Abstract
Objectives:
Outcome studies among post-menopausal females with calcified carotid artery plaque (CCAP) on their panoramic images have not been previously undertaken. We sought to compare the extent of abdominal aortic calcification (AAC) on lateral lumbar spine radiographs (LLSRs), among groups of females with (CCAP+) and without (CCAP−) carotid lesions on their panoramic images. “Severe” levels of AAC have previously been validated as a risk indicator of future adverse cardiovascular events.
Methods:
This cross-sectional case–control study included a “CCAP+ group” consisting of females more than 50 years of age having the carotid lesion diagnosed by their dentists and an atherogenic risk factor (age, body mass index, hypertension, diabetes and dyslipidaemia)-matched “CCAP− group”. A physician radiologist, using the Framingham index, evaluated the LLSRs for the magnitude of AAC. Summary statistics for key variables were computed and conditional logistic regression techniques were considered.
Results:
Members of the CCAP+ group were significantly (p = 0.038) more likely to demonstrate “severe” levels of AAC on their LLSRs than members of the CCAP group.
Conclusions:
This is the first published study demonstrating that CCAP on panoramic images of post-menopausal females is significantly associated with “severe” levels of AACs on LLSRs independent of traditional risk factors. Given that these levels of AAC are a validated risk indicator of future myocardial infarction and stroke, dentists must evaluate the panoramic images of post-menopausal females for the presence of CCAP. Patients with carotid atheromas should be referred to their physicians for further evaluation given the systemic implications.
Keywords: atherosclerosis, panoramic radiography, carotid arteries, abdominal aorta, abdominal radiography
Introduction
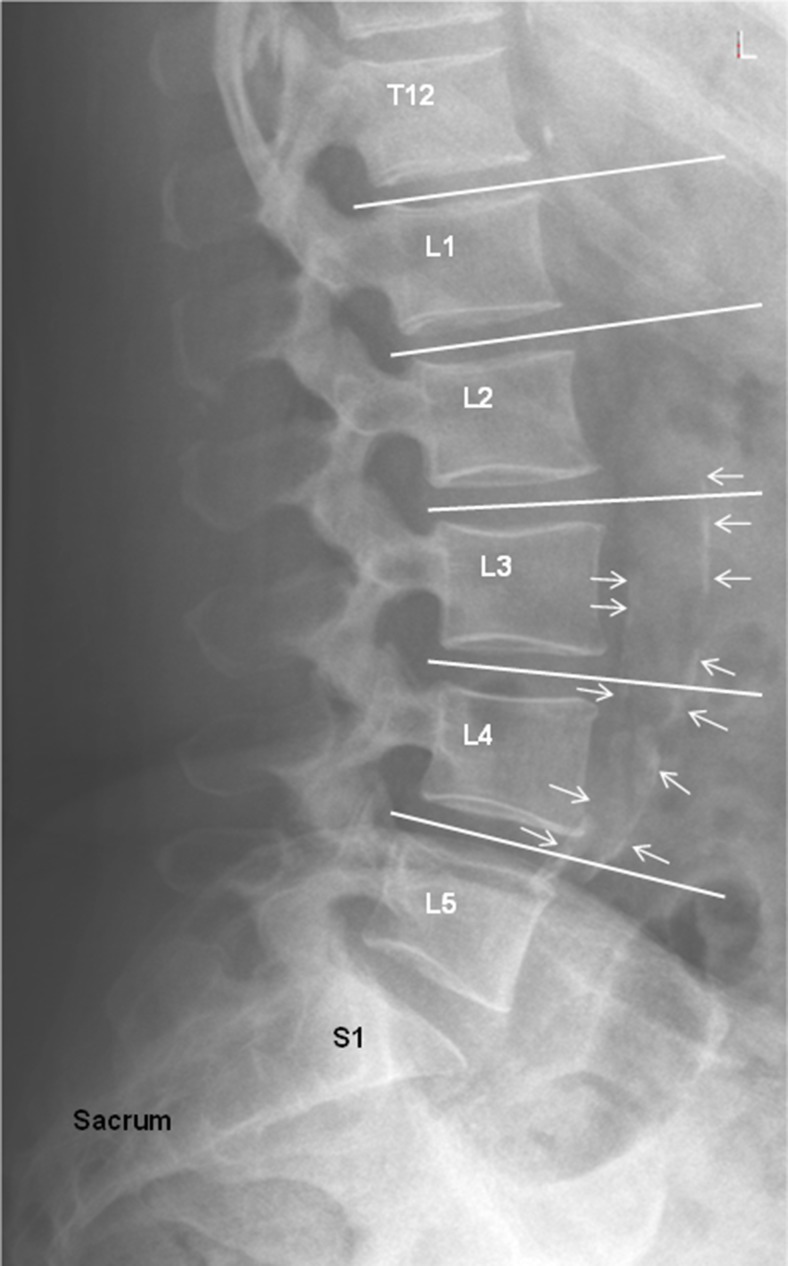
Atherosclerotic cardiovascular disease of the coronary and carotid arteries resulting in myocardial infarction (MI) and stroke is the major cause of premature death among post-menopausal females in the USA and Europe. The illness arising from a paucity of oestrogen with consequent altered fatty acid metabolism resulting in an atherogenic lipid profile is often without recognized symptoms and is of greatest concern in that the initial indication of the disease in >60% of instances is a fatal cardiovascular event.1–3 