Abstract
Objective:
To evaluate whether switching bipolar radiofrequency ablation (SB-RFA) using three internally cooled wet (ICW) electrodes can induce coagulations >5 cm in porcine livers with better efficiency than consecutive monopolar (CM) or switching monopolar (SM) modes.
Methods:
A total of 60 coagulations were made in 15 in vivo porcine livers using three 17-gauge ICW electrodes and a multichannel radiofrequency (RF) generator. RF energy (approximately 200 W) was applied in CM mode (Group A, n = 20) for 24 min, SM mode for 12 min (Group B, n = 20) or switching bipolar (SB) mode for 12 min (Group C, n = 20) in in vivo porcine livers. Thereafter, the delivered RFA energy, as well as the shape and dimension of coagulations were compared among the groups.
Results:
Spherical- or oval-shaped ablations were created in 30% (6/20), 85% (17/20) and 90% (18/20) of coagulations in the CM, SM and SB groups, respectively (p = 0.003). SB-RFA created ablations >5 cm in minimum diameter (Dmin) in 65% (13/20) of porcine livers, whereas SM- or CM-RFA created ablations >5 cm in only 25% (5/20) and 20% (4/20) of porcine livers, respectively (p = 0.03). The mean Dmin of coagulations was significantly larger in Group C than in Groups A and B (5.1 ± 0.9, 3.9 ± 1.2 and 4.4 ± 1.0 cm, respectively, p = 0.002) at a lower delivered RF energy level (76.8 ± 14.3, 120.9 ± 24.5 and 114.2 ± 18.3 kJ, respectively, p < 0.001).
Conclusion:
SB-RFA using three ICW electrodes can create coagulations >5 cm in diameter with better efficiency than do SM- or CM-RFA.
Advances in knowledge:
SB-RFA can create large, regular ablation zones with better time–energy efficiency than do CM- or SM-RFA.
Radiofrequency (RF) tumour ablation is increasingly being utilized as an alternative option in patients with unresectable primary and secondary liver malignancies.1,2 In the treatment of small hepatocellular carcinomas (HCCs), RF ablation (RFA) has been shown to yield satisfactory local tumour control, with one study pathologically demonstrating complete tumour necrosis in 83% of HCCs <3 cm.3 Indeed, according to the recent Barcelona Clinic Liver Cancer staging and treatment strategy guidelines for HCCs, RFA is favoured over surgical resection for very early stage HCCs (single nodule <2 cm) in patients with Child–Pugh A liver cirrhosis.4 Furthermore, a recent systematic review paper by Cucchetti et al5 reported that for very early HCCs (single nodule <2 cm) in Child–Pugh Class A patients, RFA provided similar life expectancy and quality-adjusted life expectancy at a lower cost than did surgical resection.
However, for single HCCs 3–5 cm in diameter, resection was shown to provide better life expectancy and to be more cost effective than RFA owing to high local tumour progression rates after RFA.5–12 This is in large part owing to the limited ability of currently available RFA devices in creating a sufficiently large ablation zone encompassing HCCs 3–5 cm in diameter along with a safety margin.7,11,13,14 Therefore, an ideal RFA system would provide the capability to create coagulations >5 cm in short-axis diameter within a reasonable time frame (<30 min) for the treatment of tumours >3 cm in diameter considering a sufficient safety margin (5–10 mm in thickness). Currently, multiple overlapping ablations are often used for the treatment of liver tumours >2 cm in order to cover the complete tumour volume as well as to create a 1-cm-thick peripheral ablation margin.15,16 However, there is considerable technical difficulty in probe repositioning during overlapping ablations, especially under ultrasound guidance, owing to gas bubble formations, ultimately resulting in incomplete ablations.17–19
Recently, multiple-electrode RFA approaches, including the switching monopolar (SM) mode, bipolar mode and multipolar mode, have been attempted with each demonstrating efficiency in creating a larger ablation zone in liver tissue than in the standard monopolar RF technique.2,20–26 Theoretically, RFA in switching bipolar (SB) mode using multiple electrodes should further improve the thermal and electronic efficiency of RFA devices compared to conventional monopolar modes. However, until now, the efficacy of SB-RFA with internally cooled wet (ICW) electrodes, which allow simultaneous internal cooling and saline infusion, in creating 3- to 5-cm coagulation areas, have not been tested in previous in vivo studies.
Therefore, the purpose of this study was to evaluate whether SB-RFA using three ICW electrodes can induce coagulations >5 cm in diameter in porcine livers with better efficiency than consecutive monopolar (CM) or SM mode.
METHODS AND MATERIALS
This study received technical support and was partially funded by a research grant from RF Medical Co. (Seoul, Republic of Korea). All authors had full control of all data and information submitted for publication at all times.
