Abstract
Objective:
To compare the performance of the 15-G internally cooled electrode with that of the conventional 17-G internally cooled electrode.
Methods:
A total of 40 (20 for each electrode) and 20 ablation zones (10 for each electrode) were made in extracted bovine livers and in in vivo porcine livers, respectively. Technical parameters, three dimensions [long-axis diameter (Dl), vertical-axis diameter (Dv) and short-axis diameter (Ds)], volume and the circularity (Ds/Dl) of the ablation zone were compared.
Results:
The total delivered energy was higher in the 15-G group than in the 17-G group in both ex vivo and in vivo studies (8.78 ± 1.06 vs 7.70 ± 0.98 kcal, p = 0.033; 11.20 ± 1.13 vs 8.49 ± 0.35 kcal, p = 0.001, respectively). The three dimensions of the ablation zone had a tendency to be larger in the 15-G group than in the 17-G group in both studies. The ablation volume was larger in the 15-G group than in the 17-G group in both ex vivo and in vivo studies (29.61 ± 7.10 vs 23.86 ± 3.82 cm3, p = 0.015; 10.26 ± 2.28 vs 7.79 ± 1.68 cm3, p = 0.028, respectively). The circularity of ablation zone was not significantly different in both the studies.
Conclusion:
The size of ablation zone was larger in the 15-G internally cooled electrode than in the 17-G electrode in both ex vivo and in vivo studies.
Advances in knowledge:
Radiofrequency ablation of hepatic tumours using 15-G electrode is useful to create larger ablation zones.
Radiofrequency ablation (RFA) is the most widely used local ablation technique for the management of primary and metastatic liver tumours. However, previous studies have reported that RFA showed a relatively higher local tumour progression rate than did hepatic resection.1,2 One of the most important factors affecting local tumour progression was insufficient tumour-free ablation margin of hepatic parenchyma around the tumour margin.3–6
Several strategies have been developed to obtain sufficient ablation margin. In the aspect of RFA techniques, overlapping technique and combined treatment with transcatheter arterial chemoembolization can be used.7–9 Another strategy is to use switching monopolar, bipolar or multipolar modes to deliver radiofrequency (RF) energy more efficiently.10,11 Sufficient ablation margin can also be achieved by more efficient electrodes: internally cooled electrode increases the size of ablation zone by preventing charring around the electrode tip.12,13 Perfusion electrodes can also enlarge the ablation zone by increasing electrical conductance and thermal conductivity.14–16
The diameter of an electrode is also known to be associated with the size of the ablation zone. Theoretically, as the diameter of an electrode becomes larger, the contact surface of the electrode with the surrounding tissue becomes bigger, thereby increasing the active electric field.17,18 As a result, an electrode with a larger diameter is likely to create a larger ablation zone. In a previous study, Goldberg et al17 reported that the extent of coagulation necrosis by RFA increases as the diameter of an electrode increases through an in vivo experimental study. However, this study was performed with an electrode without an internal cooling system. Recently, a clinical study comparing therapeutic efficacy and safety between 15-G and 17-G internally cooled electrodes of RFA for hepatocellular carcinoma was published.19 According to that study, the 15-G internally cooled electrode created a larger ablation volume than did the 17-G electrode. However, the study was limited by selection bias owing to the retrospective study design. In addition, the ablation protocol was not exactly the same between the two groups. Therefore, the issue whether an internally cooled electrode with a larger diameter creates a larger ablation volume should be verified with ex vivo and in vivo experimental studies.
The purpose of this experimental study was to compare the performance of the 15-G internally cooled RF electrode with that of the conventional 17-G electrode in both ex vivo and in vivo studies.
METHODS AND MATERIALS
Study overview
In Samsung Medical Center, Seoul, Republic of Korea, there are guidelines for maintenance and care of experimental animals to ensure humane treatment and reliable results. All authors affirmed their compliance with these guidelines. This study was approved by the Institutional Animal Care and Use Committee in Samsung Medical Center before initiating experiments. For this study, research laboratory support was provided by STARmed, Goyang, Republic of Korea. However, all authors independently and completely controlled the whole study, including the study design, data collection and analysis, and the preparation of manuscript. We performed ex vivo and in vivo studies to compare the ablation performance of the 15-G and 17-G internally cooled electrodes.