This “detection gap” has spurred physicians to evaluate routinely obtained imaging studies used for other purposes in order to determine if they can assist in the identification of advanced atherosclerotic plaques prior to manifestation of clinical symptoms or even death. This construct is exemplified by three population-based studies conducted in Framingham, MA,4−6 and two in Rotterdam, Netherlands,7,8 which have established that the magnitude of abdominal aortic calcification (AAC) seen on lateral lumbar spine radiographs (LLSRs) (Figure 1) is an indicator of the extent of systemic atherosclerotic burden and subsequent significantly increased risk of cardiovascular morbidity and mortality independent of traditional risk factors.
Figure 1.
Lateral lumbar spine radiograph of a 61-year-old female with abdominal aortic calcifications identified by arrows.
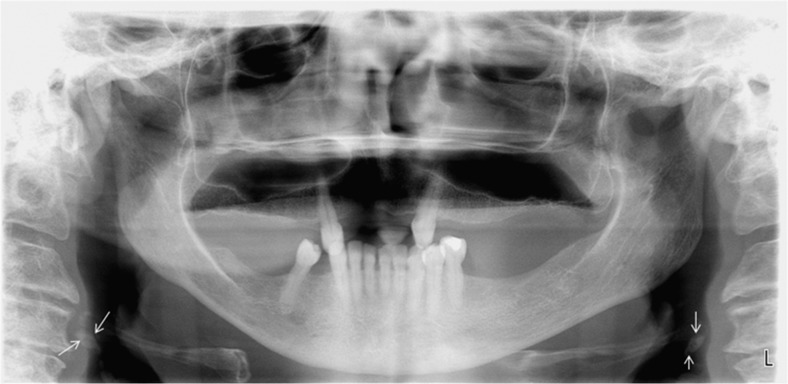
In the USA each year, approximately 15 million persons have panoramic studies.9 Images obtained from large populations of individuals attending dental school clinics reveal that approximately 5% of those in their mid-50s (gender and ethnic distribution not defined) evidence calcified atherosclerotic plaque in the region of the carotid bifurcation (Figure 2).10,11 Confirmation of the presence of such lesions by ultrasound demonstrates that panoramic imaging is highly accurate12 with an 80% sensitivity, 81% specificity and 81% accuracy.13 While longitudinal studies conducted among males have demonstrated that these panoramic detected lesions are associated with near-term MI and stroke, no such prognostic information has yet been garnered for females.14
Figure 2.
Panoramic image of the same patient in Figure 1. The maxillofacial complex has been digitally enhanced with the manufacturer-provided software and evidences bilateral calcified carotid artery plaques (arrows). Note the globular oblong atheromatous opacities lying just inferior to the greater horns of the hyoid bone. L, left.
The purpose of this study was to compare the severity levels of AAC on LLSRs among groups of atherogenic risk factor-matched post-menopausal of females with and without calcified carotid artery plaque (CCAP) on their panoramic images. We hypothesized that females with CCAP (CCAP+) on their panoramic images would have greater severity of AAC on their LLSRs than do similarly aged females free of CCAP (CCAP−).