Development of a switching bipolar radiofrequency ablation unit
Recently, a multichannel RF system (M-3004; RF Medical Co., Seoul, Republic of Korea) allowing automatic switching of RF energy among the three electrodes according to impedance changes in either monopolar or bipolar modes was developed.20 The multiRFA system includes a generator with a maximum power of 200 W at a frequency of 400 kHz and provides three separate channels for the three electrodes. It can be used in either SM or bipolar mode to heat tissue in which power is applied in alternating fashion to the three electrodes (Figure 1a). In monopolar mode, the RF system allows continuous monitoring of impedance between the active portion of the electrode and the dispersive electrode (grounding pads). The RF power is controlled by impedance changes during RFA, and the duty cycle between the electrodes is automatically controlled by continuously measured impedance values. Maximal power is switched at approximately 30 s if there is no impedance rise >40% above the baseline value. However, if the impedance of one of the electrodes rises 40% above the baseline value, the current is reduced by 10% and switched to the other electrode automatically.21,27
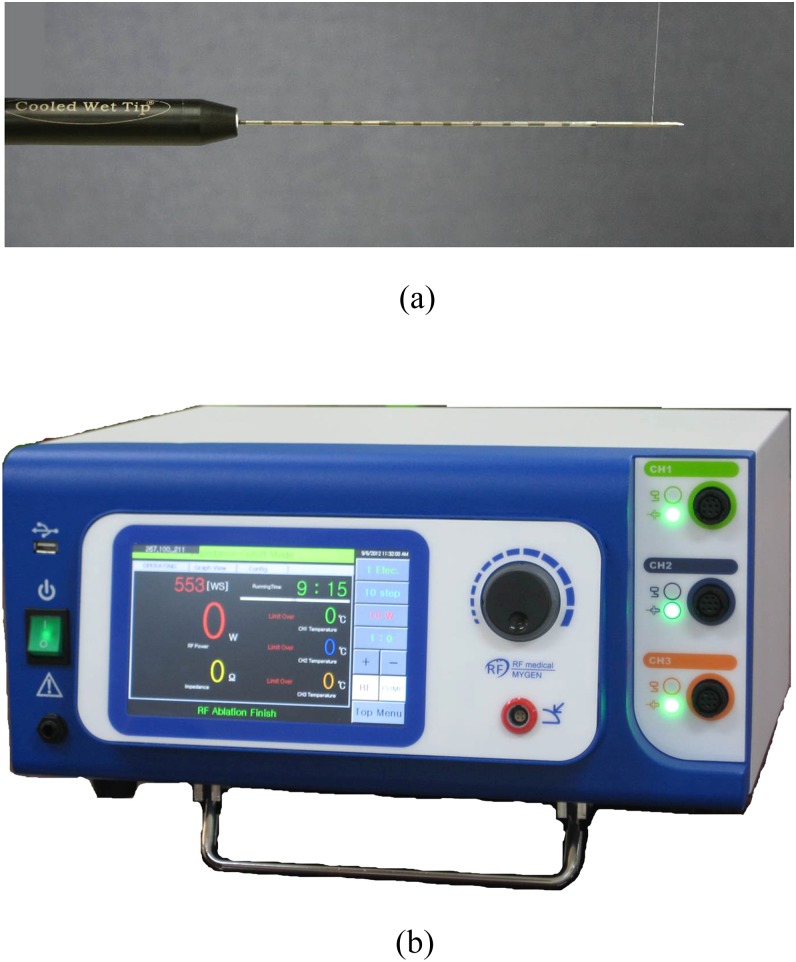
Figure 1.
(a) Photograph of an internally cooled wet electrode with a hole in the active tip. (b) A prototype three-channel radiofrequency ablation system, which allows the use of multiple electrodes in either switching monopolar or bipolar modes.
A three 17-gauge ICW electrode (RF Medical Co.) RFA system with a 3-cm exposed tip was used in this study (Figure 1b). The internal structure of the exposed tip of the ICW electrode was identical to that of a conventional internally cooled electrode except that the former contains two tiny (0.03-mm) side holes.28 Approximately, 99% of the chilled 0.9% isotonic saline was used for cooling, and 1% was used for infusion, at rates of 1.2 and 1.4 ml min−1, respectively.29 A peristaltic pump (RFP-300) was used to infuse a normal saline solution at 5–10 °C into the lumen of the three ICW electrodes at a rate sufficient enough to maintain a tip temperature of 10–25 °C.
Animals, anaesthesia and surgery
Approval for this protocol was obtained from the Animal Use and Care Administrative Advisory Committee of Seoul National University Hospital. All experiments were performed in accordance with the general guidelines issued by the National Institute of Health (NIH) of the USA for the care of laboratory animals. 15 domestic male pigs (mean weight, 65 kg; range, 60–70 kg) were anaesthetized using an intramuscular injection of 50 mg kg−1 of ketamine hydrochloride (Ketamine; Yuhan, Seoul, Republic of Korea) and 5 mg kg−1 of Xylazine (Rumpun; Bayer Korea Ltd, Ansan, Republic of Korea) and prepared for surgery. Thereafter, endotracheal intubation was performed and anaesthesia was maintained with inhaled isoflurane (1–4% IsoFlo®; Abbott Laboratories, North Chicago, IL). The pigs were then placed in the supine position and prepared at the midline and draped.
One of the three operators (SW, WSC and EJH with assistance from two technicians) performed the ablation procedures by means of open laparotomy through a midline incision, and one ablation lesion was created in each lobe of each animal. Therefore, a total of 60 ablation lesions were created in the 15 animals. After creating four ablation zones in the liver of each pig, the liver was allowed to be cooled to body temperature, and the incision was closed using non-absorbable sutures.
Radiofrequency ablation protocol
Experiments were performed with the multichannel RF generator system (M-3004; RF Medical Co.) and three ICW electrodes (RF Medical Co.). As the goal of the RFA procedure was to create coagulations >5 cm in minimum diameter (Dmin), RF energy (power output, 200 W) was applied in CM mode (Group A, n = 20) for 24 min in in vivo porcine livers, while RF energy was applied in SM mode (Group B; n = 20) or SB mode for 12 min (Group C; n = 20) each, based on our preliminary study results on ex vivo bovine livers.
