Abstract
Background
Cancer metastasis is a multi-step event including epithelial-to-mesenchymal transition (EMT). Breast cancer metastasis suppressor 1 (BRMS1) is a novel metastasis suppressor protein without anti-proliferating activity. However, a detailed underlying mechanism by which BRMS1 attenuates cancer cell EMT and invasion remained to be answered. In the present study, we report an additional mechanism by which BRMS1 attenuates Transforming growth factor-beta1 (TGF-β1)-induced breast cancer cell EMT and invasion.
Methods
Experimental analysis involving chromosome immunoprecipitation (ChIP) and luciferase reporter assays were used to validate hypoxia inducible factor-1alpha (HIF-1α) as a transcriptional regulator of TWIST1 and Snail. Quantitative RT-PCR was used to analyze transcript expression. Immunoblotting and immunofluorescence were used to analyze protein expression. Matrigel-coated in vitro invasion insert was used to analyze cancer cell invasion.
Results
BRMS1 strongly inhibited TGF-β1-induced breast cancer cell EMT and invasion. Unexpectedly, we observed that BRMS1 downregulates not only TWIST1 but also Snail expression, thereby inhibiting breast cancer cell invasion. In addition, we provide evidence that HIF-1α is required for Snail and TWIST1 expression. Further, BRMS1 reduced TGF-β1-induced HIF-1α transcript expression through inactivation of nuclear factor kappaB (NF-κB).
Conclusion
Collectively, the present study demonstrates a mechanical cascade of BRMS1 suppressing cancer cell invasion through downregulating HIF-1α transcript and consequently reducing Snail and TWIST1 expression.
Keywords: BRMS1, HIF-1α, Snail, TGF-β1, TWIST1
Background
Cancer metastasis is a multi-step event including epithelial-to-mesenchymal transition (EMT) [1]. To start invading surround extracellular matrix, tumor cells should be detached from the neighboring epithelial cells by reducing the expression of E-cadherin. Hypoxic condition and various growth factors including transforming growth factor-beta1 (TGF-β1) and epidermal growth factor (EGF) have been shown to induce EMT through upregulating the expression of transcription factors Snail and TWIST1 [2–4].
Breast cancer metastasis suppressor 1 (BRMS1) is a novel metastasis suppressor protein initially identified by differential display to compare highly metastatic breast carcinoma cells with related but metastasis-suppressed cells [5]. BRMS1 is a part of a family that includes suppressor of defective silencing 3 (SUDS3 or mSds3) and BRMS1-like (BRMS1L or p40) [6, 7] and has been shown to selectively suppress metastasis without any inhibition of tumorigenicity of multiple human cancer cells including melanoma [8], ovarian cancer [9] and non-small cell lung cancer [10]. Accumulating evidence suggests two important mechanisms for suppression of BRMS1-induced cancer metastasis: interaction with chromatin remodeling and inhibition of nuclear factor-kappaB (NF-κB) activity [11]. BRMS1 recruits histone deacetylase1 (HDAC1) to NF-κB consensus binding regions [12, 13] and upregulates miR-146a, leading to downregulation of EGFR expression in breast cancer cells [14]. In addition, BRMS1 was reported to have E3 ligase function, leading to suppressing lung metastasis [15].
Recent studies suggest that BRMS1 suppresses breast cancer cell metastasis through modulating cancer cell EMT. Loss of BRMS1 promotes EMT through NF-κB-dependent-TWIST1 expression [16]. Further, Gong et al. [17] claimed that BRMS1 epigenetically silences a receptor for Wnt signaling FZD10, leading to suppress breast cancer cell EMT. However, detailed underlying mechanism by which BRMS1 attenuates cancer cell EMT has not been fully characterized. In the present study, we observed that BRMS1 efficiently inhibited TGF-β1-induced breast cancer cell EMT and invasiveness by downregulating not only TWIST1 but also Snail expression. In addition, we provide evidence that NF-κB is implicated in TGF-β1-induced hypoxia inducible factor-1alpha (HIF-1α) expression and subsequent Snail and TWIST1 expression. Further, the present study shows that BRMS1 significantly inhibits TGF-β1-induced HIF-1α transcript, leading to downregulation of Snail and TWIST1. Therefore, our results identify mechanism by which BRMS1 attenuates cancer cell progression through downregulating HIF-1α and subsequently reducing Snail and TWIST1 expression.
Methods
Reagents
TGF-β1 was obtained from R&D systems (Minneapolis, MN). BAY11-7082 was purchased from Calbiochem (La Jolla, CA). The pcDNA3-BRMS1 plasmid was generated by subcloning EcoRI/XhoI from pOTB7-BRMS1 (clone ID: hMU011011, KRIBB, Korea). The empty pcDNA3 vector was used as a negative control.
Cell culture
All breast cancer cell lines were purchased from American Type Culture Collection (Manassas, VA). MDA-MB-231 were maintained in RPMI 1640 supplemented with 10 % fetal bovine serum (FBS) and 1 % penicillin/streptomycin. MCF-7 cells were maintained in Dulbecco’s modified Eagle’s medium, supplemented with 10 % fetal bovine serum and 1 % penicillin/streptomycin. All cells were incubated at 37 °C under 5 % CO2 in a humidified incubator.