Methods and materials/patients
Study design and patient sample
To address this research question, the investigators designed and implemented a retrospective cross-sectional case–control study. Institutional review board approval was obtained and the need for consent was obviated given the retrospective nature of the project and its compliance with the Helsinki Declaration. The medical centre's panoramic and lateral lumbar spine digital libraries and electronic medical records were accessed and the studies of all community-living females aged 50 years or over obtained between 1 October 1999 and 1 July 2013 were assessed. The panoramic images had been obtained with a Planmeca panorex unit and Dimaxis Pro v. 4.1.4 image transfer system (Planmeca Oy, Helsinki, Finland), and the LLSRs were taken in the standing position with a tube–film distance of 100 cm centred at the third lumbar vertebrae. Other parameters were: 94 kV and 33–200 mAs (depending on body habitus).
To be included in the “CCAP+ group”, the females had to have (1) a panoramic image that evidenced CCAP as jointly determined by two dentists (AHF, TIC) certified by the American Board of Oral and Maxillofacial Surgery using the American Academy of Oral and Maxillofacial Radiology-sponsored training packet for identification of carotid artery calcifications on panoramic radiographs.15 Consistent with these guidelines, heterogeneous radio-opacities in a verticolinear orientation adjacent to or inferior to the hyoid bone, epiglottis and cervical vertebrae at, above or below the intervertebral space C3–4 were diagnosed as CCAP after ruling out confounding radio-opacities that lie in close proximity to the vessel such as a calcified triticeous cartilage or superior cornu of calcified thyroid cartilage,16 (2) a LLSR obtained for evaluation of low back pain within 24 months of the panoramic image, (3) the image determined to be diagnostic for AAC by the physician radiologist provided at a minimum, an 8-cm field of view anterior to the lumbar spines.
A “CCAP− group” consisting of atherogenic risk-factor-matched females devoid of CCAP on their panoramic image and having a contemporaneous LLSR was likewise constituted. Successful matching required that the following specific elements be met: age ±3 years and body mass index (BMI) ±3 units, and that the medical record indicates prescriptions (yes or no) to control two of the three following conditions/illnesses: hypertension, diabetes mellitus and dyslipidaemia. Race was not matched. Excluded from both groups were females whose medical record identified a history of therapeutic irradiation or surgery of the abdomen. These criteria were invoked in order to obviate the confounding issues of therapeutic irradiation-induced atherosclerosis and surgical artefact.
The medical records librarian identified 628 females who had both imaging studies performed during the prescribed period of time. The panoramic images of 147 females were deemed technically unacceptable because patient positioning errors precluded viewing the area of interest that extended 2.5 cm inferior and 2.5 cm posterior to the cortical rim of the midpoint of the mandible or because they were over or under exposed. Approximately 7% of these females (n = 34) had satisfactory imaging studies demonstrating CCAP and constituted a CCAP+ group. A CCAP− group (n = 34) was developed from the cadre of 447 individuals having technically satisfactory panoramic images devoid of CCAP after being matched to the CCAP+ subjects by age and atherogenic risk factors.
Study variables and data source retrieval