Equipment
We used conventional 17-G (Cool tip®; Valleylab™, Boulder, CO) and 15-G (Proteus®; STARmed) electrodes, both internally cooled electrodes with 3-cm active tip, with a 200-W RF generator (VIVA RF generator; STARmed). The electrodes were cooled internally by delivering chilled saline to maintain the electrode temperature <25 °C with a peristaltic pump (VIVA pump; STARmed). The total ablation time was 12 min in both the ex vivo and in vivo studies. A RFA was performed by a radiologist (MWL) who had 8 years' experience with RFA for hepatic tumours.
Ex vivo study
Freshly extracted bovine livers were obtained from a local slaughterhouse (Hyupjin Center, Seoul, Republic of Korea), and they were cooled to maintain hypothermia at 4–6 °C. They were transported to our research laboratory immediately before the experiment and were cut into about 10 × 10 × 10-cm3 blocks and partially immersed in a 50 × 20 × 20-cm3 saline-filled bath. A custom-made metal plate grounding pad was placed to one sidewall of the bath. The RF electrode was inserted on the outer hepatic capsule at least 4 cm into the liver parenchyma. A total of 40 ablation zones (20 for each electrode) were alternatively made by the 17-G and 15-G internally cooled electrodes. The generators were set to deliver the maximum power in the automatic control mode.
In vivo study
Six Yorkshire pigs (about 60 kg of body weight) were used. All the pigs were initially anaesthetized by an intramuscular injection with 5 mg kg−1 body weight tiletamine hydrochloride + zolazepam hydrochloride (Zoletil 50; Virbac SA, Carros, France) and 0.5 mg kg−1 body weight xylazine (Rompun; Bayer Schering Pharma, Berlin, Germany). A 22-G intravenous catheter was inserted into the dorsal auricular vein. The pigs were intubated, and anaesthesia was maintained with inhaled isoflurane gas (Forane solution; Choongwae Pharma, Seoul, Republic of Korea). The pigs were placed in the supine position, and vital signs were monitored. Both thighs were shaved, and ground pads were placed. After sterilizing the epigastric area, the liver was exposed by laparotomy. A total of 10 RFA zones (3 or 4 ablations per pig) were created alternatively using the 2 different types of electrodes. To determine the ablation sites in the liver, ultrasonographic evaluation (1–4-MHz convex probe, Acuson Sequoia™ Gastrointestinal 512; Siemens Healthcare; formerly Siemens Medical Solutions, formerly Siemens Medical Systems, Erlangen, Germany) was performed. A RF electrode was placed in the liver under real-time ultrasound guidance away from intrahepatic vessels, interlobar fissure or the liver capsule so that the RFA zone would not be influenced by these structures. The distance between the centre of each ablation zone was kept at least 4 cm apart to avoid attachment of the ablation zones. Immediately after the in vivo experiments were completed, the animals were euthanized and the liver was extracted.
Analysis of radiofrequency ablation zones
For both the ex vivo and in vivo experiments, the liver specimens were dissected along the axis of the electrode insertion and were sliced again in the perpendicular plane to the electrode tracks. The central white area of ablation zones was considered as the zone of coagulation necrosis. The margin of the central white zone was determined based on the consensus of two radiologists (MWL and KDS). The long-axis diameter (Dl), vertical diameter (Dv) and short-axis diameter (Ds) of the ablation zones were measured. The gross images of the ablation zones were analysed by using ImageJ software v. 1.42 (National Institutes of Health, Bethesda, MD; http://rsbweb.nih.gov/ij/download.html). To minimize measurement error, the dimensions were measured three times based on the consensus of two radiologists (MWL and KDS), and the average dimensions were used. The volume of the ablation zones was estimated using the following formula: π (Dl × Dv × Ds)/6. The circularity of the ablation zones was evaluated by the ratio between the Dl and Ds.
