Abstract
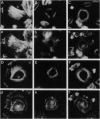
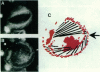
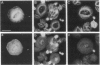
The cytoskeletal patterns of human platelets spread on a glass surface are analyzed. F-actin is arranged in patterns of parallel microfilaments, microfilaments forming triangles, or microfilaments radiating tangentially from a central ellipse or circle. Vinculin, a cytoskeletal protein, is located at both ends of the filaments. In platelets with tangentially radiating microfilaments, vinculin patches are aligned on the branches of a two-armed spiral. The spirals are always left-handed. Talin and two integrins (gpIIb-IIIa, vitronectin receptor), proteins usually associated with focal contacts in tissue culture cells, are not concentrated at the ends of microfilaments in human platelets. It is suggested that the distribution of vinculin is due to competitive aggregation of vinculin close to the inner leaflet of the ventral plasma membrane and that sites of cytoskeleton-membrane linkage are important for generating supramolecular asymmetries of biological systems.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., Zacharski L. R., Widirstky S. T., Rosenstein R., Zaitlin L. M., Burgess D. R. Transformation and motility of human platelets: details of the shape change and release reaction observed by optical and electron microscopy. J Cell Biol. 1979 Oct;83(1):126–142. doi: 10.1083/jcb.83.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avnur Z., Small J. V., Geiger B. Actin-independent association of vinculin with the cytoplasmic aspect of the plasma membrane in cell-contact areas. J Cell Biol. 1983 Jun;96(6):1622–1630. doi: 10.1083/jcb.96.6.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch R. J., Geiger B. The localization of acetylcholine receptor clusters in areas of cell-substrate contact in cultures of rat myotubes. Cell. 1980 Aug;21(1):25–35. doi: 10.1016/0092-8674(80)90111-7. [DOI] [PubMed] [Google Scholar]
- Brown N. A., Wolpert L. The development of handedness in left/right asymmetry. Development. 1990 May;109(1):1–9. doi: 10.1242/dev.109.1.1. [DOI] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Burridge K., Mangeat P. An interaction between vinculin and talin. Nature. 1984 Apr 19;308(5961):744–746. doi: 10.1038/308744a0. [DOI] [PubMed] [Google Scholar]
- Fringeli U. P., Leutert P., Thurnhofer H., Fringeli M., Burger M. M. Structure-activity relationship in vinculin: an IR/attenuated total reflection spectroscopic and film balance study. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1315–1319. doi: 10.1073/pnas.83.5.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway J. W. Reflections on the ambivalent helix. Experientia. 1989 Sep 15;45(9):859–872. doi: 10.1007/BF01954060. [DOI] [PubMed] [Google Scholar]
- Geiger B. A 130K protein from chicken gizzard: its localization at the termini of microfilament bundles in cultured chicken cells. Cell. 1979 Sep;18(1):193–205. doi: 10.1016/0092-8674(79)90368-4. [DOI] [PubMed] [Google Scholar]
- Geiger B. Cytoskeleton-associated cell contacts. Curr Opin Cell Biol. 1989 Feb;1(1):103–109. doi: 10.1016/s0955-0674(89)80045-6. [DOI] [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Buck C., Beckerle M. C., Burridge K. Interaction of plasma membrane fibronectin receptor with talin--a transmembrane linkage. Nature. 1986 Apr 10;320(6062):531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- Kalb E., Engel J. Binding and calcium-induced aggregation of laminin onto lipid bilayers. J Biol Chem. 1991 Oct 5;266(28):19047–19052. [PubMed] [Google Scholar]
- Karlsson R., Lassing I., Höglund A. S., Lindberg U. The organization of microfilaments in spreading platelets: a comparison with fibroblasts and glial cells. J Cell Physiol. 1984 Oct;121(1):96–113. doi: 10.1002/jcp.1041210113. [DOI] [PubMed] [Google Scholar]
- Meyer R. K. Vinculin-lipid monolayer interactions: a model for focal contact formation. Eur J Cell Biol. 1989 Dec;50(2):491–499. [PubMed] [Google Scholar]
- Molony L., Burridge K. Molecular shape and self-association of vinculin and metavinculin. J Cell Biochem. 1985;29(1):31–36. doi: 10.1002/jcb.240290104. [DOI] [PubMed] [Google Scholar]
- Müller S. C., Kai S., Ross J. Curiosities in periodic precipitation patterns. Science. 1982 May 7;216(4546):635–637. doi: 10.1126/science.216.4546.635. [DOI] [PubMed] [Google Scholar]
- Nachmias V. T., Golla R. Vinculin in relation to stress fibers in spread platelets. Cell Motil Cytoskeleton. 1991;20(3):190–202. doi: 10.1002/cm.970200303. [DOI] [PubMed] [Google Scholar]
- Niggli V., Dimitrov D. P., Brunner J., Burger M. M. Interaction of the cytoskeletal component vinculin with bilayer structures analyzed with a photoactivatable phospholipid. J Biol Chem. 1986 May 25;261(15):6912–6918. [PubMed] [Google Scholar]
- Niggli V., Sommer L., Brunner J., Burger M. M. Interaction in situ of the cytoskeletal protein vinculin with bilayers studied by introducing a photoactivatable fatty acid into living chicken embryo fibroblasts. Eur J Biochem. 1990 Jan 12;187(1):111–117. doi: 10.1111/j.1432-1033.1990.tb15283.x. [DOI] [PubMed] [Google Scholar]
- Otto J. J. Detection of vinculin-binding proteins with an 125I-vinculin gel overlay technique. J Cell Biol. 1983 Oct;97(4):1283–1287. doi: 10.1083/jcb.97.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenhorn A. L., Drake B., Prater C. B., Gould S. A., Hansma P. K., Ohnesorge F., Egger M., Heyn S. P., Gaub H. E. Immobilized proteins in buffer imaged at molecular resolution by atomic force microscopy. Biophys J. 1990 Nov;58(5):1251–1258. doi: 10.1016/S0006-3495(90)82465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. G. Views of the platelet cytoskeleton at rest and at work. Ann N Y Acad Sci. 1987;509:156–176. doi: 10.1111/j.1749-6632.1987.tb30993.x. [DOI] [PubMed] [Google Scholar]
- Yost H. J. Regulation of vertebrate left-right asymmetries by extracellular matrix. Nature. 1992 May 14;357(6374):158–161. doi: 10.1038/357158a0. [DOI] [PubMed] [Google Scholar]