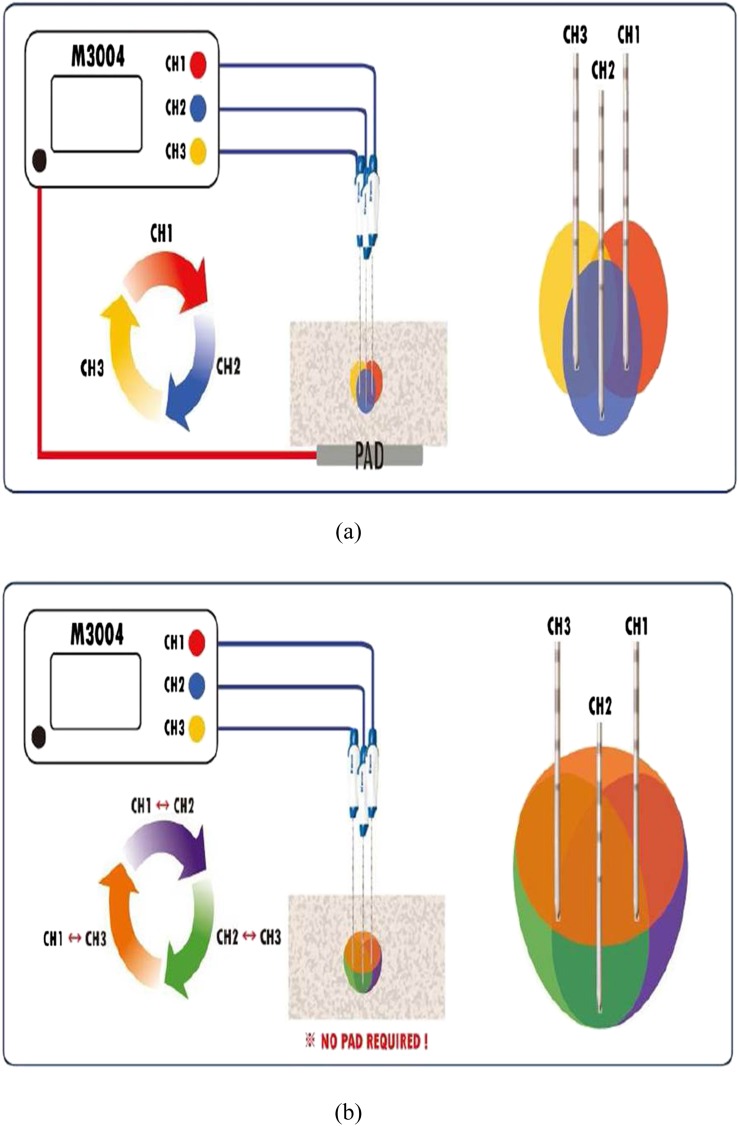
The location of the ablation lesions in the liver lobes was randomized, in order to minimize the bias from any potential variation of segmental perfusion. To avoid the influence of the increased temperature in the liver tissue, a minimum distance of 7 cm between ablation zones was maintained. RF electrodes were placed to ensure that the entire ablation zone would be within the liver parenchyma. Based on the results of our preliminary ex vivo study and considering the influence of tissue perfusion in an in vivo setting, the three cluster electrodes were placed in a triangular array with equidistant interprobe spacing of 2.5 cm using an acrylic puncture aid with multiple holes 3 mm in diameter at 5-mm intervals from the centre up to 4 cm. Ablation lesions were formed by placing the RF electrodes in the pig liver under visual guidance, and after insertion of the probes into the liver, ultrasound (7.5-MHz linear transducer; SonoAce 5500; Medison, Seoul, Republic of Korea) was used to guide and further position the probe away from vessels >5 mm in diameter. In CM mode, RF energy was consecutively applied to each of the three internally cooled electrodes for 8 min by changing the current flow to the second probe just after the ablation with the first probe. In SM mode, the RF energy was applied to one of the three ICW electrodes, and RF energy delivery was automatically changed among the three electrodes depending on impedance changes (Figure 2a). In SB mode, the RF energy was applied to a pair of the three ICW electrodes, and according to impedance changes, RF energy delivery was automatically changed between the three pairs of the three electrodes (Figure 2b). In CM and SM modes, RF power was manually increased to 200 W, and RF energy was applied consecutively or alternately to the electrode so that current flows from one electrode to the dispersive metallic pads. In SB mode, RF current flowed from one electrode to the other, and RF energy was applied alternately to the pair of electrodes.24 The applied current, power output and impedance were continuously monitored by the generator during RFA and were recorded automatically using a computer program.
Figure 2.
Diagrams showing the radiofrequency (RF) energy delivery protocol in switching monopolar (SM) mode and switching bipolar (SB) mode. (a) In SM mode, RF energy was applied to one of the three internally cooled wet (ICW) electrodes, and RF energy delivery was automatically changed among the three electrodes depending on impedance changes. (b) In SB mode, RF energy was applied to a pair of the three ICW electrodes, and according to impedance changes, RF energy delivery was automatically changed between the three pairs of three electrodes. Note that there is no grounding pad in RF current circuitry. CH, change.
Assessment of coagulation necrosis
The 15 pigs were euthanized with intravenous pentobarbital 1 h after closing the incision of the abdominal wall on the day of the procedure, and immediately after sacrificing the animals, the liver was removed en block. Segments of the livers containing RF-induced coagulations were sliced perpendicular to the electrode tracks (transverse plane) at 5–7 mm intervals, and then the slices were cut in the longitudinal plane containing one of the three electrode tracks by one of the four authors. To assess cell viability, as indicated by mitochondrial enzyme activity, the specimens obtained were stained by incubating representative tissue sections for 30 min in 2% 2,3,5-triphenyl tetrazolium chloride (TTC) (Sigma-Aldrich, St Louis, MO) at 20–25 °C,30,31 which was used to determine irreversible cellular injury during the early stages of RF-induced necrosis.19 In addition, after TTC staining, the slices were photographed using a digital camera (Canon EOS 300D; Canon Inc., Tokyo, Japan), and the images were saved to an image management software program (Photoshop®; Adobe®, San Jose, CA). All specimens containing the ablation lesions were independently examined in blinded fashion using NIH ImageJ (http://rsb.info.nih.gov) by two of the authors (JML and WSC, with >15 years' and >4 years' experience, respectively, in evaluating coagulation diameters). Using an electronic caliper, the observers measured the Dmin and the maximum diameter (Dmax) of the central, white region of the RF-induced coagulation zones in slices showing the maximal coagulation diameter along the transverse plane. The vertical axis diameter (Dv) along the electrode insertion axis was measured in the longitudinal plane. In addition, the effective volume of the ablation zone was determined as follows: 4/3π (Dmin/2)3.6 If it was central, white regions of the RF-induced coagulation zones around the electrodes were separated, then the diameters and volume of the largest region among the separated coagulation zones were calculated.
In order to analyse the shape of the ablation zone, the ablation lesions were evaluated by the isoperimetric ratio of each lesion in the most representative slice, using NIH ImageJ software.19 The closer the isoperimetric ratio to one, the more circular the lesion shape. In addition, to compare the configuration of the ablation zones in each group, the ratio of Dmin/Dmax was calculated. We also assessed whether the coagulation was confluent (round or oval shaped), partially confluent (clover or maple leaf shaped) or separated.32 The RFA zones of all cases were fixed in 10% formalin for routine histological processing and were finally processed by paraffin sectioning and haematoxylin–eosin staining.
Statistical analysis
The results of quantitative measurements of ablation lesions and electrical parameters were reported as mean ± standard deviations. The prevalence of round- or oval-shaped lesions in each group was also compared using the χ2 test. Parameters were tested using the Kolmogorov–Smirnov test for normality: the dimensions, areas, volume and effective volume of the ablation zones in the three groups were compared using the one-way analysis of variance test followed by the post hoc Tukey–Kramer test for group-to-group comparison. For all statistical analyses, a p-value of <0.05 was considered statistically significant. Statistical analyses were performed using commercially available software (MedCalc; MedCalc Software, Ostend, Belgium).
