Abstract
Objectives:
This study investigated the applicability of a Bayesian belief network (BBN) to MR images to diagnose temporomandibular disorders (TMDs). Our aim was to determine the progression of TMDs, focusing on how each finding affects the other.
Methods:
We selected 1.5-T MRI findings (33 variables) and diagnoses (bone changes and disc displacement) of patients with TMD from 2007 to 2008. There were a total of 295 cases with 590 sides of temporomandibular joints (TMJs). The data were modified according to the research diagnostic criteria of TMD. We compared the accuracy of the BBN using 11 algorithms (necessary path condition, path condition, greedy search-and-score with Bayesian information criterion, Chow–Liu tree, Rebane–Pearl poly tree, tree augmented naïve Bayes model, maximum log likelihood, Akaike information criterion, minimum description length, K2 and C4.5), a multiple regression analysis and an artificial neural network using resubstitution validation and 10-fold cross-validation.
Results:
There were 191 TMJs (32.4%) with bone changes and 340 (57.6%) with articular disc displacement. The BBN path condition algorithm using resubstitution validation and 10-fold cross-validation was >99% accurate. However, the main advantage of a BBN is that it can represent the causal relationships between different findings and assign conditional probabilities, which can then be used to interpret the progression of TMD.
Conclusions:
Osteoarthritic bone changes progressed from condyle to articular fossa and finally to mandibular bone contours. Disc displacement was directly related to severe bone changes. Early bone changes were not directly related to disc displacement. TMJ functional factors (condylar translation, bony space and disc form) and age mediated between bone changes and disc displacement.
Keywords: temporomandibular joint disorders, magnetic resonance imaging, Bayesian method, algorithms, computer simulation
Introduction
Temporomandibular disorder (TMD) is the comprehensive diagnostic name for pathological conditions that have the main symptoms of pain in the temporomandibular joints (TMJs) or masticatory muscle, abnormal sounds at the TMJ and limitations to the mouth opening or abnormal jaw movement.1 TMJ disorder classification is complex, and the research diagnostic criteria for TMDs (RDC/TMD) is currently the most widely used diagnostic protocol for TMD research.1 Its assessment components include Axis I (a clinical and imaging assessment that differentiates myofascial pain, disc displacement, arthralgia, arthritis and arthrosis) and Axis II (which evaluates psychological status and pain-related disabilities). The criteria recommended by the Japanese Society for Temporomandibular Disorders for diagnosing TMD include myalgia of the masticatory muscle, arthralgia of the TMJ, TMJ disc derangement with and without disc reduction and osteoarthrosis/osteoarthritis of the TMJ.2 These criteria were designed to be consistent with the RDC/TMD protocol. The diagnostic criteria (DC) for TMDs (DC/TMD) were released in 2014 and are DC for clinical and research applications.3 This protocol (as with all of the most common DC for intra-articular TMDs) includes functional factors such as disc displacement with intermittent locking and TMJ subluxation as new disorders with/without limited opening, coupled with disc displacement and with/without reduction in the RDC/TMD. The important relationships between bone changes, articular disc displacement of the TMJ and skeletal morphology have been studied.4–10 However, a more sophisticated study is needed to clarify how bone changes, articular disc displacement, bony space, condylar translation and disc form affect each other, especially within the role of functional factors.
The Bayesian approach11 has been extensively applied to medical image diagnoses,12–17 and more sophisticated Bayesian belief networks (BBN) have improved image diagnostic accuracy in medicine18–21 and dentistry.22–27 The naïve Bayesian approach states that the probability of a condition (diagnosis) can be estimated from a given set of observations (findings), when the relative prevalence of each diagnosis is known and the probability of the occurrence of the findings is known. A BBN expresses numerical relationships between multiple nodes (variables, in this case, “findings” and “diagnosis”) that have no logical cyclic relationships and determines the directed arcs that connect those nodes. A numerical expression using a directed acyclic graph (DAG) shows the direction of cause and effect among multiple nodes. Additionally, a BBN can model the quantitative strength of the connections between variables and can automatically update our probabilistic beliefs as new information becomes available. This study investigates the applicability of a BBN to the diagnosis (the probability of a condition) and findings (i.e. a given set of observations) in MR images to try to determine the progression of TMD.
The purpose of this study was not to statistically compare the search algorithms and information criterion of BBN. Instead, we focused on the probabilistic relationships between bone changes and disc displacement, and on how the condylar translation, bony space and disc form affect bone changes and disc displacement. A multiple regression analysis can only show the mathematical relationships between the independent and dependent variables. Causal relationships are not shown, but they are shown in the DAG of the BBN. In an artificial neural network (ANN), the complicated mathematical relationships between the inputs and outputs are shown as values from 0 to 1. The causal relationships are not shown, and the coefficients of the hidden layers have no meaning to the radiologist. Accordingly, an ANN cannot show the progression of TMD and is not suitable for a computer-aided diagnosis(CAD) model of a multivariate diagnostic analysis of TMD. Thus, the BBN is the most appropriate method for this study, because the DAG describes cause and effect relationships (which can be easily understood by a radiologist), and each relationship has an associated conditional probability distribution.
Using the BBN, we have particularly focused on how bone changes, disc displacement, condylar translation, bony space and disc form affect each other.
Methods and materials
This study was authorized by the ethics committee of Tokushima University Hospital (number 982-2010). It was based on 1.5-T MR images from patients who presented with TMD from 2007 to 2008. There were a total of 295 cases, and 590 right and left sides of TMJs (male, 54 cases; female, 241 cases; average age, 39.5 years; maximum, 86 years; minimum, 11 years). The MRI sets were obtained using a Signa EXCITE XI (GE Healthcare Japan Ltd., Tokyo, Japan) with a dual (3-inch diameter) surface coil. Each image set consisted of seven images for each side (field of view, 120 × 120 mm2; matrix, 256 × 256 pixels), oblique sagittal and oblique coronal 3-mm proton density (repetition time/echo time, 1500/22 ms), T2 weighted images (repetition time/echo time, 3500/81 ms) in the closed mouth position and oblique sagittal 3-mm proton density and T2 weighted images in the open mouth position.28 The variables and instances to be used as the findings and diagnosis were selected according to RDC/TMD1 or defined by the author (Tables 1 and 2). The cases were interpreted by a maxillofacial radiologist with more than 30 years' experience.