The primary measure was the quantification of severity of AAC on LLSRs of patients with and without CCAP on their panoramic images. A physician, certified in diagnostic radiology by the American Board of Radiology and masked to the patients' medical histories assessed the radiographs for technical adequacy and scored the severity of AAC using the Framingham Heart Study Aortic Calcification Severity (AAC-24) index scoring system.17
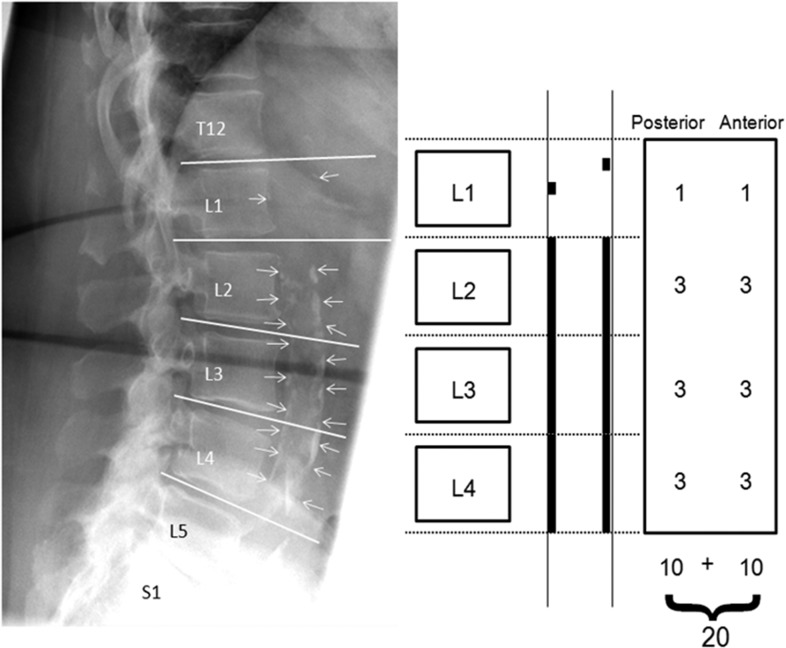
Using this index, the severity of calcified deposits was quantified along the anterior and posterior longitudinal walls of the abdominal aorta adjacent to each lumbar vertebra from L1 to L4 with the midpoint of the intervertebral space above and below serving as the boundaries.18 The developers of this system have documented intrarater correlations in the range of 0.93–0.98 for summary scores indicating excellent reproducibility. Densities overlapping the vertebrae were deemed to represent AAC only if they extended from or formed a clear pattern with those of the lower part of the aorta. Based on the aforementioned Framingham index, calcifications were graded (Figure 3) as follows: a score of 0 was given if no calcific deposits were observed; as 1, if one-third or less (small scattered calcific deposits) of the longitudinal aortic wall in that segment was calcified; as 2, if one-third or more but less than two-thirds of the longitudinal aortic wall was calcified; or 3, if two-thirds or more of the longitudinal aortic wall was calcified. Both the anterior and posterior walls were scored, thus scores could range from 0 to 6 for each vertebral level, and the total score range was 0–24. Then using the Framingham Heart Study model, we totalled and categorized the severity of aortic calcification using the variables (0–3, minimal; 4–10, moderate and ≥11, severe)4,5 with a continuous natural log-transformed variable because of the skewed distribution.
Figure 3.
Utilization of the Framingham Heart Study Aortic Calcification Severity (AAC-24) index scoring system to quantify the extent of abdominal aorta calcretions (arrows) on lateral lumbar spine radiograph of a 73-year-old female.
Demographic data and the pro-atherogenic variables: BMI, hypertension, dyslipidaemia and diabetes mellitus were obtained from the participants' medical records. Specifically, the presence of comorbid illnesses was determined by the list of physician-prescribed medications. Furthermore, the senior author (AHF) reviewed the medical records of each of the study's 68 patients and found convincing historical information and medical diagnostic testing data to substantiate the comorbid diagnoses and medication regimens.
Data analysis
Data were recorded, de-identified, entered into a standardized electronic database and imported into PASW Statistics 18, release 18.0.02009 (IBM Corporation, Somers, NY) and SAS® v. 9.1 (SAS Institute Inc., Cary, NC). Descriptive statistics included measures of central tendency and dispersion for age and BMI, and frequency distributions for the categorical atherosclerotic risk factor variables. Comparisons of frequency distributions for nominal and categorical variables were made and tested for differences in mean age, BMI and AAC scores between CCAP+ and CCAP− groups. For evaluating association between AAC categories and females with or without CCAP, we first used conditional logistic regression techniques, followed by Fisher's exact test.
Results
The 34 post-menopausal female members of the CCAP+ group had a mean age of 62 ± 9 years (Table 1), and, in the main, were obese (BMI, 32.0 ± 6.8). A review of their medical histories disclosed that a large percentage of them were taking medications to control hypertension, diabetes and dyslipidaemia. The atherogenic risk factor profile of the CCAP− group, namely age (61.5 ± 8.0 years), BMI (32.0 ± 7.0) and prevalence of hypertension, diabetes and dyslipidaemia, was found to be comparable/well-matched to the CCAP+ group (Table 1).
Table 1.