RESULTS
Electrical measurement of the three modes
The mean impedance of each RF energy delivery mode in Groups A, B and C were 57.9 ± 7.2, 62.7 ± 12.7 and 61.3 ± 11.9 Ω, respectively, with no significant differences between the groups (p = 0.394, Table 1). The mean delivered RF energy for Group C (76.8 ± 14.3 kJ) was significantly less than that of Group A (120.9 ± 24.5 kJ) and Group B (114.2 ± 18.3 kJ; p < 0.001). In addition, the average delivered RF energy during the procedure in Groups A, B and C were 84 ± 17, 158.7 ± 25.4 and 106.68 ±19.9 W, respectively (p < 0.001).
Table 1.
Measured values of technical parameters and shapes of coagulation necrosis according to radiofrequency power application modes
| Parameters | Group A Consecutive mode RFA (n = 20) |
Group B Switching monopolar RFA (n = 20) |
Group C Switching bipolar RFA (n = 20) |
p-valuea |
p-value A vs B |
p-value A vs C |
p-value B vs C |
|---|---|---|---|---|---|---|---|
| Technical parameters | |||||||
| Total delivered energy (kJ) | 120.9 ± 24.5 | 114.2 ± 18.3 | 76.8 ± 14.3 | <0.001 | 0.728 | <0.001 | <0.001 |
| Delivered energy per minute | 5.3 ± 1.1 | 6.5 ± 1.3 | 9.46 ± 1.4 | <0.001 | <0.001 | 0.006 | <0.001 |
| Average Watt | 84 ± 17 | 158.7 ± 25.4 | 106.7 ± 19.9 | <0.001 | <0.001 | 0.006 | <0.001 |
| Impedance (W) | 57.9 ± 7.2 | 62.7 ± 12.7 | 61.3 ± 11.9 | 0.394 | 1.000 | 0.550 | 1.000 |
| Qualitative analysis of coagulation necrosis | |||||||
| Confluent necrosis | 30% (6/20) | 85% (17/20) | 90% (18/20) | 0.0002b |
|||
| Round shape | 0 | 3 | 8 | ||||
| Oval shape | 6 | 14 | 10 | ||||
| Partial confluent necrosis | 55% (11/20) | 15% (3/20) | 10% (2/20) | ||||
| Clover shape | 10 | 2 | 1 | |
|||
| Maple leaf shape | 1 | 1 | 1 | ||||
| Separated necrosis | 15% (3/20) | 0% (0/20) | 0% (0/20) | ||||
| Quantitative analysis of coagulation necrosis | |||||||
| Circularity (isometric ratio) | 0.69 ± 0.12 | 0.82 ± 0.08 | 0.85 ± 0.08 | <0.001 | <0.001 | <0.001 | 0.758 |
| Dmin : Dmax ratio | 0.67 ± 0.19 | 0.79 ± 0.15 | 0.86 ± 0.13 | 0.002 | 0.057 | 0.002 | 0.643 |
Dmax, maximum diameter of the ablation zone; Dmin, minimum diameter of the ablation zone; Dv, vertical diameter of the ablation zone; RFA, radiofrequency ablation.
Values are mean ± standard deviation.
p-values indicate the statistical difference among Groups A–C.
χ2 test results indicating the difference between the three groups.
Analysis of the shape of ablation lesions
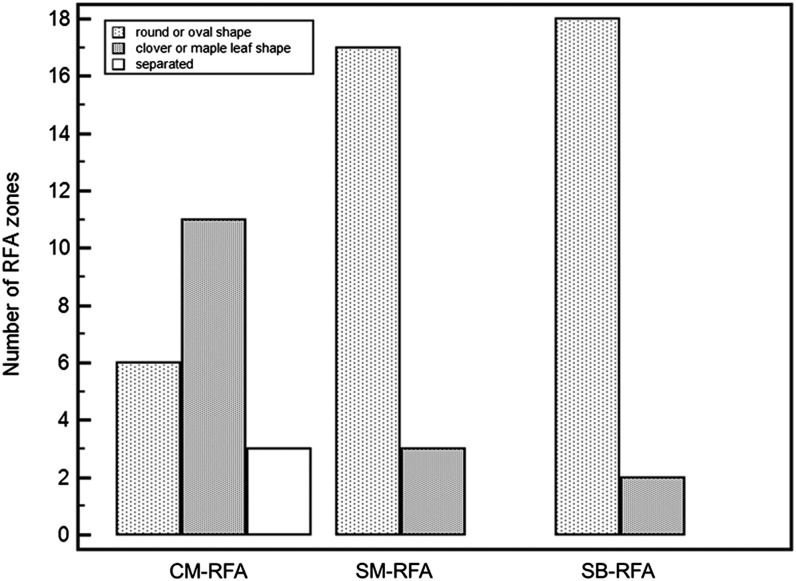
Groups B and C created 17 and 18 circular- or oval-shaped ablation lesions, respectively, whereas Group A created six circular- or oval-shaped ablation lesions (Table 1). Groups B and C showed significantly larger numbers of confluent ablation lesions than did Group A (p = 0.003, Figure 3). In addition, three separated ablation lesions were observed in Group A, whereas none of the ablation lesions was separated in Groups B and C. On quantitative measurement, the mean ratio of Dmin : Dmax of ablation lesions and the mean circularity (isometric ratio) were substantially higher in Groups B and C than in Group A (Table 1).
Figure 3.
Graph showing the distribution of coagulation necrosis shapes in the three groups according to the radiofrequency (RF) energy delivery mode. The x-axis indicates different RF energy delivery modes, and the y-axis indicates the number of RF ablation zones. Ablation zones were categorized into three shapes: round or oval shaped; partially confluent (clover leaf or maple leaf shaped); and separated. CM, consecutive monopolar; RFA, radiofrequency ablation; SB, switching bipolar; SM, switching monopolar.