Small interfering RNA (siRNA)
siRNAs of HIF-1α was obtained from Sigma-Aldrich (St. Louis, MO). Control scrambled-siRNA was obtained from Invitrogen (Carlsbad, CA). Transfection was performed by utilizing Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions.
Quantitative RT-PCR
Total cellular RNA was isolated from cultured cells using Trizol (Invitrogen, Carlsbad, CA), and 1 μg of RNA was reverse transcribed using oligo (dT) and M-MLV reverse transcriptase (Promega, Madison, WI) according to the manufacturer’s protocol. Reactions were performed as described previously [18]. The primer sequences of mutants are shown below. HIF-1α; forward 5’-GTT TAC TAA AGG ACA AGT CAC C-3’ and reverse 5’-TCC TGT TTG TTG AAG GGA G-3’, TWIST1; forward 5’-GTC CGC AGT CTT ACG AGG AG-3’ and reverse 5’-CCA GCT TGA GGG TCT GAA TC-3’, E-cadherin; forward 5’-ACA GCC CCG CCT TAT GAT T-3’ and reverse 5’-TCG GAA CCG CTT CCT TCA-3’, Snail; forward 5’-TTT ACC TTC CAG CAG CCC TA-3’ and reverse 5’-GGA CAG AGT CCC AGA TGA GC-3’, Slug; forward 5’-TCT GCA GAC CCA TTC TGA TG-3’ and reverse 5’-AGC AGC CAG ATT CCT CAT GT-3’, GAPDH; forward 5’-ACA GTC AGC CGC ATC TTC TT-3’ and reverse 5’-ACG ACC AAA TCC GTT GAC TC-3’.
Immunoblotting
The cell lysates were prepared as described [19]. Antibodies for HIF-1α (1:1000), p-p65 (1:1000), Snail (1:1000) and Slug (1:1000) were from Cell Signaling Technology (Danvers, MA). Antibodies for E-cadherin (1:1000), TWIST1 (1:1000), BRMS1 (1:500), p52 (1:500), p50 (1:1000) and GAPDH (1:3000) were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Antibody for p65 (1:1000) was obtained from BD Biosciences (San Jose, CA). Secondary antibodies for anti-rabbit (1:2000 ~ 5000) and anti-mouse (1:2000) were Thermo Fisher Scientific Inc (Rockford, IL). Secondary antibody for anti-goat (1:3000) obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). The immunoreactive bands were visualized by ECL (Thermo Fisher Scientific Inc., Rockford, IL) using ImageQuant 300 (GE Healthcare, Buckinghamshire, UK).
Luciferase assay
A TWIST1 luciferase reporter vector [3] was kindly provided from Dr. Hung, MC (M.D. Anderson Cancer Center, Houston, TX). A Snail luciferase reporter vector [20] was kindly provided from Dr. Yook, JI (Yonsei University College of Medicine, Korea). A HIF-1α luciferase reporter vector was obtained from Addgene (Cambridge, MA). The cells were prepared in 12-well plates in triplicate and transfected with the indicated reporter plasmids by utilizing Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA). After stimulation with or without TGF-β1, the cells were washed twice with ice-cold PBS and harvested with a reporter lysis buffer (Promega, Madison, WI). The luciferase activity was analyzed as described previously [21].
In vitro invasion assay
In vitro invasion assay was performed with invasion assay kit with Matrigel-coated inserts (BD Biosciences, San Jose, CA) as described previously [22]. Volume of 1 x 106 cells/well was added to the upper compartment of the invasion chamber. To the lower compartment, we added serum-free conditioned medium with or without TGF-β1. After incubation for 12 h (MDA-MB-231) or 48 h (MCF-7) at 37 °C, filters were fixed and stained with Diff-Quik reagents (Dade Behring, Inc., Newark, DE). The average numbers of six random microscopic fields (x200) was recorded in each experiment.
Immunofluorescence
Immunofluorescence assays were performed as described previously [23]. Antibodies for E-cadherin (1:100) and HIF-1α (1:50) were obtained from BD Biosciences (San Jose, CA). The cells were examined by confocal microscopy (LSM710; Carl Zeiss, Jena, Germany).
Chromatin immunoprecipitation
The ChIP assay was performed as described in the protocol from the Millipore ChIP Assay Kit (Upstate Biotechnology, Charlottesville, VA). Antibody for HIF-1α was obtained from Abcam (Cambridge, MA). The promoter-specific primers used were: TWIST1-HRE; forward 5’-GGA CTG GAA AGC GGA AAC TT-3’ and reverse 5’-CGA GGT GTC TGG GAG TTG G-3’, Snail-HRE; forward 5’-GCT GGG CCA GGC TGC TTT GCA-3’ and reverse 5’-GGA CAC CTG ACC TTC CGA CG-3’.