Statistical analysis
The three diameters, approximated volumes, ratio of Ds and Dl, and technical parameters for the ablation zones between the two groups were compared using t-test and the Mann–Whitney U test according to the normal distribution assumption. The coefficient of variation (CV) of the ablation volume (the ratio of the standard deviation to mean volume of the ablation zones) in each group was calculated to test the variability of the size of the ablation zones. Statistical significance was considered at p < 0.05. All analyses were conducted using the appropriate software (SPSS® for Windows v. 17.0.0; SPSS Inc., Chicago, IL).
RESULTS
Technical parameters
The average delivered energy of the 15-G group was higher than that of the 17-G group in both the ex vivo (8.78 ± 1.06 vs 7.70 ± 0.98 kcal, p = 0.033) and the in vivo (11.20 ± 1.13 vs 8.49 ± 0.35 kcal, p = 0.001) studies (Tables 1 and 2). The average impedance of the 15-G group was lower than that of the 17-G group (69.65 ± 7.00 vs 84.90 ± 3.06 Ω, p < 0.001) in the ex vivo study, but it was not significantly different in the in vivo study (66.30 ± 5.77 vs 67.40 ± 4.47 Ω, p = 0.172).
Table 1.
Dimensions and approximated volumes of ablation zones in the ex vivo study
| Measurements | 17-G (n = 20) | 15-G (n = 20) | p-value |
|---|---|---|---|
| Total delivered energy (kcal) | 7.70 ± 0.98 | 8.78 ± 1.06 | 0.033 |
| Impedance (Ω) | 84.90 ± 3.06 | 69.65 ± 7.00 | <0.001 |
| Diameters (cm) | |||
| Long axis (Dl) | 4.09 ± 0.18 (3.62–4.81) | 4.23 ± 0.26 (3.62–4.62) | 0.335 |
| Short axis (Ds) | 3.25 ± 0.20 (2.97–3.63) | 3.53 ± 0.36 (2.76–3.96) | 0.020 |
| Vertical axis (Dv) | 3.41 ± 0.26 (2.99–3.98) | 3.74 ± 0.46 (3.06–4.73) | 0.035 |
| Volume (cm3) | 23.86 ± 3.82 (17.72–30.33) | 29.61 ± 7.10 (16.45–39.31) | 0.015 |
| Ds/Dl | 0.79 ± 0.04 (0.71–0.88) | 0.83 ± 0.07 (0.70–0.94) | 0.155 |
| Coefficient of variation (%) | 16 | 23 | |
Values are mean ± standard deviation (range).
Table 2.
Dimensions and approximated volumes of ablation zones in the in vivo study
| Measurements | 17-G (n = 10) | 15-G (n = 10) | p-value |
|---|---|---|---|
| Total delivered energy (kcal) | 8.49 ± 0.35 | 11.20 ± 1.13 | 0.001 |
| Impedance (Ω) | 67.4 ± 4.47 | 66.30 ± 5.77 | 0.172 |
| Diameters (cm) | |||
| Long axis (Dl) | 3.67 ± 0.20 (3.34–3.95) | 3.94 ± 0.25 (3.49–4.38) | 0.020 |
| Short axis (Ds) | 1.85 ± 0.26 (1.47–2.25) | 2.01 ± 0.15 (1.71–2.17) | 0.517 |
| Vertical axis (Dv) | 2.18 ± 0.24 (1.74–2.50) | 2.45 ± 0.34 (1.93–3.07) | 0.108 |
| Volume (cm3) | 7.79 ± 1.68 (4.78–10.10) | 10.26 ± 2.28 (6.07–13.96) | 0.028 |
| Ds/Dl | 0.60 ± 0.09 (0.48–0.75) | 0.62 ± 0.08 (0.49–0.77) | 1.000 |
| Coefficient of variation (%) | 21.6 | 22.2 | |
Values are mean ± standard deviation (range).