Table 1.
Variables and instances used as findings and diagnosis (bone changes)
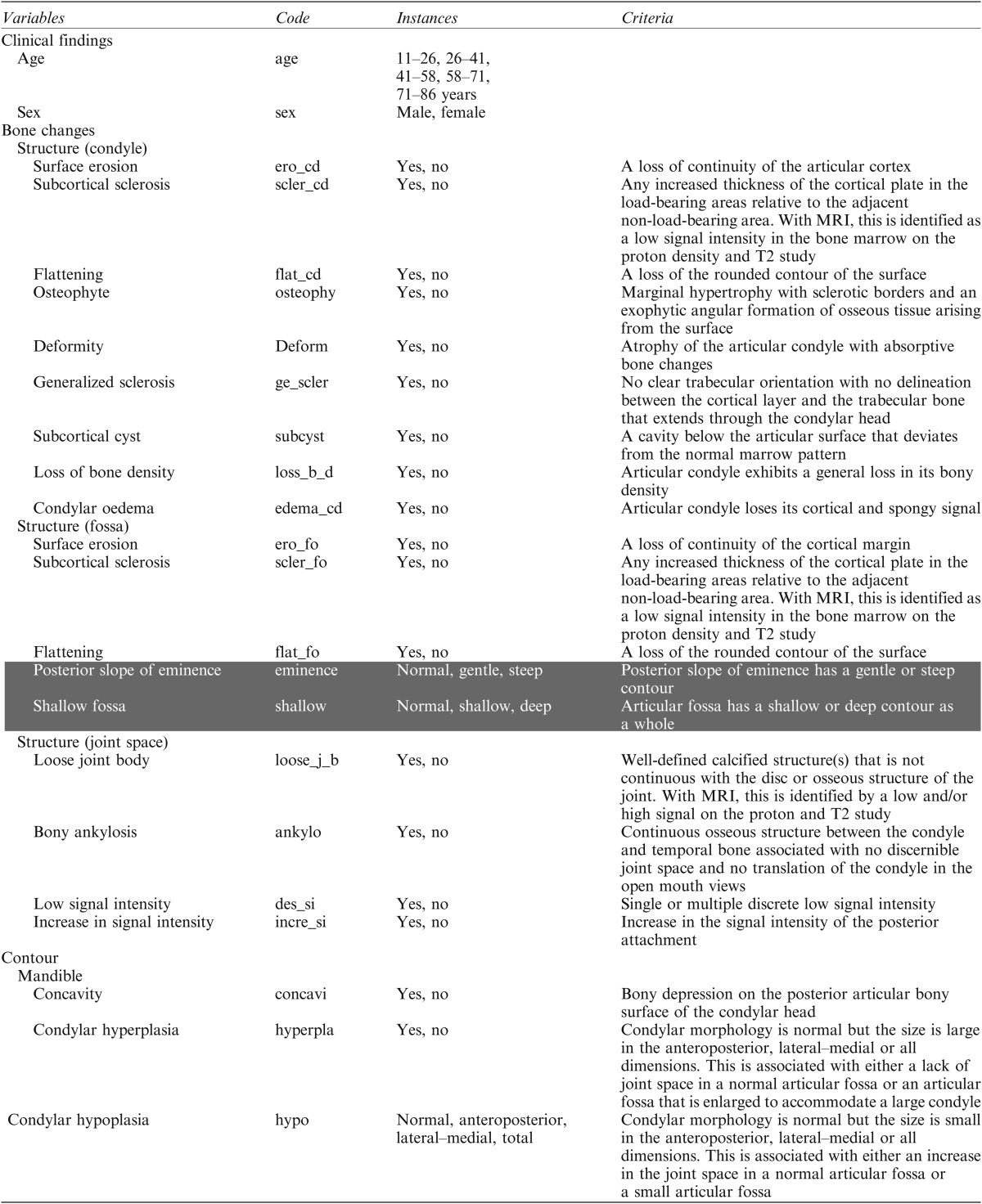
CC, concavity at the posterior slope of the condyle; CH, condylar hypoplasia; CN, condylar neck length; DF, deformity; N, normal; notN, not normal; OP, osteophyte; RL, ramus length; SE, surface erosion.
Shading represents definitions from this study. The other findings were defined by research diagnostic criteria/temporomandibular disorder.1
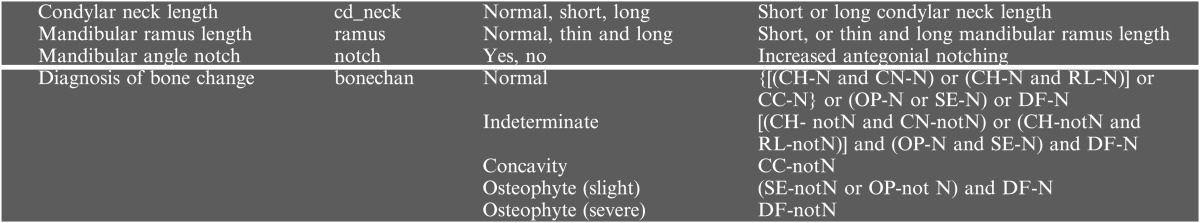
Table 2.
Variables and instances used as findings and diagnosis (disk displacement)
ADD, anterior disc displacement; Lat, lateral displacement; N, normal; notN, not normal; woR, without reduction; wR, with reduction.
Shading represents definitions from this study and the other findings were defined by research diagnostic criteria for temporomandibular disorder.1
Multicollinearity is a statistical phenomenon in which two or more independent variables in a MR model are highly correlated. This causes the estimates of the coefficients to erratically vary in response to small changes in the model or data. We tested the multicollinearity of the independent variables using Dr SPSS® for Windows v. 11.0.1.J (SPSS Inc., Tokyo, Japan). We checked threshold values such as the tolerance (not <0.1) and its reciprocal variance inflation factor (not >10), the condition index (not >15) and the variance proportion (not >0.5) to quantify the severity of the multicollinearity in the MR analysis. We set the tolerance to 1 − R2 (coefficient of determination), when regressing an independent variable on other independents. The condition index flagged excessive colinearities in the data. The most common criterion related to two or more variables with a variance proportion of 0.5 or higher on the high condition index.