Subcellular fractionation
The cells were fractionated using the ProteoExtract Subcellular Proteome Extraction Kit (Calbiochem, La Jolla, CA) according to the manufacturer’s instructions. The fractionated samples were analyzed by Immunoblotting.
Statistics
Data are shown as means ± s.d. Differences between two groups were assessed using the Student’s t-test. Differences among three or more groups were evaluated by analysis of variance, followed by Bonferroni multiple comparison tests.
Results
BRMS1 inhibits breast cancer cell EMT
Given that BRMS1 has been known to attenuate cancer cell metastasis, we first determined whether BRMS1 inhibits TGF-β1-induced breast cancer cell invasion. Indeed, ectopic expression of BRMS1 significantly inhibited TGF-β1-induced breast cancer cell invasion (Fig. 1a). Since EMT process is important for cancer cell invasion, we next determined whether BRMS1 regulates breast cancer cell EMT. Stimulation of the cells with TGF-β1 efficiently induced morphological change from epithelial to mesenchymal phenotype of breast cancer cells (Fig. 1b). However, overexpression of BRMS1 strongly inhibited TGF-β1-induced EMT. In addition, immunofluorescence analysis showed that BRMS1 restored E-cadherin expression downregulated by TGF-β1 (Fig. 1c). Consistent with these findings, we observed that BRMS1 efficiently inhibited TGF-β1-induced reduction of E-cadherin transcript (Fig. 1d). Therefore, these data strongly suggest that BRMS1 inhibits TGF-β1-induced breast cancer cell EMT.
Fig. 1.
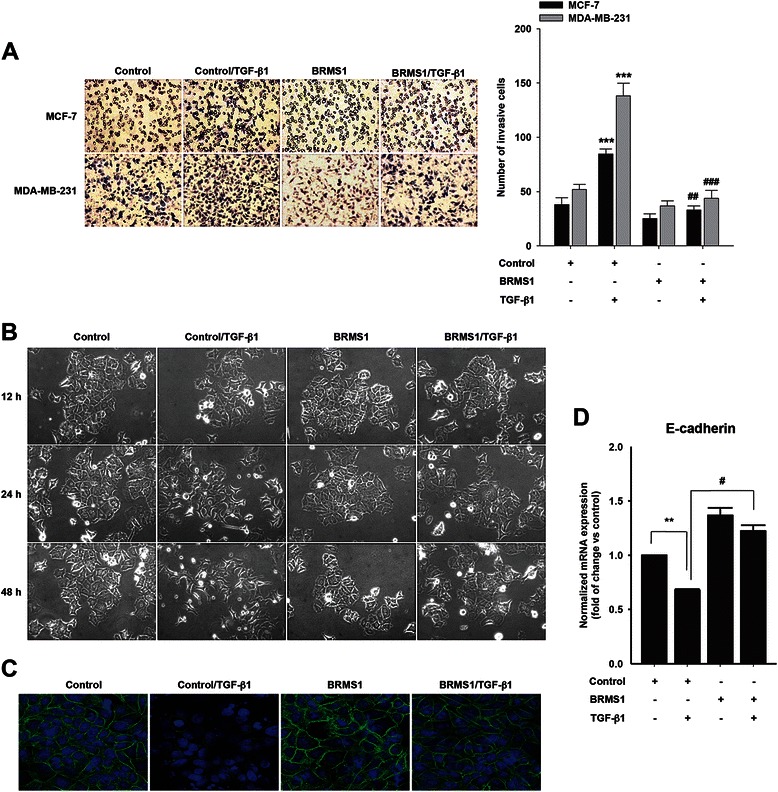
BRMS1 inhibits TGF-β1-induced EMT. a The cells were transfected with BRMS1 or empty vector (control), and in vitroinvasion was analyzed against TGF-β1 (5 ng/ml) (***P < 0.001 vs control, ##P < 0.01 and ###P < 0.001 vs control with TGF-β1). All images original magnification, x200. b The MCF-7 cells were transfected with BRMS1 or empty vector (control) and then treated with TGF-β1 (5 ng/ml) for indicated times. The morphology of the cells was examined under light microscope. All images original magnification, x200. c The MCF-7 cells were transfected with BRMS1 or empty vector (control) for 48 h and then stimulated with TGF-β1 (5 ng/ml) for 6 h. The cells were fixed and stained to assess expression of E-cadherin (green; E-cadherin, blue; Nuclei). All images original magnification, x200. d The MCF-7 cells were transfected with BRMS1 or empty vector (control) for 48 h, followed by stimulation with TGF-β1 (5 ng/ml) for 6 h. The E-cadherin transcript was analyzed by quantitative RT–PCR. Relative mRNA levels normalized to the expression of the housekeeping gene, GAPDH (**P < 0.01 vs control, #P < 0.05 vs control with TGF-β1). Representative results were presented from at least three independent experiments with similar results
BRMS1 inhibits Snail and TWIST1 expression
We next determined the underlying mechanism by which BRMS1 inhibits TGF-β1-induced breast cancer cell EMT. Stimulation of the cells with TGF-β1 significantly induced mRNA expression of EMT factors Snail, Slug and TWIST1 (Fig. 2a). However, overexpression of BRMS1 markedly inhibited Snail and TWIST1 but not Slug transcript (Fig. 2a). Immunoblotting analysis also showed that BRMS1 decreased TGF-β1-induced Snail and TWIST1 expression (Fig. 2b). In addition, BRMS1 significantly inhibited TGF-β1-induced promoter activities of Snail (Fig. 2c) and TWIST1 (Fig. 2d), confirming that BRMS1 reduces not only TWIST1 but also Snail expression. Therefore, these results strongly suggest that BRMS1 attenuates TGF-β1-induced breast cancer cell EMT through downregulation of both Snail and TWIST1.
