR Am J Neuroradiol 21: 74– 78, 2000. [PMC free article] [PubMed] [Google Scholar]
- 26). Leach JL, Fortuna RB, Jones BV, Gaskill-Shipley MF: Imaging of cerebral venous thrombosis: current techniques, spectrum of findings, and diagnostic pitfalls. Radiographics 26( Suppl 1): S19– S43; discussion S42–S43, 2006. [DOI] [PubMed] [Google Scholar]
- 27). Rollins N, Ison C, Reyes T, Chia J: Cerebral MR venography in children: comparison of 2D time-of-flight and gadolinium-enhanced 3D gradient-echo techniques. Radiology 235: 1011– 1017, 2005. [DOI] [PubMed] [Google Scholar]
- 28). Ducreux D, Oppenheim C, Vandamme X, Dormont D, Samson Y, Rancurel G, Cosnard G, Marsault C: Diffusion-weighted imaging patterns of brain damage associated with cerebral venous thrombosis. AJNR Am J Neuroradiol 22: 261– 268, 2001. [PMC free article] [PubMed] [Google Scholar]
- 29). Keller E, Flacke S, Urbach H, Schild HH: Diffusion- and perfusion-weighted magnetic resonance imaging in deep cerebral venous thrombosis. Stroke 30: 1144– 1146, 1999. [DOI] [PubMed] [Google Scholar]
- 30). Aoki N, Masuzawa H: Infantile acute subdural hematoma. Clinical analysis of 26 cases. J Neurosurg 61: 273– 280, 1984. [DOI] [PubMed] [Google Scholar]