By definition, “power” is the ability to find statistical significance when the null hypothesis is false, that is, the ability to determine real differences. The power of a study depends on the sample size, alpha level and the effect size. For this study, we performed a power analysis for the MR analysis using IBM SPSS SamplePower v. 3 (IBM Japan Ltd, Tokyo, Japan).
We implemented the BBN using commercially available machine-learning algorithms (Hugin Researcher v. 7.8; Hugin Expert A/S, Aalborg, Denmark, http://www.hugin.com/, and BayoNet System v. 5.0; Mathematical Systems Inc., Tokyo, Japan, http://www.msi.co.jp/BAYONET/index.html). These algorithms automatically learn the network structures and joint probabilities from the training data and do not require any additional user-defined parameters. We used six search algorithms in Hugin Researcher: necessary path condition (NPC),29 path condition (PC),30 greedy search-and-score31 with Bayesian information criterion,32 Chow–Liu tree,33 Rebane–Pearl poly tree34 and tree augmented naïve Bayes model.35 We used the greedy search31 algorithm by BayoNet with five information criterion: maximum log likelihood,36 Akaike information criterion,37 minimum description length,38 K239 and C4.5.40 Hugin has an expectation-maximization algorithm,41 which supports data analysis in the case of missing values (as is often the case in medical data). The MR and ANN analyses were performed using commercially available software (JMP v. 8.0.1; SAS Institute Japan Ltd., Tokyo, Japan).
We evaluated the accuracy of bone changes and disc displacement using resubstitution validation and 10-fold cross-validation.42 In resubstitution validation, the model is trained using all the available data and then tested on the same set of data. It is simple, but suffers from overfitting. 10-fold cross-validation is widely accepted in data mining and the machine-learning community. It is a standard procedure for estimating performance and selecting models. We used 10-fold cross-validation to train and test the BBN model. The data were first partitioned into 10 almost equally sized subsets. We then performed 10 iterations of training and validation. In each iteration, a different subset of the data is removed during training (leaving nine subsets in the training set) and then used for validation. 10-fold cross-validation tends to provide a less biased estimate of the accuracy because we can ensure that cases used to train the model are never used to determine its performance.
Results
Our multicollinearity testing determined that the independent variants of all indices were acceptable and did not have excessive correlations. We used a power analysis to determine if we had a large enough data set for an effective MR analysis. For our sample size of 590 and alpha = 0.05 (where alpha is the Type I error rate, i.e. the rate at which a true null hypothesis will be rejected), the SPSS sample power function determined that the MR analysis had a power of one. Therefore, we had a sufficiently large data set for MR analysis. There is no suitable and commercially available power analysis software for BBN or ANN, and there is some controversy concerning the optimal sample sizes, especially for BBN. This is a frontier research problem in the data mining and machine-learning community. Therefore, we used the MR power analysis as a substitute, in a similar way as we validated the independency of the variants using the multicollinearity test for MR analysis.
Table 3 compares incorrectly diagnosed cases of bone changes and disc displacement using the BBN, NPC, PC, greedy search-and-score with Bayesian information criterion, Chow–Liu tree, Rebane–Pearl poly tree, tree augmented naïve, maximum log likelihood, Akaike information criterion, minimum description length, K2 and C4.5 methods, and the MR and ANN analyses using resubstitution validation. Table 4 compares the incorrectly diagnosed cases of bone changes and disc displacement using the same 11 BBNs and the ANN with 10-fold cross-validation. In our data set, 191 TMJs (32.37%) had bone changes and 340 TMJs (57.62%) had disc displacement. The MR using resubstitution validation was very accurate, and the ANN was also very accurate, using resubstitution or 10-fold cross-validation. However, neither of these methods produce a suitable CAD model for a multivariate diagnostic analysis of TMD. The BBN PC algorithm had almost the same accuracy as the MR and ANN using resubstitution validation and 10-fold cross-validation. When using resubstitution validation, there were only three incorrect diagnoses of bone changes (accuracy, 99.49% = 587/590), and no incorrect disc displacement diagnoses (accuracy, 100% = 590/590). The overall accuracy was 99.75% (=1177/1180). The accuracy using 10-fold cross-validation was 97.62% (=576/590) for bone changes, 99.66% (=588/590) for disc displacement and 99.49% (=1174/1180) overall. Figure 1 shows the DAG of TMD using the BBN PC algorithm, 35 variables and resubstitution validation. This DAG shows the relationships between TMJ findings concerning bone changes, disc displacement, joint space, clinical findings, condylar translation, bony space and disc form and the MR diagnosis of bone changes and disc displacement. Figure 2 contains the conditional probability distribution tables of each node superimposed on the DAG.
Table 3.