Fig. 2.
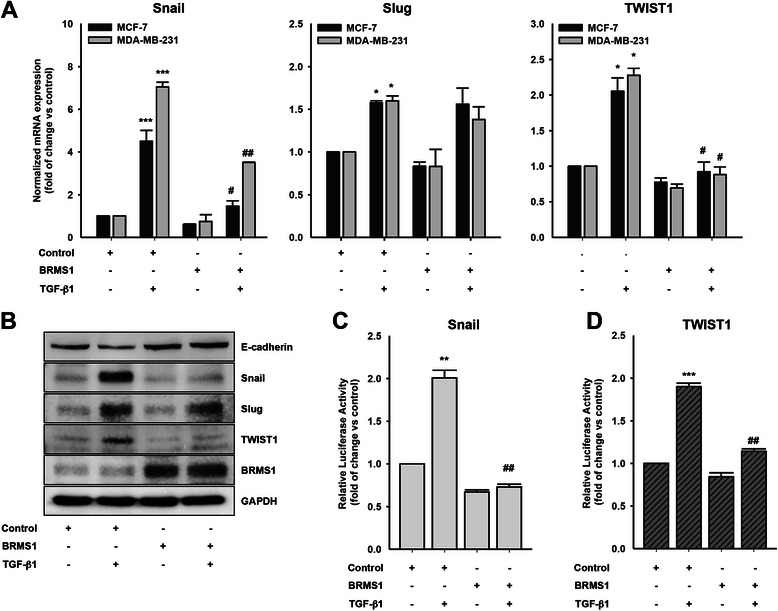
BRMS1 inhibits Snail and TWIST1 expression. a The cells were transfected with BRMS1 or empty vector (control) for 48 h and then stimulated with TGF-β1 (5 ng/ml) for 6 h. Quantitative RT–PCR. Relative mRNA levels normalized to the expression of the housekeeping gene, GAPDH (*P < 0.05 and ***P < 0.001 vs control, #P < 0.05 and ##P < 0.01 vs control with TGF-β1). b The MDA-MB-231 cells were transfected with BRMS1 or empty vector (control) for 48 h and then stimulated with TGF-β1 (5 ng/ml) for 6 h. Immunoblotting. c and d The MDA-MB-231 cells were cotransfected with the empty vector (control), BRMS1 and indicated reporter plasmids for 48 h, followed by stimulation with TGF-β1 (5 ng/ml) for 24 h. Luciferase activity (**P < 0.01 and ***P < 0.001 vs control,##P < 0.01 vs control with TGF-β1). Representative results were presented from at least three independent experiments with similar results
HIF-1α is important for both Snail and TWIST1 expression
Since hypoxia increased Snail [24] and TWIST1 [2] expression, we next determined whether HIF-1α is implicated in TGF-β1-induced Snail and TWIST1expression in breast cancer cells. Stimulation of the cells with TGF-β1 significantly upregulated Snail and TWIST1 expression, while E-cadherin expression was reduced (Fig. 3a). However, silencing HIF-1α showed opposite effects. HIF-1α siRNA reduced both Snail and TWIST1 expression concomitant with increased E-cadherin (Fig. 3a). Consistently, silencing HIF-1α abrogated TGF-β1-induced breast cancer cell invasion (Fig. 3b). Therefore, these data strongly suggest that HIF-1α is important for TGF-β1-induced Snail and TWIST1 expression and cancer cell invasion.
Fig. 3.