Analysis of the size and circularity of the ablation zone
Ex vivo experiment
The three diameters, the calculated volume, the ratio of Ds/Dl and the CV of ablated volume, are shown in Table 1. The Ds, Dv and volume of the 15-G group were greater than those of the 17-G group (p = 0.020, p = 0.035 and p = 0.015, respectively). However, the Dl and the ratio of Ds/Dl were not significantly different between the two groups (p = 0.335 and p = 0.155, respectively) (Figure 1).
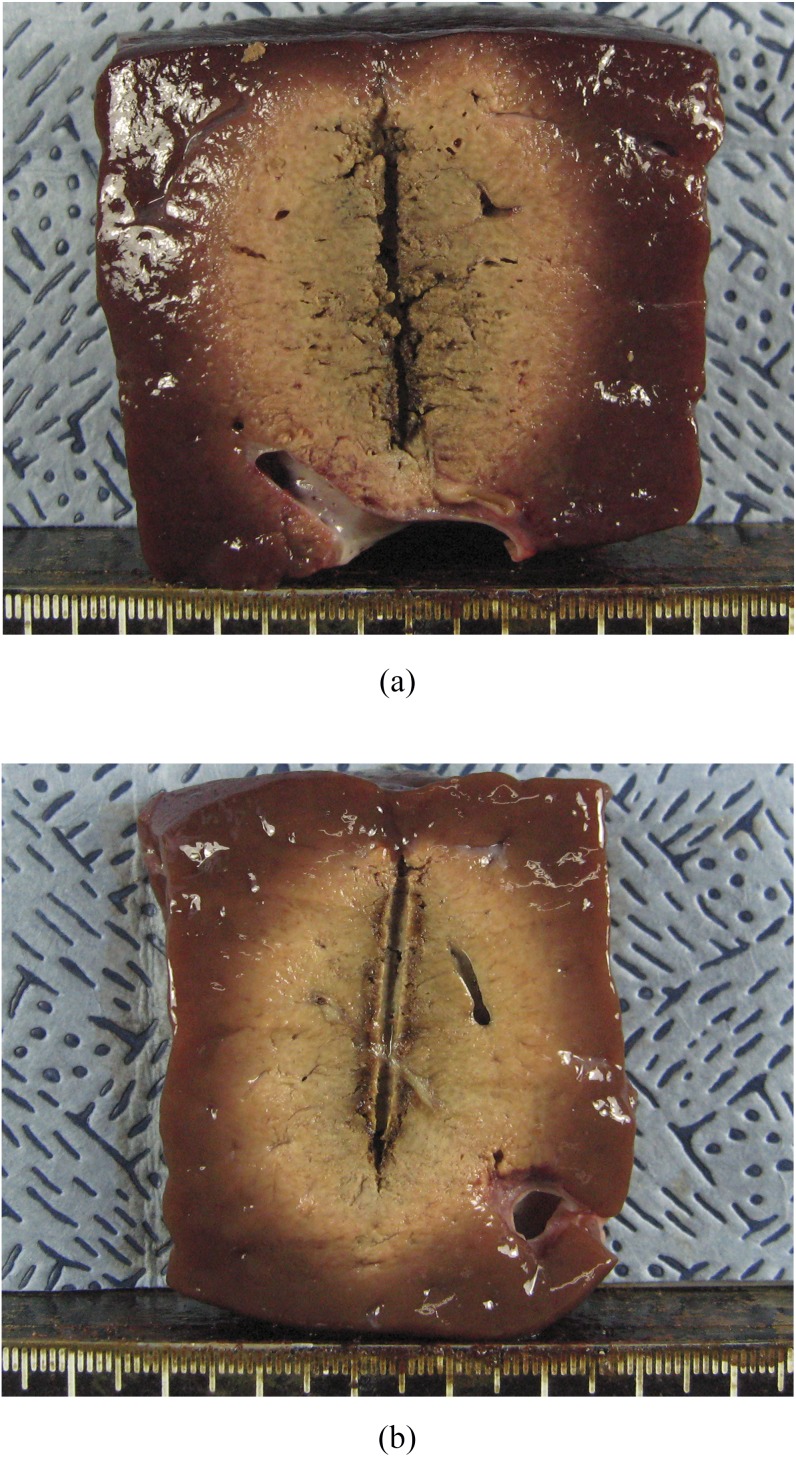
Figure 1.