Quantitative measurement of the size of coagulation necroses
RFA in SB mode for 12 min using three ICW electrodes in Group C created significantly larger effective ablation volume than those created with CM ablation for 24 min in Group A (72.8 ± 36.4 vs 39.0 ± 27.7, respectively, p = 0.002, Figure 4). At the slice showing the largest ablation zone, the mean Dmin and the mean areas of coagulations in Group C were substantially larger than those of Groups A and B (Table 2). However, there were no significant differences in effective ablation volume between Groups A and B (p = 0.49) and B and C (p = 0.062).
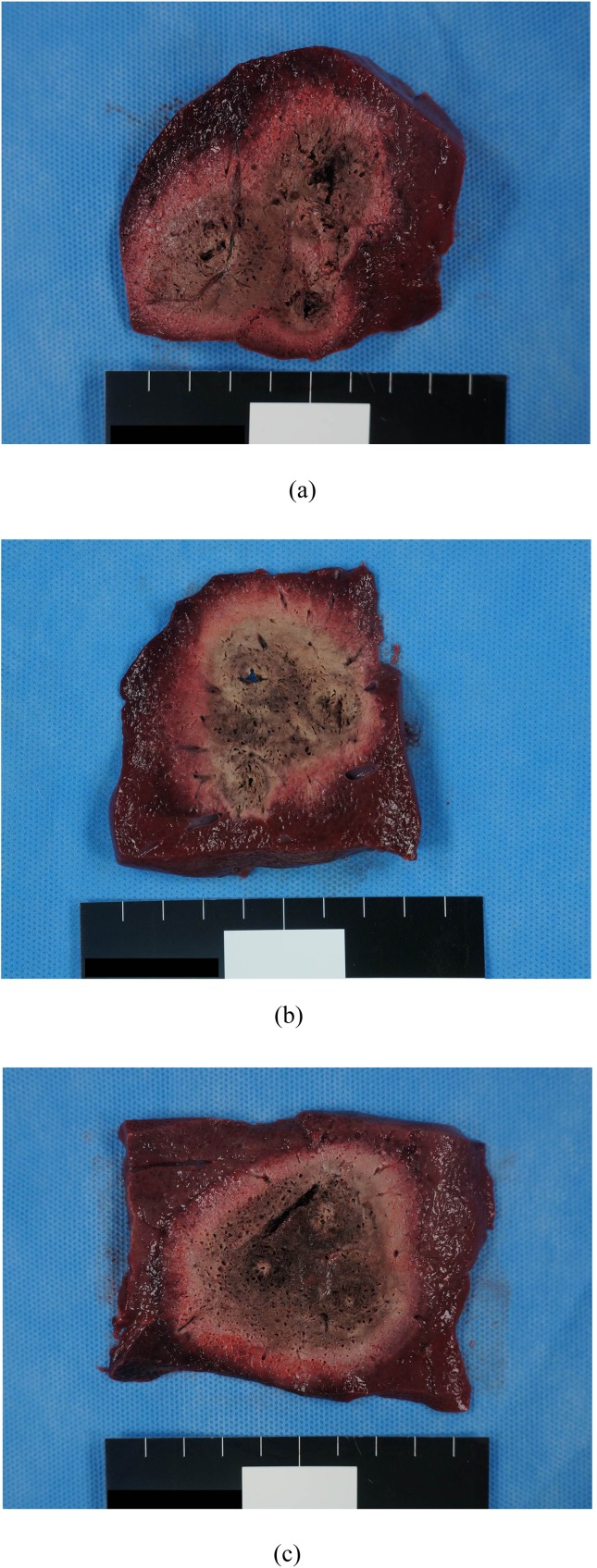
Figure 4.
Comparison of the radiofrequency (RF)-induced coagulations of the consecutive monopolar (CM), switching monopolar (SM) and switching bipolar (SB) groups. (a) Photograph of the cut surface of the coagulation created by CM-RF ablations demonstrates a 3.5 × 6.9-cm-sized, confluent ablation zone with an irregular shape. Note that the size of each ablation zone along the three electrodes is relatively different. (b) Photograph of the cut surface of the coagulation created by SM-RF ablation demonstrates an oval-shaped, 4.5 × 5.3-cm ablation area with an irregular margin. (c) Photograph of the cut surface of the coagulation created by SB-RF ablation demonstrates an oval-shaped, 6.1 × 6.5-cm ablation area with a sharp margin. Note that the size of each ablation zone along the three electrodes is relatively the same.
Table 2.
Measured values of the dimensions of coagulation necrosis according to radiofrequency (RF) power application modes
| Coagulation necrosis | Group A Consecutive mode RFA (n = 20) |
Group B Switching monopolar RFA (n = 20) |
Group C Switching bipolar RFA (n = 20) |
p-valuea | p-value A vs B | p-value A vs C | p-value B vs C |
|---|---|---|---|---|---|---|---|
| Dmin (cm) | 3.9 ± 1.2 | 4.4 ± 1.0 | 5.1 ± 0.9 | 0.002 | 0.469 | 0.004 | 0.175 |
| Dmax (cm) | 5.8 ± 0.6 | 5.5 ± 0.8 | 5.9 ± 0.7 | 0.382 | 0.679 | 1.000 | 0.356 |
| Dv (cm) | 4.6 ± 0.2 | 5.3 ± 0.4 | 5.5 ± 0.28 | 0.963 | 1.000 | 1.000 | 1.000 |
| Area (cm2) | 21.2 ± 4.3 | 21.8 ± 5.3 | 25.1 ± 5.5 | 0.040 | 1.000 | 0.047 | 0.131 |
| Effective volume (cm3) | 39.0 ± 27.7 | 50.2 ± 27.8 | 72.8 ± 36.4 | 0.004 | 0.768 | 0.003 | 0.073 |
Dmax, maximum diameter of the ablation zone; Dmin, minimum diameter of the ablation zone; Dv, vertical diameter of the ablation zone; RFA, radiofrequency ablation.
Values are mean ± standard deviation.
p-values indicate the statistical difference among Groups A–C.
In addition, SB-RFAs had a significantly higher number of coagulations >5 cm in Dmin than did CM- or SM-RFAs: 65% (13/20) in Group C, 20% (4/20) in Group A and 25% (5/20) in Group B (p = 0.03). In Group C, there were five lesions between 4 and 5 cm in Dmin (25%, 5/20), and only two lesions between 3 and 4 cm in Dmin (10%, 2/20).