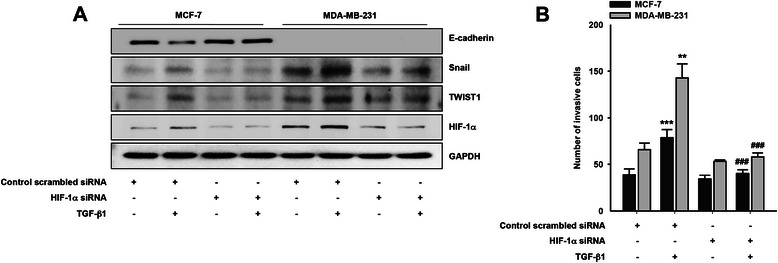
HIF-1α is important for TGF-β1-induced Snail expression. a The cells were transfected with indicated siRNAs and then stimulated with or without TGF-β1 (5 ng/ml) for 6 h. Immunoblotting. b The cells were transfected with indicated siRNAs and in vitro invasion was analyzed against TGF-β1 (5 ng/ml) (**P < 0.01 and ***P < 0.001 vs control scrambled siRNA, ###P < 0.001 vs control scrambled siRNA with TGF-β1). Representative results were presented from at least three independent experiments with similar results
BRMS1 inhibits HIF-1α expression
Given that BRMS1 inhibits Snail and TWIST1 expression and that hypoxic condition induces Snail [24] and TWIST1 [2] expression in breast cancer cells, we hypothesized that BRMS1 inhibits HIF-1α expression. Indeed, overexpression of BRMS1 strongly inhibited TGF-β1-induced HIF-1α mRNA expression (Fig. 4a). In addition, ectopic expression of BRMS1 significantly reduced TGF-β1-induced promoter activities of HIF-1α (Fig. 4b). We also observed that BRMS1 strongly reduced TGF-β1-induced HIF-1α expression (Fig. 4c). In addition, immunofluorescence analysis showed that BRMS1 downregulated TGF-β1-induced HIF-1α expression (Fig. 4d). Further, we observed that BRMS1 abrogated TGF-β1-induced binding of HIF-1α on a promoter region of Snail [25] and TWIST1 [26] (Fig. 4e). Together, these results suggest that BRMS1 inhibits TGF-β1-induced HIF-1α expression and subsequent Snail and TWIST1 expression.
Fig. 4.
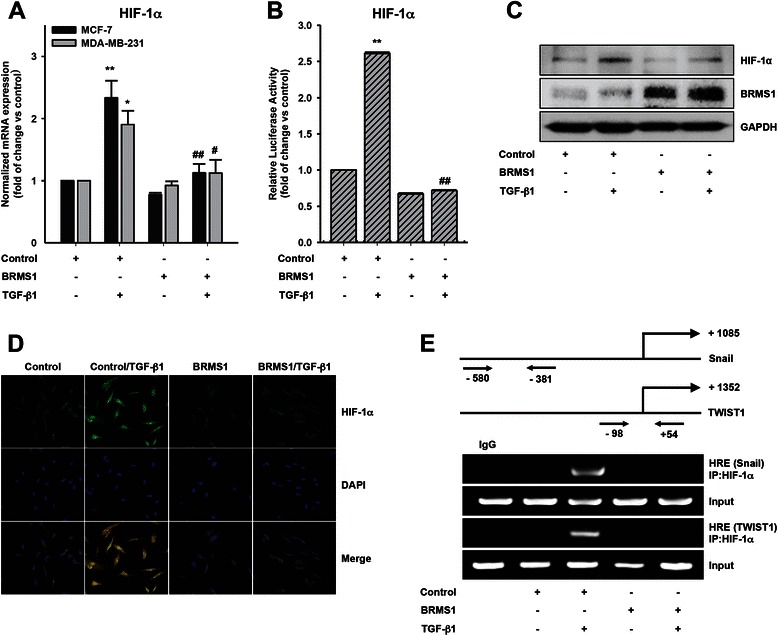
BRMS1 inhibits TGF-β1-induced HIF-1α expression. a The cells were transfected with BRMS1 or empty vector (control) for 48 h and then stimulated with TGF-β1 (5 ng/ml) for 6 h. HIF-1α mRNA expression was analyzed by quantitative RT–PCR. Relative HIF-1α mRNA levels normalized to the expression of the housekeeping gene, GAPDH (*P < 0.05 and **P < 0.01 vs control, #P < 0.05 and ##P < 0.01 vs control with TGF-β1). b The MDA-MB-231 cells were co-transfected with the empty vector (control), BRMS1 and HIF-1α reporter plasmids for 48 h, followed by stimulation with TGF-β1 (5 ng/ml) for 24 h. Luciferase activity (**P < 0.01 vs control, ##P < 0.01 vs control with TGF-β1). c The MDA-MB-231 cells were transfected with BRMS1 or empty vector (control) for 48 h and then stimulated with TGF-β1 (5 ng/ml) for 6 h. Immunoblotting. d The MDA-MB-231 cells were sequentially transfected with indicated vectors for 48 h and then stimulated with TGF-β1 (5 ng/ml) for 6 h. The cells were fixed and stained to assess expression of HIF-1α. e The MDA-MB-231 cells were transfected with indicated vectors before TGF-β1 (5 ng/ml) treatment. The ChIP assay was performed as described in Materials and Methods. Representative results were presented from at least three independent experiments with similar results
NF-κB is important for HIF-1α expression
Since NF-κB is one of important transcription factors for HIF-1α transcript [27], we next determined whether NF-κB is important for HIF-1α transcript expression. TGF-β1 induced translocation of NF-κB subunits from cytosol to nucleus (Fig. 5a). However, BRMS1 strongly inhibited TGF-β1-induced translocation of NF-κB subunits. In addition, pretreatment of the cells with a pharmacological inhibitor of NF-κB, BAY11-7082 markedly inhibited TGF-β1-induced HIF-1α promoter activity (Fig. 5b). Further, BAY11-7082 abrogated TGF-β1-induced HIF-1α and TWIST1 expression (Fig. 5c). Therefore, these data strongly suggest that NF-κB is implicated in HIF-1α expression and consequent Snail and TWIST1 expression.