Patient characteristics
| Risk factors | CCAP+ females, n = 34 | CCAP− females, n = 34 | p-value |
|---|---|---|---|
| Age (years) (mean ± SD) | 62.2 ± 9.1 | 61.5 ± 8.0 | 0.723 |
| Range (years) | 51–90 | 53–84 | |
| 95% confidence interval | 59.1–65.3 | 58.8–64.2 | |
| Body mass index (mean ± SD) | 32.0 ± 6.8 | 32.0 ± 7.0 | 0.997 |
| Range | 19.1–45.3 | 16.9–45.0 | |
| 95% confidence interval | 29.7–34.3 | 29.7–34.4 | |
| Hypertension, n (%) | 32 (94.1) | 32 (94.1) | 0.999 |
| 95% confidence interval | 86.2–100 | 86.2–100 | |
| Diabetes mellitus, n (%) | 15 (44.1) | 11 (32.4) | 0.455 |
| 95% confidence interval | 27.4–60.8 | 16.7–48.1 | |
| Dyslipidaemia, n (%) | 25 (73.5) | 24 (70.6) | 0.999 |
| 95% confidence interval | 58.7–88.3 | 55.3–85.9 | |
| Prevalence of abdominal aortic calcifications, n (%) | 19 (55.9) | 19 (55.9) | 0.999 |
| 95% confidence interval | 39.2–72.6 | 39.2–72.6 | |
| Abdominal aortic calcification severity index (mean ± SD) Framingham Index Scoring System | 4.25 ± 5.7 | 2.3 ± 3.0 | 0.084 |
| Range | 0–20 | 0–10 | |
| 95% confidence interval | 2.3–6.2 | 1.3–3.3 |
CCPA, calcified carotid artery plaque; CCAP+, patients with CCPA on their panoramic image; CCAP−, patients without CCPA on their panoramic image; SD, standard deviation.
Our analysis with conditional logistic models did not produce estimates because of the small sample size and the number of matching variable employed. We used STATA® v. 13.0 (StataCorp, College Station, TX) and other statistical packages, and all had difficulties. However, we note that using Fisher's exact test, the presence or absence of CCAP was a significant (p = 0.038) determinant of severity of aortic calcification (Table 2) adjusted for traditional risk factors (Table 1). Females with CCAP on their panoramic image were significantly more likely to demonstrate “severe” levels of AAC on their LLSRs than those without carotid plaque and were also less likely to demonstrate “minimal” levels of AAC. As can be noted in Table 2, the six cases with “severe” levels of AAC were all positive for CCAP and represented 17.7% of the CCAP+ sample. We recognize that failure to obtain convergence in the conditional regression analyses may result in our findings being viewed with some suspect from the purely statistical viewpoint; however, the implications of our results, if confirmed, can be profound. We feel further study into the association between females with CCAP or not and the degree of AAC severity is warranted because a confirmation of the association can be impactful in several meaningful ways including providing important diagnostic and preventive values to patients.
Table 2.
Comparison of the presence of calcified carotid artery plaques (CCAPs) to abdominal aortic calcification (AAC) severity index score
| Study groups | “Minimal” AAC (grade, 0–3), n (%) |
“Moderate” AAC (grade, 4–10), n (%) |
“Severe” AAC (grade ≥11), n (%) |
|---|---|---|---|
| Number of females with CCAP (CCAP+), n = 34 | 20 (58.8) | 8 (23.5) | 6 (17.6) |
| Number of females without CCAP (CCAP−), n = 34 | 25 (73.5) | 9 (26.5) | 0 |
| Fisher's exact test; p = 0.038 (distribution of AAC severity among CCAP+ vs CCAP−) | |||
CCAP+, patients with CCAP on their panoramic image; CCAP−, patients without CCAP on their panoramic image.