Accuracy comparison for the Bayesian belief networks [necessary path condition (NPC),29 path condition (PC),30 greedy search-and-score31 with Bayesian information criterion (BIC),32 Chow–Liu tree,33 Rebane–Pearl poly tree,34 tree augmented naïve (TAN) Bayes model,35 maximum log likelihood (ML),36 Akaike information criterion (AIC),37 minimum description length (MDL),38 K239 and C4.540], and multiple regression (MR) and the artificial neural network (ANN) using resubstitution validation
| Diagnosis | Incorrect cases (%) using resubstitution validation |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NPC | PC | BIC | Chow–Liu | Rabena-Pearl | TAN | ML | AIC | MDL | K2 | C4.5 | MR | ANN | |
| Bone changes | |||||||||||||
| Normal (399) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Indeterminate (13) | 2 (0.34%) | 0 (0.0%) | 2 (0.34%) | 3 (0.51%) | 2 (0.34%) | 0 (0.00%) | 9 (1.36%) | 4 (0.68%) | 2 (0.34%) | 0 (0.00%) | 5 (0.85%) | 0 (0.00%) | 0 (0.00%) |
| Concavity (31) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 2 (0.34%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Osteoarthrosis (slight) (126) | 1 (0.17%) | 1 (0.17%) | 1 (0.17%) | 1 (0.17%) | 0 (0.00%) | 0 (0.00%) | 1 (0.17%) | 1 (0.17%) | 1 (0.17%) | 1 (0.17%) | 29 (4.92%) | 1 (0.17%) | 0 (0.00%) |
| Osteoarthrosis (severe) (21) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (0.17%) | 0 (0.00%) | 0 (0.00%) | 1 (0.17%) | 0 (0.00%) | 0 (0.00%) |
| Subtotal (590) | 3 (0.51%) | 1 (0.17%) | 3 (0.51%) | 4 (0.68%) | 2 (0.34%) | 0 (0.00%) | 10 (1.69%) | 6 (1.02%) | 3 (0.51%) | 1 (0.17%) | 35 (5.93%) | 1 (0.17%) | 0 (0.00%) |
| Disc displacement | |||||||||||||
| Normal (250) | 1 (0.17%) | 1 (0.17%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| ADDwR (97) | 0 (0.00%) | 0 (0.00%) | 1 (0.17%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (0.17%) | 1 (0.17%) | 97 (16.44%) | 0 (0.00%) | 0 (0.00%) |
| ADDwoR (127) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (0.17%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 60 (10.17%) | 0 (0.00%) | 0 (0.00%) |
| Lateral (19) | 1 (0.17%) | 1 (0.17%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| ADDwR + lateral (60) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 37 (6.27%) | 0 (0.00%) | 0 (0.00%) |
| ADDwoR + lateral (37) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 19 (3.22%) | 0 (0.00%) | 0 (0.00%) |
| Subtotal (590) | 2 (0.34%) | 2 (0.34%) | 1 (0.17%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (0.17%) | 0 (0.00%) | 1 (0.17%) | 1 (0.17%) | 213 (36.10%) | 0 (0.00%) | 0 (0.00%) |
| Total (1180) | 5 (0.42%) | 3 (0.25%) | 4 (0.34%) | 4 (0.34%) | 2 (0.17%) | 0 (0.00%) | 11 (0.93%) | 6 (0.51%) | 4 (0.34%) | 2 (0.17%) | 249 (21.10%) | 1 (0.09%) | 0 (0.00%) |
ADD, anterior disc displacement; woR, without reduction; wR, with reduction.
295 cases × 2 sides = 590 temporomandibular joints.
Table 4.
Accuracy comparison for the Bayesian belief networks [necessary path condition (NPC),29 path condition (PC),30 greedy search-and-score31 with Bayesian information criterion (BIC),32 Chow–Liu tree,33 Rebane–Pearl poly tree,34 tree augmented naïve (TAN) Bayes model,35 maximum log likelihood (ML),36 Akaike information criterion (AIC),37 minimum description length (MDL)38 and K2,39], and the ANN (artificial neural network) using 10-fold cross validation
| Diagnosis | Incorrect cases (%) using 10-fold cross-validation |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NPC | PC | BIC | Chow–Liu | Rabena–Pearl | TAN | ML | AIC | MDL | K2 | ANN | |
| Bone changes | |||||||||||
| Normal (399) | 0 (0.00%) | 0 (0.00%) | 1 (0.17%) | 2 (0.34%) | 25 (4.24%) | 38 (6.44%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Indeterminate (13) | 2 (0.34%) | 2 (0.34%) | 4 (0.68%) | 8 (1.36%) | 4 (0.68%) | 12 (2.03%) | 10 (1.69%) | 5 (0.85%) | 5 (0.85%) | 1 (0.17%) | 0 (0.00%) |
| Concavity (31) | 0 (0.00%) | 0 (0.00%) | 1 (0.17%) | 8 (1.36%) | 10 (1.69%) | 19 (3.22%) | 1 (0.17%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Osteoarthrosis-slight (126) | 1 (0.17%) | 1 (0.17%) | 6 (1.02%) | 8 (1.36%) | 27 (4.58%) | 55 (9.32%) | 1 (0.17%) | 1 (0.17%) | 1 (0.17%) | 2 (0.34%) | 0 (0.00%) |
| Osteoarthrosis-severe (21) | 1 (0.17%) | 1 (0.17%) | 1 (0.17%) | 4 (0.68%) | 5 (0.85%) | 5 (0.85%) | 1 (0.17%) | 1 (0.17%) | 2 (0.34%) | 1 (0.17%) | 0 (0.00%) |
| N/A | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (0.17%) | 1 (0.17%) | 1 (0.17%) | 1 (0.17%) | 0 (0.00%) |
| Subtotal (590) | 4 (0.68%) | 4 (0.68%) | 13 (2.20%) | 30 (5.08%) | 71 (12.03%) | 129 (21.86%) | 14 (2.37%) | 8 (1.36%) | 9 (1.53%) | 5 (0.85%) | 0 (0.00%) |
| Disc displacement | |||||||||||
| Normal (250) | 1 (0.17%) | 1 (0.17%) | 1 (0.17%) | 0 (0.00%) | 0 (0.00%) | 1 (0.17%) | 0 (0.00%) | 1 (0.17%) | 1 (0.17%) | 1 (0.17%) | 0 (0.00%) |
| ADDwR (97) | 1 (0.17%) | 1 (0.17%) | 3 (0.51%) | 1 (0.17%) | 5 (0.85%) | 7 (1.19%) | 2 (0.34%) | 0 (0.00%) | 1 (0.17%) | 1 (0.17%) | 0 (0.00%) |
| ADDwoR (127) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 4 (0.68%) | 5 (0.85%) | 3 (0.51%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Lateral (19) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| ADDwR + lateral (60) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 8 (1.36%) | 3 (0.51%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (0.17%) | 0 (0.00%) |
| ADDwoR + lateral (37) | 0 (0.00%) | 0 (0.00%) | 2 (0.34%) | 0 (0.00%) | 0 (0.00%) | 3 (0.51%) | 3 (0.51%) | 0 (0.00%) | 0 (0.00%) | 1 (0.17%) | 0 (0.00%) |
| N/A | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (0.17%) | 1 (0.17%) | 1 (0.17%) | 1 (0.17%) | 0 (0.00%) |
| Subtotal (590) | 2 (0.34%) | 2 (0.34%) | 6 (1.02%) | 1 (0.17%) | 17 (2.88%) | 19 (3.22%) | 9 (1.53%) | 2 (0.34%) | 3 (0.51%) | 5 (0.85%) | 0 (0.00%) |
| Total (1180) | 6 (0.51%) | 6 (0.51%) | 19 (1.61%) | 31 (2.63%) | 88 (7.46%) | 148 (12.54%) | 23 (1.95%) | 10 (0.85%) | 12 (1.02%) | 10 (0.85%) | 0 (0.00%) |
ADD, anterior disc displacement; N/A, not applicable; woR, without reduction; wR, with reduction.