Fig. 5.
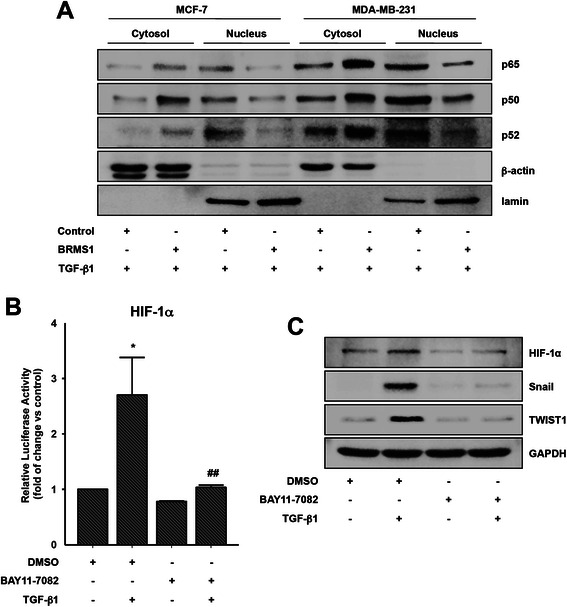
NF-κB is important for HIF-1α expression. a The cells were transfected with BRMS1 or empty vector (control) for 48 h and then stimulated with TGF-β1 (5 ng/ml) for 6 h. Immunoblotting. b The MDA-MB-231 cells transfected with a luciferase vector containing a HIF-1α promoter were pretreated with BAY11-7082 (5 μM) for 1 h, followed by stimulation with TGF-β1 (5 ng/ml) for 24 h. Luciferase activity (*P < 0.05 vs DMSO, ##P < 0.01 vs TGF-β1 treatment only). c The serum-starved MDA-MB-231 cells were pretreated with BAY11-7082 (5 μM) for 1 h and then stimulated with TGF-β1 (5 ng/ml) for 6 h. Immunoblotting. Representative results were presented from at least three independent experiments with similar results
Discussion
In our current study, we elucidate the underlying mechanism by which BRMS1 attenuates breast cancer cell invasion. We demonstrate that both Snail and TWIST1 are important for TGF-β1-induced breast cancer cell invasion. Strikingly, our present data show that BRMS1 downregulates not only TWIST1 but also Snail expression, thereby attenuating TGF-β1-induced breast cancer cell EMT and invasion. Moreover, our data show that HIF-1α mediates TGF-β1-induced Snail and TWIST1 expression and that BRMS1 inactivates NF-κB to reduce HIF-1α transcript, leading to downregulation of TGF-β1-induced Snail and TWIST1 expression. These finding suggest a critical role of HIF-1α through NF-κB activation in TGF-β1-induced Snail and TWIST1 expression and their inhibition by BRMS1 for suppressing breast cancer progression.
BRMS1 has been known to attenuate TWIST1 expression [11, 28]. More recently, Liu et al. [16] proposed that BRMS1 suppresses TWIST1 expression and subsequent NSCLC metastasis. In the present study, we provide evidence that in addition to TWIST1, BRMS1 attenuates breast cancer cell invasion through downregulating Snail expression. First, BRMS1 inhibits TGF-β1-induced Snail and TWIST1 but not Slug expression. Second, silencing either Snail or TWIST1 expression recovered TGF-β1-induced E-cadherin expression and breast cancer cell EMT. More importantly, Snail siRNA significantly inhibited TGF-β1-induced breast cancer cell invasion.
HIF-1α induced by hypoxia and growth factors has been shown to mediate EMT and metastasis of various cancer cells. HIF-1α was reported to upregulate TWIST1 expression to induce morphological change of epithelial cells to mesenchymal phenotype [2]. In addition, our recent results suggest that HIF-1α is important for TWIST1 expression and prostate cancer cell invasion [23, 26]. Further, recent study shows that HIF-1α induces histone deacetylase 3 (HDAC3) which in turn cooperate with Snail to induce EMT and metastatic phenotypes [29]. Consistent with these notions, we demonstrated in the present study that TGF-β1 induces HIF-1α expression, which in turn upregulates TWIST1 expression. Notably, we observed that HIF-1α is also implicated in Snail expression in breast cancer cells which was not detected in prostate cancer cells [23], suggesting the differential role of HIF-1α in Snail expression depending on the cellular context. Consistent with this notion, tumor hypoxia correlates with overexpression of HIF-1α, and consequently with TWIST and Snail expression [2]. Since Snail has been known to enhance TWIST1 protein stability in mouse breast epithelial NMuMG cells [30], further study to explore the crosslink between Snail and TWIST1 expression is warranted to determine the underlying mechanism of TGF-β1-induced breast cancer cell EMT and aggressiveness.
Conclusion
Our results demonstrate that BRMS1 attenuates TGF-β1-induced breast cancer cell invasion through inhibition of NF-κB and subsequent reduction of HIF-1α expression required for Snail and TWIST1 expression, uncovers a new mechanism through which BRMS1 suppresses breast cancer progression.