Discussion
The results of our cross-sectional case–control study demonstrate that the presence of CCAP on a panoramic image of post-menopausal females is highly related to the severity of AAC, adjusted for traditional risk factors. This is an important construct given that the extent of AAC on lumbar spine radiographs is a validated risk indicator among older females of future MI and ischaemic stroke as well as death from cardiovascular disease.4–6
The abdominal aorta, typically defined as the vessel segment above the iliac bifurcation and below the diaphragm, is particularly prone to the development of atherosclerotic lesions. In susceptible subjects, calcified atheromas develop earlier and more significantly than in cerebral or coronary vascular beds.19 Calcification in the aorta initially begins in the vessel's anterior and posterior walls opposite L3 and L4. These calcretions are easy to discern on the radiographs, because the vessel at this juncture is situated anterior to the spine. The vascular wall calcification process begins later in the L1/L2 region, and on occasion, these calcretions may be obscured because in this territory, the aorta may lie lateral to the spine resulting in the aortic densities overlapping the vertebrae.20 Validity of radiographic assessment is, however, demonstrated by a comparison study with CT, which demonstrated that in 97% of instances, calcifications that were detected on LLSRs were shown on CT to be located in the aorta.21 Calcifications in the abdominal aorta seen on assessment of LLSRs have at necropsy been shown to be highly specific for advanced intimal atherosclerosis.22 The development of these lesions in post-menopausal females is believed to arise in part from a paucity of oestrogen, weight gain, systemic hypertension and serum lipid abnormalities.23 Locally, the transdifferentiation of vascular smooth muscle cells into osteoblastic-like cells contributes to the development of vascular calcifications.24
Given that atherosclerosis is a systemic disorder, it occurs throughout the arterial tree. Studies using CT to quantify calcified atherosclerotic plaque report that after age 60 years there is significant correlation (Spearman r = 0.70) between those seen in the region of the carotid artery bifurcation region and the abdominal aorta.25 Furthermore, both carotid artery atherosclerosis detected on ultrasound and aortic atherosclerosis evidenced on abdominal X-ray are strongly associated with the amount of coronary artery calcification detected by electron beam CT and incident cardiovascular disease.26
The clinical implications of identifying the association between CCAP and severity of AAC are substantiated by the results of the Framingham Heart Study which validated the prognostic significance of AAC severity.5 In that project, a cohort of more than 1400 females (mean age at study entry was 61 years) from a general population sample were followed for a mean duration of 21 years. During the study period 59% of the participants demonstrated aortic calcification. Mortality was 91% in the females with aortic calcification and 60% in those without. Furthermore mortality was more than double in those females with the greatest severity of aortic calcification: hazard ratio 2.1 (95% confidence interval, 1.7–2.6) with score “≥11” relative to those with score “0”. In a second Framingham study involving an overlapping cohort of female subjects (n = 1301; mean age, 60 ± 8 years) followed for over 32 years, the severity of AAC on LLSRs was a strong predictor of increased morbidity and mortality from cardiovascular and cerebrovascular disease over and above traditional Framingham risk factors.6 In a third Framingham study also involving an overlapping cohort of female subjects (n = 1466; mean age, 60, 9 ± 8.1 years) followed for more than 20 years, the severity of AAC on LLSRs was associated with an increased risk of subsequent death from cardiovascular disease.4
In Europe, a number of studies have likewise evaluated the prognostic significance of AAC on LLSRs by using a modified form (quantifying the total length of calcification) of the Framingham Index. In the Rotterdam study among females (n = 3955; mean age, 69 ± 9.2 years), the severity of AAC on LLSRs was a significant predictor of incident MI independent of traditional cardiovascular risk factors.7 Additionally, for females with severe atherosclerosis, their hazard risk for MI exceeded that for males. In a second Rotterdam study with overlapping female cohorts (n = 4169; mean age, 69.5 ± 9.2 years) the presence and severity of AAC on LLSRs was a very strong predictor of ischaemic stroke.8
Our study's most significant weakness given its retrospective nature was that ultrasound studies were not used to confirm the presence of CCAP on the panoramic images, thus some calcifications other than those caused by carotid atheroma may have been included. Additionally, this study is an analysis of cross-sectional data, and while it demonstrates an association between CCAP on the panoramic images and AAC on LLSR, a direct link with incident cardiovascular disease remains to be established. Lastly, calcific deposits in the posterior wall of the abdominal aorta have been reported to increase a person's risk for development of ischaemic disc degeneration with resultant back pain thus possibly biasing our population sample.27
Conclusion
In summary, our research findings demonstrate that females with panoramic images evidencing carotid artery calcification may also demonstrate marked aortic calcifications, a previously validated risk indicator of future adverse cardiovascular events (MI and stroke) adjusted for traditional risk factors.4–8 We conclude that the dental profession must be uniquely vigilant for the presence of CCAP when evaluating the panoramic images of older females because there is strong evidence in the medical literature that the detection of subclinical atherosclerosis in any number of vascular beds provides prognostic information about future adverse cardiovascular events. If CCAP is identified, the clinician should consider referring the patient to their primary care physician with a detailed note describing the image findings and suggesting a comprehensive cardiovascular evaluation given the implications of our study. This is an opportune moment for cardiovascular risk reclassification in asymptomatic individuals and potentially allows for primary prevention prior to a clinical event.