295 cases × 2 sides = 590 temporomandibular joints.
Figure 1.
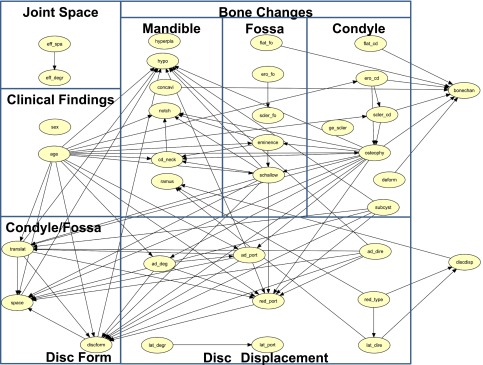
Directed acyclic graph of temporomandibular disorder using the Bayesian belief network path condition algorithm, 35 variables and resubstitution validation. Relationships between findings split into joint space, clinical findings, bone changes, disc displacement, condylar/fossa relation and disc form.
Figure 2.
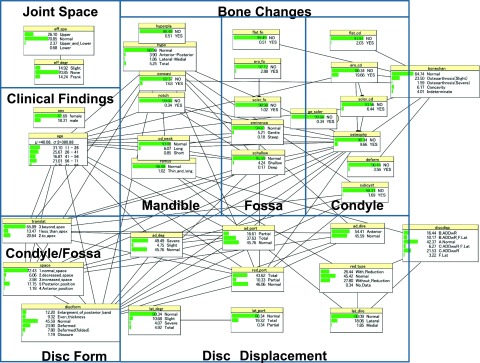
Conditional probability distribution tables superimposed on the directed acyclic graph.
We did not compare the methods using the area under the receiver operating characteristic curve. The results in Tables 3 and 4 were used to determine if the BBN produced comparable results to the MR and ANN, and to investigate the most appropriate BBN technique. The advantage of the BBN is not that it is more accurate, but rather that it gives us more information. In particular, the causal relationships between the radiographic findings and final diagnosis can be graphically represented as a DAG.
Discussion
We decided that BBN PC was suitable for determining the progression using image findings of TMD, because this algorithm was the most accurate using resubstitution validation and 10-fold cross-validation. When using 10-fold cross-validation, we produced 10 training sets and 10 test sets, which produced 10 different DAGs. The accuracies of each of the 10 models were approximately 99%, which implies that there were no differences in the causal relationships between these DAGs. We can interpret this as meaning that there were a sufficient number of data, a sufficient number of cases for each lesion (there were over 10 cases of each diagnosis and large variations within each diagnosis) or sufficient randomization. In our study, 10-fold cross-validation using the PC algorithm and 35 variables did not tend to underfit. We believe that resubstitution did not tend to overfit or overtrain. Therefore, we consider it a suitable validation procedure in this case, because we have a large number of samples that include many lesions (each with a large sample size).
White24 noted that the variables should be independent when using a Bayesian approach, to avoid placing undue emphasis on two or more related findings and degrading performance. Accordingly, our multicollinearity testing showed that the independent variants of all indices were acceptable with no excessive correlations. Cross-validation presents a potential methodological pitfall if some of the findings are related or not independent. For example, a single patient with multiple findings in both the training and test sets represents a leak of information, which can result in overoptimistic performance measures. However, we ensured the independence of independent variants by applying multicollinearity testing to the MR analysis.
The reported accuracies of diagnoses using MR images of TMJs range from 60% to 100% for bony abnormalities and from 73% to 85% for disc position.1 Although the value of MRI when detecting TMJ osseous abnormalities is considered to be limited43 (especially by a single examiner44), MRI poses no radiation threat to patients and can detect 59% of patients with bone changes and 95% with disc displacement.45 In our hospital, multidetector CT or CBCT are not used in routine examinations for TMD. Additionally, surgical interventions such as arthrocentesis, arthroscopy and open surgery are not applied. Therefore a “gold standard” histopathological diagnosis is not performed, unlike oral bone lesions. TMDs are treated relatively conservatively. In this study, 191 TMJs (32.37%) had bone changes and 340 TMJs (57.63%) had disc displacement. These proportions were almost the same as bone changes (29.9%) and disc displacement (58.6%) in 314 TMJs of 157 cases from Tokushima University hospital's TMJ clinic.28
The complex criteria for bone changes and disc displacement given in RDC/TMD will be prohibitive to daily clinical use in the future. Therefore, we have used relatively simple criteria with four levels of bone changes and six types of disc displacement (Tables 1 and 2). Figure 1 shows that bone changes, disc displacement, condyle/fossa relation (condylar translation and bony space), disc form and age have complex influences on each other. Findings among bone changes have complex interrelationships, but the relationships between disc displacement, condyle/fossa relation and disc form are relatively simple.
Joint spaces (upper, lower or both) of effusion relate to the degree of effusion (slight or frank). Spaces and degree of joint effusion do not relate to any other findings. The results of this study did not reveal statistical or significant evidence that joint effusion was an initial finding of TMJ inflammation leading to bone changes and disc displacement. Manfredini et al46 evaluated the link between TMJ effusion and disc displacement using MRI but did not reach a conclusion regarding how non-reducing displacement causes joint effusion. Disc displacement may not be the dominant factor for defining TMJ effusion, meaning that we must consider local or systemic conditions other than the disc–condyle relationship.47 Intra-articular fluid accumulation did not indicate disease progression to more chronic bone changes and disc displacement. Our results did not relate joint effusion to bone changes and disc displacement.
Considering clinical findings, we found that age was related to bone changes, disc displacement, condylar location, bony space and disc form. In Figure 1, a bone change can be directly diagnosed by five main condylar findings (flattening, surface erosion, subcortical sclerosis, osteophyte and deformity), one fossa finding (flattening) and one mandibular contour finding (concavity). These findings conclusively diagnosed bone changes. Subcortical cyst, posterior slope of eminence, shallow fossa, condylar hypoplasia, mandibular angle notch and condylar neck length were related to other fossa and mandibular findings, and indirectly related to a diagnosis of bone changes. Bone changes from surface erosion to the subcortical sclerosis in fossa were not related to other findings. Condylar hyperplasia, mandibular ramus length and generalized sclerosis were not related to other findings.