Acknowledgements
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (121182) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2013R1A1A2A10059565, 2014R1A2A1A11051091).
Abbreviations
- BRMS1
breast cancer metastasis suppressor 1
- EMT
epithelial-to-mesenchymal transition
- TGF-ß1
transforming growth factor beta 1
- EGF
epidermal growth factor
- HIF-1α
hypoxia-inducible factor 1alpha
- NF-κB
nuclear factor-kappaB
- siRNA
Small interfering RNA
- ChIP
Chromatin immunoprecipitation
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
KHY, CGP and HYL designed the experiments. KHC, SLY and DYC performed the experiments. KHC, SLY and DYC analyzed the data. KHY and HYL wrote the manuscript. All the authors approved the final draft of this manuscript. All authors read, edited and approved the final version of this manuscript.
Contributor Information
Kyung Hwa Cho, Email: sarang3580@gmail.com.
Seong-Lan Yu, Email: ysl@hanmail.net.
Do Yeun Cho, Email: dycho@kyuh.co.kr.
Chang Gyo Park, Email: aruso@konyang.ac.kr.
Hoi Young Lee, Email: hoi@konyang.ac.kr.
References
- 1.Tomaskovic-Crook E, Thompson EW, Thiery JP. Epithelial to mesenchymal transition and breast cancer. Breast Cancer Res. 2009;11(6):213. doi: 10.1186/bcr2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10(3):295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 3.Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei Y, et al. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007;67(19):9066–9076. doi: 10.1158/0008-5472.CAN-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oft M, Peli J, Rudaz C, Schwarz H, Beug H, Reichmann E. TGF-beta1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Dev. 1996;10(19):2462–2477. doi: 10.1101/gad.10.19.2462. [DOI] [PubMed] [Google Scholar]
- 5.Seraj MJ, Samant RS, Verderame MF, Welch DR. Functional evidence for a novel human breast carcinoma metastasis suppressor, BRMS1, encoded at chromosome 11q13. Cancer Res. 2000;60(11):2764–2769. [PubMed] [Google Scholar]
- 6.Silveira AC, Hurst DR, Vaidya KS, Ayer DE, Welch DR. Over-expression of the BRMS1 family member SUDS3 does not suppress metastasis of human cancer cells. Cancer Lett. 2009;276(1):32–37. doi: 10.1016/j.canlet.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikolaev AY, Papanikolaou NA, Li M, Qin J, Gu W. Identification of a novel BRMS1-homologue protein p40 as a component of the mSin3A/p33(ING1b)/HDAC1 deacetylase complex. Biochem Biophys Res Commun. 2004;323(4):1216–1222. doi: 10.1016/j.bbrc.2004.08.227. [DOI] [PubMed] [Google Scholar]
- 8.Shevde LA, Samant RS, Goldberg SF, Sikaneta T, Alessandrini A, Donahue HJ, et al. Suppression of human melanoma metastasis by the metastasis suppressor gene, BRMS1. Exp Cell Res. 2002;273(2):229–239. doi: 10.1006/excr.2001.5452. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S, Lin QD, Di W. Suppression of human ovarian carcinoma metastasis by the metastasis-suppressor gene, BRMS1. Int J Gynecol Cancer. 2006;16(2):522–531. doi: 10.1111/j.1525-1438.2006.00547.x. [DOI] [PubMed] [Google Scholar]
- 10.Smith PW, Liu Y, Siefert SA, Moskaluk CA, Petroni GR, Jones DR. Breast cancer metastasis suppressor 1 (BRMS1) suppresses metastasis and correlates with improved patient survival in non-small cell lung cancer. Cancer Lett. 2009;276(2):196–203. doi: 10.1016/j.canlet.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurst DR, Welch DR. Unraveling the enigmatic complexities of BRMS1-mediated metastasis suppression. FEBS Lett. 2011;585(20):3185–3190. doi: 10.1016/j.febslet.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cicek M, Fukuyama R, Cicek MS, Sizemore S, Welch DR, Sizemore N, et al. BRMS1 contributes to the negative regulation of uPA gene expression through recruitment of HDAC1 to the NF-kappaB binding site of the uPA promoter. Clin Exp Metastasis. 