Conflict of interest
All authors are either full-time salaried employees of the Department of Veterans Affairs or the University of California, and financial support of this project was limited to the full-time salaries paid to the authors by their respective places of employment.
Contributor Information
A H Friedlander, Email: arthur.friedlander@med.va.gov.
S M El Saden, Email: Suzie.El-saden@va.gov.
R C Hazboun, Email: rennahazboun.dmd@gmail.com.
T I Chang, Email: tina.chang2@va.gov.
W K Wong, Email: wkwong@ucla.edu.
N R Garrett, Email: ngarrett@ucla.edu.
References
- 1.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren WM, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): the Fifth Joint Task Force of the European Society of Cardiology and other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Atherosclerosis 2012; 223: 1–68. doi: 10.1016/j.atherosclerosis.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012; 125: 188–97. doi: 10.1161/CIR.0b013e31823ac046 [DOI] [PubMed] [Google Scholar]
- 3.Mosca L, Appel LJ, Benjamin EJ, Berra K, Chandra-Strobos N, Fabunmi RP, et al. Evidence-based guidelines for cardiovascular disease prevention in women. Circulation 2004; 109: 672–93. [DOI] [PubMed] [Google Scholar]
- 4.Wilson PW, Kauppila LI, O'Donnell CJ, Kiel DP, Hannan M, Polak JM, et al. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation 2001; 103: 1529–34. doi: 10.1161/01.CIR.103.11.1529 [DOI] [PubMed] [Google Scholar]
- 5.Samelson EJ, Cupples LA, Broe KE, Hannan MT, O'Donnell CJ, Kiel DP. Vascular calcification in middle age and long-term risk of hip fracture: the Framingham Study. J Bone Miner Res 2007; 22: 1449–54. doi: 10.1359/jbmr.070519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levitzky YS, Cupples LA, Murabito JM, Kannel WB, Kiel DP, Wilson PW, et al. Prediction of intermittent claudication, ischemic stroke and other cardiovascular disease by detection of abdominal aortic calcific deposit by plan lumbar radiographs. Am J Cardiol 2008; 101: 326–31. doi: 10.1016/j.amjcard.2007.08.032 [DOI] [PubMed] [Google Scholar]
- 7.van der Meer IM, Bots ML, Hofman A, del Sol AI, van der Kuip DA, Witteman JC. Predictive value of noninvansive measure of atherosclerosis for incident myocardial infarction: the Rotterdam Study. Circulation 2004; 109: 1089–94. doi: 10.1161/01.CIR.0000120708.59903.1B [DOI] [PubMed] [Google Scholar]
- 8.Hollander M, Hak AE, Koudstaal PJ, Bots ML, Grobbee DE, Hofman A, et al. Comparison between measures of atherosclerosis and risk of stroke: the Rotterdam Study. Stroke 2003; 34: 2367–72. doi: 10.1161/01.STR.0000091393.32060.0E [DOI] [PubMed] [Google Scholar]
- 9.American Dental Association. 2005-2006 survey of dental services rendered. Chicago, IL: American Dental Association; 2007. [Google Scholar]
- 10.Carter LC, Haller AD, Nadarajah V, Calamel AD, Aguirre A. Use of panoramic radiography among an ambulatory dental population to detect patients at risk of stroke. J Am Dent Assoc 1997; 128: 977–84. doi: 10.14219/jada.archive.1997.0338 [DOI] [PubMed] [Google Scholar]
- 11.Lewis DA, Brooks SL. Carotid artery calcification in a general dental population: a retrospective study of panoramic radiographs. Gen Dent 1999; 47: 98–103. [PubMed] [Google Scholar]
- 12.Romano-Sousa CM, Krejci L, Medeiros FM, Graciosa-Filho RG, Martins MF, Guedes VN. Diagnostic agreement between panoramic radiographs and color Doppler images of carotid atheroma. J Appl Oral Sci 2009; 17: 45–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ertas ET, Sisman Y. Detection of incidental carotid artery calcifications during dental examinations: panoramic radiography as an important aid in dentistry. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011; 112: e11–17. doi: 10.1016/j.tripleo.2011.02.048 [DOI] [PubMed] [Google Scholar]