Comparison of radiofrequency ablation zones created by 15-G and 17-G internally cooled electrodes in ex vivo study. (a) Cut section of the specimen created by the 15-G electrode along the electrode insertion axis shows a 4.48 × 3.70-cm-sized ablation zone. (b) Cut section of the specimen created by the 17-G electrode shows a 4.34 × 3.18-cm-sized ablation zone.
In vivo experiment
The Dl and the volume of the ablation zone of the 15-G group were greater than those of the 17-G group. However, the differences of the Ds and Dv were not statistically significant (Table 2) between the two groups. The ratio of Ds/Dl was also not significantly different (p = 1.000) (Figure 2).
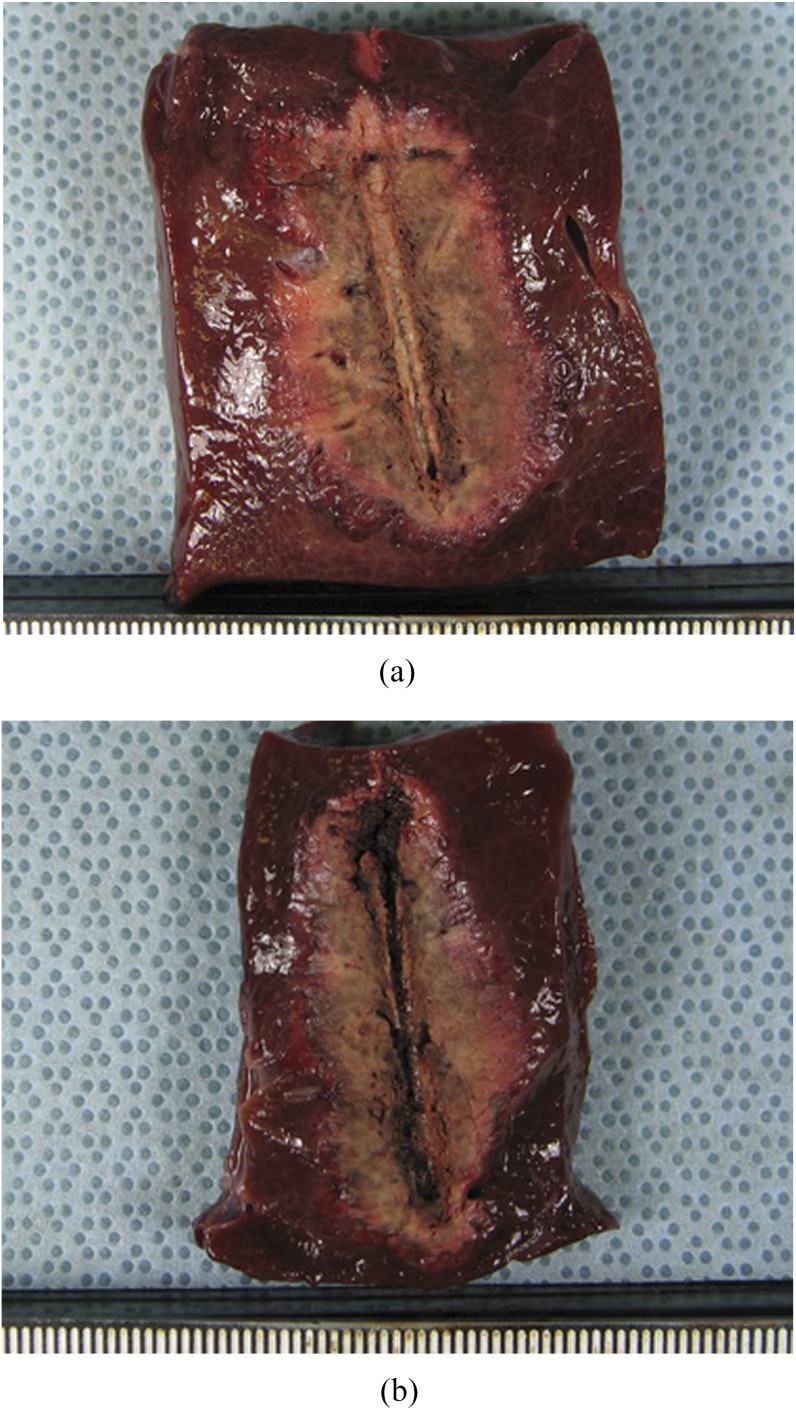
Figure 2.