2009;26(3):229–237. doi: 10.1007/s10585-009-9235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Smith PW, Jones DR. Breast cancer metastasis suppressor 1 functions as a corepressor by enhancing histone deacetylase 1-mediated deacetylation of RelA/p65 and promoting apoptosis. Mol Cell Biol. 2006;26(23):8683–8696. doi: 10.1128/MCB.00940-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurst DR, Edmonds MD, Scott GK, Benz CC, Vaidya KS, Welch DR. Breast cancer metastasis suppressor 1 up-regulates miR-146, which suppresses breast cancer metastasis. Cancer Res. 2009;69(4):1279–1283. doi: 10.1158/0008-5472.CAN-08-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Mayo MW, Nagji AS, Hall EH, Shock LS, Xiao A, et al. BRMS1 suppresses lung cancer metastases through an E3 ligase function on histone acetyltransferase p300. Cancer Res. 2013;73(4):1308–1317. doi: 10.1158/0008-5472.CAN-12-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Mayo MW, Xiao A, Hall EH, Amin EB, Kadota K, et al. Loss of BRMS1 Promotes a Mesenchymal Phenotype through NF-kappaB-Dependent Regulation of Twist1. Mol Cell Biol. 2015;35(1):303–317. doi: 10.1128/MCB.00869-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong C, Qu S, Lv XB, Liu B, Tan W, Nie Y, et al. BRMS1L suppresses breast cancer metastasis by inducing epigenetic silence of FZD10. Nat Commun. 2014;5:5406. doi: 10.1038/ncomms6406. [DOI] [PubMed] [Google Scholar]
- 18.Choi MJ, Cho KH, Lee S, Bae YJ, Jeong KJ, Rha SY, et al. hTERT mediates norepinephrine-induced Slug expression and ovarian cancer aggressiveness. Oncogene. 2015;34(26):3402–3412. doi: 10.1038/onc.2014.270. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Park SY, Lee EK, Park CG, Chung HC, Rha SY, et al. Activation of hypoxia-inducible factor-1alpha is necessary for lysophosphatidic acid-induced vascular endothelial growth factor expression. Clin Cancer Res. 2006;12(21):6351–6358. doi: 10.1158/1078-0432.CCR-06-1252. [DOI] [PubMed] [Google Scholar]
- 20.Yook JI, Li XY, Ota I, Hu C, Kim HS, Kim NH, et al. A Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast cancer cells. Nat Cell Biol. 2006;8(12):1398–1406. doi: 10.1038/ncb1508. [DOI] [PubMed] [Google Scholar]
- 21.Jeong KJ, Park SY, Cho KH, Sohn JS, Lee J, Kim YK, et al. The Rho/ROCK pathway for lysophosphatidic acid-induced proteolytic enzyme expression and ovarian cancer cell invasion. Oncogene. 2012;31:4279–4289. doi: 10.1038/onc.2011.595. [DOI] [PubMed] [Google Scholar]
- 22.Mertens PR, Harendza S, Pollock AS, Lovett DH. Glomerular mesangial cell-specific transactivation of matrix metalloproteinase 2 transcription is mediated by YB-1. J Biol Chem. 1997;272(36):22905–22912. doi: 10.1074/jbc.272.36.22905. [DOI] [PubMed] [Google Scholar]
- 23.Cho KH, Choi MJ, Jeong KJ, Kim JJ, Hwang MH, Shin SC, et al. A ROS/STAT3/HIF-1alpha signaling cascade mediates EGF-induced TWIST1 expression and prostate cancer cell invasion. Prostate. 2014;74(5):528–536. doi: 10.1002/pros.22776. [DOI] [PubMed] [Google Scholar]
- 24.Lundgren K, Nordenskjold B, Landberg G. Hypoxia, Snail and incomplete epithelial-mesenchymal transition in breast cancer. Br J Cancer. 2009;101(10):1769–1781. doi: 10.1038/sj.bjc.6605369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu GH, Huang C, Feng ZZ, Lv XH, Qiu ZJ. Hypoxia-induced snail expression through transcriptional regulation by HIF-1alpha in pancreatic cancer cells. Dig Dis Sci. 2013;58(12):3503–3515. doi: 10.1007/s10620-013-2841-4. [DOI] [PubMed] [Google Scholar]
- 26.Cho KH, Jeong KJ, Shin SC, Kang J, Park CG, Lee HY. STAT3 mediates TGF-beta1-induced TWIST1 expression and prostate cancer invasion. Cancer Lett. 2013;336(1):167–173. doi: 10.1016/j.canlet.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 27.Tsapournioti S, Mylonis I, Hatziefthimiou A, Ioannou MG, Stamatiou R, Koukoulis GK, et al. TNFalpha induces expression of HIF-1alpha mRNA and protein but inhibits hypoxic stimulation of HIF-1 transcriptional activity in airway smooth muscle cells. J Cell Physiol. 2013;228(8):1745–1753. doi: 10.1002/jcp.24331. [DOI] [PubMed] [Google Scholar]
- 28.Edmonds MD, Hurst DR, Welch DR. Linking metastasis suppression with metastamiR regulation. Cell Cycle. 2009;8(17):2673–2675. doi: 10.4161/cc.8.17.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu MZ, Tsai YP, Yang MH, Huang CH, Chang SY, Chang CC, et al. Interplay between HDAC3 and WDR5 is essential for hypoxia-induced epithelial-mesenchymal transition. Mol Cell. 2011;43(5):811–822. doi: 10.1016/j.molcel.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Dave N, Guaita-Esteruelas S, Gutarra S, Frias A, Beltran M, Peiro S, et al. Functional cooperation between Snail1 and twist in the regulation of ZEB1 expression during epithelial to mesenchymal transition. J Biol Chem. 2011;286(14):12024–12032. doi: 10.1074/jbc.M110.168625. [DOI] [PMC free article] [PubMed] [Google Scholar]