- 14.Friedlander AH, Cohen SN. Panoramic radiographic atheromas portend adverse vascular events. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007; 103: 830–5. doi: 10.1016/j.tripleo.2006.07.016 [DOI] [PubMed] [Google Scholar]
- 15.Almog DM, Tsimidis K, Moss ME, Gottlieb RH, Carter LC. Evaluation of a training program for detection of carotid artery calcifications on panoramic radiographs. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000; 90: 111–17. doi: 10.1067/moe.2000.107056 [DOI] [PubMed] [Google Scholar]
- 16.Carter LC. Discrimination between calcified triticeous cartilage and calcified carotid atheroma on panoramic radiography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000; 90: 108–10. doi: 10.1067/moe.2000.106297 [DOI] [PubMed] [Google Scholar]
- 17.Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O'Donnell CJ, Wilson PW. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham heart study. Calcif Tissue Int 2001; 68: 271–6. doi: 10.1007/BF02390833 [DOI] [PubMed] [Google Scholar]
- 18.Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PW. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis 1997; 132: 245–50. doi: 10.1016/S0021-9150(97)00106-8 [DOI] [PubMed] [Google Scholar]
- 19.Eggen DA, Strong JP, McGill HC, Jr. Calcification in the abdominal aorta; relationship to race, sex, and coronary atherosclerosis. Arch Pathol 1964; 78: 575–83. [PubMed] [Google Scholar]
- 20.Kiel DP, Menn SP, Karasik DE. Radiographic atlas of location, severity and progression of calcific lesions of in the abdominal aorta. Institute for Aging Research, Hebrew Senior Life, Boston, MA. [Updated 22 May 2013.] Available from: http://www.hebrewseniorlife.org/research-musculoskeletal-resources [Google Scholar]
- 21.Witteman JC, Grobbee DE, Valkenburg HA, van Hemert AM, Stijnen T, Burger H, et al. J-shaped relation between change in diastolic blood pressure and progression of aortic atherosclerosis. Lancet 1994; 343: 504–7. doi: 10.1016/S0140-6736(94)91459-1 [DOI] [PubMed] [Google Scholar]
- 22.Hyman JB, Epstein FH. A study of the correlation between roentgenographic and post-mortem calcifications of the aorta. Am Heart J 1954; 48: 540–3. doi: 10.1016/0002-8703(54)90119-2 [DOI] [PubMed] [Google Scholar]
- 23.Christian RC, Harrington S, Edwards WD, Oberg AL, Fitzpatrick LA. Estrogen status correlates with the calcium content of coronary atherosclerotic plaques in women. J Clin Endocrinol Metab 2002; 87: 1062–7. doi: 10.1210/jcem.87.3.8354 [DOI] [PubMed] [Google Scholar]
- 24.Abedin M, Tintut Y, Deemer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol 2004; 24: 1161–70. doi: 10.1161/01.ATV.0000133194.94939.42 [DOI] [PubMed] [Google Scholar]
- 25.Strobl FF, Rominger A, Wolpers S, Rist C, Bamberg F, Thierfelder KM, et al. Impact of cardiovascular risk factors on vessel wall inflammation and calcified plaque burden differs across vascular beds: a PET-CT study. Int J Cardiovasc Imaging 2013; 29: 1899–908. doi: 10.1007/s10554-013-0277-8 [DOI] [PubMed] [Google Scholar]
- 26.Oei HH, Vliegenthart R, Hak AE. The association between coronary calcification assessed by electron beam computed tomography and measures of extra coronary atherosclerosis: the Rotterdam Coronary Calcification Study. J Am Coll Cardiol 2002; 39: 1745–51. doi: 10.1016/S0735-1097(02)01853-3 [DOI] [PubMed] [Google Scholar]
- 27.Kauppila LI, McAlindon T, Evans S, Wilson PW, Kiel D, Felson DT. Disc degeneration/back pain and calcification of the abdominial aorta. A 25-year follow-up study in Framingham. Spine (Phila Pa 1976) 1997; 22: 1642–7. doi: 10.1097/00007632-199707150-00023 [DOI] [PubMed] [Google Scholar]