Figure 1 suggests that a main progression for bone changes may be identified by showing changes in the condyle (surface erosion through subcortical sclerosis to osteophyte) that lead to changes in the articular fossa (posterior slope of eminence to shallow fossa) and then to deformations of the mandible contour (condylar neck length and mandibular angle notch7). Changes in the condyle (surface erosion and osteophyte) are defined as osteoarthritis by RDC/TMD. Subcortical sclerosis and flattening are defined as indeterminate osteoarthrosis by RDC/TMD. We defined new findings (posterior slope of eminence, shallow fossa, condylar neck length and mandibular angle notch) that indicate severe bone changes of the fossa and mandibular contour.
The progression for bone changes from osteophyte, subcortical cyst and shallow fossa to condylar hypoplasia was recognized by the BBN (Figure 1). Condylar hypoplasia is related to bone changes (osteophyte, subcondylar cyst and shallow fossa), disc displacement (direction and portion of anterior disc displacement), condylar translation, bony space, disc form and age. Thus, we determined that this bone change was caused by disc anterior displacement, TMJ movement factors (i.e., bony space/condylar translation and disc form) and ageing. In RDC/TMD, subcortical cysts are used as criteria for osteoarthrosis. In this study, subcortical cysts were directly related to condylar hypoplasia, and severe bone changes of the condyle (osteophyte) and fossa (shallow fossa). Subcortical cysts are thought to be a severe modification of surface erosion. Deformity (which has been thought to be a final stage of osteoarthrosis of the condyle) had no relationship with the main progression in the condyle (surface erosion through subcortical sclerosis to osteophyte). Deformity may represent a static stage in the progression of bone changes, when compared with active stages such as surface erosion to the osteophyte. Changes in the articular fossa (surface erosion to subcortical sclerosis) did not lead to other bone changes. This may be caused by bone changes that occur on the condyle but not in the fossa, in the early stage of the pathological process. Bone changes in the fossa cannot be easily detected using MR images. Generalized sclerosis was not related to other findings. In RDC/TMD generalized sclerosis is used as a criterion for osteoarthrosis. The results in this study do not include generalized sclerosis in the progression of bone changes. However, there were very few cases (0.34%), so we did not have a sufficient amount of detail regarding abnormal bone changes for generalized sclerosis.
Concavity did not indicate any relationship between other bone changes and disc displacement (Figure 1). At the beginning of our study, we included concavity as “indeterminate”. Using the BBN, concavity was misdiagnosed as “normal”. We believe that condylar neck length and mandibular ramus length that were defined as “indeterminate” by the author were considered to be minor, because many findings such as concavity were diagnosed as “normal”. However, by independently considering concavity, the BBN properly diagnosed one type of bone change using simpler logical relationships. Concavity may be an independent diagnostic entity. There may be an unrecognized aetiology of “concavity on the posterior articular bony surface of the condylar head”.48 Mandibular ramus length was not related to any bone changes, but it was related to disc displacement (type of disc reduction and direction of lateral disc displacement). de Leeuw et al5 reported that unilateral disc displacement leads to osteoarthritic degeneration of the condyle and shortening of the mandibular affected side. Our BBN results are consistent with their report. Condylar hyperplasia is not related to other findings. There are no details about the abnormal bone changes of condylar hyperplasia.
In RDC/TMD, “indeterminate” TMJ osteoarthritis is determined by the presence of subcortical sclerosis and/or articular surface flattening, and “osteoarthritis” is defined by the presence of subcortical cyst(s), erosion(s), osteophyte(s) or generalized sclelosis.1 In this study, “indeterminate” osteoarthritis is determined using a simple relational expression formula (a combination of condylar hypoplasia, condylar neck length and mandibular ramus length), and “osteoarthritis” is defined by the presence of surface erosion, osteophyte and deformity (Table 1). The BBN directly diagnosed bone changes using seven variables: three indeterminate findings (flattening of condyle and fossa and subcortical sclerosis), two osteoarthritis findings (surface erosion and osteophyte) and two other findings (deformity and concavity). Indeterminate condylar hypoplasia, condylar neck length and mandibular ramus length for TMJ osteoarthritis (by the author's definition) were not related to a diagnosis. Three indeterminate findings (flattening of condyle and fossa, and subcortical sclerosis) from RDC/TMD statistically agreed with our definition of “indeterminate”. Two findings (surface erosion and osteophyte) of slight “osteoarthritis” (by our definition) were related to the same definition from RDC/TMD.
In Figure 1, the direction, portion and degree of anterior displacement relates to the portion of reduction. The degree of lateral displacement is related to the portion of lateral displacement. But these six findings were not directly related to the disc displacement diagnosis. The type of reduction was related to the direction of lateral displacement. These two findings were conclusive for disc displacement. Direction, portion and degree of anterior displacement and portion of reduction had a direct relationship with disc form (Figure 1). These four findings for disc displacement are the first factors that relate to disc form. Findings concerning disc displacement can be split into two groups according to their relationship to disc form or a diagnosis of disc displacement. In RDC/TMD, the degree and portion of anterior displacement, and portion of reduction (Table 2) are not defined as DC for disc displacement. The BBN revealed a direct relationship between disc form and these disc displacement findings but did not find a relationship between lateral disc displacement (portion and degree of lateral displacement) and other disc displacement and disc form findings.
Figure 1 shows the relationships between bone changes, clinical findings, condyle/fossa relation (condylar translation and bony space) and disc form. Osteophyte, subcortical cyst, shallow fossa, condylar hypoplasia of bone change findings and age are secondary factors that relate to disc form. Age relates to condylar translation and bony space. These three findings (age, condylar translation and bony space) are tertiary factors that relate to disc form. Condylar translation and bony space relate to osteophyte, subcortical cyst, shallow fossa, condylar hypoplasia and age. These factors were the same as the secondary factor related to disc form. Many types of disc forms were formed by combining disc displacement, bone changes, condyle/fossa relation (condylar translation and bony space) and age. Yano et al8 investigated the relationship between the appearance of double contours on the mandibular condyle, and changes in the articular disc position after splint therapy. These adaptive changes are thought to result from the displaced articular disc, which directly relates morphology to function. Busato et al9 showed that a lack of synchronous movement between disk and condyle and dislodgment of the condyle in the glenoid fossa relate to morphological changes of the condyle and articular eminence (fossa). Interarticular space reduction or enlargement and disk derangement are not associated with disk and condylar incoordination. Our results show that disc displacement (direction, portion and degree of anterior displacement, portion and type of reduction, and direction of lateral displacement) directly relates to severe bone changes (osteophyte, subcondylar cyst, shallow fossa, condylar hypoplasia and mandibular ramus length). Early bone changes were not directly related to disc displacement. Our results may not show the first cause of TMD to be disc displacement or TMJ morphological bone changes, even though TMJ movement factors (i.e., bony space/condylar translation, and disc form) and age certainly mediate both of those phenomena (bone changes and disc displacement).