DISCUSSION
Our study demonstrated that RFA in SB mode using three ICW electrodes was able to create coagulations >5 cm in 65%, and ablation zones >4 cm in 90% of cases within a relatively short duration (12 min) of energy application in perfused in vivo liver tissue. The mean Dmin of ablation lesions created by SB-RFA was 5.1 cm, which is large enough to safely treat 3-cm-sized tumours. This fact has significant clinical implications including simplification of the RFA procedure, reduction in procedure time and improvement of the therapeutic results of RFA for hepatic malignancies. Our results surpass the results of a previous study that reported an ablation zone 3.2 cm in Dmin using SM-RFA.20,33 Indeed, the bipolar mode has been shown to create larger, more regular coagulation necroses than do either monopolar simultaneous or alternating RFA methods. This is owing to its superior heat production efficiency at a given current level, since the current is confined between the tissue and the electrode.25 In addition, ICW electrodes were used in our study instead of internally cooled electrodes. ICW electrodes are able to deliver a larger amount of RF energy to the tissue, as thermal and electrical conductance are improved by avoiding tissue vaporization and charring using perfused saline in tissue.28,29,34 ICW electrodes are also helpful in avoiding tissue dehydration and charring, which have been more commonly reported with the bipolar mode.35 Considering that there is a huge clinical demand regarding ablation devices that can allow the creation of larger zones of coagulation coupled with the need to secure an adequate “ablation margin”,13 our SB-RFA system using triple ICW electrodes represents a potential advance in imaging-guided tissue coagulation, providing the ability to perform RFA in tumours >2 cm within a single application, with reduced treatment time, anaesthetic risk and treatment cost.22,36,37
Our study also demonstrated that RFA in the SB mode with electrodes spaced 2.5 cm apart for 12 min was superior to the CM-RF mode in creating more round- or oval-shaped coagulation areas. Although reports of coagulation zones much >5.0 cm created primarily by CM-RFA using cluster electrodes combined with saturated saline infusion have been published,38 the shape of the lesions was quite irregular. Previous studies using perfused electrodes demonstrated that irregularly shaped areas of coagulation have been observed at RFA and simultaneous saline injection, attributing to non-uniform saline distribution.39 Given that most tumours treatable with RFA are spherical, the shape of the ablation area should also approximate a sphere, in order to create a sufficient ablative margin while minimizing the destruction of normal surrounding tissue.17 We believe that the reason why SB-RFA created a coagulation zone more spherical and confluent in shape as well as with a larger size than the standard CM- or SM-RFA technique can be explained by the better thermal efficiency of the SB mode,25 and that this feature can help reduce recurrence following ablation therapy.
Despite of the fact that RFA using three ICW electrodes in the SB mode was able to create coagulations >5 cm in short-axis diameter in 65% of procedures, there are several technical considerations to think over prior to applying this technology for the treatment of liver malignancies percutaneously. First, since large-scale ablations may be associated with a greater risk of thermal injury to adjacent structures and have larger variability in coagulation diameters, further investigation regarding accurate titration on interprobe distance, duration and power may be necessary to avoid unnecessary injury to adjacent normal tissue, as well as to better determine the predictability of coagulation size in the liver.22,25 Second, theoretically, RFA using three ICW electrodes in the SB mode has an increasing risk of bleeding and other injuries related to multiple-electrode insertions.37 However, during our in vivo experiments, we did not observe severe bleeding from the electrode insertion sites. Last, the complexity of the SB-RFA procedure could be higher than that of CM-RFA with multitine electrodes. Based on our experience, however, although placing three probes into the index tumour under ultrasound guidance can make the RFA procedure difficult, it is only minimally more complicated for clinicians experienced in ultrasound-guided procedures than the insertion of a single probe.22
Our study has several limitations to consider. First, this study established the initial feasibility of RFA in the SB mode using three ICW electrodes and a multichannel RF system, using the intraoperative approach. Thus, we may not be able to properly assess the risks of using the percutaneous approach, as we were able to avoid unwanted thermal injury by positioning electrodes away from adjacent tissue using the intraoperative approach. However, multiple electrodes are frequently used for image-guided percutaneous RFA in routine clinical practice.22,36,40,41 Second, in 35% (7/20) of cases, SB-RFA with three ICW electrodes failed to create coagulations >5 cm in diameter in a clinically acceptable time frame. Although we believe that the improved efficiency of SB-RFA in creating large ablation zones could be valuable for the treatment of large tumours and for decreasing local recurrence, further improvement in reproducibility in creating ablations >5 cm is warranted. Third, we did not compare SB-RFA using multiple electrodes with microwave ablations with multiple antennas. However, RF systems have been shown to provide better cost effectiveness, and it is also more widely available than microwave ablations.18 Further comparison between our RF device and microwave devices may be warranted. Last, RFAs were carried out in normal porcine livers, and therefore, the thermal efficiency of the current RF system in this study may not be translated into real clinical practice owing to the different tissue texture of target tumours. Despite these shortcomings, as far as we know, pig livers may be the best in vivo model to provide a reliable basis for testing the efficacy of RFA devices.
In conclusion, our results demonstrated that SB-RFA using multiple ICW electrodes can achieve coagulations >5 cm in diameter with better efficiency than SM- or CM-RFA in a relatively short time frame in 65% of cases. In addition, we believe that this technology may ultimately result in more effective treatment of hepatic tumours 3–4 cm in diameter by reducing the treatment time and enabling more confident destruction of the entire tumour as well as the creation of a sufficient safety margin.
FUNDING
This study received technical support and research grant from RF Medical (Seoul, Republic of Korea).
Contributor Information
J H Yoon, Email: jhjhry@gmail.com.
J M Lee, Email: jmsh@snu.ac.kr.
S Woo, Email: jcrew7@hotmail.com.
E J Hwang, Email: ken921004@hotmail.com.
I Hwang, Email: mit3000kr@gmail.com.
W Choi, Email: kchoipro@gmail.com.
J K Han, Email: hanjk@snu.ac.kr.
B I Choi, Email: bichoi@snu.ac.kr.