Comparison of radiofrequency ablation zones created by 15-G and 17-G internally cooled electrodes in in vivo study. (a) Cut section of the specimen created by the 15-G electrode along the electrode insertion axis shows a 3.91 × 2.07-cm-sized ablation zone. (b) Cut section of the specimen created by the 17-G electrode along the electrode insertion axis shows a 3.71 × 1.70-cm-sized ablation zone.
DISCUSSION
In this study, we compared the ablation performances between the 15-G and 17-G internally cooled RF electrodes. Total delivered energy in the 15-G group was higher than in the 17-G group, resulting in larger ablation zones in the 15-G group in both in vivo and ex vivo studies. The circularity of the ablation zone was not significantly different between the two groups in both in vivo and ex vivo studies. Based on these results, the 15-G electrode is expected to be more useful for RFA of liver tumours than the conventional 17-G electrode owing to its higher probability of creating a sufficient tumour-free ablation margin.
According to a study by Goldberg et al,17 there was a linear correlation between the diameter of an electrode and the diameter of an ablation zone. In that study, the diameter of the ablation zones differed about 2 mm between the 18-G and 15-G electrodes with 3-cm active tip. This result is in agreement with the result of the present study: the difference of the mean Dv was 3.3 mm in the ex vivo study and 2.7 mm in the in vivo study, and the increment of the mean ablation volume in the 15-G group was 24.1% [(29.61/23.86 − 1) × 100] in the ex vivo study and 31.7% [(10.26/7.79 – 1) × 100] in the in vivo study compared with the 17-G group.
Multiple overlapping treatments with either a single electrode or multiple electrodes are the common method used to increase the size of an ablation zone.20 However, the precise re-positioning of an electrode in a contiguous fashion or the precise positioning of multiple electrodes can be technically challenging.13 For this reason, more efficient RF electrodes have been developed to decrease the need of overlapping techniques.21,22 The 15-G electrode has an advantage over the conventional 17-G electrode in this technical aspect. Since the size of the ablation zone is larger in the 15-G electrode than in the 17-G electrode, the number of overlapping treatments and consequently the overall procedure time would be decreased.
Although the 15-G electrode can create a larger ablation zone than the conventional 17-G electrode, there are few data regarding its complication. Gazelle et al23 reported that aspiration biopsy with needles larger than 18-G resulted in increased blood loss. However, unlike liver biopsy, the ablation tract is usually cauterized along the electrode path in RFA of hepatic tumours to avoid complications such as tract seeding or excessive bleeding. Therefore, the increased bleeding risk by using a 15-G electrode may not be a major problem compared with using a 17-G electrode. In fact, in a recent clinical study, the complication rate was not significantly different between the two electrode groups.19 Meanwhile, other complications such as collateral thermal injury to the surrounding tissue may be higher using the 15-G electrode owing to its larger ablation volume than when using the 17-G electrode. This concern regarding increased collateral thermal injury from a larger ablation zone also applies for microwave ablation, which is known to be able to create larger ablation zones with shorter ablation time.24 Further clinical study using 15-G electrodes is warranted to validate this issue.