This study focused on analysing relationships between MRI findings to determine the progression of TMD, rather than on providing answers to existing clinical concerns. The added benefit of our approach is not presented in the context of the existing standard of care, and this study has limited value because the reproducibility of our interpretation cannot be statistically evaluated, and MRI may not be the best imaging modality to assess bone changes. The study would be more valuable if clinical symptoms and MRI findings are correlated. TMDs are comprehensive and multifactor-related diseases and are subjects of ongoing investigation.
Conclusion
In this study, we investigated the applicability of a BBN to MRI diagnoses of TMD in an attempt to determine the progression of TMDs. We particularly focused on how bone changes, disc displacement, condylar translation, bony space and disc form affect each other. We used a BBN PC algorithm with resubstitution and 10-fold cross-validation, which was >99% accurate. Our results suggest that osteoarthritic bone changes progress from condyle to articular fossa, and finally to a mandibular bone contour. TMJ movement factors (condylar translation, bony space and disc form) and age mediate between bone changes and disc displacement.
References
- 1.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examination and specifications, critique. J Craniomandib Disord 1992; 6: 301–55. [PubMed] [Google Scholar]
- 2.Classification of TMD (verification committee for the classification of TMJ disorder and RDC/TMD by Japanese Society for Temporomandibular Joints). J Jpn Soc TMJ 2013; 25: 177–82. [Google Scholar]
- 3.Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache 2014; 28: 6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schellhas KP, Piper MA, Omlie MR. Facial skeleton remodeling due to temporomandibular joint degeneration: an imaging study of 100 patients. Cranio 1992; 10: 248–59. [DOI] [PubMed] [Google Scholar]
- 5.de Leeuw R, Boering G, Stegenga G, de Bont LG. Radiographic signs of temporomandibular joint osteoarthrosis and internal derangement 30 years after non-surgical treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995; 79: 382–92. [DOI] [PubMed] [Google Scholar]
- 6.Gidarakou IK, Tallents RH, Kyrkanides S, Stein S, Moss ME. Comparison of skeletal and dental morphology in asymomatic volunteers and symptomatic patients with bilateral disk displacement without reduction. Angle Orthod 2004; 74: 684–90. [DOI] [PubMed] [Google Scholar]
- 7.Kambylafkas P, Kyrkanides S, Tallents RH. Mandibular asymmetry in adult patients with unilateral degenerative joint disease. Angle Orthod 2005; 75: 305–10. [DOI] [PubMed] [Google Scholar]
- 8.Yano K, Nishikawa K, Sano T, Okano T. Relationship between appearance of a double contour on the mandibular condyle and the change in articular disc position after splint therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009; 108: e30–4. doi: 10.1016/j.tripleo.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 9.Busato A, Vismara V, Bertele' L, Zollino I, Carinci F. Relation between disk/condyle incoordination and joint morphological changes: a retrospective study on 268 TMJs. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 110: e34–40. doi: 10.1016/j.tripleo.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Bertram S, Moriggl A, Rudisch A, Emshoff R. Structural characteristics of bilateral temporomandibular joint disc displacement without reduction and osteoarthrosis are important determinants of horizontal mandibular and vertical ramus deficiency: a magnetic resonance imaging study. J Oral Maxillofac Surg 2011; 69: 1898–904. doi: 10.1016/j.joms.2010.12.026 [DOI] [PubMed] [Google Scholar]
- 11.Sox HC, Higgins MC, Owens DK. Understanding new information: Bayes' theorem. In: Medical decision making. Hoboken, NJ: Wiley-Blackwell; 2013. pp. 61–92. [Google Scholar]
- 12.Ledley RS, Lusted LB. Reasoning foundations of medical diagnosis; symbolic logic, probability, and value theory aid our understanding of how physicians reason. Science 1959; 130: 9–21. [DOI] [PubMed] [Google Scholar]
- 13.Warner HR, Toronto AF, Veasey LG, Stephenson R. A mathematical approach to medical diagnosis. Application to congenital heart disease. JAMA 1961; 177: 177–83. [DOI] [PubMed] [Google Scholar]
- 14.Lodwick GS, Haun CL, Smith WE, Keller RF, Robertson ED. Computer diagnosis of primary bone tumors. A preliminary report. Radiology 1963; 80: 273–5. [Google Scholar]
- 15.Collen MF, Rubin L, Neyman J, Dantzig GB, Baer RM, Seigelaub AB. Automated multiphase screening and diagnosis. Am J Public Health Nations Health 1964; 54: 741–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lodwick GS. A probabilistic approach to the diagnosis of bone tumors. Radiol Clin North Am 1965; 3: 487–97. [PubMed] [Google Scholar]
- 17.Wellwood JM, Spiegelhalter DJ. Computer and the diagnosis of acute abdominal pain. Br J Hosp Med 1989; 41: 564–7. [PubMed] [Google Scholar]
- 18.Lodwick GS, Turner AH, Jr, Lusted LB, Templeton AW. Computer-aided analysis of radiographic images. J Chronic Dis 1966; 19: 485–96. [DOI] [PubMed] [Google Scholar]
- 19.Lejbkowicz I, Wiener F, Nachtigal A, Militiannu D, Kleinhaus U, Applbaum YH. Bone browser a decision-aid for the radiographical diagnosis of bone tumors. Computer Methods Programs Biomed 2002; 67: 137–54. [DOI] [PubMed] [Google Scholar]