REFERENCES
- 1.Crocetti L, de Baere T, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc Intervent Radiol 2010; 33: 11–17. doi: 10.1007/s00270-009-9736-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin YJ, Kim KM, Hwang S, Lee SG, Ha TY, Song GW, et al. Exercise and diet modification in non-obese non-alcoholic fatty liver disease: analysis of biopsies of living liver donors. J Gastroenterol Hepatol 2012; 27: 1341–7. doi: 10.1111/j.1440-1746.2012.07165.x [DOI] [PubMed] [Google Scholar]
- 3.Lu DS, Yu NC, Raman SS, Limanond P, Lassman C, Murray K, et al. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology 2005; 234: 954–60. doi: 10.1148/radiol.2343040153 [DOI] [PubMed] [Google Scholar]
- 4.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012; 379: 1245–55. doi: 10.1016/S0140-6736(11)61347-0 [DOI] [PubMed] [Google Scholar]
- 5.Cucchetti A, Piscaglia F, Cescon M, Colecchia A, Ercolani G, Bolondi L, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol 2013; 59: 300–7. doi: 10.1016/j.jhep.2013.04.009 [DOI] [PubMed] [Google Scholar]
- 6.Kuvshinoff BW, Ota DM. Radiofrequency ablation of liver tumors: influence of technique and tumor size. Surgery 2002; 132: 605–11. doi: 10.1067/msy.2002.127545 [DOI] [PubMed] [Google Scholar]
- 7.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology 2000; 214: 761–8. doi: 10.1148/radiology.214.3.r00mr02761 [DOI] [PubMed] [Google Scholar]
- 8.Waki K, Aikata H, Katamura Y, Kawaoka T, Takaki S, Hiramatsu A, et al. Percutaneous radiofrequency ablation as first-line treatment for small hepatocellular carcinoma: results and prognostic factors on long-term follow up. J Gastroenterol Hepatol 2010; 25: 597–604. doi: 10.1111/j.1440-1746.2009.06125.x [DOI] [PubMed] [Google Scholar]
- 9.Solmi L, Nigro G, Roda E. Therapeutic effectiveness of echo-guided percutaneous radiofrequency ablation therapy with a LeVeen needle electrode in hepatocellular carcinoma. World J Gastroenterol 2006; 12: 1098–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterol 2005; 129: 122–30. doi: 10.1053/j.gastro.2005.04.009 [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y, Zhao Y, Li B, Xu D, Yin Z, Xie F, et al. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol 2010; 10: 78. doi: 10.1186/1471-230X-10-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazzaferro V, Battiston C, Perrone S, Pulvirenti A, Regalia E, Romito R, et al. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann Surg 2004; 240: 900–9. doi: 10.1097/01.sla.0000143301.56154.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberg SN, Ahmed M. Minimally invasive image-guided therapies for hepatocellular carcinoma. J Clin Gastroenterol 2002; 35: S115–29. doi: 10.1097/00004836-200211002-00008 [DOI] [PubMed] [Google Scholar]
- 14.Molinari M, Helton S. Hepatic resection versus radiofrequency ablation for hepatocellular carcinoma in cirrhotic individuals not candidates for liver transplantation: a Markov model decision analysis. Am J Surg 2009; 198: 396–406. doi: 10.1016/j.amjsurg.2009.01.016 [DOI] [PubMed] [Google Scholar]
- 15.Dodd GD, 3rd, Frank MS, Aribandi M, Chopra S, Chintapalli KN. Radiofrequency thermal ablation: computer analysis of the size of the thermal injury created by overlapping ablations. AJR Am J Roentgenol 2001; 177: 777–82. doi: 10.2214/ajr.177.4.1770777 [DOI] [PubMed] [Google Scholar]
- 16.Chen MH, Yang W, Yan K, Zou MW, Solbiati L, Liu JB, et al. Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients—mathematic model, overlapping mode, and electrode placement process. Radiology 2004; 232: 260–71. doi: 10.1148/radiol.2321030821 [DOI] [PubMed] [Google Scholar]
- 17.Hines-Peralta AU, Pirani N, Clegg P, Cronin N, Ryan TP, Liu Z, et al. Microwave ablation: results with a 2.45-GHz applicator in ex vivo bovine and in vivo porcine liver. Radiology 2006; 239: 94–102. doi: 10.1148/radiol.2383050262 [DOI] [PubMed] [Google Scholar]
- 18.Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology 2012; 262: 43–58. doi: 10.1148/radiol.11110144 [DOI] [PubMed] [Google Scholar]
- 19.Lee JM, Han JK, Kim HC, Choi YH, Kim SH, Choi JY, et al. Switching monopolar radiofrequency ablation technique using multiple, internally cooled electrodes and a multichannel generator: ex vivo and in vivo pilot study. Invest Radiol 2007; 42: 163–71. doi: 10.1097/01.rli.0000252495.44818.b3 [DOI] [PubMed] [Google Scholar]
- 20.Laeseke PF, Sampson LA, Haemmerich D, Brace CL, Fine JP, Frey TM, et al. Multiple-electrode radiofrequency ablation creates confluent areas of necrosis: in vivo porcine liver results. Radiology 2006; 241: 116–24. doi: 10.1148/radiol.2411051271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cha J, Choi D, Lee MW, Rhim H, Kim YS, Lim HK, et al. Radiofrequency ablation zones in ex vivo bovine and in vivo porcine livers: comparison of the use of internally cooled electrodes and internally cooled wet electrodes. Cardiovasc Intervent Radiol 2009; 32: 1235–40. doi: 10.1007/s00270-009-9600-0 [DOI] [PubMed] [Google Scholar]
- 22.Woo S, Lee JM, Yoon JH, Joo I, Kim SH, Lee JY, et al. Small- and medium-sized hepatocellular carcinomas: monopolar radiofrequency ablation with a multiple-electrode switching system–mid-term results. Radiology 2013; 268: 589–600. doi: 10.1148/radiol.13121736 [DOI] [PubMed] [Google Scholar]