Our study suffers from several limitations. First, we used gross specimens for the size measurement of ablation zones. Although gross appearance is known to correlate well with histological findings, the grossly measured size of ablation zones might not be exactly the same with the size of real coagulated tissue. Second, although we measured the dimensions three times for each specimen and used the average value to minimize measurement error, there was still the possibility of measurement error since dimensions of the ablation zone were measured manually. Third, we did not compare the issues related with safety between the two electrodes in this study.
In conclusion, total delivered energy and size of the ablation zone was larger in the 15-G internally cooled RF electrode than in the 17-G electrode in both the ex vivo and in vivo studies.
ACKNOWLEDGMENTS
We thank Mr Dong Un Kim (STARmed, Goyang, Republic of Korea) for his experimental assistance.
Acknowledgments
FUNDING
This study was supported by the Samsung Biomedical Research Institute grant [#CB11261].
Contributor Information
K D Song, Email: kdsong0308@gmail.com.
M W Lee, Email: leeminwoo0@gmail.com.
H J Park, Email: seolly1024@hanmail.net.
D I Cha, Email: cdisampp@gmail.com.
T W Kang, Email: kaienes.kang@samsung.com.
J Lee, Email: jisun1.lee@samsung.com.
J Y Moon, Email: jy2014.moon@samsung.com.
H Rhim, Email: hc.rhim@samsung.com.
REFERENCES
- 1.Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg 2010; 252: 903–12. doi: 10.1097/SLA.0b013e3181efc656 [DOI] [PubMed] [Google Scholar]
- 2.Pompili M, Saviano A, de Matthaeis N, Cucchetti A, Ardito F, Federico B, et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤3 cm. Results of a multicenter Italian survey. J Hepatol 2013; 59: 89–97. doi: 10.1016/j.jhep.2013.03.009 [DOI] [PubMed] [Google Scholar]
- 3.Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol 2012; 107: 569–77; quiz 78. doi: 10.1038/ajg.2011.425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 2005; 129: 122–30. doi: 10.1053/j.gastro.2005.04.009 [DOI] [PubMed] [Google Scholar]
- 5.Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology 2003; 228: 235–40. doi: 10.1148/radiol.2281020718 [DOI] [PubMed] [Google Scholar]
- 6.Kim YS, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol 2013; 58: 89–97. doi: 10.1016/j.jhep.2012.09.020 [DOI] [PubMed] [Google Scholar]
- 7.Takaki H, Yamakado K, Nakatsuka A, Fuke H, Murata K, Shiraki K, et al. Radiofrequency ablation combined with chemoembolization for the treatment of hepatocellular carcinomas 5 cm or smaller: risk factors for local tumor progression. J Vasc Interv Radiol 2007; 18: 856–61. doi: 10.1016/j.jvir.2007.04.022 [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Won HJ, Shin YM, Kim SH, Yoon HK, Sung KB, et al. Medium-sized (3.1-5.0 cm) hepatocellular carcinoma: transarterial chemoembolization plus radiofrequency ablation versus radiofrequency ablation alone. Ann Surg Oncol 2011; 18: 1624–9. doi: 10.1245/s10434-011-1673-8 [DOI] [PubMed] [Google Scholar]
- 9.Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer 2010; 116: 5452–60. doi: 10.1002/cncr.25314 [DOI] [PubMed] [Google Scholar]
- 10.Lee JM, Han JK, Lee JY, Kim SH, Choi JY, Lee MW, et al. Hepatic radiofrequency ablation using multiple probes: ex vivo and in vivo comparative studies of monopolar versus multipolar modes. Korean J Radiol 2006; 7: 106–17. doi: 10.3348/kjr.2006.7.2.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JM, Han JK, Kim HC, Choi YH, Kim SH, Choi JY, et al. Switching monopolar radiofrequency ablation technique using multiple, internally cooled electrodes and a multichannel generator: ex vivo and in vivo pilot study. Invest Radiol 2007; 42: 163–71. doi: 10.1097/01.rli.0000252495.44818.b3 [DOI] [PubMed] [Google Scholar]