- 20.Burnside ES, Davis J, Chhatwal J, Alagoz O, Lindstrom MJ, Geller BM, et al. Probabilistic computer model development from clinical data in national mammography database format to classify mammographic findings. Radiology 2009; 251: 663–72. doi: 10.1148/radiol.2513081346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stojadinovic A, Peoples GE, Libutti SK, Henry LR, Eberhardt J, Howard RS, et al. Development of a clinical decision model for thyroid nodules. BMC Surg 2009; 9: 12. doi: 10.1186/1471-2482-9-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiener F, Laufer D, Ribak A. Computer-aided diagnosis of odontogenic lesions. Int J Oral Maxillofac Surg 1986; 15: 592–6. [DOI] [PubMed] [Google Scholar]
- 23.Abby LM. An expert system for oral diagnosis. J Dent Educ 1987; 51: 475–80. [PubMed] [Google Scholar]
- 24.White SC. Computer-aided differential diagnosis of oral radiographic lesions. Dentomaxillofac Radiol 1989; 18: 53–9. [DOI] [PubMed] [Google Scholar]
- 25.Huber JS, Manson-Hing LR, Heaven T. COMRADD: computerized radiographic differential diagnosis. Oral Surg Oral Med Oral Pathol 1990; 69: 263–5. [DOI] [PubMed] [Google Scholar]
- 26.Seigel MA, Firrolo FJ, Finkelsteio MW, Computer application in oral diagnosis. Dent Clin North Am 1993; 37: 113–31. [PubMed] [Google Scholar]
- 27.White SC. Decision-support system in dentistry. J Dent Educ 1996: 61: 47–63. [PubMed] [Google Scholar]
- 28.Matsumoto M, Ishikawa T, Takeuchi H, Fujisawa K, Hosoki H, Hada M, et al. Correlation between magnetic resonance image evaluation and clinical findings in patients with temporomandibular disorder. [In Japanese.] Sikoku Dental Res 2010; 22: 167–72. [Google Scholar]
- 29.Steck H, Tresp V. Bayesian belief networks for data mining, proceedings of the second workshop on data mining und data warehousing as the basis of modern decision support systems DWDW99, Sammelband. Magdeburg, Germany: Magdeburg University; 1999. [Google Scholar]
- 30.Spirtes P, Glymour C, Scheines R. The PC algorithm. In: Causation, prediction, and search. 2nd edn. Cambridge, MA: MIT Press; 2000. pp. 84–90. [Google Scholar]
- 31.Cormen TH, Leiserson CE, Riverst RL, Steine C. Chapter 16 greedy algorithm. In: Introduction to algorithms. 3rd edn. Cambridge, MA: The MIT Press; 2009. pp. 414–50. [Google Scholar]
- 32.Schwarz G. Estimating the dimension of a model. Ann Stat 1978; 6: 461–4. [Google Scholar]
- 33.Chow CK, Liu CN. Approximating discrete probability distribution with dependence trees. IEEE Trans Inf Theory 1968; 14: 462–7. [Google Scholar]
- 34.Rebane G, Pearl J. The recovery of causal poly-tree from statistical data. Proceedings of the third conference on uncertainty in artificial intelligence; 10–12 July 1987; Seattle, WA, Corvallis, OR: AUAI Press, 1987.
- 35.Friedman N, Geiger D, Goldszmidt M. Tree-augmented naïve Bayes or tree-augmented network, Mach Learn 1997; 29: 131–63. [Google Scholar]
- 36.Aldrich J.RA. Fisher and the making of maximum likelihood 1912–1922. Stat Sci 1997; 12: 162–76. [Google Scholar]
- 37.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr 1974; 19: 716–23. [Google Scholar]
- 38.Gründal PD. The minimum description length principle. 1st edn. Cambridge, MA: MIT Press; 2007.
- 39.Cooper GF, Herskovits E. A Bayesian method for induction of probabilistic network from data. Mach Learn 1992; 9: 309–47. [Google Scholar]
- 40.Quinlan JR. C4.5: programs for machine learning. San Francisco, CA: Morgan Kaufman Publishers, Inc.; 1993. [Google Scholar]
- 41.Lauritzen SL. The EM algorithm for graphical association models with missing data. Comput Stat Data Anal 1995; 19: 191–201. [Google Scholar]
- 42.Refaeilzadeh P, Tang L, Liu H. Cross-validation. In: Ling L, Tamer ÖM, eds. Encyclopedia of database systems. Berlin, Germany: Springer Verlag; 2009. pp. 532–8. [Google Scholar]
- 43.Alkhader M, Ohbayashi N, Tetsumura A, Nakamura S, Okochi K, Momin MA, et al. Diagnostic performance of magnetic resonance imaging for detecting osseous abnormalities of the temporomandibular joint and its correlation with cone beam computed tomography. Dentomaxillofac Radiol 2010; 39: 270–6. doi: 10.1259/dmfr/25151578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Widmalm SE, Brooks SL, Sano T, Upton LG, McKay DC. Limitation of the diagnostic value of MR images for diagnosing temporomandibular joint disorders. Dentomaxillofac Radiol 2006; 35: 334–8. [DOI] [PubMed] [Google Scholar]
- 45.Ahmad M, Hollender L, Anderson Q, Kartha K, Ohrback R, Truelove EL, et al. Research diagnostic criteria for temporomandibular disorders (RDC/TMD): development of image analysis criteria and examiner reliability for image analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009; 107: 844–60. doi: 10.1016/j.tripleo.2009.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manfredini D, Basso D, Arboretti G, Guarda-Nardini L. Association between magnetic resonance signs of temporomandibular joint effusion and disk displacement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009; 107: 266–71. doi: 10.1016/j.tripleo.2008.03.033 [DOI] [PubMed] [Google Scholar]
- 47.Orlando B, Chiappe G, Landi N, Bosco M. Risk of temporomandibular joint effusion related to magnetic resonance imaging signs of disc displacement. Med Oral Patol Oral Cir Bucal 2009; 14: E188–93. [PubMed] [Google Scholar]
- 48.Uemura S, Iwasaki H, Hosoki H, Iwamoto M, Takagi Y, Sato I, et al. Adaptive bony changes in the functional temporomandibular joints—a consideration of the concavity on the posterior articular bony surface of the condylar head. [In Japanese.] Dental Radiol 1990; 30: 211–18. [Google Scholar]