- 23.Brace CL, Laeseke PF, Sampson LA, Frey TM, van der Weide DW, Lee FT, Jr. Microwave ablation with multiple simultaneously powered small-gauge triaxial antennas: results from an in vivo swine liver model. Radiology 2007; 244: 151–6. doi: 10.1148/radiol.2441052054 [DOI] [PubMed] [Google Scholar]
- 24.Mulier S, Ni Y, Frich L, Burdio F, Denys AL, De Wispelaere JF, et al. Experimental and clinical radiofrequency ablation: proposal for standardized description of coagulation size and geometry. Ann Surg Oncol 2007; 14: 1381–96. doi: 10.1245/s10434-006-9033-9 [DOI] [PubMed] [Google Scholar]
- 25.Lee JM, Han JK, Kim SH, Han CJ, An SK, Lee JY, et al. Wet radio-frequency ablation using multiple electrodes: comparative study of bipolar versus monopolar modes in the bovine liver. Eur J Radiol 2005; 54: 408–17. doi: 10.1016/j.ejrad.2004.06.004 [DOI] [PubMed] [Google Scholar]
- 26.Clasen S, Schmidt D, Boss A, Dietz K, Kröber SM, Claussen CD, et al. Multipolar radiofrequency ablation with internally cooled electrodes: experimental study in ex vivo bovine liver with mathematic modeling. Radiology 2006; 238: 881–90. doi: 10.1148/radiol.2382050571 [DOI] [PubMed] [Google Scholar]
- 27.Kim KW, Lee JM, Jeon YS, Lee IJ, Choi Y, Park J, et al. Vascular disrupting effect of CKD-516: preclinical study using DCE-MRI. Invest New Drugs 2013; 31: 1097–106. doi: 10.1007/s10637-012-9915-6 [DOI] [PubMed] [Google Scholar]
- 28.Kim JH, Kim PN, Won HJ, Shin YM. Percutaneous radiofrequency ablation using internally cooled wet electrodes for the treatment of hepatocellular carcinoma. AJR Am J Roentgenol 2012; 198: 471–6. doi: 10.2214/ajr.11.6583 [DOI] [PubMed] [Google Scholar]
- 29.Kim JH, Kim PN, Won HJ, Shin YM. Percutaneous radiofrequency ablation with internally cooled versus internally cooled wet electrodes for small subphrenic hepatocellular carcinomas. J Vasc Interv Radiol 2013; 24: 351–6. doi: 10.1016/j.jvir.2012.11.025 [DOI] [PubMed] [Google Scholar]
- 30.Goldberg SN, Gazelle GS, Compton CC, Mueller PR, Tanabe KK. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer 2000; 88: 2452–63. doi: 10.1002/1097-0142(20000601)88:113.0.CO;2-3 [DOI] [PubMed] [Google Scholar]
- 31.Goldlust EJ, Paczynski RP, He YY, Hsu CY, Goldberg MP. Automated measurement of infarct size with scanned images of triphenyltetrazolium chloride–stained rat brains. Stroke 1996; 27: 1657–62. doi: 10.1161/01.str.27.9.1657 [DOI] [PubMed] [Google Scholar]
- 32.Yoon JH, Lee JM, Hwang EJ, Hwang IP, Baek J, Han JK, et al. Monopolar radiofrequency ablation using a dual-switching system and a separable clustered electrode: evaluation of the in vivo efficiency. Korean J Radiol 2014; 15: 235–44. doi: 10.3348/kjr.2014.15.2.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laeseke PF, Sampson LA, Haemmerich D, Brace CL, Fine JP, Frey TM, et al. Multiple-electrode radiofrequency ablation: simultaneous production of separate zones of coagulation in an in vivo porcine liver model. J Vasc Interv Radiol 2005; 16: 1727–35. doi: 10.1097/01.rvi.000018362.17771.b0 [DOI] [PubMed] [Google Scholar]
- 34.Lee JM, Han JK, Chang JM, Chung SY, Kim SH, Lee JY, et al. Radiofrequency ablation of the porcine liver in vivo: increased coagulation with an internally cooled perfusion electrode. Acad Radiol 2006; 13: 343–52. [DOI] [PubMed] [Google Scholar]
- 35.Haemmerich D, Lee FT, Jr, Schutt DJ, Sampson LA, Webster JG, Fine JP, et al. Large-volume radiofrequency ablation of ex vivo bovine liver with multiple cooled cluster electrodes. Radiology 2005; 234: 563–8. doi: 10.1148/radiol.2342031122 [DOI] [PubMed] [Google Scholar]
- 36.Lee J, Lee JM, Yoon JH, Lee JY, Kim SH, Lee JE, et al. Percutaneous radiofrequency ablation with multiple electrodes for medium-sized hepatocellular carcinomas. Korean J Radiol 2012; 13: 34–43. doi: 10.3348/kjr.2012.13.1.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoon JH, Lee JM, Han JK, Choi BI. Dual switching monopolar radiofrequency ablation using a separable clustered electrode: comparison with consecutive and switching monopolar modes in ex vivo bovine livers. Korean J Radiol 2013; 14: 403–11. doi: 10.3348/kjr.2013.14.3.403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldberg SN, Ahmed M, Gazelle GS, Kruskal JB, Huertas JC, Halpern EF, et al. Radio-frequency thermal ablation with NaCl solution injection: effect of electrical conductivity on tissue heating and coagulation—phantom and porcine liver study. Radiology 2001; 219: 157–65. doi: 10.1148/radiology.219.1.r01ap27157 [DOI] [PubMed] [Google Scholar]
- 39.Lee JM, Han JK, Lee JY, Kim SH, Choi JY, Lee MW, et al. Hepatic radiofrequency ablation using multiple probes: ex vivo and in vivo comparative studies of monopolar versus multipolar modes. Korean J Radiol 2006; 7: 106–17. doi: 10.3348/kjr.2006.7.2.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park MJ, Kim YS, Rhim H, Lim HK, Lee MW, Choi D. A comparison of US-guided percutaneous radiofrequency ablation of medium-sized hepatocellular carcinoma with a cluster electrode or a single electrode with a multiple overlapping ablation technique. J Vasc Interv Radiol 2011; 22: 771–9. doi: 10.1016/j.jvir.2011.02.005 [DOI] [PubMed] [Google Scholar]
- 41.Hänsler J, Frieser M, Tietz V, Uhlke D, Wissniowski T, Bernatik T, et al. Percutaneous radiofrequency ablation of liver tumors using multiple saline-perfused electrodes. J Vasc Interv Radiol 2007; 18: 405–10. doi: 10.1016/j.jvir.2006.12.729 [DOI] [PubMed] [Google Scholar]