- 12.Goldberg SN, Gazelle GS, Solbiati L, Rittman WJ, Mueller PR. Radiofrequency tissue ablation: increased lesion diameter with a perfusion electrode. Acad Radiol 1996; 3: 636–44. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol 2000; 174: 323–31. doi: 10.2214/ajr.174.2.1740323 [DOI] [PubMed] [Google Scholar]
- 14.Miao Y, Ni Y, Yu J, Marchal G. A comparative study on validation of a novel cooled-wet electrode for radiofrequency liver ablation. Invest Radiol 2000; 35: 438–44. doi: 10.1097/00004424-200007000-00007 [DOI] [PubMed] [Google Scholar]
- 15.Lee JM, Han JK, Kim SH, Sohn KL, Choi SH, Choi BI. Bipolar radiofrequency ablation in ex vivo bovine liver with the open-perfused system versus the cooled-wet system. Eur Radiol 2005; 15: 759–64. doi: 10.1007/s00330-004-2375-4 [DOI] [PubMed] [Google Scholar]
- 16.Lee JM, Han JK, Kim SH, Lee JY, Choi SH, Choi BI. Hepatic bipolar radiofrequency ablation using perfused-cooled electrodes: a comparative study in the ex vivo bovine liver. Br J Radiol 2004; 77: 944–9. doi: 10.1259/bjr/67069976 [DOI] [PubMed] [Google Scholar]
- 17.Goldberg SN, Gazelle GS, Dawson SL, Rittman WJ, Mueller PR, Rosenthal DI. Tissue ablation with radiofrequency: effect of probe size, gauge, duration, and temperature on lesion volume. Acad Radiol 1995; 2: 399–404. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg SN, Gazelle GS, Halpern EF, Rittman WJ, Mueller PR, Rosenthal DI. Radiofrequency tissue ablation: importance of local temperature along the electrode tip exposure in determining lesion shape and size. Acad Radiol 1996; 3: 212–18. doi: 10.1016/s1076-6332(96)80443-0 [DOI] [PubMed] [Google Scholar]
- 19.Park HJ, Lee MW, Song KD, Cha DI, Rhim H, Kang TW, et al. Comparison of therapeutic efficacy and safety of radiofrequency ablation of hepatocellular carcinomas between internally cooled 15-G and 17-G single electrodes. Br J Radiol 2014; 87: 20130534. doi: 10.1259/bjr.20130534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park MJ, Kim YS, Rhim H, Lim HK, Lee MW, Choi D. A comparison of US-guided percutaneous radiofrequency ablation of medium-sized hepatocellular carcinoma with a cluster electrode or a single electrode with a multiple overlapping ablation technique. J Vasc Interv Radiol 2011; 22: 771–9. doi: 10.1016/j.jvir.2011.02.005 [DOI] [PubMed] [Google Scholar]
- 21.Rossi S, Buscarini E, Garbagnati F, Di Stasi M, Quaretti P, Rago M, et al. Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR Am J Roentgenol 1998; 170: 1015–22. doi: 10.2214/ajr.170.4.9530052 [DOI] [PubMed] [Google Scholar]
- 22.Goldberg SN, Solbiati L, Hahn PF, Cosman E, Conrad JE, Fogle R, et al. Large-volume tissue ablation with radio frequency by using a clustered, internally cooled electrode technique: laboratory and clinical experience in liver metastases. Radiology 1998; 209: 371–9. doi: 10.1148/radiology.209.2.9807561 [DOI] [PubMed] [Google Scholar]
- 23.Gazelle GS, Haaga JR, Rowland DY. Effect of needle gauge, level of anticoagulation, and target organ on bleeding associated with aspiration biopsy. Work in progress. Radiology 1992; 183: 509–13. doi: 10.1148/radiology.183.2.1561359 [DOI] [PubMed] [Google Scholar]
- 24.Lubner MG, Brace CL, Hinshaw JL, Lee FT, Jr. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol 2010; 21: S192–203. doi: 10.1016/j.jvir.2010.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]